Abstract
This study examined the toxicity of six Gambierdiscus species (Gambierdiscus belizeanus, Gambierdiscus caribaeus, Gambierdiscus carolinianus, Gambierdiscus carpenteri, Gambierdiscus ribotype 2 and Gambierdiscus ruetzleri) using a human erythrocyte lysis assay. In all, 56 isolates were tested. The results showed certain species were significantly more toxic than others. Depending on the species, hemolytic activity consistently increased by ~7–40% from log phase growth to late log – early stationary growth phase and then declined in mid-stationary growth phase. Increasing growth temperatures from 20 to 31 °C for clones of G. caribaeus showed only a slight increase in hemolytic activity between 20 and 27 °C. Hemolytic activity in the G. carolinianus isolates from different regions grown over the same 20–31 °C range remained constant. These data suggest that growth temperature is not a significant factor in modulating the inter-isolate and interspecific differences in hemolytic activity. The hemolytic activity of various isolates measured repeatedly over a 2 year period remained constant, consistent with the hemolytic compounds being constitutively produced and under strong genetic control. Depending on species, greater than 60–90% of the total hemolytic activity was initially associated with the cell membranes but diffused into solution over a 24 h assay incubation period at 4 °C. These findings suggest that hemolytic compounds produced by Gambierdiscus isolates were held in membrane bound vesicles as reported for brevetoxins produced by Karenia brevis. Gambierdiscus isolates obtained from other parts of the world exhibited hemolytic activities comparable to those found in the Caribbean and Gulf of Mexico confirming the range of toxicities is similar among Gambierdiscus species worldwide. Experiments using specific inhibitors of the MTX pathway and purified MTX, Gambierdiscus whole cell extracts, and hydrophilic cell extracts containing MTX, were consistent with MTX as the primary hemolytic compound produced by Gambierdiscus species. While the results from inhibition studies require validation by LC–MS analysis, the available data strongly suggest differences in hemolytic activity observed in this study reflect maitotoxicity.
Keywords: Cell lysis, Ciguatoxins, Ciguatera fish poisoning, Hemolytic assay, Maitotoxin, Regional differences in toxicity
1. Introduction
Dinoflagellates in the genus Gambierdiscus produce potent polyketide neurotoxins known as ciguatoxins and maitotoxins (Yasumoto, 2000). The lipophilic ciguatoxins (CTX) bioaccumulate in the food chain causing ciguatera fish poisoning (CFP) (Bagnis et al., 1980,1985). Globally, CFP is the most common cause of non-bacterial food poisoning associated with eating fish (Friedman et al., 2008). Maitotoxins (MTX) are water soluble and among the most toxic naturally occurring compounds known (Yokoyama et al., 1988). Historically, MTX was not considered to be involved in causing CFP. Recent experimental evidence, however, suggests that MTX can accumulate in the flesh of fish, at least transiently, and the role of MTX in causing CFP should be reexamined (Kohli et al., 2012). Interestingly, a great deal is known about the toxins produced by the genus Gambierdiscus, but relatively little is known about how toxicity varies among species and isolates, by geographic region, and growth conditions. Two factors have made addressing inter-isolate and interspecific toxicity difficult. First, previous studies, with the exception of Chinain et al. (2010), were conducted when the genus Gambierdiscus was considered to contain only one species, Gambierdiscus toxicus. Over the past decade, ten additional Gambierdiscus species have been described (Litaker et al., 2009; Fraga et al., 2011), suggesting that much of the inter-isolate variation in previous studies may be attributable to inherent species-specific differences in toxicity (Litaker et al., 2010). The second limitation is the lack of certified LC–MS standards for the various CTX and MTX congeners. In the case of MTX, many of these congeners have yet to be identified. For this reason, the toxicity of Gambierdiscus isolates have been primarily assessed using the mouse LC50 bioassay, receptor binding assay, or erythrocyte lysis assay (Yasumoto et al., 1979b; Nakajima et al., 1981; Holmes et al., 1990; Diogene et al., 1995; Bignami et al., 1996; Louzao et al., 2006; Caillaud et al., 2010; Chinain et al., 2010). In this study, we used the erythrocyte lysis assay developed by Eschbach et al. (2001) as modified by Tatters et al. (2009) to determine how toxicity varies among Gambierdiscus species. The assay is advantageous because it requires relatively little starting material and is quick, highly reproducible, and cost-effective to perform.
In all 56 Gambierdiscus isolates representing six species (Gambierdiscus belizeanus, Gambierdiscus caribaeus, Gambierdiscus carolinianus, Gambierdiscus carpenteri, Gambierdiscus ribotype 2 and Gambierdiscus ruetzleri) collected in the Caribbean as well as a few isolates from two other species obtained from the Pacific (Gambierdiscus australes and Gambierdiscus pacificus) were assayed. Because there were multiple isolates of each species it was possible to distinguish interspecific from intraspecific differences in toxicity. The study also examined how toxicity varied with growth phase, temperature, and by geographic region.
A second part of this work involved supplemental studies to verify that MTX produced by Gambierdiscus was the compound likely responsible for causing hemolytic activity (Yasumoto et al., 1979b; Nakajima et al., 1981; Takahashi et al., 1982,1983; Murata et al., 1992; Yasumoto, 2000). MTX alters ion transport systems causing an increase in free intracellular Ca2+ (Ohizumi and Yasumoto, 1983; Murata et al., 1994; Lundy et al., 2004; Frew et al., 2008; Wang et al., 2009). The increase in intracellular Ca2+ alters nerve transmission and triggers muscle contraction, breakdown of phosphoinositides, activation of calcium-dependent, non-lysosomal cysteine proteases (calpains), and release of arachidonic acid and neurotransmitters (Frew et al., 2008; Wang et al., 2009). Mouse LD50 bioassay studies used to assess maitotoxicity showed: (1) significant variability among isolates; (2) toxicity peaked in late log to early stationary growth phase; (3) nutrient limitation, particularly P-limitation (N:P > 30), caused maitotoxicity per cell to increase significantly, and (4) maitotoxicity per cell in isolates grown under the same conditions remained constant even after years in culture (Yasumoto et al., 1979a, 1979b; Bagnis et al., 1980; Higerd et al., 1986; McMillan et al., 1986; Bomber et al., 1989b; Holmes et al., 1990; Micouin et al., 1992; Sperr and Doucette, 1996; Chinain et al., 1999, 2010). If hemolytic activity measured by the erythrocyte lysis assay reflects maitotoxicity, then the results from our study should mirror the mouse LD50 results. Overall, this work represents the first major survey of toxicity differences among Gambierdiscus isolates where those isolates have been unambiguously identified using species-specific molecular assays (Litaker et al., 2009; Vandersea et al., 2012).
2. Materials and methods
2.1. Algal cultures
Representative isolates of each species were obtained from the Provasoli – Guillard National Center for Marine Algae and Microbiota (NCMA) or isolated directly from field material (Litaker et al., 2009). Single cell isolates were established by first drawing individual Gambierdiscus cells into a pulled Pasteur pipette and sequentially transferring them through 3–4 drops of sterile modified K medium (no TRIS, no Cu, no Si) made using Gulf Stream water with a salinity of 33 (Keller et al., 1987). After the final transfer, each cell was placed into a separate well of a 24-well tissue culture plate (TC-Plate 24 well, Costar, Lowell, Massachusetts, USA) containing 2 mL of K medium. The plates were incubated at 27 °C, 12:12 L:D cycle, 71 to 82 mE m−2 s−1 under full spectrum lights (Blue Max F20-T12, Full Spectrum Solutions, Mississippi, USA). When culture densities reached ~25–35 cells mL−1, isolates were transferred to 50 mL glass culture flasks containing 30 mL of K medium for grow out. Single cell isolates from each of the NCMA cultures were established similarly to ensure they were comparable to those isolated directly from field samples. The identity of each isolate was confirmed by sequencing the D1–D3 rDNA region and comparing the resulting sequences with those previously obtained from the known Gambierdiscus species (Litaker et al., 2009).
The cultures used to assess hemolytic activity were initiated by adding 25 mL from the single cell grow-out cultures to 125 mL of fresh K medium in a vented, polystyrene culture flask (T-75, Corning Incorporated, Illinois, USA). These cultures were then incubated under the same conditions as above. Cell counts and biovolumes for each culture were obtained every two days by removing a 10 mL aliquot from each culture for analysis using a Coulter Counter® (Beckman Coulter, Georgia, USA) cell counter equipped with a 280 μm aperture tube and set at a 1000 μL per 3.4 s flow rate. Manual counts were performed on one to two samples from each species and found to be within 3% of the Coulter Counter estimate. Growth rates were estimated by calculating the slope of the regression line between elapsed time and the log 10 of the estimated cell density for each time point collected over the previous 7–10 days. Cells for assessing hemolytic activity were harvested in log, late log - early stationary and mid-stationary phase to determine if hemolytic activity varied with growth phase. Log phase was defined as the period when the slope of the regression line between elapsed time and log10 cell density was maximal. Late log phase - early stationary phase samples were taken at the transition point where growth was slowing considerably but had not yet reached zero. Mid stationary phase samples were taken at least 5 days after the onset of zero or slightly negative growth. Negative growth was defined by a consistent decrease in cell number measured on the Coulter Counter for at least six days. During this period of negative growth, the drop in cell density from day to day was minimal.
2.2. Hemolytic assays
Human erythrocytes were obtained from the Red Cross (North Carolina, USA) and stored at 4 °C. Just before initiating the hemolytic assays, ~3 mL of erythrocytes per assay plate were centrifuged at 537 × g (1727 rpm) at 4 °C for 5 min and the supernatant was carefully removed. The pellet was then gently suspended in 10 mL of ELA buffer. The centrifugation and resuspension step was carried out two or three more times until the supernatant was completely clear, indicating that only intact erythrocytes remained. After the final resuspension, the erythrocytes were diluted to a working concentration of between 4.2 and 4.7 × 104 cells mL−1. These erythrocytes gave very consistent hemolytic responses from batch to batch when exposed to the same control concentration of saponin (20 μg per 125 μL−1 of ELA buffer), a detergent which causes erythrocytes to lyse (Tatters et al., 2009). A saponin control was included with each assay to provide an estimate of the optical density (OD) reading where complete lysis occurred.
The hemolytic assays were carried out in Fisherbrand© 96 well v-bottom plates (BD Falcon, New York, USA). Column 1 served as a negative control, where 125 μL of ELA buffer and 125 μL of erythrocytes were added to each well. The lysis of erythrocytes in these wells was minimal. Column 2 served as the positive control. Each well received 125 μL of erythrocytes and 125 μL of a saponin solution (0.08 g in 50 mL of ELA buffer). In the initial experiments, columns 3–12 were used to test dilution series of either (1) whole cell extracts from the 56 Gambierdiscus isolates sampled during log, late log – early stationary, and midstationary phase growth and (2) whole cell extracts of selected isolates grown at different temperatures. The details for the whole cell extraction protocol are given Section 2.3. Each extract tested was diluted 0, 1:10, 1:100, 1:500 and 1:1000 in ELA buffer. Every test well received 125 μL of erythrocytes and 125 μL of diluted cell extract. After all reagents were added, the plates were sealed with adhesive film (VWR, Georgia, USA) and incubated in a refrigerator for 24 h at 4 °C.
After incubation, the plates were spun at 281 × g (1250 rpm) for 10 min at 4 °C. One hundred and fifty μL of supernatant from each well was transferred to plates with round bottom (u-shaped) wells (Part number 3595, Corning Incorporated, Illinois, USA). The plate was then scanned at 415 nm using a Multiskan FC© plate reader (Thermo Scientific, Massachusetts, USA). The 415 nm wavelength was used because it is near the 414 nm maximum absorption for hemoglobin (Tatters et al., 2009). To calculate % lysis, the average negative control absorbance value (ELA and cells only) was determined, and then subtracted from the values obtained from every well in columns 2–12. Next, a mean absorbance value for the corrected saponin controls (maximal lysis) was calculated. The corrected values in columns 3–12 were next divided by the mean corrected positive control value and multiplied by 100 to estimate % lysis. The resulting cell equivalents added to each well along with their corresponding % lysis values were entered into GraphPad Prism® program (GraphPad Software, Inc., California, USA). This program is designed to calculate EC50 values based on a sigmoidal dose response curve fit analysis (GraphPad Software, Inc., 1999). The EC50 values were reported as either number of cells or equivalent cell volumes (μm3) necessary to lyse 50% of the erythrocytes. Lower EC50 values indicated more hemolytic activity.
2.3. Optimizing the extraction protocol
Our initial protocol development focused on determining if the cell lysis procedure was releasing all the hemolytic compounds into solution. To test the lysis procedure, 10 mL aliquots from log phase cultures (~ 750–1100 cells mL−1) were placed in 15 mL conical tubes and spun for 10 min at 2240 × g (3525 rpm) at 4 °C in an Eppendorf® tabletop 5810 R centrifuge (Eppendorf North America, New York, USA). The supernatant was removed and the cells were resuspended in 1.5 mL of ELA buffer (Eschbach et al., 2001). The cells were alternatively sonicated or exposed to successive freeze thaw cycles to disrupt the cell membranes and release the cytoplasmic contents into solution. The cell lysate was spun at 2240 × g at 4 °C for 10 min to pellet the cellular debris. The supernatant containing MTX in solution was removed and the remaining pellet suspended in 1.2 mL of ELA buffer. The hemolytic activity of both the supernatant and the resuspended pellet were tested using the hemolytic assay described below. Given that MTX is water soluble, it was expected that only residual hemolytic activity would be associated with the cell debris. Surprisingly, repeated testing showed that the majority of the hemolytic activity remained with the pellet and not in the aqueous cell lysate suggesting incomplete cell lysis. However, examination of the lysed cell pellet using 600 × light microscopy revealed the cells were completely disrupted.
