Abstract
Introduction
Prostate growth and androgenic alopecia are both under the influence of dihydrotestosterone. Dihydrotestosterone can make prostates larger and men balder. Therefore, we assessed the association of men suffering from lower urinary tract symptoms and androgenic alopecia.
Material and methods
We enrolled 177 subjects which underwent a Green Light Laser Vaporization Procedure of the prostate between 2006 and 2017. We identified two groups of males with different levels of androgenic alopecia, classified according to the Norwood-Hamilton scale, which we compared in this study. lower urinary tract symptoms were evaluated using the International Prostate Symptom Score questionnaire. Other parameters included demographics, urodynamics, prostate volume and prostate-specific antigen.
Results
Mean patient age was 68 (47–87) years. Mean International Prostate Symptom Score was 20 (1–35). 59.3% of subjects were classified as bald using the Norwood-Hamilton scale (androgenic alopecia score). Mean prostate size (range) was 77.5 ml (21–245), mean Qmax was 9 ml/s (1–25), and mean PSA was 4.7 ng/ml (0.3–39). The androgenic alopecia score correlated with none of the other parameters. PSA and prostate volume correlated with the Schäfer obstruction classification. Qmax correlated with the Schäfer classification and International Prostate Symptom Score. International Prostate Symptom Score correlated with QoL. Schäfer classification correlated with PSA, prostate volume, Qmax and age.
Conclusions
As expected, various lower urinary tract symptoms parameters in our study correlated with each other. However, no correlation was found between the androgenic alopecia score and LUTS or prostate volume. This score cannot be used to estimate prostate size.
Keywords: benign prostatic hyperplasia, prostatic hyperplasia, lower urinary tract symptoms, androgen receptor, androgenic alopecia
INTRODUCTION
Lower urinary tract symptoms (LUTS) are prevalent in older men and exist as storage LUTS, micturition LUTS, and post-micturition LUTS. The most common etiology of voiding LUTS is an enlarged prostate mostly caused by benign prostate hyperplasia (BPH). Well documented factors in the pathogenesis of BPH and LUTS are aging, hormonal, and genetic factors [1, 2, 3]. Prostate growth, as seen in BPH, is under the influence of dihydrotestosterone. If the effect of dihydrotestosterone is reduced or blocked e.g. by application of a 5 alpha-reductase inhibitor (5-ARI), prostate growth is stopped and prostate size can be reduced by as much as 25% [4, 5]. In Caucasian men, the prevalence of androgenic alopecia is 30% at the age of 30, which increases to 50% at the age of 50 and 80% by 70 years [6, 7, 8]. The cause of male lower urinary tract symptoms due to an enlarged prostate, as in BPH, partially overlaps with that of the occurrence of androgenic alopecia. Dihydrotestosterone is also an essential element in the physiology of androgenic alopecia. 5 Alpha-reductase converts testosterone into dihydrotestosterone which contributes to the miniaturization of hair follicles [9]. The application of 5-ARI has been shown to be effective in the treatment of androgenic alopecia [10]. Since androgenic alopecia and prostate growth causing lower urinary tract symptoms are both under the influence of dihydrotestosterone, the objective of this exploratory study is to assess the association of the androgenic alopecia baldness score and prostate size. Because digital rectal examination can be used to estimate the size of the prostate and it is less accurate than trans rectal ultrasound, the prostate size tends to be underestimated [11]. We hope that with the results of this study we can support physicians in the estimation of the prostate size without using invasive methods. Because there is an endocrinological association between baldness and a higher prostate volume, our hypothesis is that a higher baldness score could be predictive of prostate size or perhaps other parameters associated with lower urinary tract symptoms.
