Abstract
Introduction
Urologists are commonly facing the dilemma of elevating prostate-specific antigen (PSA) levels despite a series of negative prostate biopsy results. Although fusion biopsies are being used increasingly, they are not available in many centers. We evaluated the prostate cancer detection rate using transperineal magnetic resonance imaging (MRI) template-guided cognitive biopsy.
Material and methods
Twenty-two patients with a suspicious lesion on MRI were enrolled into this study and underwent a repeated biopsy. All procedures were done under anesthesia and with antibiotic prophylaxis. Brachytherapy template was applied in each case.
Results
The median age, PSA and prostate volume were 67 years, 9.2 ng/ml, and 65 ml, respectively. The average number of biopsy cores was 24. Nine patients (41%) were diagnosed with prostate cancer. The grade distribution was Gleason score 7 for 5 patients, and Gleason score 6 for 4 patients. No major complications occurred.
Conclusions
Transperineal MRI template-guided cognitive prostate biopsy appears to be a safe procedure, which helps to detect significant cancer. The biopsy-associated adverse events are negligible.
Keywords: prostate cancer, multiparametric MRI, PI-RADS, biopsy, cognitive, template, transperineal
INTRODUCTION
Prostate cancer (PCa) constitutes the most common cancer in men with a steadily rising incidence affecting on average one in six men during their lifetime. This malignancy affects mainly senior men aged 60 and above. Factors known to be associated with the development and progression of PCa are age, family history, and ethnicity. The increase in morbidity is related to the increasing overall life expectancy, prostate-specific antigen (PSA) testing, implementation of new biomarkers and the more frequent application of multiparametric magnetic resonance imaging (mpMRI).
Biopsy remains the standard tool for PCa diagnosis. Standard transrectal ultrasound guided (TRUS) biopsy has been a far from ideal cancer detection with a rate of around 25%, meaning no PCa is found in 3 of 4 patients undergoing biopsy. As a consequence, a substantial number of patients continue to be under suspicion of cancer despite multiple benign biopsies.
The improvement in imaging technology and the developments of mpMRI have increased the sensitivity of imaging for PCa [1, 2, 3]. Furthermore, to standardize mpMRI evaluation and decrease reporting ambiguity- the Prostate Imaging-Reporting and Data System (PI-RADS) classification was created. Magnetic resonance imaging (MRI) is considered as an important tool to improve the diagnostic accuracy of PCa detection. The MRI-guided biopsy can be performed using different techniques. Three techniques of MRI-guided procedures are available: in-bore MRI, MRI-ultrasound (US) software-assisted fusion and cognitive biopsy. Each method possesses its own advantages and disadvantages, but to date, no prospective comparison of the three methods has been made [4].
In cognitive biopsy, the MRI is reviewed before the intervention and the attention is focused on the area(s) described on the MRI. Cognitive fusion is the quickest and least expensive of MRI-guided options, but is the most operator-dependent. The procedure can be done transrectally or transperineally. The transperineal approach enables urologists to obtain samples from prostate areas that are difficult and frequently impossible, to obtain by the transrectal approach- mainly from the anterior region of the prostate [4, 5, 6].
In our study we wanted to assess the feasibility of the transperineal cognitive technique in the detection of PCa in a highly selected group of patients who had at least two sets of benign biopsies.