Further optimization studies determined that the cellular hemolytic activity could be most reliably estimated using whole cell extracts prepared according to the following protocol. As each culture entered log, late log – early stationary phase, and mid-stationary phase, a 10 mL aliquot was removed and concentrated by pelleting for 10 min at 2240 × g at 4 °C. The supernatant was carefully removed and the cell pellets were resuspended in 1.2 mL of ELA buffer before being transferred to 2 mL microfuge tubes. The cells were then sonicated 3 times for 30 s using a Branson Sonifier equipped with a microtip (VWR Scientific, Georgia, USA). Samples were always kept on ice to avoid overheating except when being centrifuged. Just prior to running the assay, the cell debris and lysate were mixed to form a homogenous suspension which was used to assay hemolytic activity. The variation among the four replicates for each condition tested was always <0.1% indicating that cell debris remained evenly suspended during the period when the aliquots were added to the wells.
Given that much of the hemolytic activity was associated with the cell debris immediately after cell lysis, we undertook the following experiment to determine if the hemolytic compound(s) associated with the cell debris was released into solution during the 24 h assay incubation period. Release of the compound(s) would mean that additional extraction methods were not required to estimate total hemolytic activity in the cell lysate. To test this possibility, log phase cultures of Gambierdiscus ribotype 2 (CCMP isolate 1655) were harvested, pelleted, and whole cell extracts prepared as described above. One set of three aliquots extracted from the whole cell pellet was run using the standard assay procedure using a dilution series of 0, 1:10, 1:100, 1:500 and 1:1000. A second set of triplicate aliquots was centrifuged at 4000 rpm for 10 min to remove the cell debris. The supernatant from this treatment was carefully removed without disturbing the pellet. The supernatant was then diluted and run using the same assay procedures to confirm how much activity was associated with the cell debris versus the cleared supernatant. A third set of whole cell extracts was incubated at 4 °C for 24 h along with the standard assay plates containing the first two sets of extracts. After 24 h, the first two sets of samples were analyzed for percent lysis, and the third set spun down at 4000 rpm for 10 min to remove the cell debris. The supernatant from the 24 incubation in ELA buffer was assayed to determine the amount of hemolysis relative to the whole cell and cleared supernatant assayed on the previous day. The results showed equivalent hemolysis in the whole cell extracts from day 1 and the cleared supernatant from the pellet incubated for 24 h in ELA buffer. This confirmed that the hemolytic compound was released into solution over the 24 h incubation period.
2.4. Cultures tested for hemolytic activity
Extracts from the 56 Gambierdiscus isolates listed in Table 1 were tested for hemolytic activity in log, late log – early stationary, and mid stationary phase (Fig. S1). In addition, the hemolytic activity of one G. caribaeus and two G. australes isolates from Hawaii, one G. pacificus isolate from French Polynesia, and one G. carolinianus isolate from Crete were assayed for comparative purposes (Table 1). The EC50 values for these isolates were compared to those obtained for the Caribbean, Gulf of Mexico, and North Carolina isolates.
Table 1.
Species and strain identification with relevant collection information.
| Species | Location | Strain name | Date isolated | Isolated by |
|---|---|---|---|---|
| G. carolinianus | North Carolina | Kenny 6 | 8/1/2004 | S. Kibler |
| North Carolina | Lob Rock N7 | 7/23/2005 | S. Kibler | |
| North Carolina | Ken 3 | 9/1/2010 | C. Holland | |
| North Carolina | Big Fish gam 5 | 8/1/2008 | C. Holland | |
| North Carolina | Lob Rock N3 | 8/1/2007 | C. Holland | |
| Dry Tortugas | ETB exp 28 gam 10 | 9/1/2009 | C. Holland | |
| Jupiter, Florida | Jupiter Algae gam 5 | 7/1/2009 | C. Holland | |
| Puerto Rico | PRG gam 1 | 8/1/2007 | C. Holland | |
| Puerto Rico | RROV5 | 5/1/2007 | C. Holland | |
| St Thomas | ST1 F7 | 7/29/2010 | C. Tomas | |
| Flower Gardens | WBHR21 Gam 2 | 9/23/2010 | C. Holland | |
| Mexico | Mex Algae gam 1 | 1/1/2010 | C. Holland | |
| Ocho Rios | Jamaica Algae gam 1 | 12/1/2009 | C. Holland | |
| Belize | Dive 1 gam1 | 5/1/2008 | C. Holland | |
| Belize | Elbow Cay | 5/1/2009 | C. Holland | |
| G. caribaeus | Belize | Gam19 | 5/6/2004 | S. Kibler |
| Belize | Jar 17 gam 20 | 5/1/2009 | C. Holland | |
| Belize | Gam 4 | 5/17/2004 | S. Kibler | |
| Belize | SW gam 1 | 5/1/2005 | S. Kibler | |
| Belize | NCMA 1733 | 2/1/1996 | S.L Morton | |
| Belize | TC tow Gam 3 | 5/1/2008 | C. Holland | |
| Belize | Norval Cay | 5/1/2009 | C. Holland | |
| Belize | SW gam 5 | 5/1/2005 | S. Kibler | |
| Belize | CBC gam1 | 5/1/2006 | S. Kibler | |
| Grand Cayman | NCMA 1651 | |||
| Cancun | Mexico Algae 1 gam 1 | 1/1/2010 | C. Holland | |
| Ocho Rios | Jamaica Algae 1 gam1 | 12/1/2009 | C. Holland | |
| Dry Tortugas | ETB Gam 6 | 9/1/2009 | C. Holland | |
| Dry Tortugas | Outfish 7–1 | 8/1/2008 | S. Kibler | |
| Dry Tortugas | Outfish 7–3 | 8/1/2008 | S. Kibler | |
| Long key, Florida | Jar 12 Tow 3 | 7/1/2008 | C. Holland | |
| St. Thomas | ST 1 C5 | 7/29/2010 | C. Tomas | |
| St. Johns | SJ 3 D7 | 7/29/2010 | C. Tomas | |
| Bathtub Beach, Florida | BB gam 4 | 7/1/2009 | C. Holland | |
| Jupiter, Florida | BRP gam 4 | 7/1/2009 | C. Holland | |
| Ft. Pierce, Florida | Dive 1 fa gam 1 | 7/1/2008 | C. Holland | |
| Jupiter, Florida | Coral cove gam 1 | 7/1/2009 | C. Holland | |
| Long key, Florida | Keys jar 7 gam 7 | 8/1/2010 | C. Holland | |
| Flower Gardens | WBHR 21 gam 2 | 9/23/2010 | C. Holland | |
| Flower Gardens | WBHR 26 gam 1 | 9/23/2010 | C. Holland | |
| G. belizeanus | St. Barthelemy Islands | NCMA 399 | 2/1/1990 | M. Durand-Clement |
| Florida Keys | Keys gam 1 | 11/1/2005 | C. Holland | |
| St. Thomas | ST1 gam f4 | 7/29/2010 | C. Tomas | |
| Gambierdiscus ribotype 2 | Martinique | NCMA 1655 | 4/1/1994 | J. Babinchak |
| St. Thomas | ST3 gam f2 | 7/29/2010 | C. Tomas | |
| G. carpenteri | Puerto Rico Guam | Mixed PR gam 4 NCMA 1654 | 7/1/2007 | C. Holland |
| Belize | GT4 | 8/1/2005 | T. Villareal | |
| Oahu, Hawaii | Pat HI jar 5 gam 3 | 6/1/2010 | C. Holland | |
| Flower Gardens | WBHR 21 | 9/23/2010 | C. Holland | |
| Dry Tortugas | ETB Exp 24 gam 1 | 9/1/2009 | C. Holland | |
| Ocho Rios | Jamaica Algae 2 gam 1 | 12/1/2009 | C. Holland | |
| G. ruetzleri | SW Cay Belize | Gam 1 | 5/12/2004 | S. Kibler |
| North Carolina | NC ruetz | 7/1/2006 | S. Kibler | |
| Pacific isolates | ||||
| G. australes | Waikiki Beach | HI9 gam 5 | 6/1/2010 | C. Holland |
| Waikiki Beach | WB gam 3 | 6/24/2010 | C. Holland | |
| G. pacificus | Moorea, Society Islands | NCMA 1650 | 4/1/1994 | J. Babinchak |
| G. caribaeus | Oahu, Hawaii | Pat Jar 2 Gam 2 | 7/1/2011 | C. Holland |
| Mediterranean Isolate | ||||
| G. carolinianus | Crete | Greece Gam 3 | 10/1/2010 | C. Holland |
2.5. Hemolytic activity versus growth temperature
Previous research showed the growth versus temperature relationship varies among Gambierdiscus species (Tester et al., 2010; Kibler et al., 2012). If the pattern of hemolytic activity also varies significantly among species with growth temperature, it would have important implications for determining interspecies differences in hemolytic activity. For example, 27 °C may be the optimal temperature for production of hemolytic compounds in one species, but 22 °C in another. In this case, measuring both species simultaneously at only 22 °C or 27 °C would not accurately reflect the interspecific differences in relative hemolytic activity. In contrast, if changes in growth temperature up or down regulate hemolytic activity in a consistent manner, then hemolytic activity measured at a single growth temperature provides an indicator of interspecific and inter-strain differences in toxin content.
To assess how the hemolytic activity might vary with growth temperature in Gambierdiscus species, the hemolytic activity of five G. caribaeus and six G. carolinianus isolates grown at 20, 24, 27, and 31 °C were measured. Samples were taken for analysis from log, late log – early stationary, and mid stationary phase cultures. These two species were selected because they represent the extremes in growth response to changes in temperature (Kibler et al., 2012). The growth optimum for G. caribaeus, for example, is ~31 °C compared to ~25 °C for G. carolinianus (Kibler et al., 2012). Since these optima represent the extremes measured among Gambierdiscus species to date, it is likely that their responses to changes in growth temperature will be representative of other species in the genus. The isolates used in this study were selected from different geographic regions to determine if location of origin affected the observed response. Those regions included the western Caribbean and Gulf of Mexico, the eastern Caribbean and Gulf of Mexico, and North Carolina. Within a region, the isolates with the highest and lowest EC50 values were selected for inclusion in the temperature study.
These experiments were carried out by establishing batch cultures of each isolate as described previously. Cell densities were measured on a daily basis using a Coulter Counter. When cultures entered log, late log – early stationary, or mid stationary phase, 10 mL aliquots were removed, extracted and tested using the hemolytic protocol in Section 2.2. Results were reported as EC50 cells for each isolate, at each growth temperature and growth phase.
2.6. Confirmation that MTX was responsible for the observed hemolytic activity
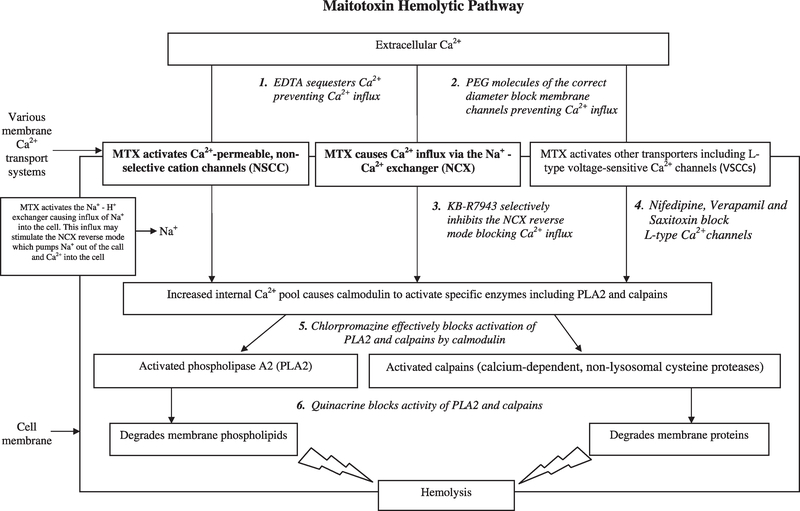
Because the definite LC–MS data are not available to confirm conclusively that the hemolytic activity measured in this study was due to MTX, we undertook a series of inhibition and heat treatment experiments further test whether MTX is the likely cause of the observed hemolytic activities. The inhibition studies can be best understood by consulting Fig. 5, which presents a proposed conceptual model of the MTX toxin pathway based on current research findings. This diagram illustrates where each inhibitor can block the MTX pathway. The initiation of the MTX pathway involves alteration of Ca2+ transport systems so that a large influx of extracellular Ca2+ enters the cell. Data indicate that multiple ion transport systems are affected by MTX (Few et al., 2008; Wang et al., 2009). Chief among these is the Na+/Ca2 exchanger (NCX), a bidirectional low-affinity high-capacity ion transporter that exchanges Na+ and Ca2+ depending on membrane potential and trans membrane ion gradients (Few et al., 2008). The NCX system is the primary mechanism by which cells regulate internal Ca2+ concentrations. In forward mode, the NCX transporter pumps Ca2+ out of the cell and Na+ into the cell (Ca2+ efflux). In reverse mode it pumps Ca2+ into and Na+ out of the cell (Ca2+ influx). MTX causes the NCX to be stuck in the reverse mode (Few et al., 2008). MTX is also known to activate Ca2+-permeable, non-selective cation channels (NSCC) (Schilling et al., 1999; Lundy et al., 2004; Wisnoskey et al., 2004), L-type voltage-sensitive Ca2+ channels (VSCCs) (Su et al., 2004), and the Na+/H+ exchanger (NHE) (Wang et al., 2009). Work on neurons suggests that the initial response to MTX involves activation of NHE and a large increase in intracellular Na+. Elevated Na+ levels would in turn foster the reverse mode of the NCX, which exchanges internal Na+ for external Ca2+ (Wang et al., 2009). What is still poorly understood is whether MTX independently affects each of these transport systems separately, or if it affects them through a common control mechanism. It is possible that MTX binds to its own receptor(s), which in turn regulates the opening of the various ion channels (Lundy et al., 2004; de la Rosa et al., 2001; Wang et al., 2009).
Fig. 5.