MATERIAL AND METHODS
Data collection
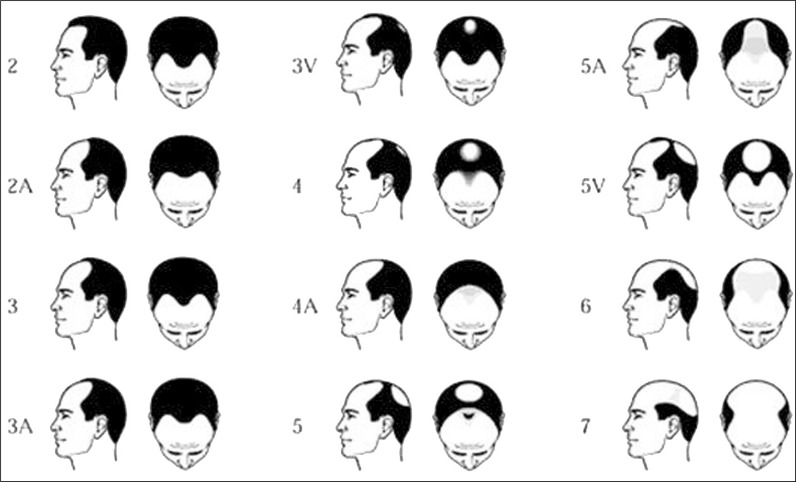
We looked at all subjects undergoing a green light laser vaporization procedure of the prostate for the treatment of male lower urinary tract symptoms. Between 2006 and 2017, 822 patients underwent this procedure in our clinic. This study used the personal identification picture of the subject in the electronic patient file. To the best of our knowledge, this method has not been used in any prior study. 177 out of 822 subjects in this database had a personal identifying photograph of the head in their electronic patient file that could be used for scoring baldness. From these photographs, we assessed the rate of hair loss according to the Norwood-Hamilton scale (Figure 1) [7, 12]. The scoring was performed by one of the researchers. For simplification and because precise scoring was difficult, the androgenic alopecia was scored in two categories: <4 and ≥4. We decided that an androgenic alopecia score ≥4 was classified as bald, whereas lower scores were regarded as not-bald [12]. The following items were collected in all patients: age, prostate volume measured by transrectal ultrasound, PSA, International Prostate Symptom Score International Prostate Symptom Score, free uroflowmetry parameters, and filling and pressure flow urodynamics. In order to analyze group means and to find a correlation, we reclassified various variables into different groups. Prostate volumes was categorized in groups of <50 ml, 50–77 ml, 78–100 ml and >100 ml. Age was dichotomized into 2 categories; <68 years and ≥68 years. International Prostate Symptom Score was put into categories of 0–7, 8–19, 20–35 according to the internationally accepted categories mild, moderate and severe symptoms. PSA was put into classes of 0–2.5 ng/ml, 2.5–5 ng/ml, 5–7.5 ng/ml, 7.5–10 ng/ml, >10 ng/ml.
Figure 1.
Norwood- Hamilton or androgenic alopecia scale for scoring baldness. For this study <4 was regarded as not bald and ≥4 was regarded as bald.
Statistical analysis
Statistical analysis was performed using SPSS 24.0.0.0 (IBM Corp). Categorical and ordinal variables where analyzed with the Chi-Squared test and Fisher Exact test.
RESULTS
A total of 177 subjects were enrolled. All subjects were Western European males between 47 and 87 years of age, with a mean age of 68 years. 2% (n = 2) presented with mild lower urinary tract symptoms (International Prostate Symptom Score <8), 45% of subjects (n = 49) with moderate lower urinary tract symptoms (International Prostate Symptom Score 8–9) and 53% of subjects (n = 57) presented with severe lower urinary tract symptoms (International Prostate Symptom Score >19). Subjects with hair loss (≥4 on the Norwood- Hamilton scale) totaled n = 105 (59.3%), and not bald totaled n = 72 (40.3%). Descriptive statistics are listed in Table 1. Mean International Prostate Symptom Score was 19, mean prostate volume was 77 ml, and mean Qmax was 8.92 ml/s.
Table 1.
