Abstract
Lentiviruses infect myeloid cells, leading to acute infection followed by persistent/latent infections not cleared by the host immune system. HIV and SIV are lentiviruses that infect CD4+ lymphocytes in addition to myeloid cells in blood and tissues. HIV infection of myeloid cells in brain, lung and heart cause tissue specific diseases that are mostly observed during severe immunosuppression, when the number of circulating CD4+ T cells declines to exceeding low levels. Antiretroviral therapy (ART) controls viral replication but does not successfully eliminate latent virus, which leads to viral rebound once ART is interrupted. HIV latency in CD4+ lymphocytes is the main focus of research and concern when HIV eradication efforts are considered. However, myeloid cells in tissues are long-lived and have not been routinely examined as a potential reservoir. Based on a quantitative viral outgrowth assay (QVOA) designed to evaluate latently infected CD4+ lymphocytes, a similar protocol was developed for the assessment of latently infected myeloid cells in blood and tissues. Using an SIV ART model, it was demonstrated that myeloid cells in blood and brain harbor latent SIV that can be reactivated and produce infectious virus in vitro, demonstrating that myeloid cells have the potential to be an additional latent reservoir of HIV that should be considered during HIV eradication strategies.
Introduction
AIDS emerged as a new disease in 1980 and was shown to be caused by a retrovirus, the human T-cell lymphotrophic virus III (HTVL-III), thought to be similar to human viruses HTLV-I and II (1–4). AIDS pathogenesis included not only immunosuppression but also infection of tissues; in particular the brain, causing encephalitis and dementia in adults and children (5, 6). Soon after the AIDS virus was isolated and molecular clones were constructed to further characterize the virus molecular hybridization studies demonstrated that HTLV-III was actually more closely related to the ungulate lentiviruses than to the human deltaretroviruses HTLV-I and II, and the virus was renamed human immunodeficiency virus (HIV) (7, 8). HIV infection in vivo has many parallels to lentivirus pathogenesis causing not only primary immunodeficiency but also CNS- and lung-specific diseases. In contrast to most lentiviruses, the cellular tropism of HIV included not only macrophage lineage cells but also CD4+ lymphocytes (9). While classic lentiviruses like visna virus do not infect lymphocytes, infection of macrophages does cause lymphocyte activation and lymphocytic proliferation in infected tissues such as brain, lung, and joints (10, 11). Lentivirus infections are characterized by an acute phase followed by suppression of virus replication in blood and tissues that led to a state of undetectable virus in most animals (12). Despite lack of detectable viral RNA, cells from infected, suppressed animals can be activated to produce virus in vitro. This was also an early observation in studies of the ovine (visna virus) and caprine (caprine arthritis-encephalitis virus, CAEV) viruses in which monocytes from infected animals without detectable viral RNA mature in vitro into macrophages with subsequent reactivation and detection of viral cellular RNA and virus in the culture supernatant (13). Viral latency in myeloid lineage cells in lentiviruses in vivo is also a feature of non-primate lentiviruses shared by SIV and HIV.
The pathogenesis of HIV during the early AIDS epidemic and before the development of antiretroviral therapy was characterized by immunodeficiency disease as a result of loss of circulating CD4+ T cells and by subsequent opportunistic infections. CNS neurologic disease accompaniea these infections and infectious virus is detected in the cerebrospinal fluid (CSF) (5, 14, 15). HIV infection in brain of infected adults and children was shown to be responsible for the neurologic disease and was called AIDS Dementia Complex (ADC). This neurologic syndrome, ADC, was the cause of mortality in HIV infected individuals. Although HIV enters the CNS during acute infection, CNS disease manifested mainly during later stages of infection, when individuals were immunosuppressed (16).
Initially, the cause for the late stage development of ADC was not clear, however, later studies of the replication and regulation of HIV in macrophages, the cells infected in brain demonstrated differential regulation of HIV in myeloid cells as compared to lymphocytes (17–19). HIV transcription in macrophages is regulated by the transcription factor c/EBPβ and its isoforms (17–22), in contrast to transcriptional regulation of HIV by NF-kΒ in CD4+ lymphocytes (23, 24). The differential expression of c/EBPβ isoforms is modulated in macrophages by IFNβ (17–19). Presence of this cytokine in brain causes the translation of a dominant negative form of c/EBPβ that down-regulates viral transcription and histone acetylation of the HIV LTR, resulting in transcriptional silencing of HIV in vitro and SIV in vitro and in vivo (20–22, 25, 26). Thus, regulation of HIV transcriptional activation and suppression by IFNβ may be one mechanism for establishing HIV latency in macrophages in tissues. Viral latency is a state of reversibly nonproductive infection of individual cells and provides an important mechanism for viral persistence and escape from immune recognition and drug pressure.
In the era of ART, fully suppressed HIV-infected individuals usually control virus replication in blood controlling viral levels below 50 copies of HIV/ml. The occurrence of systemic immunosuppression and HIV-associated dementia has been greatly diminished by treatment. ART does not eliminate the viral provirus from tissues but suppresses virus that becomes latent, the latent reservoir is recognized as a major barrier to curing HIV-1 infection. HIV research is mainly focused on the suppression of virus replication in CD4+ lymphocytes (CD4+T) and on mechanisms of virus latency and the formation of long-lived CD4+T reservoirs (27). The dramatic decrease in CNS dementia suggests that the infected brain macrophages (microglia and perivascular macrophages) are no longer actively infected during ART. In contrast to the availability of CD4+T cells in blood, however, brain macrophages cannot be directly studied in humans because of the difficulty of analysis in situ. To circumvent this hurdle, HIV studies performed in cerebrospinal fluid (CSF) and brain (post-mortem) of HIV infected individuals on ART demonstrated that HIV is present in brain despite undetectable virus in the plasma (28, 29). The identification of HIV-neurocognitive disorders (HAND) and HIV RNA in the CSF in HIV-infected individuals on suppressive ART further demonstrates that HIV infection persists in brain in either a latent or persistent form (28, 29). SIV-infected macaques on ART regimens, similar to those used in humans, provide the opportunity to study longitudinal progression of AIDS, CNS infection, disease pathogenesis and viral latency of both CD4+T and myeloid cells in blood, CSF, and tissues, including brain.
HIV & SIV Infection in the CNS
Both HIV and SIV infect the CNS as early as the first week after infection, and both viruses are detectable in CSF as well as in the blood of infected individuals during acute, chronic, and late stage disease. Infection of the CNS is caused by entry of infected CD4+ T cells and monocytes trafficking across the blood brain barrier (BBB). HIV and SIV infection is then spread to perivascular macrophages that line the BBB and to microglia, the resident brain macrophages. Microglia are embryonically derived cells that self-renew rather than being replenished from circulating monocytes (30, 31). Infected microglial cells have been identified in HIV-infected humans and SIV-infected non-human primates (32–37) However, the role of microglia infection in long-term HIV latency and persistence is controversial despite the detection of HIV DNA in post-mortem brain of ART-suppressed individuals. In SIV infection, microglia isolated from both viremic and ART suppressed macaques contain SIV DNA and RNA (see below for detailed studies).