Conceptual diagram showing the maitotoxin (MTX) hemolytic pathway. Binding of MTX opens certain ion channels allowing a large Ca2+ influx into cells. This influx causes the signaling molecule calmodulin to activate phospholipase A2 and calpains which degrade the cell membrane resulting in cell lysis. The specific pathway inhibitors used in this study to confirm that MTX is the primary compound responsible for the observed hemolytic activity are indicated as numbered, italicized text. The two ion transporter systems shown in bold font are the primary known ion channel targets of MTX.
The inhibitors selected this study were EDTA, polyethylene glycol (PEG), KB-R7943, nifedipine, verapamil, and saxitoxin. EDTA tightly binds cations such as calcium making them unavailable for entry into the cells. Polyethylene glycol is a flexible, water-soluble polymer, which comes in a number of different molecular weights. When PEG molecules with sufficient effective diameter are present, they will enter and block membrane ion channels preventing influx of Ca2+ into cells (Deeds, 2003; Movileanu et al., 2003; Hromada et al., 2008; Hilf et al., 2010). KB-R7943 selectively inhibits the reverse mode of the NCX (Frew et al., 2008). Nifedipine is a dihydropyridine L-type voltage sensitive calcium channel blocker that inhibits trans membrane influx of calcium ions into cells. Verapamil is a similar L-type calcium channel modulator that acts by plugging up the channels and limiting the entry of calcium into cells at higher doses. Saxitoxin (STX) also blocks L-type Ca2+ channels as well as sodium channels which are its primary target (Su et al., 2004).
The other two inhibitors examined, chlorpromazine hydrochloride and quinacrine, affect the downstream portion of the hemolytic pathway (Fig. 5). Chlorpromazine hydrochloride specifically blocks the activity of calmodulin thereby preventing the activation of phospholipase A2 (PLA2) and calpains (Zhang and Yuan, 1998), the enzymes which cause the breakdown of the cell membrane. Quinacrine acts by directly inhibiting the activity of both PLA2 and calpains (Igarashi et al., 1999; Farooqui et al., 2006). The suite of inhibitors used in this study would fail to consistently inhibit the activity of reactive oxygen species (ROS) (Chiu et al., 1982; Marshall et al., 2003), polyunsaturated fatty acids (PUFAs) (Yasumoto et al., 1990; Landsberg, 2002; de Boer et al., 2009), or glycolipids (Marshall et al., 2003; Emura et al., 2004; Ma et al., 2011; Pagliara and Caroppo, 2011) which are produced by other phytoplankton species and known to cause hemolysis (Damude and Kinney, 2008; de Boer et al., 2009; Law et al., 1980).
The inhibition experiments were initiated by preparing a standard whole cell extract from 10 mL aliquots of log phase cultures of Gambierdiscus ribotype 2 (CCMP isolate 1655) containing ~8650 to 11,080 total cells as described above. This isolate was selected because it had been shown to constitutively produce both MTX and CTX (Lartigue et al., 2009; Litaker et al., 2010). The extracts were serially diluted in ELA buffer and assayed using the standard hemolytic protocol to establish the dilution which was just sufficient to cause complete hemolysis. That dilution was then used as the amount added to each test well used in determining how the differing inhibitor concentrations would affect hemolysis. In each of the inhibition experiments, eight replicates were run for each inhibitor dilution tested.
As a control, purified MTX (Enzo Life Sciences, formerly Alexis Biochemicals, California, USA) was diluted in ELA buffer to establish the concentration sufficient to cause complete hemolysis. This concentration was then used to determine whether increasing concentrations of the specific inhibitors suppressed hemolysis. Partially purified MTX and CTX extracts were also prepared from ~ 8.9 × 106 late log phase Gambierdiscus ribotype 2 cells following the protocol of Lewis et al. (2009). In this procedure, MTX partitions into the hydrophilic phase and CTX into the hydrophobic phase. The reason for preparing these extracts was two-fold. First, the extraction process eliminates the PUFAs (Yasumoto et al., 1990; de Boer et al., 2009), glycolipids (Law et al., 1980; Emura et al., 2004; Ma et al., 2011; Pagliara and Caroppo, 2011), and reactive oxygen species (Chiu et al., 1982; Marshall et al., 2003; Damude and Kinney, 2008) known to cause hemolysis in other phytoplankton species, but not MTX. By diluting the partially purified MTX extract based on the number of cells extracted it was possible to prepare a stock solution which contained the same number of extracted cell equivalents as used in the whole cell inhibition experiments to cause complete hemolysis. An equivalent suppression of hemolysis in by various inhibitors in the presence of pure MTX, whole cell extracts, and partially purified extracts containing MTX would be consistent with MTX as the primary compound responsible for hemolysis in Gambierdiscus species. Second, the purification process provided a way to determine whether the CTX-containing fraction had measurable hemolytic activity. Tests using the CTX fraction showed only minimal lysis even at a 2.5-fold dilution of the full strength extract in ELA buffer plus 5% methanol. This concentration was a 100-fold higher than the diluted MTX fraction which caused complete lysis. These data indicated that the CTX fraction cause <1% of any total observed hemolytic activity. Consequently, the CTX containing extracts were not tested further.
The methods used for the various inhibition studies were as follows. In the EDTA experiments, 125 μL aliquots containing 0, 0.20, 0.25, 0.50, or 5 mM EDTA were added to erythrocytes suspended in ELA buffer containing 5% methanol and either purified MTX, whole cell extract, or partially purified MTX extract as described above. Five percent methanol was added to ensure that MTX remained fully dissolved. Preliminary studies showed that five to ten percent methanol did not to increase hemolysis relative to saponin controls containing no methanol. Two negative controls (125 μL ELA buffer, 5% methanol, no EDTA and 125 μL ELA buffer, no methanol, no EDTA) and one positive control (125 μL ELA buffer, 20 μg per saponin, 5% methanol, no EDTA) were included with each assay.
The (PEG) experiments were done by first preparing 60 mM solutions of 300, 600, 1450, 4000, and 8000 MW PEG in ELA buffer containing 5% methanol. Eight 125 μL replicates of each MW PEG were then added to 96-well plates. Next, a mixture of ELA buffer, erythrocytes, 5% methanol, and a volume of purified MTX, whole cell extract, or partially purified MTX extract sufficient to cause 100% lysis were combined. One hundred twenty five μL aliquots were then added to the wells containing the various MW PEG solutions and gently mixed at 400 rpm for 10 min to ensure thorough mixing. The remainder of the assay was carried out as for EDTA including the use of the two positive and one negative control. The final PEG concentration in each well was 30 mM. To determine if any of the observed responses to PEG was likely due to osmotic differences among the various PEG solutions, the osmolality of each PEG solution was determined using a Wescor 5500 Vapor Pressure Osmometer (Wescor, Inc., Utah, USA) and expressed in mmol/kg. Any inhibition in hemolysis which occurred between different MW PEG treatments having similar osmolalities was assumed to be due to blockage of the ion channels and not to osmotic stabilization of the cell membrane.
The KB-R7943 assays were carried out using a 10 mg mL−1 (23.4 mM) stock dissolved in dimethyl sulfoxide. The stock was diluted in ELA buffer so that the final concentrations were 100,10,1, 0.1, 0.01, and 0.001 μM. One hundred twenty five μL aliquots were then used to test hemolytic activity as described above.
For the initial nifedipine and verapamil experiments, 100 mM solutions were prepared by dissolving nifedipine in DMSO and verapamil in deionized water. The final concentrations tested were 200, 100, 50, 20, 10, 5, and 1 μM. These experiments showed that these concentrations suppressed hemolytic activity to only 75–80% of the control. The same results were observed when a 334 μM stock of saxitoxin was diluted to final concentrations of 1.9, 0.94, 0.47, 0.23, 0.12, and 0 μM (Fig. S3). These results are similar to those of Igarashi et al. (1999) who noted that L-type channel blockers only minimally inhibited MTX activity. It is also consistent with the fact that saxitoxins are known to bind less readily to Ca2+ channels than Na+ channels, which are their primary target (Su et al., 2004). In order to substantially inhibit hemolytic activity with all three of these inhibitors, it was necessary to pre-incubate the erythrocytes with each inhibitor prior to adding MTX. In order to carry out these experiments the procedure was altered slightly. The erythrocytes were diluted in ELA buffer +5% methanol as before except that allowances were made in the total volume so that small aliquots containing each inhibitor could be added to produce the final concentrations listed above. The volume added did not significantly change the concentration of erythrocytes in the final 125 μL aliquots. Once the inhibitors were added to the cells, the 96-well plate was placed on an orbital ELISA plate shaker (Lab Line, Illinois, USA) at 400 rpm for 10 min at room temperature. After incubation, 125 μL aliquots containing purified MTX, whole cell extract, or partially purified MTX cell extract diluted in ELA buffer were added to their respective sample wells and the assays were processed as described previously.
The chlorpromazine hydrochloride and quinacrine inhibition experiments were carried out in the same manner as the EDTA experiment except that the replicate 125 μL aliquots of erythrocyte + whole cell extracts were incubated with either 0, 0.035, 0.07, 0.14, 0.7, or 2.8 mM chlorpromazine or 2.2, 4.4, 17.6, 52.8, 105.6, or 176 μM quinacrine.
To further confirm that other non-MTX related hemolytic compounds known to be present in other phytoplankton were not present in Gambierdiscus extracts, whole cell extracts from log phase cultures of Gambierdiscus ribotype 2, G. belizeanus, and G. ruetzleri were prepared as described above. Triplicate 1.2 mL aliquots of each extract were heated to 100 °C for 10 min on a PTC 150 Minicycler (MJ Research, Massachusetts, USA) while corresponding control aliquots were held on ice. Both sets of aliquots were then tested for hemolytic activity. High temperatures are known to cause the loss of hemolytic activity by PUFAs (Yasumoto et al., 1990; de Boer et al., 2009), glycolipids (Law et al., 1980; Emura et al., 2004; Ma et al., 2011; Pagliara and Caroppo, 2011), and reactive oxygen species (Chiu et al., 1982; Marshall et al., 2003; Damude and Kinney, 2008), but not MTX (Burke and Tester, 2002).
2.7. Statistical analysis
The EC50 data normalized on a per cell and a per biovolume basis were analyzed using a Levene homogeneity of variance test (Zar, 1999). The specific data sets included the hemolytic activity among (a) different species, (b) various growth phases, (c) regional variations and (d) the effects of temperature on growth of G. caribaeus and G. carolinianus isolates. For each data set with homogeneous variance, differences in hemolytic activity were evaluated using a parametric analysis of variance (ANOVA) test. Those data sets which failed the homogeneity of variance test were analyzed using a non-parametric one way analysis of variance test from Kruskal and Wallis (1952). Nonparametric multiple comparison tests were used to analyze statistical differences among treatment groups such as the pairwise differences in hemolytic activity among individual species. The multiple comparisons tests were performed to examine statistical differences among treatments (e.g., among different species in an experiment) used a modified Kruskal-Wallis approach (Siegel and Castellan, 1988).
3. Results
3.1. Species-specific hemolytic activity
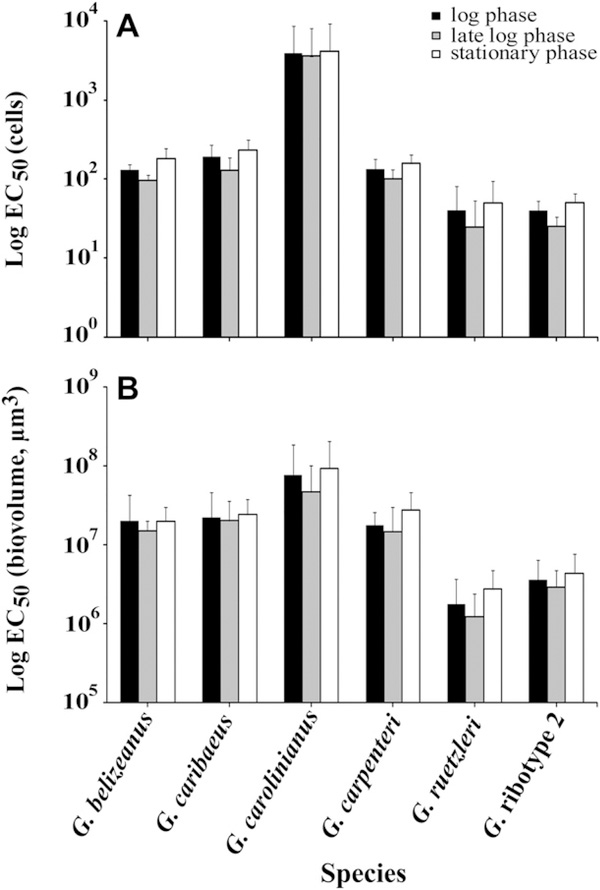
Hemolytic activity averaged across all three growth phases varied significantly among species and fell into three different groups (Tables S1 and S2, p < 0.001) (H-statistic = 49.461, df = 5, p < 1.79 × 10−9). The most hemolytic species on a per cell basis were Gambierdiscus ribotype 2 and G. ruetzleri, which required only 39 ± 26 or 39 ± 31 cells respectively to produce 50% hemolysis in 24 h (Fig. 1A). The species with intermediate hemolytic activity included G. belizeanus (127 ± 42 cells), G. caribaeus (183 ± 81 cells) and G. carpenteri (131 ± 45 cells). The least toxic species was G. carolinianus (3854 ± 4628 cells). A multiple comparison test (Zar, 1999) was performed to determine which species had statistically different hemolytic activity. The results indicated the significant differences among species were driven by the results for G. caribaeus, G. carolinianus, Gambierdiscus ribotype 2 and G. ruetzleri (Table S3A). When the EC50 data were normalized to biovolume, the same three relative toxicity groupings were observed (Fig. 1B; Table S3B). The only notable variation in the biovolume normalized data was that G. ruetzleri was slightly more toxic relative to the other species on a per μm3 basis than on a per cell basis.
