Abstract
The importance of Ca2+ signalling in key events of cancer cell function and tumour progression, such as proliferation, migration, invasion and survival, has recently begun to be appreciated. Many cellular Ca2+-stimulated signalling cascades utilise the intermediate, calmodulin (CaM). The Ca2+/CaM complex binds and activates a variety of enzymes, including members of the multifunctional Ca2+/calmodulin-stimulated protein kinase (CaMK) family. These enzymes control a broad range of cancer-related functions in a multitude of tumour types. Herein, we explore the cancer-related functions of these kinases and discuss their potential as targets for therapeutic intervention.
Keywords: CaMKK, CaMKI, CaMKII, CaMKIV, anti-cancer drugs
1. Introduction
Ca2+ is a major second messenger in cells and is essential for a variety of important signalling pathways. Alterations in intracellular Ca2+ signalling regulate many biological processes, including gene transcription, exocytosis, the cell cycle, migration and apoptosis. Cytoplasmic Ca2+ concentrations rise in response to a variety of stimuli, which activate Ca2+-channels in the plasma membrane, or by release from intracellular stores. It is increasingly being realised that disruption of normal Ca2+ signalling contributes to the development of tumourigenic phenotypes [1], and aberrant Ca2+ signalling has been implicated in each of the hallmarks of cancer originally identified by Hanahan and Weinberg [2].
Ca2+ signals in the form of spikes or oscillations and is tightly regulated. The decoding of this is achieved by several downstream effectors, including calmodulin (CaM). Binding of Ca2+ dramatically changes the conformation of CaM and increases its affinity for a large number of CaM-binding proteins, including the multifunctional CaM kinases (CaMKK, CaMKI, CaMKII and CaMKIV). These multifunctional kinases are widely expressed and control a variety of cancer related functions in a range of cancer types. Their potential as targets for anti-cancer therapeutic intervention have recently begun to be appreciated.
2. Structure and Regulation of Calcium/Calmodulin-Stimulated Protein Kinase (CAMK) Family Members
2.1. CaMKK
Ca2+/calmodulin stimulated protein kinase kinase (CaMKK) is a multifunctional protein kinase encoded by two genes (CAMKK1 and CAMKK2) that produce CaMKKα or CaMKKβ [3], respectively. The CAMKK2 gene produces several splicing isoforms depending on cell type [4,5]. CaMKKα was originally identified in rat brain as an activating kinase for CaMKIV [6] and CaMKI [7], and an additional β isoform was later identified [8]. CaMKK is primarily expressed in the brain, including the olfactory bulb, hypothalamus, hippocampus, dentate gyrus, cerebellum and amygdala, and at low levels in peripheral tissues (such as the thymus, spleen, lung, and testis) [9,10,11].
CaMKK phosphorylates CaMKI and CaMKIV [3,11], AMP activated protein kinase (AMPK) [12] and protein kinase B (PKB/Akt) [13]. CaMKK, CaMKI and CaMKIV form a signalling pathway termed the Ca2+/CaM-dependent kinase cascade, which has been implicated in several cellular processes, including regulating dendritic spine morphology, hematopoietic stem cell maintenance, cell proliferation, apoptosis, glucose uptake, adipogenesis, and normal immune cell function [14,15,16,17,18,19,20,21].
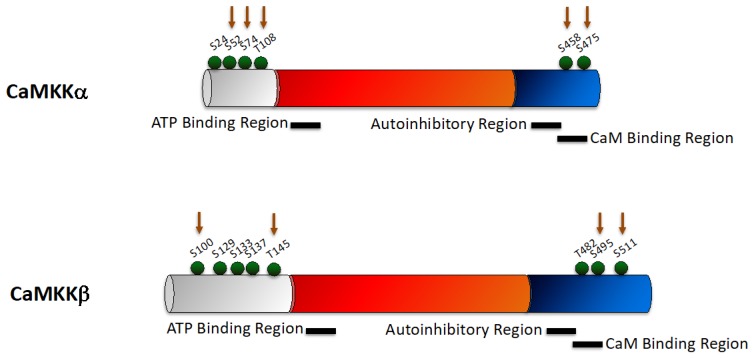
Although CaMKKα and CaMKKβ share high sequence homology (Figure 1) and possess a common domain structure, with a catalytic domain adjacent to an autoregulatory domain containing an autoinhibitory region that overlaps with the CaM-binding region, they differ in their biochemical properties. Whilst CaMKKα requires Ca2+/CaM to relieve the autoinhibitory mechanism [22], CaMKKβ exhibits partially autonomous activity in the absence of Ca2+/CaM [8,11], which is regulated by phosphorylation by glycogen synthase kinase 3β (GSK3β) and cyclin-dependent kinase 5 (CDK5) [23]. CDK5 phosphorylates CaMKKβ at S137, thereby priming CaMKKβ for phosphorylation by GSK-3β at S129 and S133 [23]. Both CaMKK isoforms are partly inhibited following cAMP-dependent protein kinase (PKA) phosphorylation [24,25], and PKA can phosphorylate CaMKKα on S52, S74, T108, S458, and S475 [25], and CaMKKβ on S100, S495 and S511 [10]. The major site of autophosphorylation of CaMKKα is S24 [25], and for CaMKKβ is T482 [26]. T482 generates partial autonomous activity, which results in partial disruption of the autoinhibitory mechanism [26]. As CaMKKβ is not dependent on rapid fluxes in intracellular Ca2+ for basal activity, it can respond to other stimuli of longer duration and can phosphorylate AMPK.
Figure 1.
Schematic representing the domain structure of CaMKK. There are two CaMKK isoforms—CaMKKα and CaMKKβ. CaMKK consists of a unique N-terminal domain (grey), a catalytic domain (red) which contains an ATP binding region, and a regulatory domain (blue) containing overlapping autoinhibitory and calmodulin (CaM) binding regions. Phosphorylation sites are indicated by green balls, with protein kinase A (PKA) phosphorylation sites indicated with red arrows.
Binding of Ca2+/CaM to CaMKKα prevents phosphorylation at S52, S74, T108, and S458, but enhances phosphorylation at S475 [25]. Additionally, phosphorylation of CaMKKα on S74, T108 and S458 negatively regulates activity [24,27,28] and phosphorylation of S458 blocks Ca2+/CaM binding.
2.2. CaMKI
The Ca2+/calmodulin-stimulated protein kinase I (CaMKI) family is composed of four members, which are coded for by four different genes: CAMK1, PNCK, CAMK1G and CAMK1D, which produce CaMKIα, CaMKIβ/Pnck, CaMKIγ/CLICK3, or CaMKIδ/CKLiK, respectively. The PNCK gene produces several splicing isoforms depending on cell type and developmental stage [29]. The various isoforms of CaMKI are ubiquitously expressed at low levels [30], and expressed at high levels in several brain regions, including the cortex, hippocampus, thalamus, hypothalamus, midbrain, and olfactory bulb, with each isoform exhibiting distinct spatiotemporal expression during neuronal development [31,32]. CaMKI has been implicated in the control of a variety of cellular functions, including long term potentiation, the control of synapsin in nerve terminals, axon/dendritic outgrowth and growth cone motility, aldosterone synthase expression, osteoclast differentiation and bone resorption and proliferation [32,33,34,35,36,37,38,39].
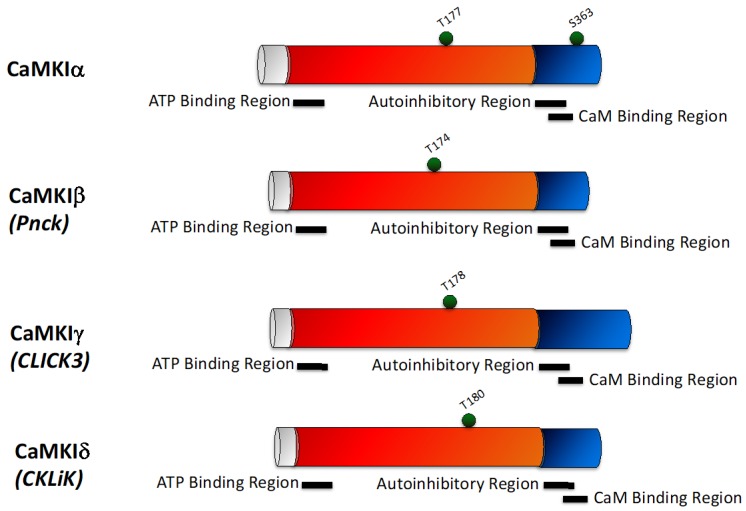
Similar to CaMKK, the four CaMKI isoforms share a common domain structure (Figure 2), with a catalytic domain adjacent to an autoregulatory domain containing an autoinhibitory region that overlaps with the CaM-binding region. Binding of Ca2+/CaM to CaMKI causes a conformational change that relieves the autoinhibition and allows phosphorylation by CaMKK (T174 for CaMKIα), which is required for maximal CaMKI activity [40,41]. Additionally, once CaMKIδ is phosphorylated by CaMKK, it becomes resistant to protein phosphatases, which induces a ‘primed’ state, where it can more readily be activated in response to Ca2+ signals than other CaMKI enzymes [42].
Figure 2.
Schematic representing the domain structure of the CaMKI family. There are four CaMKI isoforms—CaMKIα, CaMKIβ, CaMKIγ, and CaMKIδ, with each isoform sharing a similar structure. CaMKI consists of a unique N-terminal domain (grey), adjacent to a catalytic domain (red) which contains an ATP binding region, and a regulatory domain (blue) containing overlapping autoinhibitory and calmodulin (CaM) binding regions. Phosphorylation sites are indicated by green balls.