New Insights on Macrophage Origin and Phenotypes
Depletion of CD4+ T cells is the hallmark of HIV-1 infection, and most studies of pathogenesis and latency of HIV and SIV have focused on lymphocytes. Nonetheless, macrophages are a natural host cell for lentiviruses (13, 38–40) and multiple lines of evidence point to the importance of macrophages during HIV infection: 1) The accessory protein Vpx (HIV-2) specifically enhances viral replication in macrophages (41, 42), but not in CD4+ T cells. Comparably, Vpr (HIV-1) recruits UNG2 into virions and modulates viral mutation rates in macrophages (43)(2) Many HIV-1 strains replicate efficiently in macrophages, independent of the presence of Vpx (44, 45); 3) AIDS is characterized by dramatic depletion of CD4+ T cells, however, despite depletion of these cells high plasma viral load persists, suggesting that viral replication is occurring in cells other than CD4+ lymphocytes. In the macaque models for SIV infection, experimental depletion of CD4+ T cells results in an increase in viral load and selection in vivo of CD4-independent macrophage-tropic SIV phenotypes (46–48)(4). Damage to both lung and brain (interstitial pneumonia and encephalitis) are directly associates with infections of macrophages (49, 50); 5) Finally, activation of monocytes and macrophages during cART suppression in HIV is associated with higher morbidity (51–53).
In the last several years, advances in myeloid cell biology have shown that every tissue harbors distinct populations of macrophages: those arriving during embryogenesis (both yolk-sac and fetal liver-derived), and post-natal bone marrow-derived blood monocytes (31). A similar classification can be extended to humans based on transcriptomic and phenotypic profiling (30). Most resident tissue mononuclear phagocytes - including Kupffer cells in the liver, Langerhans cells in the skin, microglia in brain, and alveolar and periotoneal macrophages –originate from Myb-independent progenitor cells that migrated directly or indirectly from the yolk sac to their respective tissues during embryogenesis (30, 54). They are predominantly maintained through self-renewal during steady state, independently of adult hematopoiesis (55). These cells have only recently been thoroughly characterized as distinct from monocyte-derived macrophages, and little is known about their in vitro function (54). Resident tissue macrophages, infected with HIV or SIV, have the potential to divide and expand the viral reservoirs in tissues. In addition, HIV and SIV infected macrophages are not efficiently killed by CD8+ T cells unlike infected CD4+ T cells (56, 57). Thus, resident tissue macrophages remain in tissues long-term, are capable of self-renewal, are relatively resistant to the cytopathic effects of HIV infection compared to CD4+ T cells, and may serve as stable viral reservoirs.
Some tissue macrophages are directly derived from blood monocytes, which arise from common monoblasts in bone marrow. Especially during infection or inflammation, circulating monocytes infiltrate tissues via pro-inflammatory mediators, including chemokine gradients, and differentiate into cells with a broad range of functions, depending on the microenvironment (58, 59). Although morphologically similar, macrophages originating from monocytes have distinct transcriptomes, surface markers, and phenotypic profiles from those of embryonic origin (31, 60). Macrophages with the same ontogeny can express different sets of transcripts and may respond differently to pathogens, depending on the tissue location (61).
In addition to distinct ontogenies, in vitro studies have demonstrated that monocyte-derived macrophages (MDM) can dramatically change their phenotype, pattern of gene expression, and functionality under different culture conditions (62). Generally, macrophages matured in CSF-1 are the starting point for many murine and human experiments. Any alteration to these culture conditions will present specialized functional properties, referred to as polarization. Historically, post-differentiated MDMs treated with IFN-γ were defined as M1, or classically activated, and MDMs treated with IL-4 termed M2, or alternatively activated. Current perspective acknowledges a spectrum of polarization, with large shifts in gene expression based on stimuli used in culture, and suggests terminology specific to the activator, such as M(IFN-γ + LPS). MDMs activated by IFN-γ alone or in combination with microbial stimuli (LPS) or cytokines (TNF) express copious amounts of inflammatory and effector molecules (IL-6, IL-2, CCL2, CXCL10, iNOS, and ROS), contribute to the induction and maintenance of TH1 and TH17 responses. They also have enhanced complement- and antibody-mediated phagocytosis with microbicidal capacity (63) Conversely, MDMs exposed to anti-inflammatory stimuli such as IL-4, IL-13, IL-10, TGF-beta, immune complexes, or glucocorticoids are associated with TH2 responses, high levels of Fc receptors (CD16, CD32, CD64), and the resolution of inflammatory responses (64, 65). These phenotypes are likely to be less distinctive in vivo since the microenvironments that macrophages inhabit are exposed to a broad and changing range of signaling molecules. Also, in vitro studies show that macrophages, even after full polarization, can rapidly change phenotypes when exposed to a novel stimulus (66).
In the context of HIV infection, polarization studies demonstrate the antiviral features of M1 MDM (67). This inflammatory phenotype has poor surface expression of CD4 and DC-SIGN, which are important receptors for viral binding. They also inhibit intracellular steps of viral replication due to high levels of APOBEC3A, tetherin, and TRIM22 (68, 69). These cells present a transcription profile (65), which, at least in theory, should support HIV RNA expression (70, 71). Thus, while cell activation is directly related to increased viral transcription in CD4+ T lymphocytes, this is not the case in macrophages. Polarization has been mainly explored in bone marrow-derived MDMs in vitro; while in vivo macrophages are incredibly heterogeneous, and likely exist along a continuum of the M1-M2 spectrum.. During inflammatory and infectious processes, there is a major influx of monocytes into tissues (75, 76), making it difficult to demonstrate that shifts in polarization are occurring in resident cells and not in recently arrived monocytes. Recent studies on brain macrophages show that microglia respond to cytokine stimulation similar to MDMs (77–79). While the concepts described by in vitro studies have been useful in tissue macrophages (72–74), direct translation of these studies has not been comprehensively explored in vivo (80).
SIV Macaque Models
SIV infection in macaques comprehensively reproduces the immunodeficiency symptoms observed in HIV-infected humans, with infection of CD4+T cells and monocytes in blood, and of macrophages in tissues such as lymph nodes, bowel, brain, lung, spleen, and heart (81, 82). Antiretroviral drugs have been shown to fully suppress SIV replication in blood (82) and, in limited studies, CSF (83–85) to levels comparable to those in ART-suppressed HIV-infected individuals. SIV-infected macaques carry latently infected CD4+T cells that harbor replication competent virus, as shown by quantitative viral outgrowth assays (QVOA) (86) and by the rapid rebound of SIV in plasma when ART is discontinued (87). The role of infection and latency in monocytes and tissue macrophages in ART-suppressed macaques has only been recently addressed. This is important to pursue because, in the era of ART and potential HIV cure approaches, fully characterizing all latently infected cells that may contribute to viral rebound after cessation of ART has become a priority. Initial trials of HIV eradication strategies have focused on viral load (VL) in plasma as an indication of HIV reactivation or change in the latent reservoir, although there is evidence from the “Boston Patients” that virus rebound occurred not only in the blood but also in the brain, based on CNS symptoms prior to virus rebound and presence of HIV in CSF (88). However, the mechanisms that drive latency in macrophages remain unclear and, probably, are distinct from those in CD4+ T-cells. Also, new evidence indicates that many latent SIV genomes located in tissues may respond differently to latency reversing agents (LRA) (84).