Fig. 1.
A.) The average EC50 values normalized to the number of cells extracted (±1 standard deviation) in log, late log – early stationary, and mid-stationary phase for each of the six species tested. The number of isolates used to calculate these averages varied among species (see Table 1). B) Same as A except theEC50EC50 values were normalized to cell biovolume (μm3).
Overall, cell size varied extensively among species and was not significantly correlated with either growth rate or hemolytic activity (Fig. S2, Table S4). Cell size tended to increase slightly during late log to stationary phase with the exception of G. caribaeus, which decreased in size (Fig. S2). Regardless of cell size, hemolytic activity on a per cell basis was observed to increase consistently in late log – early stationary phase in each species (Fig. 1). The fact that hemolytic activity showed the same pattern normalized on a per cell or per biovolume basis, indicates that the observed changes in hemolytic activity in late log – early stationary phase were not due to changes in cell volume, but rather to real differences in the amount of hemolytic compound(s) per mm3 of biomass (Fig. 1, S2).
In addition to the Caribbean isolates, we examined the hemolytic activity of two strains of G. australes (EC50 cells, 69 ± 31) and one strain of G. caribaeus (EC50 cells, 168) from Hawaii; one strain of G. pacificus from the Society Islands, French Polynesia (EC50 cells, 34); and one isolate of G. carolinianus from Crete (EC50 cells, 183). These resulting EC50 estimates fell within the range observed for species from the Caribbean.
3.2. Regional differences in hemolytic activity
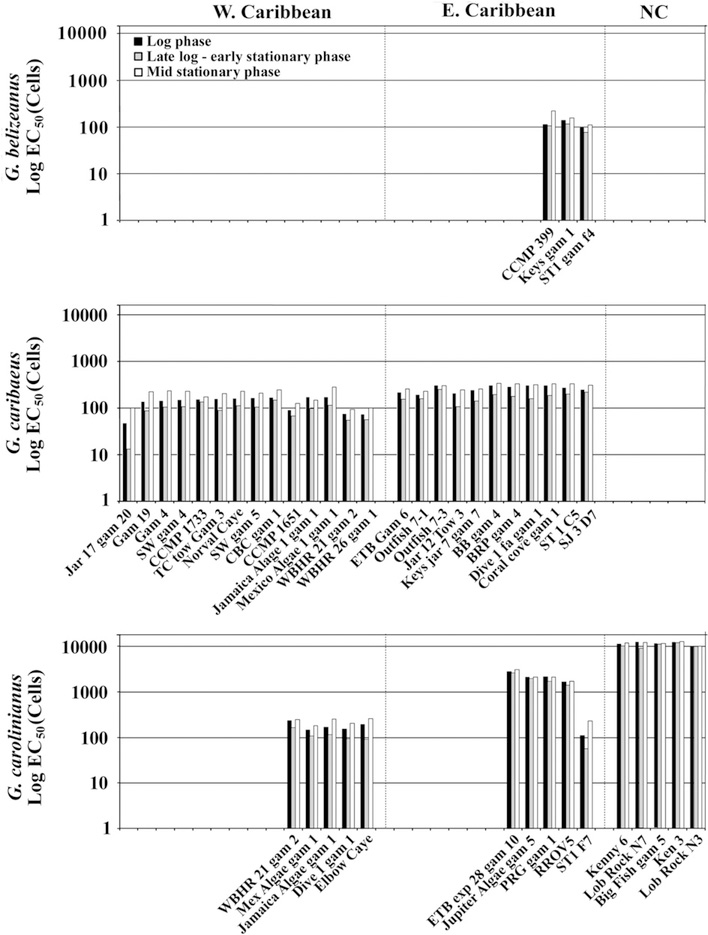
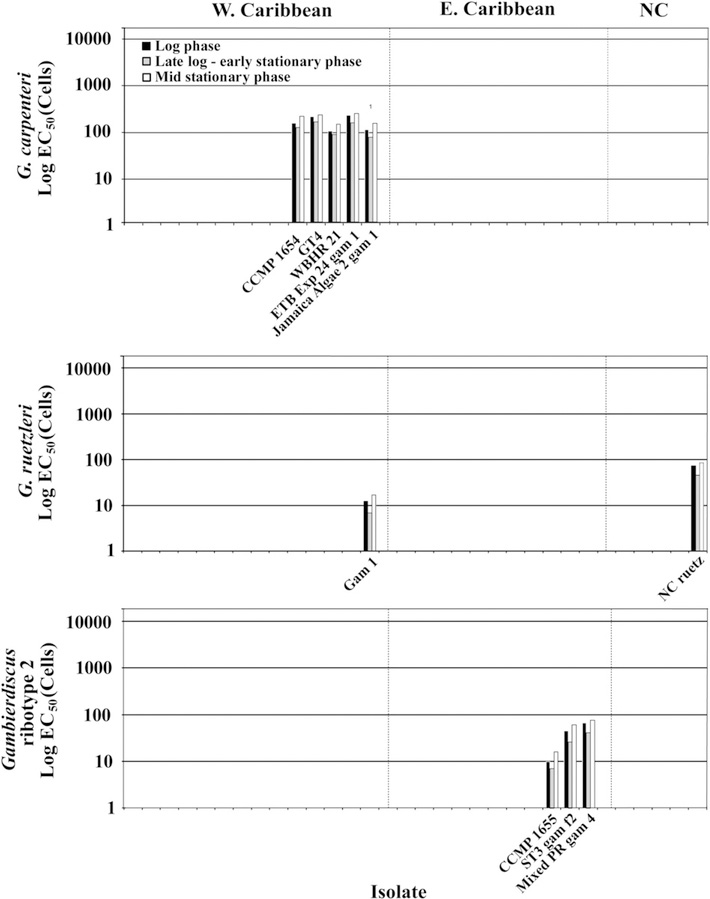
When we examined the data for G. carolinianus from throughout the Caribbean and Gulf of Mexico, it was clear that regional hemolytic activity differences existed, but that those differences were not correlated with latitude. Hemolytic activity of G. carolinianus from the Gulf of Mexico and western Caribbean (EC50 = 167 ± 31 cells, n = 5), were higher than those from the eastern Caribbean and West Indies (EC50 = 1638 ± 848 cells, n = 5). Isolates from North Carolina, USA contained no appreciable hemolytic activity (EC50 = >10,621 ± 938 cells, n = 5) (Fig. 2). Isolates of G. caribaeus from the Gulf of Mexico and western Caribbean Sea were again higher (EC50 values of 136 ± 37 cells, n = 14) compared to those from the eastern Caribbean (245 ± 41 cells, n = 12). The only other species where isolates from multiple regions were available was G. ruetzleri where the strain collected in the western Caribbean had an EC50 of 13 cells averaged over all three growth phases compared to 64 cells for the isolate from North Carolina, USA (Fig. 3). An analysis of variance indicated that regional differences observed for G. caribaeus were significant (p < 0.001; Tables S5 and S6). A non-parametric ANOVA for the G. carolinianus data showed a significant difference in hemolytic activity with region (Tables S5, S7 and S8). Equivalent trends were observed for EC50 normalized to biomass.
Fig. 2.
Hemolytic activity for G. belizeanus, G. caribaeus, and G. carolinianus in log (black bars), late log – early stationary (gray bars) and mid-stationary phases (white bars) (Table 1). The data are expressed as EC50 values normalized on a per cell basis and plotted on a log scale. The data for the representative isolates of each species were divided into geographic regions (the western Caribbean and Gulf of Mexico, the eastern Caribbean and Gulf, and from off the coast of North Carolina, USA) based on the observed differences in EC50 values.
Fig. 3.
The legend is the same as Fig. 2 except the species shown are G. carpenteri, G. ruetzleri, and Gambierdiscus ribotype 2.
3.3. Variations in hemolytic activity with growth phase
As noted previously, hemolytic activity was observed to increase in late log phase (Figs. 1–3). To determine if these changes were statistically significant the data for G. caribaeus and G. carolinianus were analyzed. These two species were selected because of the large number of isolates available in our culture collection. The ANOVA for G. caribaeus indicated that hemolytic activity varied significantly with growth phase (p < 0.001; Table S9). A multiple comparison test indicated that log and mid-stationary phases were not significantly different, despite the fact that both of these phases had consistently lower hemolytic activity than late log -early stationary phase (Table S10). The differences in hemolytic activity between growth phases were not significant for G. carolinianus (Table S11). When the corresponding data for EC50 normalized to biomass were analyzed, the overall variation was higher and no significant differences were observed between growth phases even though hemolytic activity consistently increased in late log – early stationary phase.
3.4. Effect of growth temperature on hemolytic activity
Isolates of G. caribaeus and G. carolinianus were grown at 20, 24, 27, and 31 °C and the hemolytic activity determined in log, late log – early stationary and mid-stationary phase (Table 2). A major goal of this experiment was to help establish whether or not the temperature where maximal hemolytic activity occurred varied significantly between species. The results indicated that G. caribaeus hemolytic activity increased between 20 and 27 °C, but that the hemolytic activity did not continue to increase from 27 to 31 °C. A Kruskal-Wallis (1952) non-parametric ANOVA showed the increase in hemolytic activity with temperature was significant (p < 0.03; Tables S12 and S13A). A multiple comparisons test indicated this difference was solely driven by the difference in temperature between 20 and 27 °C (Table S13B). Hemolytic activity was therefore maximal at 27 °C for the representative isolates tested. This result indicates that measuring hemolytic activity of isolates grown at 27 °C provides a good comparative measure of inter-isolate and interspecific difference in MTX toxicity.
Table 2.
Hemolytic activity of G. caribaeus isolates from the eastern and western Caribbean Gulf of Mexico (EC/EGOM and WC/WGOM) and G. carolinianus from the EC/EGOM, WC/WGOM, and patch reefs of the coast of North Carolina, USA (NC). The isolates which were selected for the highest and lowest toxicities in a region based on the initial screening of all 56 isolates (Fig. 4). Cultures were grown at 20, 24, 27, and 31 °C. The resulting EC50 values were normalized on a per cell basis. At each temperature, the EC50 values were measured while the cells were in log, late log – early stationary, and in mid-stationary phase growth (Fig. S1).
| Temperature | Region | Isolate | EC50 cells log phase | EC50 cells late log – early stationary phase | EC50 cells mid-stationary phase |
| Gambierdiscus carolinianus | |||||
| 20 °C | EC/EGOM | Mex Algae gam 1 | 201 | 179 | 222 |
| EC/EGOM | WBHR21 Gam 2 | 284 | 266 | 301 | |
| WC/WGOM | RROV5 | 1799 | 1584 | 1816 | |
| WC/WGOM | ST 1 F7 | 255 | 209 | 281 | |
| NC | Kenny6 | 12205 | 11999 | 12596 | |
| NC | Big Fish gam 5 | 11856 | 11663 | 12001 | |
| 24 °C | EC/EGOM | Mex Algae gam 1 | 189 | 162 | 243 |
| EC/EGOM | WBHR 21 Gam 2 | 259 | 178 | 279 | |
| WC/WGOM | RROV5 | 1672 | 1434 | 1737 | |
| WC/WGOM | ST 1 F7 | 201 | 132 | 269 | |
| NC | Kenny6 | 11887 | 10059 | 12333 | |
| NC | Big Fish gam 5 | 11553 | 11074 | 12887 | |
| 27 °C | EC/EGOM | Mex Algae gam 1 | 149 | 110 | 189 |
| EC/EGOM | WBHR 21 Gam 2 | 236 | 167 | 257 | |
| WC/WGOM | RROV5 | 1587 | 1352 | 1605 | |
| WC/WGOM | ST 1 F7 | 113 | 58 | 236 | |
| NC | Kenny6 | 11212 | 10212 | 12000 | |
| NC | Big Fish gam 5 | 12658 | 12002 | 12888 | |
| 31 °C | EC/EGOM | Mex Algae gam 1 | 181 | 134 | 249 |
| EC/EGOM | WBHR 21 Gam 2 | 175 | 153 | 184 | |
| WC/WGOM | RROV5 | 1331 | 1183 | 1549 | |
| WC/WGOM | ST 1 F7 | 99 | 77 | 123 | |
| NC | Kenny6 | 10008 | 9608 | 12750 | |
| NC | Big Fish gam 5 | 9902 | 9810 | 10100 | |
| Temperature | Region | Isolate | EC50 cells Log | EC50 cells Late log | EC50 cells Stationary |
| Gambierdiscus caribaeus | |||||
| 20 °C | EC/EGOM | Mexico Algae 1 gam 1 | 212 | 206 | 294 |
| EC/EGOM | WBHR 26 gam 1 | 208 | 198 | 273 | |
| EC/EGOM | Gam 19 | 234 | 191 | 251 | |
| WC/WGOM | Keys jar 7 gam 7 | 245 | 232 | 265 | |
| WC/WGOM | Dive 1 fa gam 4 | 301 | 274 | 317 | |
| 24 °C | EC/EGOM | Mexico Algae 1 gam 1 | 205 | 181 | 234 |
| EC/EGOM | WBHR 26 gam 1 | 216 | 190 | 259 | |
| EC/EGOM | Gam 19 | 202 | 196 | 238 | |
| WC/WGOM | Keys jar 7 gam 7 | 239 | 221 | 269 | |
| WC/WGOM | Dive 1 fa gam 4 | 273 | 256 | 304 | |
| 27 °C | EC/EGOM | Mexico Algae 1 gam 1 | 209 | 140 | 194 |
| EC/EGOM | WBHR 26 gam 1 | 91 | 69 | 121 | |
| EC/EGOM | Gam 19 | 58 | 16 | 121 | |
| WC/WGOM | Keys jar 7 gam 7 | 246 | 172 | 262 | |
| WC/WGOM | Dive 1 fa gam 4 | 263 | 231 | 284 | |
| 31 °C | EC/EGOM | Mexico Algae 1 gam 1 | 198 | 176 | 267 |
| EC/EGOM | WBHR 26 gam 1 | 94 | 77 | 125 | |
| EC/EGOM | Gam 19 | 64 | 31 | 112 | |
| WC/WGOM | Keys jar 7 gam 7 | 253 | 233 | 331 | |
| WC/WGOM | Dive 1 fa gam 4 | 250 | 229 | 319 | |
The data for changes in hemolytic activity with growth phase were also analyzed to determine if the trends observed at 27 °C stayed the same over a range of temperatures. The data for G. caribaeus showed the increase in hemolytic activity in late log – early stationary phase was again significant (p < 0.02; Table S14A). The comparison test showed the significant difference was driven by the differences between late log – early stationary phase and mid-stationary phase (p < 0.01; Table S14B). As previously observed, the differences for G. carolinianus were not significant (Table 2 and S15).