2.3. CaMKII
The Ca2+/calmodulin-stimulated protein kinase II (CaMKII) family is encoded by four genes: CAMK2A, CAMK2B, CAMK2G, CAMK2D, which produce CaMKIIα, CaMKIIβ, CaMKIIγ, and CaMKIIδ, respectively. Alternative splicing within the variable linker-region produces multiple isoforms [43]. One or more members of the CaMKII family are found in every tissue. CaMKIIα and CaMKIIβ are most highly expressed in neurons and are involved in regulating a variety of neuronal functions, including neurotransmitter synthesis and release, neurite extension, and synaptic plasticity that underlies learning and memory [44,45,46,47,48]. CaMKII has also been implicated in the regulation of non-neuronal processes, including fertilisation, the maintenance of vascular tone, osteogenic differentiation, normal cardiac function, apoptosis and excitotoxicity/ischaemic-induced cell death and cell proliferation [34,49,50,51,52,53,54,55,56].
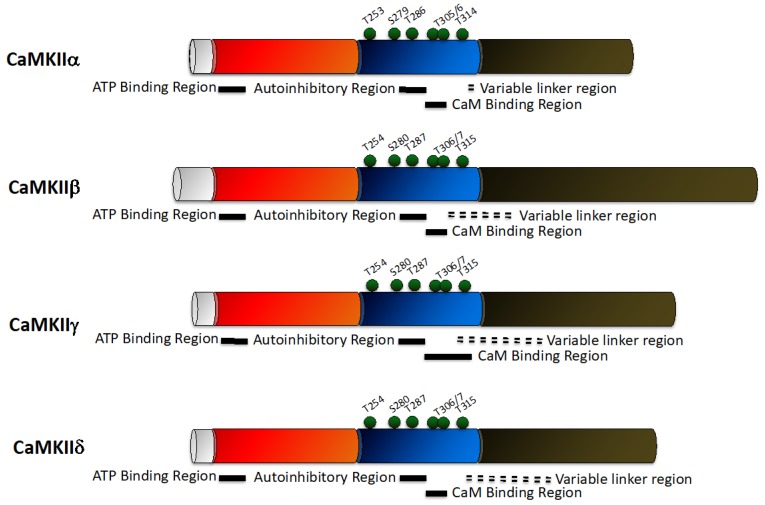
Similar to the other CaMK family members, CaMKII subunits (Figure 3) have an N-terminal catalytic domain, and a regulatory domain (which contains autoinhibitory and CaM binding domains). In contrast to the other CaMK family members, CaMKII possesses a C-terminal association domain and associates into a multimeric form [57]. The crystalline structure of CaMKIIα shows that it consists of two autoinhibited catalytic domains in a symmetric dimer. The regulatory domain is joined to the C-terminus of the catalytic domain [57], which functions as a gate (with T286 as its hinge), so that it blocks the protein substrate and adenosine triphosphate (ATP) binding sites when CaMKII is autoinhibited and is ‘open’ following autophosphorylation at T286. CaMKII is therefore comprised of six mutually inhibited dimers.
Figure 3.
Schematic representing the domain structure of the CaMKII family. There are four CaMKII isoforms—CaMKIIα, CaMKIIβ, CaMKIIγ, and CaMKIIδ, with each isoform sharing a similar structure. CaMKII consists of a unique N-terminal domain (grey), adjacent to a catalytic domain (red) which contains an ATP binding region, and a regulatory domain (blue) containing overlapping autoinhibitory and calmodulin (CaM) binding regions. Phosphorylation sites are indicated by green balls. All isoforms also contain a C-terminal association domain (brown), which is involved in the formation of CaMKII multimers.
CaMKII requires Ca2+/CaM for initial enzyme activity. Binding of CaM molecules to adjacent subunits within a holoenzyme allows autophosphorylation at T826 of one or both of these subunits to occur [58]. Autophosphorylation of T286 in CaMKIIα (T287 in CaMKIIβ, γ, and δ) occurs rapidly and alters the affinity for Ca2+/CaM and enzyme activity [59,60]. CaMKII phosphorylation at T286 generates an autonomously active kinase that remains active even following CaM dissociation. Phosphorylation at T286 can also regulate the function of CaMKII by altering its binding to specific subcellular locations [61,62,63]. Once CaMKII is Ca2+-independent, and Ca2+/CaM is no longer bound, secondary sites within the CaM-binding site can be phosphorylated (T305/306 in CaMKIIα, and T306/307 in CaMKIIβ, γ, and δ) [64,65]. Phosphorylation at these sites prevents CaM binding and CaMKII subsequently becomes insensitive to changes in Ca2+/CaM [66].
Other forms of post-translational modification have also been demonstrated to alter CaMKII activity. Specifically, a pair of methionine residues (M281/282), present in the β, γ, and δ, but not α, isoforms [67], can become oxidised and produce a conformational change in CaMKII, similar to that produced following T286 phosphorylation, leading to an autonomous activation of CaMKII [68]. However, not all CaMKII phosphorylation sites modulate Ca2+/CaM binding and kinase activity. Phosphorylation at several sites that have no direct effect on kinase activity, but alter molecular targeting, have been identified in vivo, including T253, S279 and S314 [69,70,71,72].
2.4. CaMKIV
Ca2+/calmodulin-stimulated protein kinase IV (CaMKIV) is encoded by the CAMK4 gene. Two different isoforms (CaMKIVα and CaMKIVβ) are produced by alternative processing [73,74]. The CaMKIV expression pattern is similar to that of CaMKKβ, with both primarily being expressed in the brain, however, CaMKIV is also present in immune cells, the testes and ovaries [9,16,75,76]. It is particularly enriched in cerebellar granule cells, and subsequently, has previously been referred to as CaMK-Gr. The CAMK4 gene also encodes calspermin, a Ca2+/CaM binding protein of unknown function that is exclusively expressed in spermatids in the testes [76]. CaMKIV has been implicated in the regulation of homeostatic plasticity, neurite outgrowth, fear memory, immune and inflammatory responses, the regulation of cyclic AMP element binding protein (CREB) and cell proliferation [34,77,78,79,80,81].
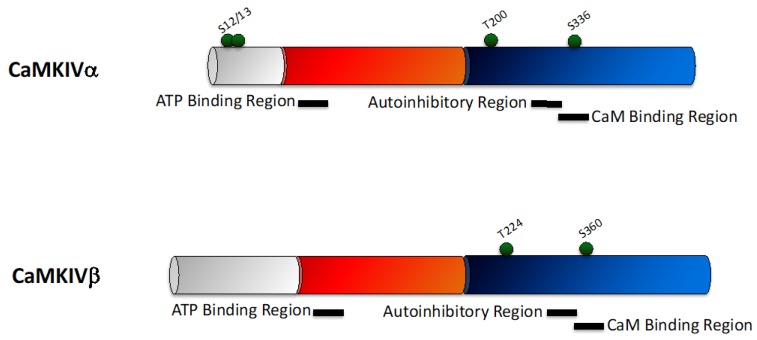
CaMKIVβ is identical to CaMKIVα, except for the addition of 28 amino acids at the N-terminus. Similar to the other CaMK family members, CaMKIV has a catalytic domain adjacent to an autoregulatory domain containing an autoinhibitory region that overlaps with the CaM-binding region (Figure 4). CaMKIV requires Ca2+/CaM to become active, as well as phosphorylation of the conserved T in the activation loop (T200 in human CaMKIV and T196 in the rat enzyme) by CaMKK [82], which generates an autonomously active kinase. Following this, CaMKIV then autophosphorylates at S12 and S13, which enable Ca2+/CaM independent activity. Double S11A/S12A phospho-null mutants lack Ca2+/CaM-dependent basal activity and are unresponsive to CaMKK [83], indicating that S12 and S13 mediate an intrasteric inhibition, and are essential for full activation of CaMKIV. Similar to T305/6 in CaMKII, phosphorylation of CaMKIV within the CaM binding region (S336 in human CaMKIV and S332 in the rat enzyme) prevents CaM binding and inactivates CaMKIV [84].
Figure 4.
Schematic representing the domain structure of the CaMKIV family. The two CaMKIV isoforms—CaMKIVα and CaMKIIβ differ only at their N-terminus. CaMKIV consists of a unique N-terminal domain (grey), adjacent to a catalytic domain (red) which contains an ATP binding region, and a regulatory domain (blue) containing overlapping autoinhibitory and calmodulin (CaM) binding regions. Phosphorylation sites are indicated (green balls).
3. The Role of CaMK Family Members in Cancer
Due to their role as mediators of Ca2+-signalling, it is not surprising that the CaMK family members have been identified as being overexpressed or aberrantly activated in a wide variety of cancer types. Additionally, each have also been implicated in controlling cellular processes important in cancer-related functions, such as cell proliferation, apoptosis, motility and invasion.
3.1. CaMKK
Despite the restricted expression of CaMKKβ in normal cells, it is overexpressed in several types of cancer compared to adjacent benign tissue (Table 1), including gastric tumours [85], hepatocellular carcinoma [86], high-grade gliomas [87], ovarian [88] and prostate cancer [89,90,91]. Androgen stimulation of androgen-dependent and castration-resistant prostate cancer cell lines results in upregulation of CaMKKβ [89,91,92], indicating direct regulation by the androgen receptor. Subsequent studies have shown that CaMKKβ is a key effector of the androgen receptor in stimulating glycolysis through the activation of AMPK and phosphofructokinase (PFK), which drives anabolism and proliferation of prostate cancer cells [91]. Furthermore, CaMKKβ expression increases with human prostate cancer progression and Gleason score [89,91], and with tumour progression in a transgenic adenocarcinoma of the mouse prostate (TRAMP) mouse model of prostate cancer [89], suggesting that it may also play a role in prostate cancer progression.