Several well-characterized SIV macaque models have been used mainly to study the development of AIDS and the pathogenesis of infection using a variety of SIV viral strains and molecular clones. The most commonly used strains are cloned SIVmac239 and SIVmac251 strains, or viruses derived from these strains. Also, there are SIV models focused on the study of infection and disease progression in the CNS (81). Each model uses distinct mechanisms to achieve SIV encephalitis, which includes infecting macaques with naturally occurring neurotropic and immunosuppressive virus swarms, neurotropic virus adapted by in vivo passage of SIV, and non-neurotropic strains in association with CD8+ lymphocyte depletion (81). A recent review has compared these SIV models concluding that all the models include monocyte/macrophage infection and activation, and increased number of macrophages in the brain of macaques that develop encephalitis (81).
This review focuses on studies using an SIV macaque model in which animals are inoculated with a viral strain swarm (SIVdelta/B670) that contains 22 SIV env-defined genotypes and a neurovirulent, molecular clone (SIV/17E) that consistently causes AIDS in 90 days with a high incidence of CNS infection and encephalitis (89–91). This SIV model has been characterized longitudinally, demonstrating that SIV infection in brain occurred in the first week of infection (by 4 d p.i.) and that virus infection in brain was differentially regulated from the periphery (89, 92). Macrophages in brain, including resident microglia and perivascular macrophages are the major target cell in the CNS; SIV and HIV infection of macrophages has been shown to be transcriptionally regulated by C/EBPβ isoforms (17–22), which are regulated by innate immune responses as discussed previously in this review. The regulation of SIV transcription in brain macrophages provides a mechanism for silencing of the viral genome in macrophages and is likely to contribute to mechanism of SIV and HIV latency in tissue resident macrophages, particularly in the CNS.
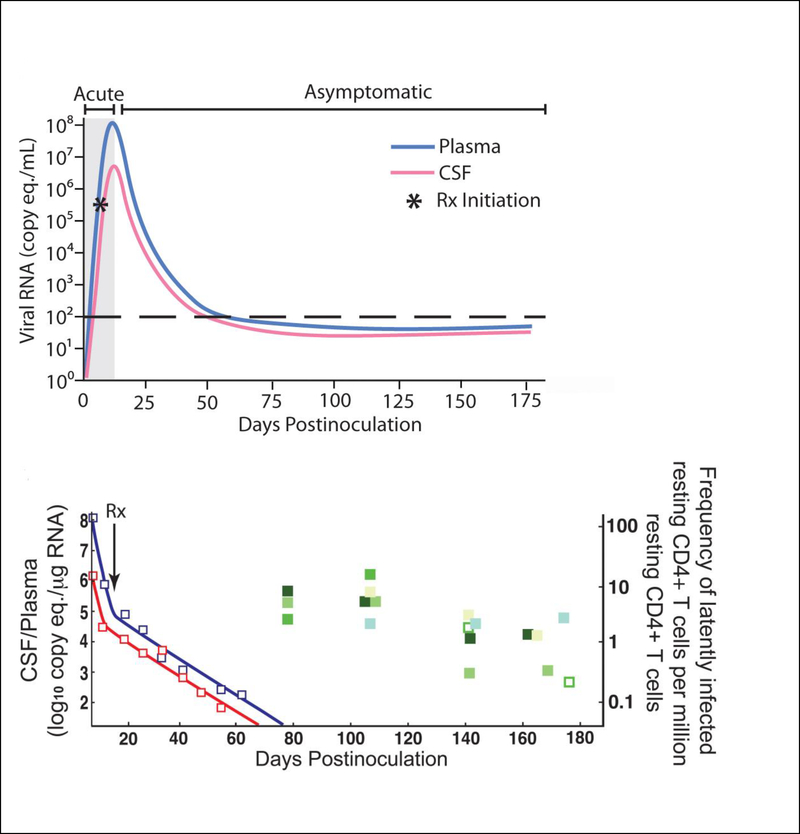
ART regimens in this dual-infection SIV model result in suppression of viral load to undetectable levels in the plasma and CSF (86), There is an extensive literature that addresses the frequency of HIV infection and latency in CD4+T cells in ART suppressed humans, but this same rigorous analysis had not been applied to the ART-suppressed SIV macaque models. Therefore, we developed an SIV rCD4+ QVOA analogous to the HIV quantitative viral outgrowth assay (QVOA) (86) and used it to measure the frequency of rCD4+ cells harboring replication competent SIV latent genomes, not only plasma but also in spleen and multiple lymph nodes and spleen (FIGURE 1) (86). These studies demonstrated that the frequencies of latently infected rCD4+ cells in blood, lymph nodes, and spleen are very similar to those in ART-suppressed HIV-infected individuals. In another study using the same macaque model, it was shown that SIV DNA persists in the brain despite undetectable levels of SIV cellular RNA in the CNS (83).
Figure 1.
(a) Viral RNA levels in plasma and CSF increase rapidly during the first 7–10 days prior to ART treatment. Within a few days after initiation of ART, plasma and CSF viral load decline. By approximately 60 days p.i., plasma and CSF viral load have declined to below the level of detection (<50 copy eq./ml) and viral loads remain low during ART. (c) The decline in plasma and CSF viral RNA occurred in two phases: an initial short-term rapid decline followed by a longer term slower decline similar to the two-phase decline seen in the plasma of HIV-infected individuals on HAART. At 80 days p.i., there were 8–10 latently infected resting CD4+ T cells per million resting CD4+ T cells in the blood. These numbers declined gradually to ~ one latently infected resting CD4+ T cells per million by 175 days p.i. Abs., absolute; CSF, cerebrospinal fluid; p.i., post-inoculation; Rx, therapy; vRNA, viral RNA., plasma vRNA;, CSF vRNA (severe/moderate encephalitis);, CSF vRNA (no/mild encephalitis);, Abs. CD4+ cell counts in blood.
Evidence for a Functional Viral Reservoir in Brain Macrophages
Our well-characterized and consistent macaque model for AIDS and CNS disease was also used to evaluate the contribution of brain macrophages in SIV latency and reactivation during ART (84). In this study, SIV-infected macaques were fully suppressed with ART for over one year (<30 copies of SIV RNA per ml of plasma). To induce in vivo activation of latent reservoirs we tested a combination of two synergistic LRAs: the protein kinase C (PKC) activator ingenol-B and the histone deacetylase (HDAC) inhibitor vorinostat. We had previously shown that the ingenol-B reactivated HIV-1 genomes in two different in vitro HIV-1 latency models as well as in CD4+T isolated from HIV-infected individuals (93). Our results show that LRA administration led to an increase in VL in cerebrospinal fluid (CSF) in one of two SIV-infected macaques. The increase in virus in the CSF was 10-fold higher than virus rebound in the plasma and phylogenetic analyses of viruses demonstrated distinct genotypes in the plasma and CSF, suggesting compartmentalization of virus in the brain. These findings suggest that the CNS harbors latent SIV genomes despite long-term ART suppression and that these reservoirs can be activated with LRAs. Although a small number of animals were assessed, this study is the first in vivo demonstration that the brain represents a consequential viral reservoir (84).