3.5. Membrane associated hemolytic activity
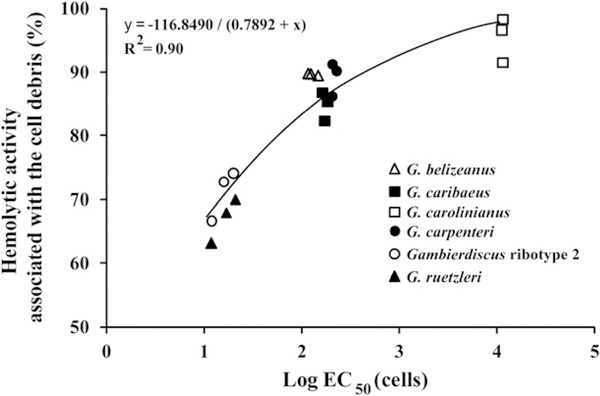
Preliminary experiments indicated a majority of the hemolytic activity remained associated with the cell pellet. This observation implied that a majority of the hemolytic activity was membrane associated. To determine how the amount of membrane-associated activity varied with species, the hemolytic activity was determined for a representative isolate of all six species in log, late log – early stationary and mid-stationary phases. The results again showed that species fell into three distinct hemolytic activity groups. These findings also indicated that the greater the hemolytic activity of the isolate, the smaller the percentage of total activity that was initially membrane bound (Fig. 4).
Fig. 4.
Percent of total hemolytic activity associated with the cell debris after the cells had been disrupted using sonication. These data were determined using a representative clone for each of the six species. For each isolate, the % hemolysis was determined when the cells were in log, late log – early stationary and mid-stationary phase (3 data points for each species).
To determine if the hemolytic activity associated with the cell debris solubilized over the 24 h incubation period, the hemolytic activity of freshly prepared whole cell and cleared lysate were compared with a whole cell preparation which was incubated at 4 °C for 24 h, then cleared by centrifugation prior to assaying the supernatant. The whole cell extract and matching cleared lysate obtained on the same day had estimated EC50 values of 17.5 ± 0.9 and 27.6 ± 1.9 cells, respectively. The cleared lysate incubated for 24 h prior to assay had an estimated EC50 of 20.36 ± 2.6. These data showed that MTX associated with the cell debris solubilized during the 24 h incubation period.
3.6. Inhibition and heat treatment studies
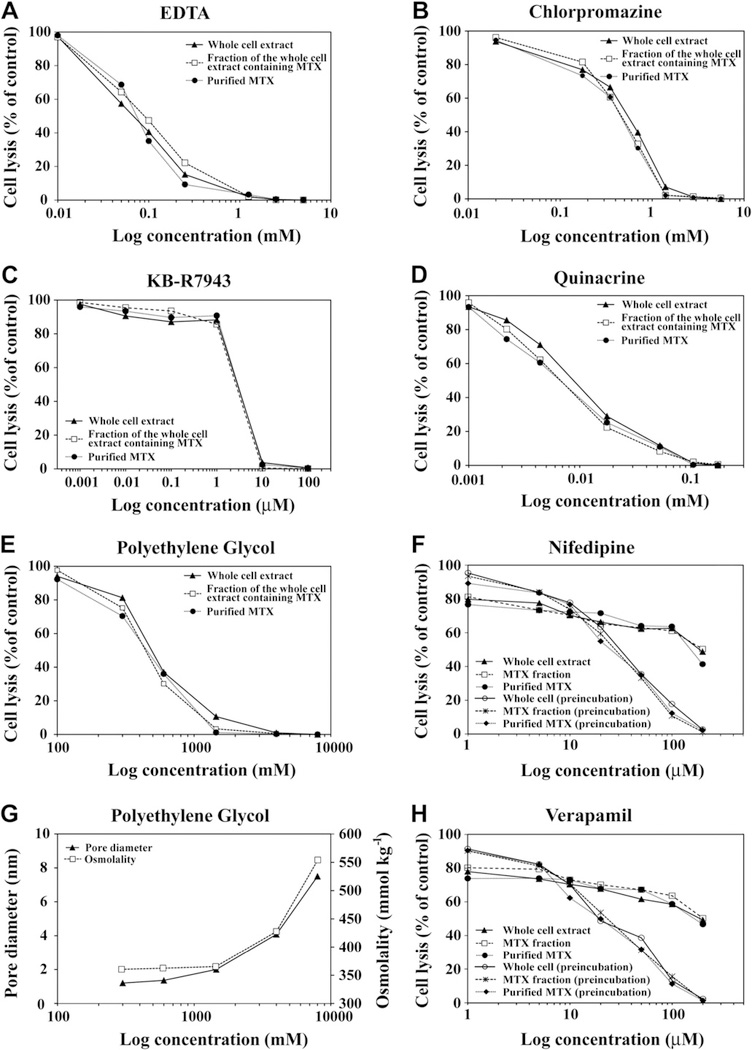
Increasing concentrations of EDTA, PEG, KB-R7943, saxitoxin, verapamil, nifedipine, chlorpromazine, and quinacrine all equally inhibited hemolytic activity of purified MTX, Gambierdiscus whole cell extracts, and partially purified MTX-containing extracts (Fig. 6). These results are consistent with MTX as the cause of the observed hemolytic activity in Gambierdiscus species. The inhibition of lysis caused by PEG began as the effective diameter of the PEG molecules increased from ~0.5 nm to 0.95 nm (PEG 300, 600, and 1450) (Lee et al., 2008). PEG molecules with an effective radius of >2 nm, completely suppressed hemolytic activity (Fig. 6E, G). The estimated radius of the various Ca2+ channels, including NSCCs, range from 0.1 to 0.6 nm, with most estimates in the 0.5–0.6 nm range (McClesky and Almers, 1985; Ternovsky and Berestovsky, 1998; Cataldi et al., 2002). Over the PEG MW size range where the bulk of inhibition occurred, there was little change in osmolality (Fig. 6). This suggested that the reduction in hemolysis was due to non-specific blockage of the ion channels rather than osmotic changes which stabilize the membrane.
Fig. 6.
The effect of increasing concentrations of A) EDTA, B) chlorpromazine hydrochloride, C) KB-R7943, D) quinacrine, E) PEG, F) nifedipine, and H) verapamil on the hemolytic activity of whole cell extracts, partially purified maitotoxin (MTX)-containing cell extracts, and purified MTX G) The relationship between osmolality (mmol kg−1 ) and the approximate molecular diameter (nm) for the various MW PEG solutions used in the experiment. The results were expressed as a percent of total cell lysis compared to a saponin control. Each of the inhibitor dilutions was incubated with a dilution of the whole cell extract or purified MTX just sufficient to cause complete lysis. For the treatments receiving the partially purified MTX-containing extracts, each extract was diluted so the same number of extracted cell equivalents were being added as in the whole cell treatment. The purification process used to prepare the MTX-containing extracts eliminated compounds produced by other phytoplankton and known to cause hemolysis. In the case of nifedipine and verapamil, incubation carried out as in the other treatments only inhibited hemolytic activity by about 60% at the highest concentrations. In contrast, pre-incubation of the erythrocytes with nifedipine and verapamil for 10 min at room temperature prior to adding the MTX containing solutions produced a complete suppression of hemolytic activity.
Results using eight MTX-specific pathway inhibitors were consistent with MTX as the primary hemolytic compound in Gambierdiscus species (Figs. 5 and 6; Yasumoto et al., 1979b; Nakajima et al., 1981; Igarashi et al., 1999; Frew et al., 2008). This conclusion was supported by the finding that extracts heated to 100 °C retained full hemolytic activity.
4. Discussion
4.1. Species-specific differences in hemolytic activity
Gambierdiscus species varied significantly in toxicity (Fig. 1; Table 1 and S1–3). The highest hemolytic activities were observed in Gambierdiscus ribotype 2 isolates and the lowest in G. carolinianus (Figs. 1–3). Limited data on ciguatoxins (CTX), which are related polyketide toxins produced by Gambierdiscus species, also indicated that some species are significantly more toxic than others (Lartigue et al., 2009; Chinain et al., 2010; Litaker et al., 2010; Fraga et al., 2011). The similarity in hemolytic activity normalized on both a per cell and a per biovolume basis showed that differences in hemolytic activity were due to changes in the amount or type of hemolytic compound per unit biovolume and not simply changes in cell size. On a per cell basis, the hemolytic activities measured for the Gambierdiscus species examined in this study were much higher than observed in other dinoflagellates. For example, G. ruetzleri and Gambierdiscus ribotype 2 were four to six-fold more hemolytic than Amphidinium, Coolia, Ostreopsis, and Prorocentrum species (Nakajima et al., 1981; de Motta et al., 1986). The extent to which the higher hemolytic activity was due to the larger cell size of Gambierdiscus, however, could not be determined because the cell volumes of species used in the other studies were not reported.
An inverse relationship between growth rate and CTX toxicity has been reported for Gambierdiscus species (Durand-Clement,1986; Lartigue et al., 2009; Caillaud et al., 2010; Chinain et al., 2010). In contrast, in this study no significant relationship was observed between growth rates and hemolytic activity per cell or per biovolume at any growth phase (Table S4).
Evidence suggests that the hemolytic activity observed in this study is caused by MTX. Consistent with this conclusion, studies where MTX or MTX + CTX-containing cell extracts from different Gambierdiscus cultures were injected into mice revealed large inter-isolate variations in toxicity (Figs. 2 and 3; Yasumoto et al., 1979a; Bagnis et al., 1980; Higerd et al., 1986; McMillan et al., 1986; Durand-Clement, 1987; Bomber et al., 1989b; Holmes et al., 1990; Lartigue et al., 2009; Chinain et al., 2010). Whether the toxicity variations represented species or isolate differences, however, could not be resolved because most isolates were not identified to species.
The assays performed in this study measured total toxicity. So, it was not possible to distinguish if differences in toxicity were due to variations in amount of hemolytic compound per cell or to production of hemolytic compounds with varying toxicities. Work by Holmes et al. (1990) and Holmes and Lewis (1994) suggests if maitotoxin is responsible for hemolytic activity, then inter-isolate differences in hemolytic activity were due, in part, to the specific congeners produced by each isolate. They showed that Gambierdiscus isolates produce at least three unique MTX congeners, maitotoxins 1–3 (MTX-1, MTX-2, MTX3). MTX-1 and MTX-3 are disulphated polyethers whereas MTX-2 is monosulphated. The degree of sulphanation appears to affect the toxicity of the congener (Yokoyama et al., 1988; Murata et al., 1991). MTX-2, for example, resulted in a more rapid time to death in mice compared to an equivalent amount of MTX-1 (Holmes et al., 1990). If related polyketide toxins such as CTX (Yasumoto, 2000), saxitoxins (Mihali et al., 2011) and brevetoxins (Walsh et al., 2008; Watkins et al., 2008) are representative, it is likely that each species produces more than one MTX congener and that the suite of congeners produced by each species will vary. Whether species produce discernibly different congener suites is unknown. Detailed LC-MS studies of multiple Gambierdiscus species will be needed to resolve this question.
4.2. Regional and inter-isolate differences in hemolytic activity
Bomber et al. (1989a, 1989b, 1990) noted an intriguing negative correlation between the latitude where a Gambierdiscus isolate originated and its toxicity (r2 = 0.67, n = 17). Low latitude isolates were generally found to be more toxic than those isolated from higher latitudes. The sample size supporting this trend, was small (17 isolates) and none of the isolates were identified to species. Bomber et al. (1989b) hypothesized this gradient might explain the higher frequency of ciguatera fish poisoning (CFP) at lower latitudes and the relatively low occurrence of CFP despite the presence of abundant Gambierdiscus populations in the Florida Keys. The methanol-based extraction procedure used in the Bomber et al. (1989b) study likely solubilized both MTXs and CTXs. This suggests the observed latitudinal gradient may have been heavily influenced by MTX as well as CTX (Bagnis et al., 1980; Durand-Clement, 1987; Yasumoto et al., 1987; Holmes et al., 1990; Chinain et al., 2010). Our results indicated major regional differences in hemolytic activity exist for isolates originating from (a) the western Caribbean and Gulf of Mexico, (b) the eastern Caribbean north to the Florida Keys and (c) the continental shelf off North Carolina, USA (Figs. 2 and 3; Tables S5–8). Isolates in this study from the eastern Caribbean and Gulf of Mexico, which are representative of the geographic range of the unidentified isolates examined by Bomber et al. (1989a, 1989b, 1990), showed no indication of a latitudinal gradient in toxicity (Figs. 2 and 3; Table 1). Isolates of the same species from this region (as was the case for the other two regions), exhibited very similar hemolytic activities. These data suggested the original observation of an inverse toxicity versus latitudinal gradient was probably due to an inadvertent selection of species with inherently different toxicities.
These results, however, do not exclude the possibility of a different type of latitudinal gradient. All of the G. carolinianus isolates from North Carolina, USA, the most northerly region sampled, were essentially non-toxic whereas those from the rest of the Gulf of Mexico and Caribbean were toxic (Figs. 2 and 3). Adachi et al. (2012) observed a similar pattern in Japan, where the species from the cooler, more northern locations exhibited little toxicity whereas species from the warmer regions to the south were highly toxic.