MATERIAL AND METHODS
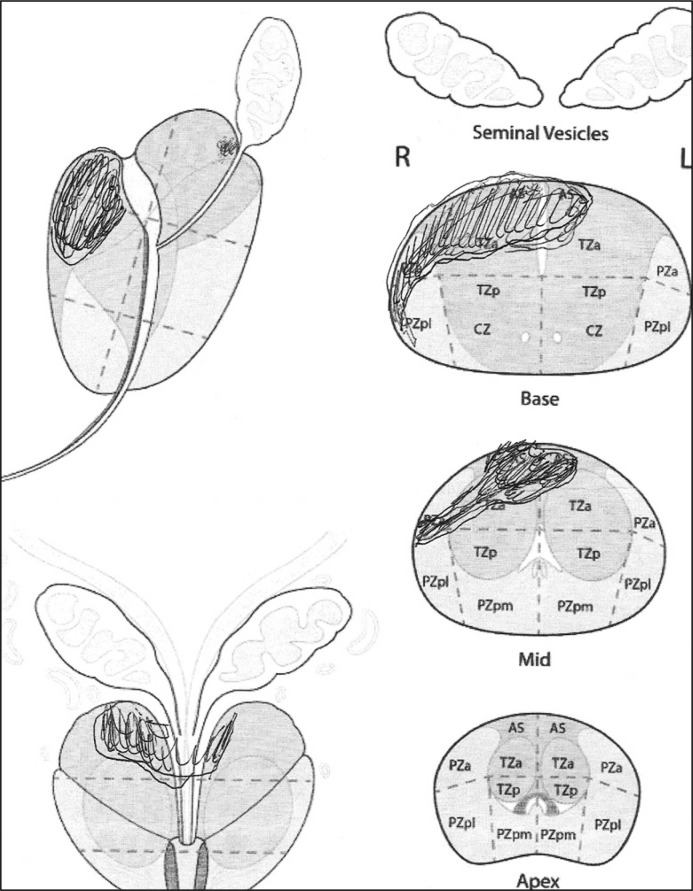
Twenty-two patients with a history of at least two negative biopsies and positive MRI results (PI-RADS 4 and 5 lesions) were enrolled to this study and underwent a repeated biopsy (Table 1). Biopsies were performed under general anesthesia with the patient in the dorsal lithotomy position using brachytherapy template in each case. A 16 French (Fr) Foley catheter was inserted for the intervention and only for the visualization of the urethra and removed directly after biopsy. The number of biopsy cores was defined by MRI result and the MRI protocol with a diagram. Senior radiologists with a relevant experience in PI-RADS reporting, evaluated suspicious lesions using T2-weighted imaging, diffusion-weighted imaging (DWI) and dynamic contrast-enhanced MRI. The region of interest found on the MRI scan diagram as depicted by the radiologist (suspicious lesions) was projected (cognitively) prior to the procedure (Figure 1). PI-RADS > 3 were considered suspicious.
Table 1.
Clinical features of patients who underwent transperineal biopsy (TPB)
| Number of patients | 22 |
| Age at TPB biopsy, years (median, range) | 67 (56–75) |
| Pre-TPB PSA level, ng/ml (median, range) | 9.2 (6.0–18.4) |
| Prostate volume, ml (median, range) | 65 (47–130) |
| No. of biopsy cores (median, range) | 24 (18–30) |
| No. of previous biopsies (median, range) | 3 (2–4) |
| Gleason score of TPB cancer (3+4) | 4 |
| Gleason score of TPB cancer (4+3) | 1 |
| Gleason score of TPB cancer (3+3) | 4 |
PSA – prostate-specific antigen
Figure 1.
mpMRI diagram for cognitive biopsy.
The biopsy procedure was performed after a thorough study of the mpMRI, with an 18G, 20 cm long automatic biopsy needle (Achieve, MeritMedical Systems Inc. Utah, USA), which was placed transperineally through the template apertures to correspond with the regional biopsy locations as described by Bott et al. [7]. A biplanar transrectal ultrasound probe was attached to a brachytherapy stepping unit and a standard 5-mm brachytherapy template grid was positioned over the perineum. In the transverse image, the prostate was divided into right and left and anterior, middle, and posterior regions. At least 2 targeted biopsy cores were taken from the suspicious lesions on a cognitive basis. Additionally, depending on prostate size, 2–3 biopsy cores depending on the prostate size, were obtained for each of the six regional biopsy locations extending from the apex toward the base. The procedure was performed with prophylactic antimicrobial cover (Ciprofloxacin). Patients were instructed to continue oral antibiotics for another 4 days.
RESULTS
Clinical features of patients who underwent transperineal cognitive prostate biopsy are presented in Table 1. Nine patients (41%) had detectable prostatic carcinoma on cognitive biopsy. The Gleason score was 6 in four and 7 in five patients, respectively. The catheter was removed directly after the biopsy. The procedure was well tolerated. Acute urinary retention was not observed in our series. No major bleeding, infection or urinary retention occurred. One patient developed scrotal pain and hematoma which resolved spontaneously within 2 weeks.