Relative Levels of Infection of CD4+ T Lymphocytes and Macrophages in SIV infected Macaques
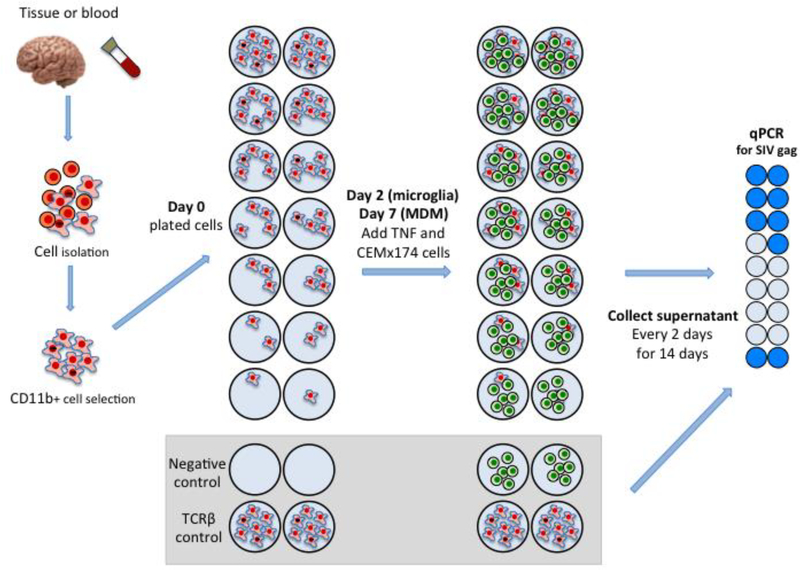
The frequency of HIV or SIV infection of macrophages in tissues has been examined previously in a number of studies by measuring viral DNA in cells isolated from tissues (94). However, this approach overestimates the number of productively infected CD4+ T cells due to the presence of a large proportion of defective proviruses in vivo (95). Thus, we developed a quantitative viral outgrowth assay similar to the CD4+ T cell assay for HIV and for SIV to estimate the size of the potential latent reservoir of monocytes and macrophages (MΦ-QVOA) (94) (Figure 2 Macrophage QVOA). To validate this assay, we first examined the number of macrophages and CD4+ T cells in in blood and tissues of viremic SIV-infected macaques (94). To eliminate the potential contribution of CD4+ T cells to the quantitation of infected macrophages, we also assessed the number of CD3+ T cells in each assay by measuring TCRβ RNA.
Figure 2.
MΦ-QVOA. Monocytes from blood and macrophages from brain were collected from SIV-infected animals and purified by CD11b-specific bead selection. Macrophages expressing CD11b were plated in serial dilutions in triplicate wells. Cells were cultured with zidovudine (AZT) and darunavir (DRV). Nonadherent cells and the antiretrovirals were removed prior to activation with TNF and co-culture with CEMx174 cells (85, 94).
The MΦ-QVOA utilized the expression of the integrin CD11b (96) on monocytes and tissue macrophages and separated these myeloid cells from other cell types by sorting with CD11b Miltenyi magnetic beads (94). Like the CD4+ T cell QVOA, the MΦ-QVOA involved a serial dilution of selected cells. Antiretroviral drugs were added to the culture to prevent virus spread from any CD4+ T cells that might be in the culture during the first couple of days, while macrophage differentiated and matured in vitro. Unlike T cells, macrophages do not divide exponentially when activated in culture and strongly adhere to culture plates when grown in vitro. Cell supernatants were collected from the MΦ-QVOA wells after 12 days of cultivation (Figure 2). Viral RNA was isolated from replicate wells and quantitated individually by qRT-PCR. The frequency of infectious virus per million (IUPM) was calculated using limiting dilution statistical analyses (97).
Quantitating macrophages with the QVOA from SIV-chronically and late-stage infected macaques demonstrated that the number of productively infected macrophages in a given tissue was surprisingly similar from macaque to macaque, whereas the number of productively infected macrophages varied widely across different tissues from the same SIV-infected macaque. The highest number of infected macrophages (424 IUPM) was measured in spleen demonstrating that splenic macrophages are highly susceptible to SIV infection and harbor high levels of productive genomes (Figure 3 IUPM CD4+ T cells, monocytes, macrophages). This suggests a role for tissue microenvironments in mediating virus infection of macrophages, since populations of macrophages that reside in each tissue may be differentially susceptible to SIV/HIV infection based on the cytokine profiles of the organs (98).
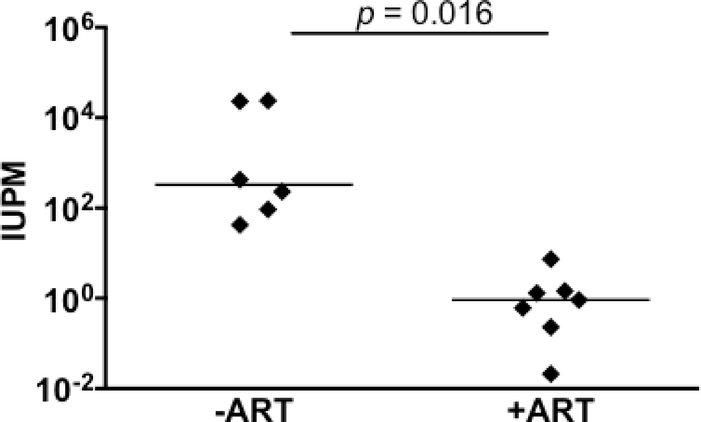
Figure 3.
Quantitation of latently infected brain macrophages in ART-treated macaques by MΦ-QVOA. Quantitation of infected brain macrophages from ART-treated macaques (85). Comparison between the numbers of SIV-infected brain macrophages isolated from animals that were not given ART (-ART) and the numbers isolated from animals that were treated with ART and with viral suppression <10 copies SIV RNA/ml plasma. The horizontal black line represents the median IUPM values. The MΦ QVOA results from SIV-infected animals with and without ART have been reported (85, 94). Significance was determined by Mann-Whitney nonparametric t test; a P of <0.05 was considered significant.
The number of infected brain macrophages, including both microglia and perivascular macrophages, were quantitated by MΦ-QVOA in SIV infected macaques during both the chronic and late stage disease. Brain sections of these animals were examined for pathological changes associated with SIV encephalitis and were scored as none, mild, moderate, or severe disease. It was found that the brain of animals with mild to severe CNS disease contained the next highest level of infected cells (median 231 IUPM) compared to spleen. The two macaques with the most productively infected cells brain macrophages s(Pm3 and Pm4 with 24,000 IUPM) had severe encephalitis and high levels of viral RNA in brain. The macaques without CNS disease had undetectable numbers of infected microglia/macrophages and little or no detectable viral RNA in the brain. Thus, the number of productively infected cells in the brain correlated with the severity of disease and the level of viral RNA detected in brain by qPCR. This study provided the first estimate of productively infected CD4+ T cells and myeloid cells in SIV-infected tissues in vivo.
Quantitation of Latently Infected Brain Macrophages in ART Suppressed SIV Macaques
ART has dramatically reduced the severe forms of HAND, but milder forms of neurologic impairment are still observed in HIV-infected individuals virally suppressed on ART. HAND is thought to be a result of chronic central nervous system (CNS) inflammation in the brain (99–102). It is unclear whether inflammation is caused by incomplete penetrance of antiretroviral drugs into the CNS or the persistence of virus in brain macrophages (BrMΦ) in a latent state that reactivate causing sporadic inflammatory responses (103). Indeed, some HIV-infected individuals on ART have no detectable virus in the plasma but have measurable levels of HIV RNA in the CSF (104, 105). Also, HIV was detected after rebound in the CSF of the Boston patients, who had undetectable plasma HIV during ART interruption for several months (88). There is a continuing debate on the sources of virus in the CSF and the cause of the chronic inflammation in brain that leads to HAND.