The low within region variation in hemolytic activity, and the differences between regions, suggest the potential for differentiated Gambierdiscus populations (Figs. 2 and 3; Table 1, S6). This idea, however, should be viewed as a testable hypothesis until a more representative sampling of isolates from other regions of the Caribbean and Gulf of Mexico can be undertaken. Additional data will be necessary to determine if the observed regional variation is due to sampling error or to true differences in hemolytic activity. Ling and Trick (2010) noted similar regional differences in hemolytic activity in the raphidophyte Heterosigma akashiwo, but did not speculate on the underlying selective mechanism responsible for these differences. Similarly, lytic activity varies considerably among different Alexandrium species as well as strains of the same species isolated from different geographic regions (Tillman et al., 2008).
Gambierdiscus caribaeus and G. carolinianus, the species with the most isolates in this study, were used to assess intraspecific variation in hemolytic activities (Fig. 2). For G. caribaeus, the intraspecific variation was approximately 1.8 fold. In contrast, the intraspecific variations in EC50 values for G. carolinianus are approximately 1,000-fold across regions. Whether the inter-isolate variation in other Gambierdiscus species is more like G. caribaeus or G. carolinianus has yet to be determined.
The EC50 values for isolates obtained from other parts of the world also allowed a limited assessment of how hemolytic activity among Gambierdiscus species varies globally. The Pacific isolate of G. caribaeus from Hawaii had an EC50 value of 168 cells, similar to that for the eastern Caribbean and Gulf of Mexico isolates. This is of particular interest because G. caribaeus is one of the species known to have a worldwide distribution (Litaker et al., 2010). Whether regional differences in hemolytic activity similar to those occurring in the Caribbean exist in the Pacific is not known. The isolate of G. carolinianus from Crete had an estimated EC50 value of 183 cells which was comparable to the western Caribbean and Gulf of Mexico isolates (Fig. 2). This is the first report for an isolate of G. carolinianus outside the Atlantic. The other two species examined in this study were G. australes (isolated from Hawaii, EC50 cells = 69 ± 31) and G. pacificus (isolated from the Society Islands, French Polynesia, EC50 cells = 34). To date these species only have been identified as occurring in the Pacific and Indian Oceans (Litaker et al., 2010; Munir et al., 2011). Both species had hemolytic activities comparable to the two most toxic Caribbean species, Gambierdiscus ribotype 2 (EC50 cells = 39 ± 26) and G. ruetzleri (EC50 cells = 39 ± 31 cells) (Fig. 3). In combination, these data show hemolytic activity estimates for isolates obtained from other parts of the world fall within the range of values observed in the Caribbean with some species in each region having significantly higher hemolytic activity than others.
4.3. Variations in hemolytic activity with growth phase
The batch cultures investigated in this study represent progressively more nutrient limited growth conditions. Hemolytic activity normalized on a per cell basis increased in all species from log to late log – early stationary phase (7–39%), but then declined again in mid-stationary phase (14–66% relative to the log phase) (Figs. 1–3). When normalized to biovolume, the same relative changes in hemolytic activity per cell were observed. Studies have shown that toxin levels per cell tend to increase as cells transition from nutrient sufficient to nutrient limited growth, provided the toxin does not contain a growth limiting nutrient (Granéli and Flynn, 2006). In phytoplankton, this increase in toxin content with nutrient-limited growth is best explained by the carbon: nutrient balance hypothesis (CNBH) (Bryant et al., 1983; Ianora et al., 2006; Hardison et al., 2012). This hypothesis predicts that as growth slows under nutrient-limitation, plants divert a greater portion of their fixed carbon from growth to defense, often in the form of increased levels of anti-grazing and allelopathic compounds (e.g. toxins). Diverting carbon into these compounds allows plants to protect the photosynthetic electron transport chain against the damaging buildup of electrons and free radicals. This happens until the photosynthetic apparatus can be down regulated so that the rate of C-fixation more closely matches that needed to sustain slower growth (Sakshaug et al., 1989; Sunda et al., 2007). The increased toxicity also deters grazing losses as cell division rates decline. Under this scenario, toxicity increases into early stationary phase then declines as the photosynthetic capacity and growth rate become more balanced. The best illustration of this phenomenon in Gambierdiscus is the study by Chinain et al. (2010) where a batch culture of Gambierdiscus polynesiensis was sampled at five day intervals for forty days. At each sampling interval, cells were removed and extracted to obtain the MTX or CTX fractions. These extracts were then assayed using the mouse bioassay. As cells entered log phase growth both MTX and CTX toxicity per cell declined, then increased steadily in late log and early stationary phase reaching their highest concentration around day 25. By mid to late stationary phase (day 30), MTX and CTX toxicity per cell both declined significantly. A similar increase in MTX or total MTX + CTX toxicity per cell in late log – early stationary phase has been noted in other mouse LC50 bioassay studies (Yasumoto, 1979b; Bomber et al., 1989b; Sperrand Doucette, 1996; Lartigue et al., 2009). This increase was particularly apparent when the cells were P-limited versus N-limited (Sperr and Doucette, 1996; Lartigue et al., 2009). These changes in MTX toxicity with growth phase were consistent with those observed for hemolytic activity in this study (Figs. 1–3). The only exception to this general trend was the report of Durand-Clement (1987), which did not find a change in toxicity as cultures aged.
4.4. The effect of growth temperature on hemolytic activity
The hemolytic activities of the 56 isolates surveyed in this study were all grown at 27 °C. Whether the results truly represent interspecific and intraspecific differences in hemolytic activity depends on whether toxicity was measured at optimal temperatures for toxin production. To address this issue, hemolytic activity was determined for G. caribaeus and G. carolinianus isolates from different geographic regions grown at 20, 24, 27, and 31 °C. For G. caribaeus, hemolytic activity increased on average by about 5% from 20 to 24 °C and by 29% from 24 to 27 °C (Table 2). Only the increase from 20 to 27 °C was significant (Tables S12–14). In contrast, G. carolinianus hemolytic activity showed a non-significant increase of only 3–5% between 20 and 27 °C (Table S15). Elevated growth temperatures therefore either failed to increase hemolytic activity, or to increase it only slightly. Durand-Clement (1986) similarly reported that total toxicity measured using mouse bioassays for two different Gambierdiscus isolates grown at 20 and 26 °C were the same. These data revealed hemolytic activity for each species was maximal when growth temperatures were ~27 °C and the resulting EC50 data for isolates grown at 27 °C could be used to establish differences in hemolytic activity among isolates and species. It also indicates that temperature plays a minor role in regulating the toxicity of Gambierdiscus species.
4.5. Stability of hemolytic activity through time
Another striking feature of individual isolates is the stability of hemolytic activity through time. The hemolytic activity of isolates measured as much as two years apart yielded EC50 estimates which were within 5% of one another (Figs. 2 and 3, Table 1). Durand-Clement (1987) and Chinain et al. (2010) observed a similar pattern where CTX per Gambierdiscus cell remained constant over 15 years. These observations suggest that production of both CTX and MTX are under strong genetic control and the amount of toxin produced remains constant through time (Holmes et al., 1990; Lartigue et al., 2009; Chinain et al., 2010).
4.6. The amount of hemolytic activity associated with the membrane fraction
Depending on the species, ~ 60–90% of the hemolytic activity was associated with the cell wall and cellular debris and not immediately released into solution when cells were lysed in ELA buffer (Fig. 4). A recent report indicates that brevetoxins, which are structurally related to MTX, are retained in membrane lined vesicles (Vigna et al., 2012). If hemolytic compounds produced by Gambierdiscus are sequestered in similar structures or bound to proteins it could account for why they are not immediately released into the aqueous phase when cells are disrupted, but are instead released over a 24 incubation period. A slow release of hemolytic compounds from the membrane is consistent with what is known about MTX. Though MTX is water soluble, <3% of total cellular MTX is released into the culture media, except during periods when cells begin to senesce and lyse (Holmes et al., 1990; Chinain et al., 2010; Caillaud et al., 2011).
4.7. Inhibition studies support MTX as the dominant hemolytic compound produced by Gambierdiscus species
The inhibition and heat treatment studies were all consistent with MTX as the primary hemolytic compound produced by Gambierdiscus species (Figs. 5 and 6). The MTX pathway inhibitors consistently suppressed hemolytic activity. These same inhibitors would not consistently interfere with activity of the hemolytic reactive oxygen species (Chiu et al., 1982; Marshall et al., 2003), polyunsaturated fatty acids (Yasumoto et al., 1990; Landsberg, 2002; de Boer et al., 2009), and glycolipids (Marshall et al., 2003; Emura et al., 2004; Ma et al., 2011; Pagliara and Caroppo, 2011) produced by other phytoplankton species. This conclusion is further bolstered by the results of the cell extraction experiments where the polyunsaturated fatty acids, glycolipids, and proteins critical to the various hemolytic pathways cited above had been removed. The hydrophilic fraction from whole cell extracts containing MTX exhibited the same hemolytic activity on a per cell basis as the whole cell extracts (Fig. 6). Similarly, heating samples to 100 °C for 10 min, which deactivates hemolytic polyunsaturated fatty acids, glycolipids, and proteins failed to reduce hemolytic activity (Marshall et al., 2003; de Boer et al., 2009; Pagliara and Caroppo, 2011). MTX, in contrast is heat stable.
The one set of hemolytic compounds produced by dinoflagellates, which would not have been eliminated during the extraction process were the pore forming polyketides, such as karlotoxins (KmTx) (Deeds, 2003; Deeds and Place, 2006; Sinkins et al., 2009). KmTxs partition into the same hydrophilic fraction as MTX and are heat stable even when heated to 100 °C. Thus, a contribution by these compounds is possible but unlikely. Extensive fractionation studies over 30 years have consistently shown Gambierdiscus species only produce CTX, MTX and gambierol and no other polyketide toxins analogous to karlotoxins (Yasumoto et al., 1976,1977,1979a, 1979b; Yasumoto, 1985; Holmes et al., 1990; Chinain et al., 2010). The preponderance of evidence from these experiments therefore confirms the earlier work of Igarashi et al. (1999) who suggested that hemolytic activity in Gambierdiscus extracts is caused by MTX. Given that the Ca2+ transport systems in erythrocytes have homologues in other mammalian tissues, hemolytic activity is likely to be a good predictor of relative MTX toxicity.
Interestingly, KB-R7943, which selectively prevents the Na+–Ca2+ exchanger (NCX) from transporting Ca2+ out of the cell, completely suppressed hemolysis (Frew et al., 2008). This result confirmed the NCX is a major target of hemolytic compounds produced by Gambierdiscus species. The role of the Ca2+-permeable, non-selective cation channels was not directly addressed in this study, but numerous reports have confirmed that MTX induces calcium flux into all cell types via these pores (Schilling et al., 1999; Lundy et al., 2004; Wisnoskey et al., 2004). In contrast, results from Igarashi et al. (1999) indicated that L-type Ca2+ channels were not the major target of MTX. The hemolytic activity results obtained in this study using the L-type Ca2+ channel blockers nifedipine, verapamil, and saxitoxin (Su et al., 2004) were consistent with Igarashi et al. (1999). All these inhibitors only partially suppressed hemolytic activity when erythrocytes were simultaneous incubated with an inhibitor and purified MTX, whole cell extracts, or partially purified MTX-containing cell extracts (Fig. 6). In contrast, hemolytic activity was completely suppressed when the erythrocytes were pre-incubated with sufficiently high concentrations of nifedipine, verapamil, and saxitoxin for 10 min prior to addition of either purified MTX, whole cell extracts, or partially purified (MTX-containing) cell extracts (Fig. 5, Fig. 6F, H; S3). It is possible that, given a head start, these inhibitors bind and block a common MTX receptor which subsequently prevents MTX from triggering a Ca2+ influx through the other ion channels. The existence of such a receptor has been postulated but not substantiated (Lundy et al., 2004; de la Rosa et al., 2001; Konoki et al., 2009; Wang et al., 2009). Another possibility is that given a head start, these molecules function like PEG to plug the various calcium channels thereby preventing MTX from triggering a Ca2+ influx of into the cell.
Incubation of erythrocytes with the CTX-containing whole cell extract resulted in negligible hemolysis, even when the extracts were used at a100-fold higher concentration than the corresponding MTX fraction which produced 100% lysis. This finding contrasts with an early study of Nakajima et al. (1981) who reported Gambierdiscus CTX extracts had hemolytic activity. This discrepancy could have been due to the extraction procedure used in the original study which allowed carryover of some MTX into the “CTX” fraction. The lack of significant hemolytic activity is also consistent with the fact the CTX activates Na+ rather than Ca2+ channels (Gawley et al., 1992; Strachan et al., 1999; Ghiaroni et al., 2006) (Fig. 5).
In summary, this study showed that Gambierdiscus species varied significantly in average toxicity as measured using a hemolytic assay. Toxicity consistently increased from log to late log – early stationary phase and then declined in mid-stationary phase (Figs. 1–3; SI). This increase in toxicity was consistent with the carbon: nutrient balance hypothesis which predicts that cellular carbon will be diverted to defensive compounds as growth slows. Growth temperature did not dramatically affect hemolytic activity. Strong regional differences in toxicity were observed, but did not conform to the latitudinal toxicity gradient previously reported for Gambierdiscus species. The strong regional differences in hemolytic activity indicate there may be regionally differentiated populations, but the selective pressure that could account for these differences is unknown. Toxin production was consistent over multiple years indicating these toxins are constitutively produced and under strong genetic regulation. Data from various inhibition, cell extraction and heat treatment experiments were all consistent with maitotoxin as the primary hemolytic compound produced by Gambierdiscus species.