In this study, cognitive transperineal biopsy based on MRI diagrams enabled a satisfactory detection rate of PCa which appears particularly important for this highly pre-study biopsied population. In most patients, adenocarcinoma was found in the cores coming from anterior and apical zones of the gland. Cancer detection led to the initiation of therapy. As a consequence, 3 patients underwent radical prostatectomy, 4 radiotherapy treatment and 2 were under active surveillance for low-risk disease.
DISCUSSION
A systematic review of the recent literature revealed that among men with no previous biopsy, MRI-US fusion biopsy demonstrates up to a 20% increase in detection on of clinically significant PCa compared to systematic biopsy while avoiding a significant proportion of low risk disease. These data according to Bjurlin and his colleagues suggest a potential role in reducing overdetection and, ultimately, over-treatment. According to the authors, among men with previous negative biopsy, 72–87% of cancers detected by MRI targeted biopsy are clinically significant [4]. In our small series, all patients with clinically significant PCa, i.e. Gleason 7, patients received treatment – radical prostatectomy or radiotherapy.
The recent metanalysis compared direct in-bore guidance, MRI/ ultrasound (US) fusion and cognitive guidance. No clear superiority of one technique over the others was found [5]. Two studies compared cognitive guidance and MRI/US fusion in the same series of patients. One did not find any difference [6]. The other found a trend towards a higher tumor detection rates in favor of MRI/US fusion [8].
Importantly, the experience of biopsy operators plays a major role in the precision of targeted biopsies [9]. Cognitive approach is the simplest, but most operator-dependent one. Furthermore, the procedure is safe, quick, and requires no additional software. However, there is a realistic possibility of error due to the difficulties in extrapolation of the US imaging plane from MRI images. It is imperative for the operator to have a good understanding of the position of the lesion in the prostate to make a precise biopsy. Unfortunately, relying only on a narrative MR report or even on a schematic diagram is far from satisfactory and the operator must be aware of systematic biases induced by the fact that so-called ‘axial’ MRI and US images are not obtained along the same plane.
In bore MRI, and MRI/US fusions require dedicated expensive equipment and, especially the latter systems, are being used increasingly [4, 10]. Unfortunately, they are not available in many centers and their cost-effectiveness is disputable. Moreover, they are not refunded by most of the European healthcare systems.
The major advantage of transperineal biopsy compared to standard transrectal approach is related to the improved sampling of anterior and apical regions of the prostate, decreased risk of underestimation of PCa volume and grade and negligible rates of post-biopsy urosepsis. It is also a useful options for patients where rectal access is difficult or impossible [4, 10, 11]. In our study, in most patients adenocarcinoma was found in the cores coming from the anterior and apical zones of the gland. Importantly, we did not report any major urinary tract infection. The reason why we did not observe any urinary retention might be related to the fact that our group was not large enough and/or most of our patients were on alpha-blockers.
Several studies evaluated the use of cognitive transperineal biopsies. Valerio et al. compared software-assisted versus visually directed targeted transperineal prostate biopsies and found that both methods were almost comparable with only slightly and not statistically significant better results using the software based approach (64% vs. 68% detection rate, respectively) [12].
In our study, patients were selected for biopsies based on mpMRI result and increasing PSA test result. Among patients with a suspicious lesion on mpMRI, the detection rate was of 41% which is slightly lower to that than described in the literature. Dekalo and co-authors were able to diagnose cancer in 43 (52%) of 82 patients who had abnormal mpMRI result [13]. The selection bias might have impacted on lower than in other studies detection rate. Importantly, in our group all patients diagnosed with cancer had at least two false negative biopsy results.
In our opinion cognitive biopsy is fast and cheap, but requires experience in mpMRI of the prostate and proper biopsy technique. If these conditions are met, the results are satisfactory. However, the accuracy of cognitive biopsy is probably slightly inferior to that of fusion or in-bore biopsy. We consider the cognitive approach as appropriate especially for the large evident lesions described on mpMRI scan where the target is easily accessible. We advocate transperineal biopsy as a safe and non-expensive procedure especially for anteriorly located tumors. Moreover, the transperineal access appears to be the most appropriate for allowing for sampling accuracy with a minimal risk of complications. Of note, cognitive biopsies can be also helpful in men with low-risk disease who meet the criteria of active surveillance (AS) protocol. Two patients in our group with low-risk disease were offered surveillance approach.