Using our SIV macaque model with SIV-infected macaques suppressed with four antiretroviral drugs for 100–500 days, we evaluated whether infected cells persist in brain despite ART. SIV-infected pigtailed macaques were virally suppressed with ART, and plasma and CSF VL were analyzed longitudinally to demonstrate viral suppression in the peripheral blood and the CNS. To assess whether virus persisted in brain macrophages (BrMΦ) in these long-term ART suppressed macaques, we used MΦ-QVOA, qPCR, and in situ hybridization (ISH) to measure the frequency of infected cells and levels of viral RNA and DNA in brain. Viral RNA in brain tissue of suppressed macaques was undetectable, although viral DNA was observed in all animals. The MΦ-QVOA demonstrated that the majority of suppressed animals contained latently infected BrMΦ. We also showed that virus produced in the MΦ-QVOAs was replication competent, suggesting that latently infected BrMΦ are capable of re-establishing productive infection upon ART interruption. This study provides the first confirmation of replication-competent SIV in BrMΦ of ART-suppressed macaques and suggests that the highly debated question of viral latency in macrophages, at least in brain, has been addressed in SIV-infected macaques treated with ART.
In this study, we identified latently infected BrMΦ in brain samples containing fewer than 10 copies of SIV DNA per million cells. In animals suppressed for more than 500 days, the number of infected macrophages measured in the Mø-QVOA ranged from 3.6 to 15 in a hundred million cells, supporting the low level of DNA quantitated by qPCR. Thus, the quantitation of SIV DNA or RNA by PCR in brain tissue does not fully reflect the size of the latent functional reservoir, which is the main target in eradication strategies. (FIGURE 3 Brain Macrophage IUPM Frequency)
Most animals in the study harbored latently infected macrophages in regions of the brain that contained no detectable viral RNA. After 1.7 years of viral suppression, three macaques in the study showed no viral RNA in basal ganglia and parietal cortex. Nevertheless, all three macaques had replication competent virus produced in the isolated BrMΦ. Of note, we detected viral RNA by ISH in the brain of one of the macaques in the study treated with a LRA. However, the RNA was detected the occipital cortex, a brain section not used for the BrMΦ QVOA. These results corroborate findings showing that SIV, and potentially HIV, infection in brain is highly focal (84) and can provide variable results depending on the brain region analyzed for each specific assay.
Also, the results from the MΦ-QVOA showing that a small number of replication competent viruses are sporadically released in some latent BrMΦ indicate that parameters we used to define a positive QVOA well supernatants with > 50 SIV RNA copies/mL) underestimate the number of latently infected cells that produce replication competent virus, at least in macrophages. Indeed, viruses collected from most QVOA assay supernatants were able to spread in healthy PBMC.
The demonstration that there is latent replication-competent virus in SIV-infected ART suppressed macaque brain provides a mechanism for the ongoing macrophage activation observed both in the macaques and HIV individuals suppressed on ART. Recent studies have suggested that, while virus does not spread during ART suppression, there is ongoing stochastic activation of virus genomes in latently infected cells (106, 107). Reactivation of virus without spread in the macrophage is likely to induce innate immune responses and cellular activation. Thus, productively infected latent macrophages in brain provide a mechanism for the ongoing inflammation of HIV in a fully suppressed individual. Also, it has been recently demonstrated that defective provirus expressed in rCD4s could be recognized by adaptive immune responses, shaping the proviral landscape (95). It is possible that similar responses might happen with viral proteins generated from defective proviruses in BrMΦ.
Conclusions
The presence of a long-term functional reservoir of SIV in brain macrophages that parallels the biologic and pathologic features of infected individuals with HIV encephalitis suggests that the HIV in brain may be a formidable barrier to strategies to decrease or eliminate latent reservoirs. Further, the presence of low levels of viral DNA in brain of ART-suppressed macaques can contribute to virus spread in brain and potentially in the periphery during cessation of ART or eradication treatments. While the brain is protected by the blood brain barrier and eradication approaches may not penetrate the brain, immune activation in the periphery could potentially activate virus in the CNS. On the other hand, the lack of CNS penetrance of such eradication therapies would potentially leave the CNS functional reservoir intact and undermine virus eradication. Strategies that include activation of virus in brain may have the effect of increasing inflammation and neuronal toxicity due to increased macrophage activation and production of cytokines, as we observed in a suppressed macaque treated with two cycles of LRAs (84). Our studies demonstrating the presence of a functional latent reservoir in brain macrophages have major implications for SIV eradication studies used to model treatment for HIV individuals. Examining recrudescence of virus in plasma but not CSF may overlook a source of virus that significantly contributes to the virus rebound.
Acknowledgements
These studies were funded by NIH awards R01NS089482, R01NS077869, P40OD0131117, R01NS055651, R56AI118753, R01AI127142, P01MH070306, P01AI131306, and the Johns Hopkins University Center for AIDS Research P30AI094189.
Anti-retroviral compounds for these studies were kindly donated by Gilead, ViiV Healthcare, Bristol-Meyers Squibb, Merck, Abbvie,
Janssen, and Roche. These studies were supported by the excellent technical staff in the Retrovirus Lab at Johns Hopkins.
References
- 1.Masur H, Michelis MA, Greene JB, Onorato I, Stouwe RA, Holzman RS, Wormser G, Brettman L, Lange M, Murray HW, Cunningham-Rundles S. 1981. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med 305:1431–1438. [DOI] [PubMed] [Google Scholar]
- 2.Gottlieb MS, Schroff R, Schanker HM, Weisman JD, Fan PT, Wolf RA, Saxon A. 1981. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med 305:1425–1431. [DOI] [PubMed] [Google Scholar]
- 3.Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vezinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. 1983. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220:868–871. [DOI] [PubMed] [Google Scholar]
- 4.Popovic M, Sarngadharan MG, Read E, Gallo RC. 1984. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224:497–500. [DOI] [PubMed] [Google Scholar]
- 5.Shaw GM, Harper ME, Hahn BH, Epstein LG, Gajdusek DC, Price RW, Navia BA, Petito CK, O’Hara CJ, Groopman JE, et al. 1985. HTLV-III infection in brains of children and adults with AIDS encephalopathy. Science 227:177–182. [DOI] [PubMed] [Google Scholar]
- 6.Epstein LG, Sharer LR, Cho ES, Myenhofer M, Navia B, Price RW. 1984. HTLV-III/LAV-like retrovirus particles in the brains of patients with AIDS encephalopathy. AIDS Res 1:447–454. [DOI] [PubMed] [Google Scholar]
- 7.Gonda MA, Braun MJ, Clements JE, Pyper JM, Wong-Staal F, Gallo RC, Gilden RV. 1986. Human T-cell lymphotropic virus type III shares sequence homology with a family of pathogenic lentiviruses. Proc Natl Acad Sci U S A 83:4007–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonda MA, Wong-Staal F, Gallo RC, Clements JE, Narayan O, Gilden RV. 1985. Sequence homology and morphologic similarity of HTLV-III and visna virus, a pathogenic lentivirus. Science 227:173–177. [DOI] [PubMed] [Google Scholar]