Supplementary Material
Acknowledgements
We would like to thank Sherwood Hall for providing the saxitoxin and John Ramsdell for the purified maitotoxin. Patrice Mason ran the osmolality analysis. Richard Lewis kindly provided toxin extraction protocols. We would also like to thank the follow individuals from the National Ocean Service, National Centers for Coastal Ocean Science, Center for Coastal Fisheries and Habitat Research and colleagues assisting CCFHR on dive operations for their willingness to collect samples from various locations in the Gulf of Mexico and the Caribbean: Paula Whitfield, Christine A. Buckel, Brian Degan, Don Field, Jenny VanderPlyum, Roldan Muñoz, John Burke, Dave Cerino, Michael Dowgiallo, Wilson Freshwater, David Johnson, Brett Harrison, Doug Kesling, William Lee, Roger Mays, James Morris, Brandon Puckett, and Sherry Reed. We also kindly thank Frankie and Chris Hill for providing samples. Avery Tatters and Joanne Kelly from the Tomas lab at UNCW kindly provided instruction and helped conduct some to the initial hemolytic assays. Reference to trade names does not imply product endorsement by the National Ocean Service, NOAA. Partial support was provided by the Ecology and Oceanography of Harmful Algal Blooms (ECOHAB) program, contribution #724. This is contribution #4715 from the University of Maryland Center for Environmental Sciences and contribution #13102 from the Institute of Marine and Environmental Technology.
Footnotes
The U.S. Government has the right to retain a nonexclusive, royalty-free license in and to any copyright covering this paper.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.toxicon.2012.12.016.
Definitions: Calpains, calcium-dependent, non-lysosomal cysteine proteases; Hemolytic assay, the abbreviated name for erythrocyte lysis assay; LC–MS, Liquid chromatography-mass spectrometry; PLA2, phospholipase A2.
References
- Adachi M, Yoshimatsu T, Iwamoto H, Nishimura T, Yamaguchi H, 2012. Effect of temperature change on the dominant species of Gambierdiscus in Japan - from a non-toxic species to a toxic species. Abstract. 15th International Conference on Harmful Algae October 29–November 2, 2012, Gyeongnam, Korea, p. 70. [Google Scholar]
- Bagnis R, Chanteau S, Chungue E, Hurtel JM, Yasumoto T, Inoue A, 1980. Origins of ciguatera fish poisoning: a new dinoflagellate, Gambierdiscus toxicus Adachi and Fukuyo, definitively involved as a causal agent. Toxicon 18, 199–208. [DOI] [PubMed] [Google Scholar]
- Bagnis R, Bennett J, Prieur C, Legrand AM, 1985. The dynamics of three toxic benthic dinoflagellates and the toxicity of ciguateric surgeonfish in French Polynesia In: Anderson DM, White AW, Baden DG (Eds.), Toxic Dinoflagellates. Elsevier Scientific Publishing Co., New York, pp. 177–182. [Google Scholar]
- Bignami G, Waller D, Yasumoto T, 1996. Antibodies to maitotoxin elicited by immunization with toxin-fragment conjugates. Toxicon 34, 1393–1397. [DOI] [PubMed] [Google Scholar]
- Bomber JW, Rubio MG, Norris DR, 1989a. Epiphytism of dinoflagellates associated with the disease ciguatera: substrate specificity and nutrition. Phycologia 28, 360–368. [Google Scholar]
- Bomber JW, Tindall DR, Miller DM, 1989b. Genetic variability in toxin potencies among seventeen clones of Gambierdiscus toxicus (Dinophyceae). J. Phycol 25, 617–625. [Google Scholar]
- Bomber JW, Tindall DR, Venable CW, Miller DM, 1990. Pigment composition and low-light response of fourteen clones of Gambierdiscus toxicus In: Graneli E (Ed.), Toxic Marine Phytoplankton. Elsevier, New York, pp. 263–268. [Google Scholar]
- Bryant J, Chapin F III, Klein D, 1983. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40, 357–368. [Google Scholar]
- Burke WA, Tester PA, 2002. Skin problems related to noninfectious coastal microorganisms. Dermatol. Ther 15,10–17. [Google Scholar]
- Caillaud A, Yasumoto T, Diogene J, 2010. Detection and quantification of maitotoxin-like compounds using a neuroblastoma (Neuro-2a) cell based assay. Application to the screening of maitotoxin-like compounds in Gambierdiscus spp. Toxicon 56, 36–44. [DOI] [PubMed] [Google Scholar]
- Caillaud A, Iglesia P, Barber E, Eixarch H, Mohammad-Noor N, Yasumoto T, Diogene J, 2011. Monitoring of dissolved ciguatoxin and maitotoxin using solid-phase adsorption toxin tracking devices: application to Gambierdiscus pacificus in culture. Harmful Algae 10,433–446. [Google Scholar]
- Cataldi M, Perez-Reyes E, Tsien RW, 2002. Differences in apparent pore sizes of low and high voltage-activated Ca2+ channels. J. Biol. Chem 76, 45969–45976. [DOI] [PubMed] [Google Scholar]
- Chinain M, Darius HT, Ung A, Cruchet P, Wang Z, Ponton D, Laurent D, Pauillac S, 2010. Growth and toxin production in the ciguatera-causing dinoflagellate Gambierdiscus polynesiensis (Dinophyceae) in culture. Toxicon 56, 739–750. [DOI] [PubMed] [Google Scholar]
- Chinain M, Faust MA, Pauillac S, 1999. Morphology and molecular analyses of three toxic species of Gambierdiscus (Dinophyceae): G. pacificus, sp. nov., G. australes, sp. nov., and G. polynesiensis, sp. nov. J. Phycol 35, 1282–1296. [Google Scholar]
- Chiu D, Lubin B, Shohet SB, 1982. Peroxidative reactions in red cell biology In: Pryor WA (Ed.), Free Radicals in Biology, vol. V Academic Press, New York. [Google Scholar]
- Damude HG, Kinney AJ, 2008. Enhancing plant seed oils for human nutrition. Plant Physiol. 147, 962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer K,M, Tyl MR, Fu M, Kulk G, Liebezeit G, Tomas CR, Lenzi A, Naar J, Vrieling EG, van Rijssel M, 2009. Haemolytic activity within the species Fibrocapsa japonica (Raphidophyceae). Harmful Algae 8, 699–705. [Google Scholar]
- de la Rosa LA, Alfonso A, Vilarino N, Vieytes MR, Yasumoto T, Botana LM, 2001. Maitotoxin-induced calcium entry in human lymphocytes: modulation by yessotoxin, Ca2+ channel blockers and kinases. Cell. Signal 13, 711–716. [DOI] [PubMed] [Google Scholar]
- de Motta GE, Rodriguez-Costas I, Tosteson TR, Ballantine DL, Durst HD, 1986. Lysis of red blood cells by extracts from benthic dinoflagellates. PRHSJ 5, 133–136. [PubMed] [Google Scholar]
- Deeds JR, 2003. Toxins and toxicity from the cosmopolitan, bloom-forming dinoflagellate Karlodinium micrum. Doctoral dissertation. University of Maryland, College Park. [Google Scholar]
- Deeds JR, Place AR, 2006. Sterol specific membrane interactions with the toxins from Karlodinium micrum (Dinophyceae) a strategy for self-protection. Afr. J. Mar. Sci 28, 421–427. [Google Scholar]
- Diogène G, Fessard V, Dubreuil A, Puiseux-Dao S, 1995. Comparative studies of the actin cytoskeleton response to maitotoxin and okadaic acid. Toxicol. In Vitro 9, 1–10. [DOI] [PubMed] [Google Scholar]
- Durand-Clement M, 1986. A study of toxin production by Gambierdiscus toxicus in culture. Toxicon 24,1153–1157. [DOI] [PubMed] [Google Scholar]
- Durand-Clement M, 1987. Study of production and toxicity of cultured Gambierdiscus toxicus. Biol. Bull 172,108–121. [Google Scholar]
- Emura A, Matsuyama Y, Oda T, 2004. Evidence for the production of a novel proteinaceous hemolytic exotoxin by dinoflagellate Alexandrium taylori. Harmful Algae 3, 29–37. [Google Scholar]
- Eschbach E, Scharsack JP, John U, Medlin LK, 2001. Improved erythrocyte lysis assay in microtitre plates for sensitive detection and efficient measurement of hemolytic compounds from ichthyotoxic algae. J. Appl. Toxicol 21, 513–519. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Ong W-Y, Horrocks LA, 2006. Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol. Rev 58, 591–620. [DOI] [PubMed] [Google Scholar]
- Few R, Wang Y, Weiss TM, Nelson P, Sawyer TW, 2008. Attenuation of matitoxin-induced cytotoxicity in rate aortic smooth muscle cells by inhibitors of Na+/Ca2+ exchange, and calpain activation. Toxicon 51, 1400–1408. [DOI] [PubMed] [Google Scholar]
- Fraga S, Rodríguez F, Caillaud A, Diogène J, Raho N, Zapata M, 2011. Gambierdiscus excentricus sp. nov. (Dinophyceae), a benthic toxic dinoflagellate from the Canary Islands (NE Atlantic Ocean). Harmful Algae 11, 10–22. [Google Scholar]
- Frew R, Wang Y, Weiss TM, Nelson P, Sawyer TW, 2008. Attenuation of maitotoxin-induced cytotoxicity in rat aortic smooth muscle cells by inhibitors of Na+/Ca2+ exchange, and calpain activation. Toxicon 51, 1400–1408. [DOI] [PubMed] [Google Scholar]
- Friedman MA, Fleming LE, Fernandez M, Bienfang P, Schrank K, Dickey R, Bottein M-Y, Backer L, Ayyar R, Weisman R, Watkins S, Granade R, Reich A, 2008. Ciguatera fish poisoning: treatment, prevention and management. Mar. Drugs 6 (3), 456–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawley RE, Rein KS, Kinoshita M, Baden DG, 1992. Binding of brevetoxins and ciguatoxin to the voltage-sensitive sodium channel and conformational analysis of brevetoxin B. Toxicon 30, 780–785. [DOI] [PubMed] [Google Scholar]
- Ghiaroni V, Fuwa H, Inoue M, Sasaki M, Miyazaki K, Hirama M, Yasumoto T, Rossini GP, Scalera G, Bigiani A, 2006. Effect of Ciguatoxin 3C on voltage-gated Na+ and K+ currents in mouse taste cells. Chem. Senses 31, 673–680. [DOI] [PubMed] [Google Scholar]
- Granéli E, Flynn K, 2006. Chapter 18. Chemical and physical factors influencing toxin content In: Granéli E, Turner JT (Eds.), Ecology of Harmful Algae. Ecological Studies, vol. 189, pp. 299–309. [Google Scholar]
- Graphpad Software, Inc, 1999. GraphPad Prism 4. http://www.graphpad.rcom/curvefit/introduction89.htm.