Various epidemiological LUTS and Androgenic alopecia parameters
| N | Range | Minimum | Maximum | Mean | Std. deviation | |
|---|---|---|---|---|---|---|
| Age (years) | 177 | 41 | 47 | 87 | 68 | 7.47 |
| International Prostate Symptom Score | 108 | 34 | 1 | 35 | 19 | 6.18 |
| Prostate volume (ml) | 170 | 224 | 21 | 245 | 77 | 37.97 |
| PSA (ng/l) | 163 | 38.7 | ,30 | 39.0 | 4.7 | 5.4 |
| Qmax (ml/s) | 128 | 24.0 | 1,0 | 25.0 | 8.9 | 4.9 |
| QoL preoperative | 107 | 5 | 1 | 6 | 3.84 | 1.15 |
| Schäfer bladder outlet obstruction grade | 112 | 5 | 1 | 6 | 4.19 | 1.09 |
| Androgenic alopecia Score | 177 | 1.00 | 0.00 | 1.00 | .59 | .49 |
Qmax – peak urinary flow rate; QoL – quality of life score
With chi-square analyses we looked at correlations of the androgenic alopecia score. The results are listed in Table 2. There was a non-significant tendency that associated androgenic alopecia score with prostate volume (p = 0.125). Androgenic alopecia score had the lowest correlation with age (p = 0.169). When analyzing prostate volume, we found a significant correlation between prostate volume and PSA (P <0.001). The higher the PSA, the bigger the prostate. Prostate volume was also correlated with the Schäfer obstruction grade; the bigger the prostate, the higher the obstruction (p = 0.011). Prostate size was not correlated with age (p = 0.111), International Prostate Symptom Score (p = 0.39) or Qmax (p = 0.377). Table 3 lists the correlations of the parameters that were used in for analysis.
Table 2.
Chi square correlation results based in reclassification of the analyzed parameters
| Androgenic alopecia Score | Total | |||
|---|---|---|---|---|
| <4 not bald | ≥4 bald | |||
| Prostate volume | <50 gram | 17 | 33 | 50 |
| 50–77 gram | 20 | 31 | 51 | |
| 78–100 gram | 14 | 8 | 22 | |
| >100 gram | 19 | 28 | 47 | |
| Total | 70 | 100 | 170 | |
| Pearson Chi square 0.125 | ||||
| Age (years) | <68 | 43 | 51 | 94 |
| ≥68 | 29 | 54 | 83 | |
| Total | 72 | 105 | 177 | |
| Pearson Chi Square 0.144 | ||||
| Qmax before GREEN LIGHT LASER (ml/s) | ≤6 | 15 | 33 | 48 |
| 6–12 | 26 | 29 | 55 | |
| ≥12 | 11 | 14 | 25 | |
| Total | 52 | 76 | 128 | |
| Pearson Chi Square 0.238 | ||||
| PSA (ng/ml) | 0–2.5 | 22 | 43 | 65 |
| 2.5–5 | 26 | 30 | 56 | |
| 5–7.5 | 5 | 13 | 18 | |
| 7.5–10 | 3 | 3 | 6 | |
| >10 | 10 | 8 | 18 | |
| Total | 66 | 97 | 163 | |
| Pearson Chi Square 0.273 | ||||
| International Prostate Symptom Score | mild (<8) | 1 | 1 | 2 |
| moderate (8–19) | 21 | 28 | 49 | |
| Severe (>19) | 23 | 34 | 57 | |
| Total | 45 | 63 | 108 | |
| Pearson Chi Square 0.939 | ||||
Table 3.
Correlations of the parameters used for analysis
| Variables | Pearson Correlation (sig 2-tailed) | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| 1. Androgenic alopecia Score | 1 | -.080 (.302) | .32 (.741) | -.110 (.160) | -.116 (.193) | .110 (.146) | |
| 2. Prostate Volume | 1 | .089 (.364) | .458** (.000) | -.034 (0.703) | .068 (0.382) | ||
| 3. International Prostate Symptom score | 1 | .089 (0.36) | .237* (0.017) | .174 (.072) | |||
| 4. prostate specific antigen | 1 | -.177* (.047) | .049 (.531) | ||||
| 5. Qmaxpre | 1 | .134 (.132) | |||||
| 6. Age | 1 | ||||||
Correlation is significant at the 0.01 level (2-tailed)
Correlation is significant at the 0.05 level (2-tailed)
Qmaxpre – maximal flow (ml/s) before treatment
DISCUSSION
A relationship between prostate growth and baldness might be expected based on the same endocrinological pathway, dihydrotestosterone, that induces prostate growth as well as baldness. Prostate growth and androgenic alopecia have long been linked together since androgens are essential in both pathophysiologies. Male pattern balding occurs in a couple of steps. First, hair cycle dynamics change. During passages of the cycle, the anagen phase (responsible for the length of the hair) shortens causing the successive hairs to be shorter with each successive cycle until they do not reach the surface of the skin. The telogen phase elongates, producing hairs that are less well anchored in their follicles, which is responsible for the increased shedding of hair. Androgen mediated changes in the hair cycle affect the size of the hair follicles on the scalp, reducing them in diameter with each successive cycle, as well cause a decreasing pigment production [9]. This typical pattern of baldness can be measured using the Norwood-Hamilton scale. The growth of the prostate is stimulated by dihydrotestosterone. Testosterone is converted to dihydrotestosterone by 5 Alpha-reductase. This may suggest that a higher level of dihydrotestosterone induces a bigger prostate and a higher level of androgenic alopecia. A possible hypothesis