- 9.Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. 1986. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233:215–219. [DOI] [PubMed] [Google Scholar]
- 10.Craig LE, Sheffer D, Meyer AL, Hauer D, Lechner F, Peterhans E, Adams RJ, Clements JE, Narayan O, Zink MC. 1997. Pathogenesis of ovine lentiviral encephalitis: derivation of a neurovirulent strain by in vivo passage. J Neurovirol 3:417–427. [DOI] [PubMed] [Google Scholar]
- 11.Zink MC, Narayan O, Kennedy PG, Clements JE. 1987. Pathogenesis of visna/maedi and caprine arthritis-encephalitis: new leads on the mechanism of restricted virus replication and persistent inflammation. Vet Immunol Immunopathol 15:167–180. [DOI] [PubMed] [Google Scholar]
- 12.Gendelman HE, Narayan O, Molineaux S, Clements JE, Ghotbi Z. 1985. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci U S A 82:7086–7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narayan O, Kennedy-Stoskopf S, Sheffer D, Griffin DE, Clements JE. 1983. Activation of caprine arthritis-encephalitis virus expression during maturation of monocytes to macrophages. Infect Immun 41:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho DD, Rota TR, Schooley RT, Kaplan JC, Allan JD, Groopman JE, Resnick L, Felsenstein D, Andrews CA, Hirsch MS. 1985. Isolation of HTLV-III from cerebrospinal fluid and neural tissues of patients with neurologic syndromes related to the acquired immunodeficiency syndrome. N Engl J Med 313:1493–1497. [DOI] [PubMed] [Google Scholar]
- 15.Chiodi F, Albert J, Olausson E, Norkrans G, Hagberg L, Sonnerborg A, Asjo B, Fenyo EM. 1988. Isolation frequency of human immunodeficiency virus from cerebrospinal fluid and blood of patients with varying severity of HIV infection. AIDS Res Hum Retroviruses 4:351–358. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy PG. 1988. Neurological complications of human immunodeficiency virus infection. Postgrad Med J 64:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honda Y, Rogers L, Nakata K, Zhao BY, Pine R, Nakai Y, Kurosu K, Rom WN, Weiden M. 1998. Type I interferon induces inhibitory 16-kD CCAAT/ enhancer binding protein (C/EBP)beta, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication. J Exp Med 188:1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiden M, Tanaka N, Qiao Y, Zhao BY, Honda Y, Nakata K, Canova A, Levy DE, Rom WN, Pine R. 2000. Differentiation of monocytes to macrophages switches the Mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and CCAAT/enhancer binding protein beta expression. J Immunol 165:2028–2039. [DOI] [PubMed] [Google Scholar]
- 19.Descombes P, Schibler U. 1991. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 67:569–579. [DOI] [PubMed] [Google Scholar]
- 20.Henderson AJ, Zou X, Calame KL. 1995. C/EBP proteins activate transcription from the human immunodeficiency virus type 1 long terminal repeat in macrophages/monocytes. J Virol 69:5337–5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson AJ, Connor RI, Calame KL. 1996. C/EBP activators are required for HIV-1 replication and proviral induction in monocytic cell lines. Immunity 5:91–101. [DOI] [PubMed] [Google Scholar]
- 22.Henderson AJ, Calame KL. 1997. CCAAT/enhancer binding protein (C/EBP) sites are required for HIV-1 replication in primary macrophages but not CD4(+) T cells. Proc Natl Acad Sci U S A 94:8714–8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinoshita S, Su L, Amano M, Timmerman LA, Kaneshima H, Nolan GP. 1997. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity 6:235–244. [DOI] [PubMed] [Google Scholar]
- 24.Tong-Starksen SE, Luciw PA, Peterlin BM. 1987. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc Natl Acad Sci U S A 84:6845–6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barber SA, Gama L, Dudaronek JM, Voelker T, Tarwater PM, Clements JE. 2006. Mechanism for the establishment of transcriptional HIV latency in the brain in a simian immunodeficiency virus-macaque model. J Infect Dis 193:963–970. [DOI] [PubMed] [Google Scholar]
- 26.Barber SA, Gama L, Li M, Voelker T, Anderson JE, Zink MC, Tarwater PM, Carruth LM, Clements JE. 2006. Longitudinal analysis of simian immunodeficiency virus (SIV) replication in the lungs: compartmentalized regulation of SIV. J Infect Dis 194:931–938. [DOI] [PubMed] [Google Scholar]
- 27.Pierson T, McArthur J, Siliciano RF. 2000. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu Rev Immunol 18:665–708. [DOI] [PubMed] [Google Scholar]
- 28.Spudich SS, Nilsson AC, Lollo ND, Liegler TJ, Petropoulos CJ, Deeks SG, Paxinos EE, Price RW. 2005. Cerebrospinal fluid HIV infection and pleocytosis: relation to systemic infection and antiretroviral treatment. BMC Infect Dis 5:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson AM, Munoz-Moreno JA, McClernon DR, Ellis RJ, Cookson D, Clifford DB, Collier AC, Gelman BB, Marra CM, McArthur JC, McCutchan JA, Morgello S, Sacktor N, Simpson DM, Franklin DR, Heaton RK, Grant I, Letendre SL, Group C. 2017. Prevalence and Correlates of Persistent HIV-1 RNA in Cerebrospinal Fluid During Antiretroviral Therapy. J Infect Dis 215:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. 2014. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 14:571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. 2012. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336:86–90. [DOI] [PubMed] [Google Scholar]
- 32.Cosenza MA, Zhao ML, Si Q, Lee SC. 2002. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol 12:442–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou L, Rua R, Ng T, Vongrad V, Ho YS, Geczy C, Hsu K, Brew BJ, Saksena NK. 2009. Evidence for predilection of macrophage infiltration patterns in the deeper midline and mesial temporal structures of the brain uniquely in patients with HIV-associated dementia. BMC Infect Dis 9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuen-Jacob E, Arendt G, Wendtland B, Jacob B, Schneeweis M, Wechsler W. 1993. Frequency and topographical distribution of CD68-positive macrophages and HIV-1 core proteins in HIV-associated brain lesions. Clin Neuropathol 12:315–324. [PubMed] [Google Scholar]
- 35.Lamers SL, Gray RR, Salemi M, Huysentruyt LC, McGrath MS. 2011. HIV-1 phylogenetic analysis shows HIV-1 transits through the meninges to brain and peripheral tissues. Infect Genet Evol 11:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brinkmann R, Schwinn A, Muller J, Stahl-Hennig C, Coulibaly C, Hunsmann G, Czub S, Rethwilm A, Dorries R, ter Meulen V. 1993. In vitro and in vivo infection of rhesus monkey microglial cells by simian immunodeficiency virus. Virology 195:561–568. [DOI] [PubMed] [Google Scholar]
- 37.Clements JE, Babas T, Mankowski JL, Suryanarayana K, Piatak M Jr., Tarwater PM, Lifson JD, MC Zink. 2002. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J Infect Dis 186:905–913. [DOI] [PubMed] [Google Scholar]
- 38.Gendelman HE, Moench TR, Narayan O, Griffin DE, Clements JE. 1985. A double labeling technique for performing immunocytochemistry and in situ hybridization in virus infected cell cultures and tissues. J Virol Methods 11:93–103. [DOI] [PubMed] [Google Scholar]
- 39.Peluso R, Haase A, Stowring L, Edwards M, Ventura P. 1985. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology 147:231–236. [DOI] [PubMed] [Google Scholar]