Table 1.
The Ca2+/calmodulin-stimulated protein kinase (CaMK) family is overexpressed in a range of cancer types. AML acute myeloid leukaemia; BPH Benign prostatic hyperplasia; ccRCC clear cell renal cell carcinoma; CML chronic myeloid leukaemia; GBM glioblastoma multiforme; GOBO gene expression based outcome for breast cancer; GWAS Genome Wide Association Study; HBV Hepatitis B virus; HCC hepatocellular carcinoma; NHT Neoadjuvant hormone therapy; NSCLC non-small cell lung cancer; PIN Prostate intraepithelial neoplasia; TCGA the cancer genome atlas; TMA tissue microarray.
| CaMK Family Member | Cancer | Sample Type | Expression | Reference |
|---|---|---|---|---|
| CaMKKβ | Prostate | Prostate cancer TMA (n = 84); NHT TMA with hormone naïve, NHT <3 months, 3–6 months, or >6 months, or castrate-resistant (n = 107) | Increased protein expression in prostate cancer compared to PIN and BPH and in castrate-resistant cancer. Reduced expression following NHT. | [91] |
| Prostate cancer progression (n = 5) | Increased protein expression in prostate cancer compared to normal prostate and with increasing Gleason score | [89] | ||
| Normal prostate and prostate cancer TMA (n = 80), cancer, adjacent normal and metastases TMA (n = 95) | Increased protein expression in primary prostate cancer and bone metastasis compared to normal prostate | [90] | ||
| Gastric | Gastric adenocarcinoma and normal oesophagus TMA (n = 98) | Increased protein expression in gastric tumours compared to normal oesophagus | [85] | |
| Liver | Hepatocellular carcinoma transcriptome profile microarray (n = 247); matched normal and tumour (n = 22). | Increased expression in liver cancer and CAMKK2high correlates with poor disease-free survival. CaMKKβ protein upregulated in tumour compared to adjacent normal tissue. | [86] | |
| Glioma | Human glioma and normal brain tissue (n = 147 for expression and n = 101 for methylation); Whole genome mRNA expression microarray (n = 305 diffuse glioma samples, n = 151 methylation microarray, n = 275 GBM) | CAMKK2 mRNA and protein is more highly expressed in high-grade gliomas compared to low-grade. Increased expression and CAMKK2high correlates with poor overall survival. CAMKK2 is differentially methylated between low and high grade glioma. | [87] | |
| Ovarian | High grade serous papillary ovarian cystadenocarcinoma and high-grade ovarian carcinoma with mucinous features (n = 4) | Increased protein expression in high grade serous papillary cystadenocarcinoma and high-grade ovarian cancer with mucinous features compared to non-malignant stromal tissue. | [88] | |
| CaMKI | AML | TCGA AML database (n = 186) | CAMK1Dhigh correlates with poor overall survival | [93] |
| Endometrial cancer | Endometrial carcinoma and normal endometria (n = 31 and n = 20) | Protein expression is associated with PCNA-labeling, stage, histological grade, the presence of invasion and outcome | [94] | |
| Breast cancer | Primary breast ductal carcinoma (n = 35) | PNCK mRNA is more highly expressed in a subset (8/23) of human breast tumours compared to benign breast tissue | [95] | |
| ccRCC | ccRCC and adjacent normal tissue (n = 92) and primary ccRCC tissue (n-248) | PNCK mRNA and protein expression higher in tumour compared to normal. Patients with PNCKhigh have shorter overall survival | [96] | |
| CaMKII | CML | Peripheral blood (n = 15 at diagnosis; n = 30 in chronic phase with remission; n = 26 in chronic phase treatment-resistant; n = 30 in advanced phase; n = 20 healthy) | CaMKIIγ upregulated at diagnosis and in treatment resistance | [97] |
| AML | Peripheral blood samples (n = 16) | Total and phosphorylation of CaMKIIγ at T287 increased in AML | [98] | |
| Endometrial cancer | Endometrial carcinoma and normal endometria (n = 31 and n = 20) | Protein expression is associated with PCNA-labeling, stage, histological grade, the presence of invasion and outcome | [94] | |
| Colon cancer | Paracancerous tissue, well-differentiated and poorly differentiated colon cancer (n = 5, n = 6, n = 6) | CaMKII protein expression increased in colon cancer compared to paracancerous tissue, and increased with poor differentiation | [99] | |
| Breast cancer | GOBO Breast Cancer Database (n = 1881); Normal, primary and metastatic breast cancer TMA (n = 40, n = 70, and n = 10) | CAMK2high associated with worse overall and distant metastasis free survival. Total CaMKII protein and T286/7 phosphorylation is increased in primary breast cancer and metastases | [100] | |
| Osteosarcoma | Chondroblastic, osteoblastic and fibroblastic subtypes (n = 114) | Phosphorylation of αCaMKII at T286 is increased in osteosarcoma compared to normal osteoblasts and mesenchymal stromal cells | [101] | |
| Primary osteosarcoma tumours (n = 4) | Phosphorylation of αCaMKII at T286 is increased in osteosarcoma | [102] | ||
| Lung cancer | Oncomine databases (n = 187, n = 226, n = 130) | CAMK2Ghigh associated with worse overall survival | [103] | |
| GWAS in NSCLC patients (n = 354) | Rs10023113 in CAMK2D associated with survival | [104] | ||
| Gastric cancer | Non-metastatic and metastatic gastric cancer tissues (n = 10, and n = 10) | Phosphorylation at T286 is increased in metastatic compared to non-metastatic tissue | [105] | |
| CaMKIV | AML | TCGA AML database (n = 186) | CAMK4high correlates with poor overall survival | [93] |
| HCC | Normal liver, chronic hepatitis, cirrhosis, and HCC (n = 4, n = 6, n = 4, n = 12) | CaMKIV protein expression and activation increased in HCC compared to normal liver and cirrhosis | [106] |
Increased CaMKKβ expression also correlates with poor patient survival in additional tumour types (Table 1). High CaMKKβ expression is associated with poor disease-free survival in hepatic cancer patients [86], and poor overall survival in glioma patients [87]. These studies suggest that CaMKKβ may be a useful prognostic marker for liver and brain cancers.
CaMKKβ positively regulates cancer cell proliferation, migration and invasion in a variety of cell types in vitro (Table 2). Downregulation of CaMKKβ expression using small interfering RNA (siRNA) or pharmacological inhibition inhibits prostate cancer cell proliferation [89,91,92,107], migration and invasion [92]. Conversely, CaMKKβ overexpression in LNCaP prostate cancer cells increases cell migration, further supporting a role for CaMKKβ overexpression in prostate cancer progression [92]. By contrast, Shima et al. showed that CaMKKβ overexpression in LNCaP cells decreases cell proliferation and tumour growth in vivo [90], indicating that further examination of the role of CaMKKβ in prostate cancer cell proliferation is required. Downregulation or pharmacological inhibition of CaMKKβ also decreases proliferation, migration and invasion of glioma [87], gastric [85,108] and liver cancer [86] cells and expression of a dominant negative CaMKK mutant suppresses medulloblastoma cell migration [109], suggesting that CaMKK activity is essential for this process. Taken together, these studies highlight the importance of CaMKK in controlling cancer cell proliferation and metastatic processes in a range of cancer types, indicating that its role in these functions is not cell-type specific, and also suggesting that CaMKKβ may be a valid anti-cancer target for a variety of cancer types.
Table 2.
The calcium/calmodulin-stimulated protein kinase family mediate a variety of cancer-related functions in multiple cancer types in vitro. AML acute myeloid leukaemia; AIP autocamtide-2 inhibitory peptide; HCC hepatocellular carcinoma; siRNA small interfering RNA; shRNA short hairpin RNA; WT wild-type.