for the lack of correlation in our study between hair loss and prostate size could be that the effect of dihydrotestosterone is dependent on localized enzyme activity of 5-alpha-reductase. When the concentration of testosterone is similar throughout the body, the localized difference in enzyme activity could be a possible explanation for the lack of correlation. In our study, no significant statistical correlation was found between androgenic alopecia and prostate size. However, as expected, we have found a significant correlation between prostate size and PSA and obstruction grade according to the Schäfer classification in our population. There have been other attempts to elucidate the relationship between androgenic alopecia and prostate parameters. Barbosa et al. [1] investigated 907 male patients with lower urinary tract symptoms. The mean patient age was 61.0 years; 58% of subjects had moderate to severe lower urinary tract symptoms and 54% were classified as bald. However, they had to conclude that Alopecia was not an independent predictor of LUTS. The drawback of their study was that they only looked at symptoms and not at prostate size. Since androgens also play a role in the development of prostate cancer, this could imply that a correlation between androgenic alopecia and prostate cancer exists. Lis-Święty et al. [13] examined the pathophysiological differences between frontal and vertex balding and their impact on the incidence of prostate cancer. In their study, they found that dihydrotestosterone serum levels where higher in subjects with vertex baldness (P <0.05). Another study by He et al. [14] performed a systematic review and meta-analysis regarding male pattern baldness and incidence of prostate cancer. They found no significant association between baldness of any pattern and prostate cancer (RR: 1.03, 95% CI 0.96–1.11). However, during subgroup analysis, they found a statistically significant association for vertex baldness (RR 1.24, 95% CI 1.05–1.46). Our study has not found a significant correlation between androgenic alopecia and lower urinary tract symptoms. This might imply that there is no correlation or that the study design has to be different. In the way in which this exploratory study was performed, there are some confounders. Age can be one since more men become bald at a higher age [7, 12]. Therefore, correction for age has to be done. Moreover, we looked at a population that underwent a Green Light Laser procedure. This procedure is mostly performed in males with bigger prostates. Therefore, we could not examine male patients with smaller prostates which could definitely influence the results. Moreover, all patients encountered subjective complaints before presentation at our clinic, which can be selective too. Not all data were available for all subjects, e.g. the pre-procedural International Prostate Symptom Score was missing in 68 subjects. An important aspect can be the scoring of the androgenic alopecia by the Norwood-Hamilton score. The photos used in assessing the scale of androgenic alopecia are photographs with a frontal view of the patient, they were not taken specifically to score baldness using the Norwood-Hamilton scale. This made scoring the degree of baldness difficult in some cases because the vertex and temples were not always visible. In Barbosa et. al. 2013 [1], hair loss was evaluated and classified according to the Norwood-Hamilton Scale by the attending physician. In Cremers et. al. 2010 [15], hair loss was assessed using a questionnaire where the subjects were asked to assess their baldness pattern at the age of 20, 40 and at the moment of filling out the questionnaire. An adapted model of the Norwood-Hamilton scale was used. Furthermore, it is unknown in our study if any of the subjects underwent a hair transplant, used a hair piece or shaved their head as a hairstyle. Finally, this was of course a retrospective exploratory study. A prospective study that could control and avoid the confounders in a more general population is perhaps a better way of finding the answer to the question expressed in the title of this manuscript.
CONCLUSIONS
In our study, no correlation was found between androgenic alopecia and lower urinary tract symptoms. Androgenic alopecia score can't be used to estimate prostate size. Therefore, skipping digital rectal examination, which can be embarrassing for the doctor and for the patient, is not yet possible based on the results of this study.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical statement
This study complied with the hospital guidelines on retrospective file studies, ethical review board approval was not required.