- 40.Gendelman HE, Narayan O, Kennedy-Stoskopf S, Kennedy PG, Ghotbi Z, Clements JE, Stanley J, Pezeshkpour G. 1986. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol 58:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westmoreland SV, Converse AP, Hrecka K, Hurley M, Knight H, Piatak M, Lifson J, Mansfield KG, Skowronski J, Desrosiers RC. 2014. SIV vpx is essential for macrophage infection but not for development of AIDS. PLoS One 9:e84463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharova N, Wu Y, Zhu X, Stranska R, Kaushik R, Sharkey M, Stevenson M. 2008. Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction. PLoS Pathog 4:e1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen R, Le Rouzic E, Kearney JA, Mansky LM, Benichou S. 2004. Vpr-mediated incorporation of UNG2 into HIV-1 particles is required to modulate the virus mutation rate and for replication in macrophages. J Biol Chem 279:28419–28425. [DOI] [PubMed] [Google Scholar]
- 44.Gorry PR, Churchill M, Crowe SM, Cunningham AL, Gabuzda D. 2005. Pathogenesis of macrophage tropic HIV-1. Curr HIV Res 3:53–60. [DOI] [PubMed] [Google Scholar]
- 45.Karita E, Nkengasong JN, Willems B, Vanham G, Fransen K, Heyndrickx L, Janssens W, Piot P, van der Groen G. 1997. Macrophage-tropism of HIV-1 isolates of different genetic subtypes. AIDS 11:1303–1304. [DOI] [PubMed] [Google Scholar]
- 46.Ortiz AM, Klatt NR, Li B, Yi Y, Tabb B, Hao XP, Sternberg L, Lawson B, Carnathan PM, Cramer EM, Engram JC, Little DM, Ryzhova E, Gonzalez-Scarano F, Paiardini M, Ansari AA, Ratcliffe S, Else JG, Brenchley JM, Collman RG, Estes JD, Derdeyn CA, Silvestri G. 2011. Depletion of CD4(+) T cells abrogates post-peak decline of viremia in SIV-infected rhesus macaques. J Clin Invest 121:4433–4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Francella N, Elliott ST, Yi Y, Gwyn SE, Ortiz AM, Li B, Silvestri G, Paiardini M, Derdeyn CA, Collman RG. 2013. Decreased plasticity of coreceptor use by CD4-independent SIV Envs that emerge in vivo. Retrovirology 10:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Igarashi T, Imamichi H, Brown CR, Hirsch VM, Martin MA. 2003. The emergence and characterization of macrophage-tropic SIV/HIV chimeric viruses (SHIVs) present in CD4+ T cell-depleted rhesus monkeys. J Leukoc Biol 74:772–780. [DOI] [PubMed] [Google Scholar]
- 49.Mankowski JL, Carter DL, Spelman JP, Nealen ML, Maughan KR, Kirstein LM, Didier PJ, Adams RJ, Murphey-Corb M, Zink MC. 1998. Pathogenesis of simian immunodeficiency virus pneumonia: an immunopathological response to virus. Am J Pathol 153:1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mankowski JL, Flaherty MT, Spelman JP, Hauer DA, Didier PJ, Amedee AM, Murphey-Corb M, Kirstein LM, Munoz A, Clements JE, Zink MC. 1997. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J Virol 71:6055–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hearps AC, Martin GE, Angelovich TA, Cheng WJ, Maisa A, Landay AL, Jaworowski A, Crowe SM. 2012. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell 11:867–875. [DOI] [PubMed] [Google Scholar]
- 52.McArthur JC, Steiner J, Sacktor N, Nath A. 2010. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann Neurol 67:699–714. [DOI] [PubMed] [Google Scholar]
- 53.Burdo TH, Lackner A, Williams KC. 2013. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev 254:102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. 2013. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. 2013. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 38:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rainho JN, Martins MA, Cunyat F, Watkins IT, Watkins DI, Stevenson M. 2015. Nef Is Dispensable for Resistance of Simian Immunodeficiency Virus-Infected Macrophages to CD8+ T Cell Killing. J Virol 89:10625–10636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vojnov L, Martins MA, Bean AT, Veloso de Santana MG, Sacha JB, Wilson NA, Bonaldo MC, Galler R, Stevenson M, Watkins DI. 2012. The majority of freshly sorted simian immunodeficiency virus (SIV)-specific CD8(+) T cells cannot suppress viral replication in SIV-infected macrophages. J Virol 86:4682–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, Pollet E, Ardouin L, Luche H, Sanchez C, Dalod M, Malissen B, Henri S. 2013. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39:925–938. [DOI] [PubMed] [Google Scholar]
- 59.Gordon S, Taylor PR. 2005. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964. [DOI] [PubMed] [Google Scholar]
- 60.Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ, Immunological Genome C. 2012. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol 13:1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okabe Y, Medzhitov R. 2014. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 157:832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gordon S 2003. Alternative activation of macrophages. Nat Rev Immunol 3:23–35. [DOI] [PubMed] [Google Scholar]
- 63.Anonymous. !!! INVALID CITATION !!! {Murray, 2011 #39;Martinez, 2014 #40}.
- 64.Martinez FO, Gordon S. 2014. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez FO, Gordon S, Locati M, Mantovani A. 2006. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177:7303–7311. [DOI] [PubMed] [Google Scholar]
- 66.Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA. 2013. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio 4:e00264–00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alfano M, Graziano F, Genovese L, Poli G. 2013. Macrophage polarization at the crossroad between HIV-1 infection and cancer development. Arterioscler Thromb Vasc Biol 33:1145–1152. [DOI] [PubMed] [Google Scholar]
- 68.Cobos Jimenez V, Booiman T, de Taeye SW, van Dort KA, Rits MA, Hamann J, Kootstra NA. 2012. Differential expression of HIV-1 interfering factors in monocyte-derived macrophages stimulated with polarizing cytokines or interferons. Sci Rep 2:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cassetta L, Kajaste-Rudnitski A, Coradin T, Saba E, Della Chiara G, Barbagallo M, Graziano F, Alfano M, Cassol E, Vicenzi E, Poli G. 2013. M1 polarization of human monocyte-derived macrophages restricts pre and postintegration steps of HIV-1 replication. AIDS 27:1847–1856. [DOI] [PubMed] [Google Scholar]
- 70.Sirois M, Robitaille L, Allary R, Shah M, Woelk CH, Estaquier J, Corbeil J. 2011. TRAF6 and IRF7 control HIV replication in macrophages. PLoS One 6:e28125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sgarbanti M, Remoli AL, Marsili G, Ridolfi B, Borsetti A, Perrotti E, Orsatti R, Ilari R, Sernicola L, Stellacci E, Ensoli B, Battistini A. 2008. IRF-1 is required for full NF-kappaB transcriptional activity at the human immunodeficiency virus type 1 long terminal repeat enhancer. J Virol 82:3632–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K. 2013. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis 4:e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, Pecker F, Tran A, Gual P, Mallat A, Lotersztajn S, Pavoine C. 2014. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology 59:130–142. [DOI] [PubMed] [Google Scholar]
- 74.Redente EF, Higgins DM, Dwyer-Nield LD, Orme IM, Gonzalez-Juarrero M, Malkinson AM. 2010. Differential polarization of alveolar macrophages and bone marrow-derived monocytes following chemically and pathogen-induced chronic lung inflammation. J Leukoc Biol 88:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai Y, Sugimoto C, Arainga M, Alvarez X, Didier ES, Kuroda MJ. 2014. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: implications for understanding lung disease in humans. J Immunol 192:2821–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ginhoux F, Jung S. 2014. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 14:392–404. [DOI] [PubMed] [Google Scholar]