| Target | Cancer | Cell Line(s) | Method of Manipulation | Effect | Reference |
|---|---|---|---|---|---|
| CaMKK | Prostate | LNCaP | Pharmacological inhibition (STO-609) | Decreased proliferation | [89] |
| LNCaP, VCqP, C4-2B, 22Rv1 | siRNA and pharmacological inhibition (STO-609) | Decreased proliferation | [91] | ||
| LNCaP | siRNA and pharmacological inhibition (STO-609) | Decreased migration and invasion | [92] | ||
| LNCaP | CaMKKβ overexpression | Increased migration | [92] | ||
| LNCaP | CaMKKβ overexpression | Decreased proliferation | [90] | ||
| DU145 | CaMKKβ siRNA | Decreased proliferation | [107] | ||
| Gastric | AGS, KATO-III, SNU-16, N87 | CaMKKβ siRNA | Decreased proliferation | [85] | |
| SNU-1, N87 | CaMKKβ siRNA and pharmacological inhibition (STO-609) | Decreased proliferation and induced apoptosis | [108] | ||
| HCC | PHM1, SK-Hep1, HepG2 | CaMKKβ siRNA and pharmacological inhibition (STO-609) | Decreased proliferation | [86] | |
| Glioma | U-87MG | CaMKKβ siRNA | Decreased proliferation, migration and invasion | [87] | |
| Ovarian | SKOV-3, OVCAR-3 | CaMKKβ siRNA and pharmacological inhibition (STO-609) | Decreased proliferation and induced apoptosis | [88] | |
| Breast cancer | MCF-7 | CaMKKα and CaMKKβ siRNA | Arrested cells in G1 | [110] | |
| Medulloblastoma | DOAY | Expression of dominant negative CaMKK mutant | Decreased migration | [109] | |
| CaMKI | AML | MV-4-11, Kasumi-1 | shRNA and CaMKI overexpression | Downregulation decreased proliferation; Overexpression of kinase dead mutant decreased colony formation | [93] |
| Breast cancer | MCF-7 | siRNA | Arrested cells in G1 | [110] | |
| Medulloblastoma | DOAY | Expression of dominant negative CaMKI mutant | Decreased migration | [109] | |
| CaMKII | Osteosarcoma | MG-63, 143B, HOS | CaMKIIα shRNA and overexpression | Knockdown decreased proliferation, migration and invasion. Overexpression increased proliferation, migration, invasion | [101] |
| MG-63, 154B | Wild-type and K42M kinase dead CaMKIIα overexpression | K42M kinase dead overexpression reduced growth | [102] | ||
| AML | KG1, KCL22, THP-1, Kasumi-1 | Overexpression of kinase dead truncated CaMKIIγ, CaMKIIγ shRNA, pharmacological inhibition (KN-62, KN-93, KN-92) | Kinase dead overexpression, shRNA and pharmacological inhibition decreased colony formation and proliferation. | [98] | |
| Lung cancer | SCC-9, NCI-H345, NCI-H128, NCI-H146, NCI-H69 | Pharmacological inhibition (KN-62) | Slowed progression through S phase and decreased proliferation | [111] | |
| Medullary thyroid cancer | TT, MZ-CRC1 | Pharmacological inhibition (antCaNtide) | Decreased cell proliferation | [112] | |
| Colon cancer | HCT116 | Pharmacological inhibition (KN-92, KN-93) | Decreased proliferation, migration and invasion | [99] | |
| Gastric cancer | BGC-823 | Pharmacological inhibition (KN-93) and CaMKIIβ shRNA | Decreased cell proliferation and migration, induced apoptosis | [113] | |
| BGC-823 | Pharmacological inhibition (KN-62) and H282R constitutively active CaMKIIα overexpression | Pharmacological inhibition decreased cell proliferation. Overexpression of constitutively active increased cell proliferation, migration and invasion | [105] | ||
| Prostate cancer | C4-2B, LNCaP, PC3, DU145 | Pharmacological inhibition (KN-93) | Decreased proliferation | [114] | |
| 1542-CP3TX | Pharmacological inhibition (AIP) | Decreased cell migration | [115] | ||
| T cell lymphoma | H9 | CaMKIIγ knockout by CRISPR/Cas | Decreased proliferation and colony formation | [116] | |
| Breast cancer | MDA-MB-231, MCF-7 | Overexpression of CaMKIIα, T286D (phosphomimic) and T286V (phosphonull), Pharmacological inhibition (KN-92, KN-93, AIP) | Overexpression of WT and phosphomimic forms increased cell proliferation, migration and invasion. Pharmacological inhibition decreased migration and invasion | [100] | |
| MDA-MB-231 | Overexpression of CaMKIIα, T286D (phosphomimic) and T253D (phosphomimic) | Overexpression of WT and T286D increased proliferation. Overexpression of T253D decreased proliferation | [117] | ||
| Glioma | C6, U-251MG | Pharmacological inhibition (KN-93) | Decreased migration | [118] | |
| D54, H8a | Pharmacological inhibition (AIP) | Decreased migration | [119] | ||
| U-87MG | CaMKIIγ siRNA, pharmacological inhibition (KN-93) | Decreased proliferation, invasion and neurosphere formation | [120] | ||
| CaMKIV | AML | Lin− AML, MV-4-11, Kasumi-1 | CaMKIV and K75M overexpression and CaMKIV shRNA | CaMKIV-K75M overexpression and shRNA knockdown decreased colony formation. shRNA knockdown induced apoptosis and decreased proliferation. | [93] |
| U937 | CaMKIV wild-type and K71M kinase dead mutant overexpression | Cells arrested in G0/G1 following WT, but not K71M, overexpression | [121] | ||
| HCC | PHM1, SK-Hep1 | CaMKIV siRNA | Inhibited colony formation and proliferation | [86] |
3.2. CaMKI
The various CaMKI isoforms have been shown to be overexpressed in several different cancer subtypes (Table 1). CaMKI is more highly expressed in stage III and IV endometrial carcinomas, when compared to stage I and II, and is associated with proliferating cellular nuclear antigen (PCNA)-labelling, clinical state, histological grade, and the presence of invasion, indicating that CaMKI may play a role in endometrial carcinoma progression [94]. Expression of several of the specific CaMKI isoforms have also been examined in specific cancer types. For example, CaMKIγ/PNCK is overexpressed in a subset of primary breast cancers compared to benign mammary tissue [95], and in clear renal cell carcinoma compared to adjacent non-tumour tissues [96]. Furthermore, a significant correlation between PNCK expression and Fuhrman grade, tumour size, T and N stage was observed in these clear cell renal carcinoma samples, and high levels of PNCK is an independent predictor for poor patient survival [96]. CAMKID has also been identified as a potential prognostic marker in acute myeloid leukaemia (AML), as high levels of CAMKID mRNA are associated with significantly worse AML patient survival [93].
Unsurprisingly, CaMKI has been implicated in a variety of cancer-related cellular processes (Table 2). CaMKI controls the progression of MCF-7 breast cancer of cells through G1 [110], potentially by regulating cdk4 and retinoblastoma protein (Rb) phosphorylation, as overexpression of the kinase-inactive CaMKI K49A mutant prevents cdk4 activation and Rb hyperphosphorylation in WI-38 fibroblasts [17]. Indeed, knockdown of CaMKI expression in MV-4-11 and Kasumi-1 AML cells significantly decreases cell proliferation and inhibits leukaemia development and prolongs the survival of AML xenografted mice [93]. By contrast, CaMKI overexpression does not affect leukaemia development, but overexpression of the kinase-inactive CaMKI K49E mutant significantly decreases AML colony formation and increases mouse survival in AML transplantation studies [93], demonstrating the importance of CaMKI activity in controlling cell proliferation. Similar to expression of a dominant negative CaMKK mutant, expression of a dominant negative CaMKI mutant significantly decreases medulloblastoma cell migration [109], highlighting the importance of the CaMKK-CaMKI cascade in this cellular process. Murine CKLiK is upregulated by the haematopoietic cell-specific ETS family transcription factor, PU.1, in murine erythroleukemia cells, and is involved in apoptosis [122], suggesting that the human homolog of CKLiK may function in a similar fashion. These studies demonstrate that CaMKI can control cell proliferation, migration, and survival in medulloblastoma, breast and haematopoietic cancers, and therefore, may be a potential anti-cancer target for these cancer types.
3.3. CaMKII
Tumour cells express an entirely different spectrum of CaMKII isozymes than adult neuronal tissue or their non-transformed tissue counterparts. Eight distinct β, γ and δ CaMKII isozymes have been identified in human mammary tumour and neuroblastoma cell lines [123]. In addition to expression of these novel tumour variants, CaMKII is also overexpressed in a variety of cancer types (Table 1). CaMKII is more highly expressed in stage III and IV endometrial carcinomas when compared to stage I and II, and is associated with PCNA-labelling, clinical state, histological grade, and the presence of invasion, indicating that similar to CaMKI, CaMKII may play a role in endometrial carcinoma progression [94]. CaMKII is overexpressed in colon cancer compared to adjacent normal tissue and increases with poor differentiation [99], and in primary breast cancer compared to adjacent normal breast, as well as in lymph node metastases [100], indicating that CaMKII may play a role in cancer progression. In regards to specific isoforms, CaMKIIγ is increased in AML patient blasts compared to normal peripheral blood cells [98], in lung cancer compared to normal lung tissue [103] and is upregulated at diagnosis in chronic myeloid leukaemia (CML) [97]. Additionally, CaMKIIγ expression is increased in CML blasts resistant to tyrosine kinase inhibitors [97], glioma cells resistant to the Fas agonistic antibody (CH-11) [124] and also in TRAIL-resistant melanoma cells [125], suggesting that it may play a role in chemoresistance.
The single-nucleotide polymorphism (SNP) rs10023113 in CAMK2D is associated with poor survival of early-stage non-small cell lung cancer patients [104], and high expression of CAMK2G in lung cancers is associated with significantly worse overall survival in 3 different cohorts [103]. Additionally, breast cancer samples that express high levels of CAMK2 exhibit significantly worse overall and distant metastasis-free survival compared to patients with low CAMK2 expression [100]. These studies suggest that CaMK2 genes are potential prognostic biomarkers for a range of cancer types.
Furthermore, not only is CaMKII expression increased in a variety of cancer types, enhanced autophosphorylation at T286 is also frequently observed (Table 1). Increased T286/7 phosphorylation of CaMKII is noted in TRAIL-resistant melanoma cells [125], lung cancer oncospheres [103], osteosarcoma [101,102], leukaemia stem cells [126], AML patient blasts [98], primary and metastatic breast tumours compared to normal adjacent breast tissue [100] and in metastatic gastric cancers compared to non-metastatic tissues [105], indicating that enhanced CaMKII activity is a feature of cancers, and is also associated with metastatic disease.