References
- 1.Barbosa JA, Muracca E, Nakano E, et al. Risk factors for male lower urinary tract symptoms: the role of metabolic syndrome and androgenetic alopecia in a Latin American population. Urology. 2013;82:182–188. doi: 10.1016/j.urology.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Yip L, Rufaut N, Sinclair R. Role of genetics and sex steroid hormones in male androgenetic alopecia and female pattern hair loss: an update of what we now know. Australas J Dermatol. 2011;52:81–88. doi: 10.1111/j.1440-0960.2011.00745.x. [DOI] [PubMed] [Google Scholar]
- 3.Neuhouser ML, Kristal AR, Penson DF. Steroid hormones and hormone-related genetic and lifestyle characteristics as risk factors for benign prostatic hyperplasia: review of epidemiologic literature. Urology. 2004;64:201–211. doi: 10.1016/j.urology.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Nickel JC, Gilling P, Tammela TL, Morrill B, Wilson TH, Rittmaster RS. Comparison of dutasteride and finasteride for treating benign prostatic hyperplasia: the Enlarged Prostate International Comparator Study (EPICS) BJU Int. 2011;108:388–394. doi: 10.1111/j.1464-410X.2011.10195.x. [DOI] [PubMed] [Google Scholar]
- 5.Montorsi F, Roehrborn C, Garcia-Penit J, et al. The effects of dutasteride or tamsulosin alone and in combination on storage and voiding symptoms in men with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH): 4-year data from the Combination of Avodart and Tamsulosin (CombAT) study. BJU Int. 2011;107:1426–1431. doi: 10.1111/j.1464-410X.2011.10129.x. [DOI] [PubMed] [Google Scholar]
- 6.Severi G, Sinclair R, Hopper JL, et al. Androgenetic alopecia in men aged 40-69 years: prevalence and risk factors. Br J Dermatol. 2003;149:1207–1213. doi: 10.1111/j.1365-2133.2003.05565.x. [DOI] [PubMed] [Google Scholar]
- 7.Norwood OT. Male pattern baldness: classification and incidence. South Med J. 1975;68:1359–1365. doi: 10.1097/00007611-197511000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton JB. Patterned loss of hair in man; types and incidence. Ann N Y Acad Sci. 1951;53:708–728. doi: 10.1111/j.1749-6632.1951.tb31971.x. [DOI] [PubMed] [Google Scholar]
- 9.Sinclair R. Male pattern androgenetic alopecia. BMJ. 1998;317:865–869. doi: 10.1136/bmj.317.7162.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi A, Cantisani C, Scarno M, Trucchia A, Fortuna MC, Calvieri S. Finasteride, 1 mg daily administration on male androgenetic alopecia in different age groups: 10-year follow-up. Dermatol Ther. 2011;24:455–461. doi: 10.1111/j.1529-8019.2011.01441.x. [DOI] [PubMed] [Google Scholar]
- 11.Roehrborn CG, Girman CJ, Rhodes T, et al. Correlation between prostate size estimated by digital rectal examination and measured by transrectal ultrasound. Urology. 1997;49:548–557. doi: 10.1016/s0090-4295(97)00031-9. [DOI] [PubMed] [Google Scholar]
- 12.Cremers RG, Aben KK, Vermeulen SH, den Heijer M, van Oort IM, Kiemeney LA. Androgenic alopecia is not useful as an indicator of men at high risk of prostate cancer. Eur J Cancer. 2010;46:3294–3299. doi: 10.1016/j.ejca.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Guarrera M, Cardo P, Arrigo P, Rebora A. Reliability of hamilton-norwood classification. Int J Trichology. 2009;1:120–122. doi: 10.4103/0974-7753.58554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He H, Xie B, Xie L. Male pattern baldness and incidence of prostate cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e11379. doi: 10.1097/MD.0000000000011379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cremers RG, Aben KK, Vermeulen SH, den Heijer M, van Oort IM, Kiemeney LA. Androgenic alopecia is not useful as an indicator of men at high risk of prostate cancer. Eur J Cancer. 2010;46:3294–3299. doi: 10.1016/j.ejca.2010.05.020. [DOI] [PubMed] [Google Scholar]