- Hardison DR, Sunda WG, Litaker RW, 2012. Nitrogen limitation increases brevetoxins in Karenia brevis (Dinophyceae): implications for bloom toxicity. J. Phycol 48 (4), 844–858. [DOI] [PubMed] [Google Scholar]
- Higerd TB, Babinchak JA, Scheuer PJ, Jollow DJ, 1986. Resolution of ciguatera-associated toxins using high-performance liquid chromatography (HPLC). Mar. Fish. Rev 48 (4), 23–28. [Google Scholar]
- Hilf RJC, Bertozzi C, Zimmermann I, Reiter A, Trauner D, Dutzler R, 2010. Structural basis of open channel block in a prokaryotic pentameric ligand-gated ion channel. Nature Struc. Mole. Biol 17,1330–1336. [DOI] [PubMed] [Google Scholar]
- Holmes MJ, Lewis RJ, 1994. Purification and characterisation of large and small maitotoxins from cultured Gambierdiscus toxicus. Nat. Toxin 2 (2), 64–72. [DOI] [PubMed] [Google Scholar]
- Holmes MJ, Lewis RJ, Gillespie NC, 1990. Toxicity of Australian and French Polynesian strains of Gambierdiscus toxicus (Dinophyceae) grown in culture: characterization of a new type of maitotoxin. Toxicon 28, 1159–1172. [DOI] [PubMed] [Google Scholar]
- Hromada LP, Nablo BJ, Kasianowicz JJ, Gaitan MA, DeVoe DL, 2008. Single molecule measurements within individual membrane-bound ion channels using a polymer-based bilayer lipid membrane chip. Lab. Chip 8, 602–608. [DOI] [PubMed] [Google Scholar]
- Ianora A, Boersma M, Casotti R, Fontana A, Harder J, Hoffmann F, Pavia H, Potin P, Poulet SA, Toth G, 2006. New trends in marine chemical ecology. Estuar. Coast 29, 531–551. [Google Scholar]
- Igarashi T, Aritake S, Yasumoto T, 1999. Mechanisms underlying the hemolytic and ichthyotoxic activities of maitotoxin. Nat. Toxin, 71–79. [DOI] [PubMed] [Google Scholar]
- Keller MD, Slevin RC, Claus W, Guillard RRL, 1987. Media for the culture of oceanic ultraphytoplankton. J. Phycol 23, 633–638. [Google Scholar]
- Kibler SR, Litaker RW, Holland WC, Vandersea MW, Tester PA, 2012. Growth of Gambierdiscus (Dinophyceae): Effects of temperature, salinity and irradiance. Harmful Algae. 10.1016/j.hal.2012.04.007. [DOI] [Google Scholar]
- Kohli GS, Rhodes L, Harwood T, Selwood A, Jerrett A, Neilsen B, Murray S, 2012. A feeding study to probe the uptake of Maitotoxin by snapper (Pagrus auratus). Abstract. In: 15th International Conference on Harmful Algae October 29-November 2, 2012, Gyeongnam, Korea, p. 79. [Google Scholar]
- Konoki K, Hashimoto M, Honda K, Tachibana K, Tamate R, Hasegawa F, Oishi T, Murata M, 2009. Maitotoxin-photoactive probe binds to membrane proteins in blood cells. Heterocycles 79, 1007–1017. [Google Scholar]
- Kruskal WH, Wallis WA, 1952. Use of ranks in one-criterion variance analysis. J. Amer. Stat. Assoc 47, 583–621. [Google Scholar]
- Landsberg JH, 2002. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci 10, 113–390. [Google Scholar]
- Lartigue J, Jester ELE, Dickey RW, Villareal TA, 2009. Nitrogen source effects on the growth and toxicity of two strains of the ciguatera-causing dinoflagellate Gambierdiscus toxicus. Harmful Algae 8, 781–791. [Google Scholar]
- Law SK, Lichtenberg NA, Levine RP, 1980. Covalent binding and hemolytic activity of complement proteins. Proc. Natl. Acad. Sci 77, 7194–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Venable RM, MacKerell AD Jr., Pastor RW, 2008. Molecular dynamics studies of polyethylene oxide and polyethylene glycol: hydrodynamic radius and shape anisotropy. Biophys. J 95, 1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RJ, Yang A, Jones A, 2009. Rapid extraction combined with LC-tandem mass spectrometry (CREM-LC/MS/MS) for the determination of ciguatoxins in ciguateric fish flesh. Toxicon 54, 62–66. [DOI] [PubMed] [Google Scholar]
- Ling C, Trick CG, 2010. Expression and standardized measurement of hemolytic activity in Heterosigma akashiwo. Harmful Algae 9, 522–529. [Google Scholar]
- Litaker RW, Vandersea MW, Faust MA, Kibler SR, Chinain M, Holmes MJ, Holland WC, Tester PA, 2009. Taxonomy of Gambierdiscus including four new species, Gambierdiscus caribaeus sp. nov., Gambierdiscus carolinianus sp. nov., Gambierdiscus carpenteri sp. nov. and Gambierdiscus ruetzleri sp. nov. (Gonyaulacales, Dinophyceae). Phycologia 48, 344–390. [Google Scholar]
- Litaker RW, Vandersea MW, Faust MA, Kibler SR, Nau AW, Holland WC, Chinain M, Holmes MJ, Tester PA, 2010. Global distribution of ciguatera causing dinoflagellates in the genus Gambierdiscus. Toxicon 56, 711–730. [DOI] [PubMed] [Google Scholar]
- Louzao M, Vieytes M, Yasumoto T, Yotsu-Yamashita M, Botana L, 2006. Changes in membrane potential: an early signal triggered by neurologically active phycotoxins. Chem. Res. Toxicol 19, 788–e793. [DOI] [PubMed] [Google Scholar]
- Lundy PM, Nelson P, Mi L, Frew R, Minaker S, Vair C, Sawyer TW, 2004. Pharmacological differentiation of the P2X7 receptor and the maitotoxin-activated cationic channel. Eur. J. Pharm 487, 17–28. [DOI] [PubMed] [Google Scholar]
- Ma H, Krock B, Tillmann U, Bickmeyer U, Graeve M, Cembella A, 2011. Mode of action of membrane-disruptive lytic compounds from the marine dinoflagellate Alexandrium tamarense. Toxicon 58, 247–258. [DOI] [PubMed] [Google Scholar]
- Marshall JA, Nichol PD, Hamilton B, Lewis RJ, Hallegraeff GM, 2003. Ichthyotoxicity of Chattonella marina (Raphidophyceae) to damselfish (Acanthochromis polycanthus): the synergistic role of reactive oxygen species and free fatty acids. Harmful Algae 2, 273–281. [Google Scholar]
- McClesky EW, Almers W, 1985. The Ca2+ channel in skeletal muscle is a large pore. Proc. Natl. Acad. Sci 82, 7149–7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan JP, Hoffman PA, Granade HR, 1986. Gambierdiscus toxicus from the Caribbean: a source of toxins involved in ciguatera. Mar. Fish. Rev 48 (4), 48–52. [Google Scholar]
- Micouin L, Chinain M, Asin P, Legrand AM, 1992. Toxicity of French Polynesian strains of Gambierdiscus toxicus in cultures. Bull. Soc. Path. Exot 85, 474–477. [PubMed] [Google Scholar]
- Mihali TK, Carmichael W, Neilan BA, 2011. A putative gene cluster from a Lyngbya wollei bloom that encodes paralytic shellfish toxin biosynthesis. PLoS ONE 6 (2), el4657 10.1371/journal.pone.001465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movileanu L, Cheley S, Bayley H, 2003. Partitioning of individual flexible polymers into a nanoscopic protein pore. Biophys. J 85, 897–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir S, Siddiqui PJA, Morton S, 2011. The occurrence of the ciguatera fish poisoning producing dinoflagellate genus Gambierdiscus in Pakistan waters. Algae 26, 317–325. [Google Scholar]
- Murata M, Gusovsky F, Sasaki M, Yokoyama A, Yasumoto T, Daly J, 1991. Effect of maitotoxin analogues on calcium influx and phosphoinositide breakdown in cultured cells. Toxicon 29, 1085–1096. [DOI] [PubMed] [Google Scholar]
- Murata M, Gusovsky G, Yasumoto T, Daly JW, 1992. Selective stimulation of Ca2+ flux in cells by maitotoxin. Eur. J. Pharm 227, 43–49. [DOI] [PubMed] [Google Scholar]
- Murata M, Naoki H, Matsunaga S, Satake M, Yasumoto T, 1994. Structure and partial stereochemical assignments for maitotoxin, the most toxic and largest natural Non-biopolymer. J. Amer. Chem. Soc 116, 7098–7107. [Google Scholar]
- Nakajima I, Yaukatsu O, Yaumoto T, 1981. Toxicity of benthic dinoflagellates in Okinawa. Bull. Jpn. Soc. Fish 47,1029–1033. [Google Scholar]
- Ohizumi Y, Yasumoto T, 1983. Contraction and increase in tissue calcium content induced by maitotoxin, the most potent known marine toxin, in intestinal smooth muscle. Br. J. Pharmacol 79, 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliara P, Caroppo C, 2011. Cytotoxic and antimitotic activities in aqueous extracts of eight cyanobacterial strains isolated from the marine sponge Petrosia ficiformis. Toxicon 57, 889–896. [DOI] [PubMed] [Google Scholar]
- Sakshaug E, Andresen K, Kiefer D, 1989. A steady state description of growth and light absorption in the marine planktonic diatom Skeletonema costatum. Limnol. Oceanogr 34,198–205. [Google Scholar]
- Schilling W, Wasylyna T, Dubyak G, Humphreys B, Sinkins W, 1999. Maitotoxin and P2Z/P2X7 purinergic receptor stimulation activate a common cytolytic pore. Am. J. Physiol. Cell. Physiol 277, 766–776. [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan J, 1988. Non Parametric Statistics for the Behavioural Sciences. MacGraw Hill Int., New York, pp. 213–214. http://pagesperso-orange.fr/giraudoux/#pgirmess. [Google Scholar]
- Sinkins WG, Estacion M, Prasad V, Goe M, Shull GE, Kunze DL, Schilling WP, 2009. Maitotoxin converts the plasmalemmal Ca2+ pump into a Ca2+-permeable nonselective cation channel. Amer. J. Physiol. Cell. Physiol 297, C1533–C1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperr AE, Doucette GJ, 1996. Variation in growth rate and ciguatera toxin production among geographically distinct isolates of Gambierdiscus toxicus In: Yasumoto T, Oshima Y, Fukuyo Y (Eds.), Harmful and Toxic Algal Blooms. Intergovernmental Oceanographic Commission of UNESCO, pp. 309–312. [Google Scholar]
- Strachan LC, Lewis RJ, Nicholson GM, 1999. Differential actions of pacific ciguatoxin-1 on sodium channel subtypes in mammalian sensory neurons. J. Pharmacol. Exp. Ther 288, 379–388. [PubMed] [Google Scholar]
- Su Z, Sheets M, Ishida H, Li F, Barry WH, 2004. Saxitoxin blocks L-type I Ca2+. J. Pharmacol. Exp. Ther 308, 324–329. [DOI] [PubMed] [Google Scholar]
- Sunda WG, Hardison R, Kiene RP, Bucciarelli E, Harada H, 2007. The effect of nitrogen limitation on cellular DMSP and DMS release in marine phytoplankton: climate feedback implications. Aquat. Sci 69, 341–351. [Google Scholar]
- Takahashi M, Ohizumi Y, Yaumoto T,1982. Maitotoxin, a Ca2+ channel activator candidate. J. Biol. Chem 257, 7287–7289. [PubMed] [Google Scholar]
- Takahashi M, Tatsumi M, Ohizumi Y, Yaumoto T, 1983. Ca2+ channel activating function of maitotoxin, the most potent marine toxin known, in clonal rat pheochromocytoma cells. J. Biol. Chem 258 (18), 10,944–10,949. [PubMed] [Google Scholar]
- Tatters AO, Muhlstein HI, Tomas CR, 2009. The hemolytic activity of Karenia selliformis and two clones of Karenia brevis throughout a growth cycle. J. Appl. Phycol 22, 435–442. [Google Scholar]
- Ternovsky VI, Berestovsky GN, 1998. Effective diameter and structural organization of reconstituted calcium channels from the Characeae algae Nitellopsis. Membr. Cell. Biol 12, 79–88. [PubMed] [Google Scholar]
- Tester PA, Feldman RL, Nau AW, Kibler SR, Litaker RW, 2010. Ciguatera fish poisoning and sea surface temperatures in the Caribbean Sea and the West Indies. Toxicon 5, 698–710. [DOI] [PubMed] [Google Scholar]
- Tillmann U, Alpermann T, John U, Cembella A, 2008. Allelochemical interactions and short-term effects of the dinoflagellate Alexandrium on selected photoautotrophic and heterotrophic protists. Harmful Algae 7, 52–64. [Google Scholar]
- Vandersea MW, Kibler SR, Holland WC, Tester PA, Schultz TF, Faust MA, Holmes MJ, Chinain M, Litaker RW, 2012. Development of semi-quantitative PCR assays for the detection and enumeration of Gambierdiscus species (Gonyaulacales, Dinophyceae). J. Phycol. 10.1111/j.1529-8817.2012.1146-11-026.x [DOI] [PubMed] [Google Scholar]
- Vigna KL, Quesada I, Verdugo P, 2012. Exocytic mechanisms of storage and release of brevetoxin in the dinoflagellate Karenia brevis. Biophys. J 102, 319a–320a. 10.1016/j.bpj.2011.11.1755. [DOI] [Google Scholar]
- Walsh CJ, Leggett SR, Strohbehn K, Pierce RH, Sleasman JW, 2008. Effects of in vitro brevetoxin exposure on apoptosis and cellular metabolism in a leukemic T cell line (Jurkat). Mar. Drugs 6, 291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Weiss MT, Yin J, Frew R, Tenn C, Nelson PP, Vair C, Sawyer TW, 2009. Role of the sodium hydrogen exchanger in maitotoxin-induced cell death in cultured rat cortical neurons. Toxicon 54, 95–102. [DOI] [PubMed] [Google Scholar]
- Watkins SM, Reich A, Fleming LE, Hammond R, 2008. Neurotoxic shellfish poisoning. Mar. Drugs 6, 431–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnoskey BJ, Estacion M, Schilling WP, 2004. Maitotoxin-induced cell death cascade in bovine aortic endothelial cells: divalent cation specificity and selectivity. Am. J. Physiol. Cell. Physiol 287, C345–C356. [DOI] [PubMed] [Google Scholar]
- Yasumoto T, 1985. Recent progress in the chemistry of dinoflagellate toxins. In: Anderson DM, White AW, Baden DG (Eds.), Toxic Dinoflagellates, Proceedings of the Third International Conference Elsevier, New York, pp. 259–270. [Google Scholar]
- Yasumoto T, 2000. The chemistry and biological function of natural marine toxins. Chem. Rec 1, 228–242. [DOI] [PubMed] [Google Scholar]
- Yasumoto T, Bagnis R, Vernoux JP, 1976. Toxicity of surgeonfishes. II-Properties of the principal water-soluble toxin. Bull. Jpn. Soc. Sci. Fish 42, 359–365. [Google Scholar]
- Yasumoto T, Nakajima I, Bagnis R, Adachi R, 1977. Finding a dinoflagellate as a likely culprit of ciguatera. Bull. Jpn. Soc. Sci. Fish 43, 1021–1026. [Google Scholar]
- Yasumoto T, Inoue A, Bagnis R, 1979a. Ecological survey of a toxic dinoflagellate associated with ciguatera In: Taylor DL, Seliger HH (Eds.), Toxic Dinoflagellate Blooms. Elsevier, New York, pp. 221–224. [Google Scholar]
- Yasumoto T, Nakajima I, Oshima Y, Bagnis R, 1979b. A new toxic dinoflagellate in association with ciguatera In: Taylor DL, Seliger HH (Eds.), Toxic Dinoflagellate Blooms. Elsevier, New York, pp. 65–70. [Google Scholar]
- Yasumoto T, Seino N, Murakami Y, Murata M, 1987. Toxins produced by benthic dinoflagellates. Biol. Bull 172, 128–131. [Google Scholar]
- Yasumoto T, Underal B, Aune T, Hormazabal V, Skulberg OM, Oshima Y, 1990. Screening for hemolytic and ichthyotoxic components of Chrysochromulina polyepsis and Gyrodinium aureolum for Norwegian coastal waters In: Graneli E, Sundstrom B, Edler L, Anderson DM (Eds.), Toxic Marine Phytoplankton; Elsevier, New York, pp. 436–440. [Google Scholar]
- Yokoyama A, Murata M, Oshima Y, Iwashita T, Yasumoto T, 1988. Some chemical properties of maitotoxin, a putative calcium channel agonist isolated from a marine dinoflagellate. J. Biochem 104, 184–187. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yuan T, 1998. Molecular mechanisms of calmodulin’s functional versatility. J. Biochem. Cell. Biol 76, 313–323. [DOI] [PubMed] [Google Scholar]
- Zar JH, 1999. Biostatistical Analysis, fourth ed. Prentice Hall, Upper Saddle River, New Jersey. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.