Each of the CaMKII isoforms has been implicated in the control of a variety of cancer-related functions. In vitro, CaMKII controls cellular differentiation in AML differentiation [98] and cell proliferation in smooth muscle cells [127,128,129,130], lung cancer [111], medullary thyroid cancer [112], AML [98], glioma [120], T cell lymphoma [116], osteosarcoma [101], and colon [99], gastric [113] and prostate cancers [114]. In vivo, CaMKII controls osteosarcoma [101,102,131] and T cell lymphoma [116] tumour growth. CaMKII is essential for metastatic processes, including cell migration and invasion in osteosarcoma [101,102,131], glioma [118,119,120], and gastric [105,113], colon [99], breast [100], and prostate cancers [114,115]. CaMKII activity is essential for this process, as expression of a constitutively active (H282R) mutant enhances gastric cancer cell migration and invasion by upregulating matrix metalloproteinase-9 (MMP-9) [105].
Furthermore, CaMKIIγ has been implicated in tumourigenesis in a variety of cancer types. CaMKIIγ deletion suppresses T cell lymphomagenesis in vivo [116]. CaMKIIγ knockout mice develop fewer tumours, that are smaller than their wild-type counterparts, in a dextran sodium sulfate (DSS) and azoxymethane (AOM) model of colitis-associated tumourigenesis. Furthermore, only knockout in colonic tissue-resident cells, and not in bone marrow-derived immune cells, is involved in this suppressive effect [132]. By contrast, CaMKIIγ overexpression increases colon proliferation rates, decreases cell death and increases distal colitis-associated cancer compared to control mice [132]. CaMKIIγ−/− mice exhibit increased tumour number and volume in diethylnitrosamine (DEN) and DEN followed by tumour promotor models of hepatic cancer [133]. Additionally, CaMKIIγ knockdown inhibits lung cancer tumourigenicity, and overexpression enhances tumourigenicity in vitro and in vivo [103].
Differentially phosphorylated CaMKII also controls different cellular functions. For example, dephosphorylation of CaMKII at T253 controls the metaphase-anaphase transition in neuroblastoma (SHSY5Y) and breast cancer (MDA-MB-231) cell lines [117]. Overexpression of wild-type or a T286 phospho-mimic mutant (T286D) of CaMKIIα increases cancer cell proliferation [117], migration and invasion [100]. By contrast, overexpression of a T253 phospho-mimic mutant (T253D) prevents cancer cell proliferation [117]. Taken together, these studies highlight the importance of CaMKII in controlling cancer cell proliferation and metastatic processes in a range of cancer types, indicating that its role in these functions is not cell-type specific but broadly applicable, and indicates that CaMKII may be a valid anti-cancer target for a variety of cancer types.
3.4. CaMKIV
Despite the restricted expression of CaMKIV in normal tissues, CaMKIV is overexpressed in several different types of cancer (Table 1). CaMKIV expression and activity is increased in hepatocellular carcinoma [106], small cell lung cancer [111], and is significantly associated with clinical stage, myometrial invasion and clinical outcome in endometrial carcinoma [134]. Furthermore, high CAMK4 expression is associated with significantly worse overall survival for AML patients [93], and in endometrial carcinoma [135].
As CaMKIV is expressed in immune cells, it is not surprising that it has been shown to regulate haematopoietic stem cell homeostasis [16]. Additionally, CaMKIV has been implicated in cell proliferation and cell cycle regulation. Decreasing CaMKIV expression inhibits AML development in vitro and in vivo [93], and decreases hepatic cancer cell proliferation [86]. By contrast, overexpression of CaMKIV in AML cells arrests cells in G0/G1, in an activity dependent manner [121], suggesting that CaMKIV may have cell line dependent effects, even within the same cancer subtype.
4. The CaMK Family Are Potential Anti-Cancer Therapeutic Targets
Due to the importance of CaMK family members in controlling cancer-related functions, their suitability as anti-cancer targets have begun to be explored. Several pharmacological inhibitors that inhibit the activity of these enzymes have been developed, and their usefulness as anti-cancer treatments in a variety of cancer types has been examined.
4.1. STO-609
7-Oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid (STO-609, Figure 5) is a selective CaMKK antagonist that inhibits autophosphorylation of both CaMKKα and CaMKKβ [136], without any significant effect on CaMKI and CaMKIV. However, at doses ~100-fold higher than the half maximal inhibitory concentration (IC50) for CaMKK, CaMKII and myosin light chain kinase (MLCK) are also inhibited. Additionally, STO-609 has been demonstrated to bind to and activate the aryl hydrocarbon receptor (AhR) [137], indicating that STO-609 may not be as CaMKK selective as initially believed. As CaMKK controls cancer cell proliferation, migration and survival in a variety of cancer types in vitro (Table 2), inhibiting CaMKK activity may be a valid anti-cancer therapeutic strategy in these cancer types.
Figure 5.
Structures of STO-609, KN-62, KN-93, berbamine dihydrochloride and bbd24.
Indeed, STO-609 decreases AML [93], prostate [89,91], gastric [108], hepatocellular [86] and ovarian cancer cell proliferation [88] in vitro, and induces apoptosis in ovarian [88] and gastric cancer lines [108]. Furthermore, treatment with STO-609 significantly reduces tumour burden in prostate and hepatocellular cancer mouse models in vivo (Table 3). Systemically administered STO-609 decreases tumour growth, both as a single agent and additively in combination with AR inhibition, in a C4-2B prostate cancer xenograft model [91], and in the DEN-induced hepatocellular carcinoma mouse model [86], demonstrating that CaMKK inhibition is a valid strategy for the treatment of prostate and hepatic cancer, and based on the in vitro studies, may potentially be suitable for other cancer types, including AML, gastric and ovarian cancers.
Table 3.
Effects of pharmacological inhibitors of CaMK family members on tumour burden in in vivo animal models of cancer. AR androgen receptor; CML chronic myeloid leukaemia; DEN diethylnitrosamine; HCC hepatocellular carcinoma; MNU N-methyl-N-nitrosourea; NOD-SCID non-obese diabetic severe combined immunodeficient; NSG nod scid gamma.
| Pharmacological Agent | Cancer | Model | Treatment Schedule | Outcome | Reference |
|---|---|---|---|---|---|
| STO-609 | Prostate | Subcutaneous C4-2B xenograft in full and castrated nude mice | 10 µmol/kg STO-609 or vehicle intraperitoneally three times/week | Reduction in tumour growth, which was enhanced in castrated mice | [91] |
| HCC | DEN-induced hepatic cancer model | 30 µg/kg STO-609 or vehicle intraperitoneally twice/week for 4 weeks | Reduction in tumour growth | [86] | |
| KN-93 | Osteosarcoma | Subcutaneous and intratibial MG-63 xenograft in nude mice | 1 mg/kg saline or KN-93 intraperitoneally every other day for 6 weeks | Reduction in tumour growth | [102] |
| Intratibial 143B xenograft in nude mice | Osmotic pump delivery of 5 µg/µL KN-93, 10 µg/µL CBO-P11 or vehicle set to release 0.25 µL/h for 2 weeks | Reduction in tumour growth alone and in combination with CBO-P11 | [131] | ||
| Berbamine | HCC | Subcutaneous Huh7 or SK-Hep-1 xenograft in NOD-SCID mice | 100 mg/kg berbamine orally twice day for 5 consecutive days, 2 days withdrawal, and then repeated once | Reduction in tumour growth | [139] |
| CML | Subcutaneous K562 and primary CML cells from a patient at blast crisis xenograft in nude mice | 100 mg/kg berbamine, imatinib or vehicle orally three time daily for 10 days | Reduction in tumour growth | [126] | |
| T cell lymphoma | MNU-induced lymphoma model and subcutaneous H9 xenograft in NSG mice | 50 m 100 or 150 mg/kg berbamine, or vehicle, orally administered to mice 2 times a day for 14 days, 14 days withdrawal, cycle repeated; Xenograft study: 150 mg/kg berbamine or vehicle oral twice a day | Reduction in tumour growth in both models | [116] |
Intraperitoneal doses of STO-609 up to 300 µM/kg, or daily injections of 30 µM/kg for 4 weeks, have been shown to be well-tolerated in C57Bl/6 J mice, and did not induce parameters of liver or kidney toxicity [138]. However, the primary limitation of the use of STO-609 as an anti-cancer treatment is its poor solubility, therefore improved derivatives that increase solubility, whilst increasing efficacy will need to be developed.
4.2. KN-62/KN-93
4-[(2S)-2-[(5-Isoquinolinylsulfonyl)methylamino]-3-oxo-3-(4-pheynl-1-piperazinyl)propyl] phenylisoquinolinesulfonic acid ester (KN-62) and N-[2-[[[3-(4′-chlorophenyl)-2-propenyl]-methylamino]methyl]phenyl]-N-(2-hydroxyethyl)-4′-methoxybenzenesulfonamide phosphate salt (KN-93) (Figure 5), are membrane permeable CaMKII inhibitors that are competitive for CaM [140]. They were originally described as CaMKII specific inhibitors, as they do not affect other CaM-dependent enzymes, such as AMPK and MLCK [141].
However, subsequent studies have shown that they also inhibit CaMKI and CaMKIV and other molecules unrelated to the CaMK family, including ion channels [142,143,144,145,146]. Both KN-62 and KN-93 prevent the activation of CaMKII, but do not inhibit autonomously active CaMKII [142]. As CaMKI, CaMKII and CaMKIV control cancer cell proliferation, migration and survival in a variety of cancer types in vitro (Table 2), inhibiting the activity of these kinases using KN-62 and KN-93 may be a valid anti-cancer therapeutic strategy for a range of cancer types.