- 77.Orihuela R, McPherson CA, Harry GJ. 2016. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173:649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin Y, Sun X, Shao X, Cheng C, Feng J, Sun W, Gu D, Liu W, Xu F, Duan Y. 2015. Macrophage-Microglia Networks Drive M1 Microglia Polarization After Mycobacterium Infection. Inflammation 38:1609–1616. [DOI] [PubMed] [Google Scholar]
- 79.Xu H, Wang Z, Li J, Wu H, Peng Y, Fan L, Chen J, Gu C, Yan F, Wang L, Chen G. 2017. The Polarization States of Microglia in TBI: A New Paradigm for Pharmacological Intervention. Neural Plast 2017:5405104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cherry JD, Olschowka JA, O’Banion MK. 2014. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams K, Lackner A, Mallard J. 2016. Non-human primate models of SIV infection and CNS neuropathology. Curr Opin Virol 19:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kumar N, Chahroudi A, Silvestri G. 2016. Animal models to achieve an HIV cure. Curr Opin HIV AIDS 11:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zink MC, Brice AK, Kelly KM, Queen SE, Gama L, Li M, Adams RJ, Bartizal C, Varrone J, Rabi SA, Graham DR, Tarwater PM, Mankowski JL, Clements JE. 2010. Simian immunodeficiency virus-infected macaques treated with highly active antiretroviral therapy have reduced central nervous system viral replication and inflammation but persistence of viral DNA. J Infect Dis 202:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gama L, Abreu CM, Shirk EN, Price SL, Li M, Laird GM, Pate KA, Wietgrefe SW, O’Connor SL, Pianowski L, Haase AT, Van Lint C, Siliciano RF, Clements JE, Group L-SS. 2017. Reactivation of simian immunodeficiency virus reservoirs in the brain of virally suppressed macaques. AIDS 31:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Avalos CR, Abreu CM, Queen SE, Li M, Price S, Shirk EN, Engle EL, Forsyth E, Bullock BT, Mac Gabhann F, Wietgrefe SW, Haase AT, Zink MC, Mankowski JL, Clements JE, Gama L. 2017. Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: a Functional Latent Reservoir. MBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dinoso JB, Rabi SA, Blankson JN, Gama L, Mankowski JL, Siliciano RF, Zink MC, Clements JE. 2009. A simian immunodeficiency virus-infected macaque model to study viral reservoirs that persist during highly active antiretroviral therapy. J Virol 83:9247–9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, Smith JY, Brinkman AL, Peter LE, Mathew SI, Smith KM, Borducchi EN, Rosenbloom DI, Lewis MG, Hattersley J, Li B, Hesselgesser J, Geleziunas R, Robb ML, Kim JH, Michael NL, Barouch DH. 2014. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature 512:74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Henrich TJ, Hanhauser E, Marty FM, Sirignano MN, Keating S, Lee TH, Robles YP, Davis BT, Li JZ, Heisey A, Hill AL, Busch MP, Armand P, Soiffer RJ, Altfeld M, Kuritzkes DR. 2014. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med 161:319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clements JE, Babas T, Mankowski JL, Suryanarayana K, Piatak M Jr., Tarwater PM, Lifson JD, Zink MC. 2002. The central nervous system as a reservoir for simian immunodeficiency virus (SIV): steady-state levels of SIV DNA in brain from acute through asymptomatic infection. J Infect Dis 186:905–913. [DOI] [PubMed] [Google Scholar]
- 90.Zink MC, Clements JE. 2002. A novel simian immunodeficiency virus model that provides insight into mechanisms of human immunodeficiency virus central nervous system disease. J Neurovirol 8 Suppl 2:42–48. [DOI] [PubMed] [Google Scholar]
- 91.Zink MC, Suryanarayana K, Mankowski JL, Shen A, Piatak M, Jr., Spelman JP, Carter DL, Adams RJ, Lifson JD, Clements JE. 1999. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol 73:10480–10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Witwer KW, Gama L, Li M, Bartizal CM, Queen SE, Varrone JJ, Brice AK, Graham DR, Tarwater PM, Mankowski JL, Zink MC, Clements JE. 2009. Coordinated regulation of SIV replication and immune responses in the CNS. PLoS One 4:e8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abreu CM, Price SL, Shirk EN, Cunha RD, Pianowski LF, Clements JE, Tanuri A, Gama L. 2014. Dual role of novel ingenol derivatives from Euphorbia tirucalli in HIV replication: inhibition of de novo infection and activation of viral LTR. PLoS One 9:e97257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Avalos CR, Price SL, Forsyth ER, Pin JN, Shirk EN, Bullock BT, Queen SE, Li M, Gellerup D, O’Connor SL, Zink MC, Mankowski JL, Gama L, Clements JE. 2016. Quantitation of Productively Infected Monocytes and Macrophages of Simian Immunodeficiency Virus-Infected Macaques. J Virol 90:5643–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pollack RA, Jones RB, Pertea M, Bruner KM, Martin AR, Thomas AS, Capoferri AA, Beg SA, Huang SH, Karandish S, Hao H, Halper-Stromberg E, Yong PC, Kovacs C, Benko E, Siliciano RF, Ho YC. 2017. Defective HIV-1 Proviruses Are Expressed and Can Be Recognized by Cytotoxic T Lymphocytes, which Shape the Proviral Landscape. Cell Host Microbe 21:494–506 e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arnaout MA. 1990. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood 75:1037–1050. [PubMed] [Google Scholar]
- 97.Rosenbloom DI, Elliott O, Hill AL, Henrich TJ, Siliciano JM, Siliciano RF. 2015. Designing and Interpreting Limiting Dilution Assays: General Principles and Applications to the Latent Reservoir for Human Immunodeficiency Virus-1. Open Forum Infect Dis 2:ofv123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mulder R, Banete A, Basta S. 2014. Spleen-derived macrophages are readily polarized into classically activated (M1) or alternatively activated (M2) states. Immunobiology 219:737–745. [DOI] [PubMed] [Google Scholar]
- 99.Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C. 2010. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 75:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C, Group H. 2011. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rao VR, Ruiz AP, Prasad VR. 2014. Viral and cellular factors underlying neuropathogenesis in HIV associated neurocognitive disorders (HAND). AIDS Res Ther 11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rappaport J, Volsky DJ. 2015. Role of the macrophage in HIV-associated neurocognitive disorders and other comorbidities in patients on effective antiretroviral treatment. J Neurovirol 21:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zayyad Z, Spudich S. 2015. Neuropathogenesis of HIV: from initial neuroinvasion to HIV-associated neurocognitive disorder (HAND). Curr HIV/AIDS Rep 12:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, Peytavin G, Tubiana R, Pialoux G, Katlama C. 2010. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 50:773–778. [DOI] [PubMed] [Google Scholar]
- 105.Eden A, Fuchs D, Hagberg L, Nilsson S, Spudich S, Svennerholm B, Price RW, Gisslen M. 2010. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis 202:1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weinberger LS, Burnett JC, Toettcher JE, Arkin AP, Schaffer DV. 2005. Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 Tat fluctuations drive phenotypic diversity. Cell 122:169–182. [DOI] [PubMed] [Google Scholar]
- 107.Dar RD, Hosmane NN, Arkin MR, Siliciano RF, Weinberger LS. 2014. Screening for noise in gene expression identifies drug synergies. Science 344:1392–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]