Unfortunately, we did not use molecular markers as they are not refunded by our healthcare system, which apart from a small number of participants, is another limitation of our study.
CONCLUSIONS
This study showed that among a group of multi-biopsied patients, the presence of multiparametric magnetic resonance imaging (mpMRI) lesions helped to detect clinically significant prostate cancer using visually directed transperineal biopsies.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gayet M, van der Aa A, Beerlage H, et al. The value of magnetic resonance imaging and ultrasonography (MRI/US)-fusion biopsy platforms in prostate cancer detection: a systematic review. BJU Int. 2016;117:392–400. doi: 10.1111/bju.13247. [DOI] [PubMed] [Google Scholar]
- 3.Schoots IG, Roobol MJ, Nieboer D, et al. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol. 2015;68:438–450. doi: 10.1016/j.eururo.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Bjurlin M, Mendhiratta N, Wysock J, Taneja S. Multiparametric MRI and targeted prostate biopsy: Improvements in cancer detection, localisation, and risk assessment. Cent European J Urol. 2016;69:9–18. doi: 10.5173/ceju.2016.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wegelin O, van Melick HH, Hooft L, et al. Comparing Three Different Techniques for Magnetic Resonance Imaging-targeted Prostate Biopsies: A Systematic Review of In-bore versus Magnetic Resonance Imaging-transrectal Ultrasound fusion versus Cognitive Registration. Is There a Preferred Technique? Eur Urol. 2017;71:517–531. doi: 10.1016/j.eururo.2016.07.041. [DOI] [PubMed] [Google Scholar]
- 6.Puech P, Rouviere O, Renard-Penna R, et al. Prostate Cancer Diagnosis: Multiparametric MRtargeted Biopsy with Cognitive and Transrectal US-MR Fusion Guidance versus Systematic Biopsy - Prospective Multicenter Study. Radiology. 2013;268:461–469. doi: 10.1148/radiol.13121501. [DOI] [PubMed] [Google Scholar]
- 7.Bott SR, Henderson A, Halls JE, Montgomery BS, Laing R, Langley SE. Extensive transperineal template biopsies of prostate: modified technique and results. Urology. 2006;68:1037–1041. doi: 10.1016/j.urology.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 8.Wysock JS, Rosenkrantz AB, Huang WC, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MRtargeted prostate biopsy: the PROFUS trial. Eur Urol. 2014;66:343–351. doi: 10.1016/j.eururo.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 9.Moldovan P, Udrescu C, Ravier E, et al. Accuracy of Elastic Fusion of Prostate Magnetic Resonance and Transrectal Ultrasound Images under Routine Conditions: A Prospective Multi Operator Study. PloS one. 2016;11:e0169120. doi: 10.1371/journal.pone.0169120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt NR, Breen K, Haroon UM, et al. Patient experience after transperineal template prostate biopsy compared to prior transrectal ultrasound guided prostate biopsy. Cent European J Urol. 2018;71:43–47. doi: 10.5173/ceju.2017.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muthuveloe D, Telford R, Viney R, Patel P. The detection and upgrade rates of prostate adenocarcinoma following transperineal template-guided prostate biopsy – a tertiary referral centre experience. Cent European J Urol. 2016;69:42–47. doi: 10.5173/ceju.2016.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valerio M, McCartan N, Freeman A, et al. Visually directed vs. software-based targeted biopsy compared to transperineal template mapping biopsy in the detection of clinically significant prostate cancer. Urol Oncol. 2015;33:e9–1. doi: 10.1016/j.urolonc.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Dekalo S, Matzkin H, Mabjeesh NJ. High cancer detection rate using cognitive fusion - targeted transperineal prostate biopsies. Int Braz J Urol. 2017;43:600–606. doi: 10.1590/S1677-5538.IBJU.2016.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]