Indeed, KN-93 decreases proliferation in osteosarcoma [131], AML [98], T cell lymphoma [116], prostate cancer [114], endometrial cancer [94], glioma [120], colon cancer [99], breast cancer [110] and medullary thyroid cancer cells [112], induces apoptosis in prostate cancer [147,148], but not AML cells [98], and resensitises resistant melanoma cells to TRAIL-induced apoptosis [125] and resistant glioma cells to CH-11 [124,149] in vitro. Whilst KN-62 treatment does not result in apoptosis in cancer cell lines, it induces differentiation in AML cell lines [150], suppresses hypoxia inducible factor (HIF)-1α in hepatoma cells [151], potentiates the effects of etoposide in head and neck squamous cell carcinoma [152] and AML cell lines [153], and reverses adriamycin resistance in human ovarian cancer cells [154], indicating that it may be useful when combined with additional therapies. Additionally, KN-93 decreases migration and invasion in osteosarcoma [131], breast [100], prostate [114], colon [99] and endometrial [94] cancer cells. Similar findings have been observed with KN-62, as it inhibits gastric cancer cell proliferation, invasion and migration in vitro [105]. Taken together, these studies indicate that inhibiting CaMK family members using KN-93 and/or KN-62 may be suitable for the treatment of metastatic cancer in a range of solid tumours.
Furthermore, KN-93 decreases tumour burden in osteosarcoma xenograft models in vivo (Table 3). Systemically administered KN-93 decreases tumour growth in subcutaneous and orthotopic (intratibial) MG-63 osteosarcoma models in vivo, both alone [102] and in combination with CBO-P11 (a vascular endothelial growth factor receptor (VEGFR) inhibitor) [131].
Whilst in vivo studies using these inhibitors do not describe any side-effects, KN-62 and KN-93 have been widely used to examine a variety of heart and brain-related functions. For example, KN-62 and KN-93 can depress the rate of beating of cultured myocytes [155] and afterdepolarisation in the heart [156], respectively, and can block long-term potentiation (LTP) in rat hippocampus [157], indicating that they are likely to affect cardiac, learning and memory processes. Due to these potential side effects, as well as the range of off-target effects using these inhibitors, these inhibitors are unlikely to be translated into clinical use without modification or the use of alternative more cancer-selective modes of drug delivery (e.g., nanoparticles or liposomes).
4.3. Substrate Based Inhibitors: Autocamtide-3 Derived Peptide Inhibitor (AC3-I) and Autocamtide-2-Related Inhibitory Peptide (AIP)
Long inhibitory peptides based on the autoinhibitory domain of CaMKIIα have been developed. The N-terminus of this peptide contains the autophosphorylation site forming the basis for peptide substrates such as autocamtide-2 and -3 [158] and the development of a non-phosphorylatable analogue of autocamtide-2 generated the peptide inhibitors AIP (KKALRRQEAVDAL) [140] and AC3-I (KKALHRQEAVDCL) [159]. AIP competes with the active site of CaMKII, inhibiting activity regardless of how CaMKII was activated, and inhibits CaMKII with over 100-fold selectivity relative to protein kinase C (PKC), PKA and CaMKIV. However, altered selectivity can also occur when peptides are fused to GFP or modified by lipids to increase membrane permeability. For example, myristoylated AIP has been shown to have effects unrelated to CaMKII inhibition [160], indicating the presence of an additional non-CaMKII-related target, and green fluorescent protein (GFP)-AC3-I can inhibit cellular actions of protein kinase D1 (PKD1) [161]. AIP significantly attenuates glioma [119,162] and breast cancer [100] migration and invasion. Furthermore, AC3-I treatment arrests ovarian carcinoma cells in G2 and stops proliferation [163]. These studies indicate that CaMKII inhibition may be a viable therapeutic option for the treatment of metastatic disease.
4.4. CaMKIIN Derived Peptides (CaMKIINtide)
Small endogenous proteins that inhibit CaMKII (CaMKIIN) have been identified in rat brain [164] and there are two known rat isoforms—CaMKIINα [164] and CaMKIINβ [165]. Human CaMKIIN has also been identified in human dendritic cells [166]. CaMKIIN is implicated in the control of progression of cells through S phase. Overexpression of hCaMKIIN in colon cancer cells decreases cell proliferation, whereas silencing increases cell growth rates [166,167]. Furthermore, hCaMKIIN overexpression in ovarian cancer cells decreases proliferation and tumourigenicity [168,169], and also reduces migration and colony formation [169]. This implies a potential application of hCaMKIIN in the treatment of colon and ovarian cancers.
Identification of the core inhibitory domain of CaMKIIN led to the generation of a 28 amino acid peptide inhibitor called CaMKIINtide [164]. CaMKIINtide only inhibits activated CaMKII [164], and does not inhibit PKC, PKA, CaMKI, CaMKIV or CaMKK [164]. CaMKIINtide has also been modified to increase potency [170,171]. A cell permeable form, antCaNtide, decreases medullary thyroid cancer [112], and AML cell proliferation [121] indicating that the development of additional CaMKII specific inhibitors may provide viable therapeutic options for the treatment of haematological and thyroid cancers.
4.5. Berbamine Dihydrochloride
Berbamine (Figure 5) is a natural bis-benzylisoquinoline alkaloid, isolated from the traditional Chinese herbal medicine Berberis amurensis. Berbamine exhibits potent antitumour activities with low toxicity in a variety of cancer types, including melanoma, hepatocellular carcinoma, breast cancer, leukaemia and lung cancer [126,172,173,174,175,176,177,178]. Recently, berbamine was shown to produce its anti-cancer effects by blocking the ATP binding pocket of CaMKIIγ [126], however, berbamine also inhibits molecules unrelated to CaMKII, including mechano-electrical transducer channels, the Bcr/Abl fusion gene, and the NF-κB pathway [175,179,180]. 2-methylbenzoyl berbamine (bbd24) (Figure 5), a derivative of berbamine and a more potent CaMKII inhibitor, has been identified [126]. Berbamine inhibits the growth and reduces the viability of liver cancer [139] and CML cells [126] in a CaMKIIγ dependent manner. Bbd24 also kills liver cancer cells in vitro [139].
Berbamine has been shown to reduce tumour burden in several different animal models (Table 3). Berbamine decreases liver cancer [139] and CML [126] tumour burden in vivo and decreases tumour burden and significantly increases survival in an N-methyl-N-nitrosurea (MNU)-induced lymphoma model [116].
The main limitation for the use of berbamine clinically as an anti-cancer agent is its short plasma half-life and poor bioavailability at the tumour site after systemic administration. To circumvent this, lipid-based nanoparticles loaded with berbamine have been developed and have been shown to decrease primary tumour growth in a B16F10 mouse melanoma model and also suppress the incidence of lung metastases in vivo [181]. This highlights that newer mechanisms of drug delivery may be useful clinically to increase the cancer-specificity of these drugs, without enhancing toxicity.
5. Concluding Remarks and Perspectives
The CaMK family members, particularly CaMKII, are attractive anti-cancer targets as they are overexpressed in a plethora of cancer types, compared to adjacent normal tissue, and are vital in the modulation of cancer cell proliferation, survival, invasion and migration. Several targeted and broad-acting CaMK inhibitors have demonstrated pre-clinical anti-cancer efficacy in vivo. Many of the previously believed ‘CaMK-specific’ inhibitors have been shown to have a variety of off-target effects, which further limits their clinical usefulness. By contrast, CaMKIIN-tide, and its modified derivatives, based on the endogenous CaMKII inhibitory protein (CaMKIIN) have no described off-target effects, and are the most promising lead compounds for further development described here-in, however, their usefulness in vivo remains to be investigated. Additionally, as the CaMK family members are also involved in highly important, non-cancer related functions, direct inhibition using these existing CaMK inhibitors are likely to produce a range of deleterious side-effects if used clinically. Therefore, to be useful therapeutically, cancer-specific inhibitors or more cancer-specific modes of drug delivery would be required to be developed. One such potential strategy would be to encapsulate these CaMK inhibitors in nanoparticles or liposomes that specifically target cancer cells for the delivery of the inhibitor. Whilst this has been examined in other disease models for CaMKIIN inhibitory peptides, such as heart disease and asthma [182,183], they remain to be tested in cancer.
Author Contributions
All authors contributed to the drafting and editing of this manuscript.
Funding
This work was funded by research funds received from the Hunter Medical Research, Mark Hughes Foundation, Hunter Children’s Research Foundation, Hunter Cancer Research Alliance, University of Newcastle and the Cure Cancer Australia Foundation/Cancer Australia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Stewart T.A., Yapa K.T., Monteith G.R. Altered calcium signaling in cancer cells. Biochim. Biophys. Acta. 2015;1848:2502–2511. doi: 10.1016/j.bbamem.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D., Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Okuno S., Kitani T., Fujisawa H. Studies on the substrate specificity of Ca2+/calmodulin-dependent protein kinase kinase alpha. J. Biochem. 1997;122:337–343. doi: 10.1093/oxfordjournals.jbchem.a021758. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa Y., Tokumitsu H., Inuzuka H., Murata-Hori M., Hosoya H., Kobayashi R. Identification and characterization of novel components of a Ca2+/calmodulin-dependent protein kinase cascade in HeLa cells. FEBS Lett. 2003;550:57–63. doi: 10.1016/S0014-5793(03)00817-2. [DOI] [PubMed] [Google Scholar]
- 5.Hsu L.S., Chen G.D., Lee L.S., Chi C.W., Cheng J.F., Chen J.Y. Human Ca2+/calmodulin-dependent protein kinase kinase beta gene encodes multiple isoforms that display distinct kinase activity. J. Biol. Chem. 2001;276:31113–31123. doi: 10.1074/jbc.M011720200. [DOI] [PubMed] [Google Scholar]
- 6.Tokumitsu H., Brickey D.A., Glod J., Hidaka H., Sikela J., Soderling T.R. Activation mechanisms for Ca2+/calmodulin-dependent protein kinase IV. Identification of a brain CaM-kinase IV kinase. J. Biol. Chem. 1994;269:28640–28647. [PubMed] [Google Scholar]
- 7.Lee J.C., Edelman A.M. A protein activator of Ca(2+)-calmodulin-dependent protein kinase Ia. J. Biol. Chem. 1994;269:2158–2164. [PubMed] [Google Scholar]
- 8.Edelman A.M., Mitchelhill K.I., Selbert M.A., Anderson K.A., Hook S.S., Stapleton D., Goldstein E.G., Means A.R., Kemp B.E. Multiple Ca(2+)-calmodulin-dependent protein kinase kinases from rat brain. Purification, regulation by Ca(2+)-calmodulin, and partial amino acid sequence. J. Biol. Chem. 1996;271:10806–10810. doi: 10.1074/jbc.271.18.10806. [DOI] [PubMed] [Google Scholar]
- 9.Ohmstede C.A., Jensen K.F., Sahyoun N.E. Ca2+/calmodulin-dependent protein kinase enriched in cerebellar granule cells. Identification of a novel neuronal calmodulin-dependent protein kinase. J. Biol. Chem. 1989;264:5866–5875. [PubMed] [Google Scholar]
- 10.Racioppi L., Means A.R. Calcium/calmodulin-dependent protein kinase kinase 2: Roles in signaling and pathophysiology. J. Biol. Chem. 2012;287:31658–31665. doi: 10.1074/jbc.R112.356485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson K.A., Means R.L., Huang Q.H., Kemp B.E., Goldstein E.G., Selbert M.A., Edelman A.M., Fremeau R.T., Means A.R. Components of a calmodulin-dependent protein kinase cascade. Molecular cloning, functional characterization and cellular localization of Ca2+/calmodulin-dependent protein kinase kinase beta. J. Biol. Chem. 1998;273:31880–31889. doi: 10.1074/jbc.273.48.31880. [DOI] [PubMed] [Google Scholar]
- 12.Hawley S.A., Selbert M.A., Goldstein E.G., Edelman A.M., Carling D., Hardie D.G. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J. Biol. Chem. 1995;270:27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- 13.Yano S., Tokumitsu H., Soderling T.R. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 14.Anderson K.A., Means A.R. Defective signaling in a subpopulation of CD4(+) T cells in the absence of Ca(2+)/calmodulin-dependent protein kinase IV. Mol. Cell. Biol. 2002;22:23–29. doi: 10.1128/MCB.22.1.23-29.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neal A.P., Molina-Campos E., Marrero-Rosado B., Bradford A.B., Fox S.M., Kovalova N., Hannon H.E. CaMKK-CaMKI signaling pathways differentially control axon and dendrite elongation in cortical neurons. J. Neurosci. 2010;30:2807–2809. doi: 10.1523/JNEUROSCI.5984-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitsos C.M., Sankar U., Illario M., Colomer-Font J.M., Duncan A.W., Ribar T.J., Reya T., Means A.R. Calmodulin-dependent protein kinase IV regulates hematopoietic stem cell maintenance. J. Biol. Chem. 2005;280:33101–33108. doi: 10.1074/jbc.M505208200. [DOI] [PubMed] [Google Scholar]
- 17.Kahl C.R., Means A.R. Regulation of cyclin D1/Cdk4 complexes by calcium/calmodulin-dependent protein kinase I. J. Biol. Chem. 2004;279:15411–15419. doi: 10.1074/jbc.M312543200. [DOI] [PubMed] [Google Scholar]
- 18.Anderson K.A., Ribar T.J., Illario M., Means A.R. Defective survival and activation of thymocytes in transgenic mice expressing a catalytically inactive form of Ca2+/calmodulin-dependent protein kinase IV. Mol. Endocrinol. 1997;11:725–737. doi: 10.1210/mend.11.6.0011. [DOI] [PubMed] [Google Scholar]
- 19.Wayman G.A., Lee Y.S., Tokumitsu H., Silva A.J., Soderling T.R. Calmodulin-kinases: Modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin F., Ribar T.J., Means A.R. The Ca2+/calmodulin-dependent protein kinase kinase, CaMKK2, inhibits preadipocyte differentiation. Endocrinology. 2011;152:3668–3679. doi: 10.1210/en.2011-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witczak C.A., Fujii N., Hirshman M.F., Goodyear L.J. Ca2+/calmodulin-dependent protein kinase kinase-alpha regulates skeletal muscle glucose uptake independent of AMP-activated protein kinase and Akt activation. Diabetes. 2007;56:1403–1409. doi: 10.2337/db06-1230. [DOI] [PubMed] [Google Scholar]
- 22.Tokumitsu H., Soderling T.R. Requirements for calcium and calmodulin in the calmodulin kinase activation cascade. J. Biol. Chem. 1996;271:5617–5622. doi: 10.1074/jbc.271.10.5617. [DOI] [PubMed] [Google Scholar]
- 23.Green M.F., Scott J.W., Steel R., Oakhill J.S., Kemp B.E., Means A.R. Ca2+/Calmodulin-dependent Protein Kinase Kinase beta Is Regulated by Multisite Phosphorylation. J. Biol. Chem. 2011;286:28066–28079. doi: 10.1074/jbc.M111.251504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wayman G.A., Tokumitsu H., Soderling T.R. Inhibitory cross-talk by cAMP kinase on the calmodulin-dependent protein kinase cascade. J. Biol. Chem. 1997;272:16073–16076. doi: 10.1074/jbc.272.26.16073. [DOI] [PubMed] [Google Scholar]
- 25.Okuno S., Kitani T., Fujisawa H. Regulation of Ca(2+)/calmodulin-dependent protein kinase kinase alpha by cAMP-dependent protein kinase: I. Biochemical analysis. J. Biochem. 2001;130:503–513. doi: 10.1093/oxfordjournals.jbchem.a003013. [DOI] [PubMed] [Google Scholar]
- 26.Tokumitsu H., Hatano N., Fujimoto T., Yurimoto S., Kobayashi R. Generation of autonomous activity of Ca(2+)/calmodulin-dependent protein kinase kinase beta by autophosphorylation. Biochemistry. 2011;50:8193–8201. doi: 10.1021/bi201005g. [DOI] [PubMed] [Google Scholar]
- 27.Davare M.A., Saneyoshi T., Guire E.S., Nygaard S.C., Soderling T.R. Inhibition of calcium/calmodulin-dependent protein kinase kinase by protein 14-3-3. J. Biol. Chem. 2004;279:52191–52199. doi: 10.1074/jbc.M409873200. [DOI] [PubMed] [Google Scholar]
- 28.Matsushita M., Nairn A.C. Inhibition of the Ca2+/calmodulin-dependent protein kinase I cascade by cAMP-dependent protein kinase. J. Biol. Chem. 1999;274:10086–10093. doi: 10.1074/jbc.274.15.10086. [DOI] [PubMed] [Google Scholar]
- 29.Loseth O.P., de Lecea L., Calbet M., Danielson P.E., Gautvik V., Hovring P.I., Walaas S.I., Gautvik K.M. Developmental regulation of two isoforms of Ca(2+)/calmodulin-dependent protein kinase I beta in rat brain. Brain Res. 2000;869:137–145. doi: 10.1016/S0006-8993(00)02359-3. [DOI] [PubMed] [Google Scholar]
- 30.Picciotto M.R., Zoli M., Bertuzzi G., Nairn A.C. Immunochemical localization of calcium/calmodulin-dependent protein kinase I. Synapse. 1995;20:75–84. doi: 10.1002/syn.890200111. [DOI] [PubMed] [Google Scholar]
- 31.Kamata A., Sakagami H., Tokumitsu H., Owada Y., Fukunaga K., Kondo H. Spatiotemporal expression of four isoforms of Ca2+/calmodulin-dependent protein kinase I in brain and its possible roles in hippocampal dendritic growth. Neurosci. Res. 2007;57:86–97. doi: 10.1016/j.neures.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Nairn A.C., Greengard P. Purification and characterization of Ca2+/calmodulin-dependent protein kinase I from bovine brain. J. Biol. Chem. 1987;262:7273–7281. [PubMed] [Google Scholar]
- 33.Joseph J.D., Means A.R. Identification and characterization of two Ca2+/CaM-dependent protein kinases required for normal nuclear division in Aspergillus nidulans. J. Biol. Chem. 2000;275:38230–38238. doi: 10.1074/jbc.M006422200. [DOI] [PubMed] [Google Scholar]
- 34.Skelding K.A., Rostas J.A., Verrills N.M. Controlling the cell cycle: The role of calcium/calmodulin-stimulated protein kinases I and II. Cell Cycle. 2011;10:631–639. doi: 10.4161/cc.10.4.14798. [DOI] [PubMed] [Google Scholar]
- 35.Wayman G.A., Kaech S., Grant W.F., Davare M., Impey S., Tokumitsu H., Nozaki N., Banker G., Soderling T.R. Regulation of axonal extension and growth cone motility by calmodulin-dependent protein kinase I. J. Neurosci. 2004;24:3786–3794. doi: 10.1523/JNEUROSCI.3294-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Condon J.C., Pezzi V., Drummond B.M., Yin S., Rainey W.E. Calmodulin-dependent kinase I regulates adrenal cell expression of aldosterone synthase. Endocrinology. 2002;143:3651–3657. doi: 10.1210/en.2001-211359. [DOI] [PubMed] [Google Scholar]
- 37.Takemoto-Kimura S., Ageta-Ishihara N., Nonaka M., Adachi-Morishima A., Mano T., Okamura M., Fujii H., Fuse T., Hoshino M., Suzuki S., et al. Regulation of dendritogenesis via a lipid-raft-associated Ca2+/calmodulin-dependent protein kinase CLICK-III/CaMKIgamma. Neuron. 2007;54:755–770. doi: 10.1016/j.neuron.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt J.M., Guire E.S., Saneyoshi T., Soderling T.R. Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation. J. Neurosci. 2005;25:1281–1290. doi: 10.1523/JNEUROSCI.4086-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ang E.S., Zhang P., Steer J.H., Tan J.W., Yip K., Zheng M.H., Joyce D.A., Xu J. Calcium/calmodulin-dependent kinase activity is required for efficient induction of osteoclast differentiation and bone resorption by receptor activator of nuclear factor kappa B ligand (RANKL) J. Cell. Physiol. 2007;212:787–795. doi: 10.1002/jcp.21076. [DOI] [PubMed] [Google Scholar]
- 40.Haribabu B., Hook S.S., Selbert M.A., Goldstein E.G., Tomhave E.D., Edelman A.M., Snyderman R., Means A.R. Human Calcium-Calmodulin Dependent Protein-Kinase-I—Cdna Cloning, Domain-Structure and Activation by Phosphorylation at Threonine-177 by Calcium-Calmodulin Dependent Protein-Kinase-I Kinase. EMBO J. 1995;14:3679–3686. doi: 10.1002/j.1460-2075.1995.tb00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takemoto-Kimura S., Terai H., Takamoto M., Ohmae S., Kikumura S., Segi E., Arakawa Y., Furuyashiki T., Narumiya S., Bito H. Molecular cloning and characterization of CLICK-III/CaMKIgamma, a novel membrane-anchored neuronal Ca2+/calmodulin-dependent protein kinase (CaMK) J. Biol. Chem. 2003;278:18597–18605. doi: 10.1074/jbc.M300578200. [DOI] [PubMed] [Google Scholar]
- 42.Senga Y., Ishida A., Shigeri Y., Kameshita I., Sueyoshi N. The Phosphatase-Resistant Isoform of CaMKI, Ca(2)(+)/Calmodulin-Dependent Protein Kinase Idelta (CaMKIdelta), Remains in Its “Primed” Form without Ca(2)(+) Stimulation. Biochemistry. 2015;54:3617–3630. doi: 10.1021/bi5012139. [DOI] [PubMed] [Google Scholar]
- 43.Cook S.G., Bourke A.M., O’Leary H., Zaegel V., Lasda E., Mize-Berge J., Quillinan N., Tucker C.L., Coultrap S.J., Herson P.S., et al. Analysis of the CaMKIIalpha and beta splice-variant distribution among brain regions reveals isoform-specific differences in holoenzyme formation. Sci. Rep. 2018;8:5448. doi: 10.1038/s41598-018-23779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giese K.P., Fedorov N.B., Filipkowski R.K., Silva A.J. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 45.Soderling T.R., Derkach V.A. Postsynaptic protein phosphorylation and LTP. Trends Neurosci. 2000;23:75–80. doi: 10.1016/S0166-2236(99)01490-3. [DOI] [PubMed] [Google Scholar]
- 46.Von Hertzen L.S., Giese K.P. Alpha-isoform of Ca2+/calmodulin-dependent kinase II autophosphorylation is required for memory consolidation-specific transcription. Neuroreport. 2005;16:1411–1414. doi: 10.1097/01.wnr.0000175244.51084.bb. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z.W. Regulation of synaptic transmission by presynaptic CaMKII and BK channels. Mol. Neurobiol. 2008;38:153–166. doi: 10.1007/s12035-008-8039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fink C.C., Bayer K.U., Myers J.W., Ferrell J.E., Schulman H., Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the of isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/S0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 49.Jones K.T. Intracellular calcium in the fertilization and development of mammalian eggs. Clin. Exp. Pharmacol. Physiol. 2007;34:1084–1089. doi: 10.1111/j.1440-1681.2007.04726.x. [DOI] [PubMed] [Google Scholar]
- 50.Shin M.K., Kim M.K., Bae Y.S., Jo I., Lee S.J., Chung C.P., Park Y.J., Min do S. A novel collagen-binding peptide promotes osteogenic differentiation via Ca2+/calmodulin-dependent protein kinase II/ERK/AP-1 signaling pathway in human bone marrow-derived mesenchymal stem cells. Cell. Signal. 2008;20:613–624. doi: 10.1016/j.cellsig.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 51.Munevar S., Gangopadhyay S.S., Gallant C., Colombo B., Sellke F.W., Morgan K.G. CaMKIIT287 and T305 regulate history-dependent increases in alpha agonist-induced vascular tone. J. Cell. Mol. Med. 2008;12:219–226. doi: 10.1111/j.1582-4934.2007.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maier L.S., Bers D.M. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc. Res. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Liu W., Xu C., Ran D., Wang Y., Zhao H., Gu J., Liu X., Bian J., Yuan Y., Liu Z. CaMK mediates cadmium induced apoptosis in rat primary osteoblasts through MAPK activation and endoplasmic reticulum stress. Toxicology. 2018;406–407:70–80. doi: 10.1016/j.tox.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Rostas J.A., Hoffman A., Murtha L.A., Pepperall D., McLeod D.D., Dickson P.W., Spratt N.J., Skelding K.A. Ischaemia- and excitotoxicity-induced CaMKII-Mediated neuronal cell death: The relative roles of CaMKII autophosphorylation at T286 and T253. Neurochem. Int. 2017;104:6–10. doi: 10.1016/j.neuint.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Rostas J.A.P., Spratt N.J., Dickson P.W., Skelding K.A. The role of Ca2+-calmodulin stimulated protein kinase II in ischaemic stroke—A potential target for neuroprotective therapies. Neurochem. Int. 2017;107:33–42. doi: 10.1016/j.neuint.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Skelding K.A., Spratt N.J., Fluechter L., Dickson P.W., Rostas J.A.P. alpha CaMKII is differentially regulated in brain regions that exhibit differing sensitivities to ischemia and excitotoxicity. J. Cerebr. Blood Flow Metab. 2012;32:2181–2192. doi: 10.1038/jcbfm.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenberg O.S., Deindl S., Sung R.J., Nairn A.C., Kuriyan J. Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell. 2005;123:849–860. doi: 10.1016/j.cell.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 58.Hanson P.I., Meyer T., Stryer L., Schulman H. Dual Role of Calmodulin in Autophosphorylation of Multifunctional Cam Kinase May Underlie Decoding of Calcium Signals. Neuron. 1994;12:943–956. doi: 10.1016/0896-6273(94)90306-9. [DOI] [PubMed] [Google Scholar]
- 59.Rostas J.A., Skelding K.A., Verrills N.M., Suzuki P.W., Dickson T. Camkii Binding Partners Vary with Cell Type and Phosphorylation State. J. Neurochem. 2009;110:40–41. [Google Scholar]
- 60.Skelding K.A., Suzuki T., Gordon S., Xue J., Verrills N.M., Dickson P.W., Rostas J.A. Regulation of CaMKII by phospho-Thr253 or phospho-Thr286 sensitive targeting alters cellular function. Cell. Signal. 2010;22:759–769. doi: 10.1016/j.cellsig.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 61.Meyer T., Hanson P.I., Stryer L., Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- 62.Strack S., Colbran R.J. Autophosphorylation-dependent targeting of calcium/ calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl- D-aspartate receptor. J. Biol. Chem. 1998;273:20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- 63.Strack S., Choi S., Lovinger D.M., Colbran R.J. Translocation of autophosphorylated calcium/calmodulin-dependent protein kinase II to the postsynaptic density. J. Biol. Chem. 1997;272:13467–13470. doi: 10.1074/jbc.272.21.13467. [DOI] [PubMed] [Google Scholar]
- 64.Hanson P.I., Schulman H. Inhibitory autophosphorylation of multifunctional Ca2+/calmodulin-dependent protein kinase analyzed by site-directed mutagenesis. J. Biol. Chem. 1992;267:17216–17224. [PubMed] [Google Scholar]
- 65.Patton B.L., Miller S.G., Kennedy M.B. Activation of type II calcium/calmodulin-dependent protein kinase by Ca2+/calmodulin is inhibited by autophosphorylation of threonine within the calmodulin-binding domain. J. Biol. Chem. 1990;265:11204–11212. [PubMed] [Google Scholar]
- 66.Meador W.E., Means A.R., Quiocho F.A. Modulation of calmodulin plasticity in molecular recognition on the basis of x-ray structures. Science. 1993;262:1718–1721. doi: 10.1126/science.8259515. [DOI] [PubMed] [Google Scholar]
- 67.Braun A.P., Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: From form to function. Annu. Rev. Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 68.Erickson J.R., Joiner M.L., Guan X., Kutschke W., Yang J., Oddis C.V., Bartlett R.K., Lowe J.S., O’Donnell S.E., Aykin-Burns N., et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jaffe H., Vinade L., Dosemeci A. Identification of novel phosphorylation sites on postsynaptic density proteins. Biochem. Biophys. Res. Commun. 2004;321:210–218. doi: 10.1016/j.bbrc.2004.06.122. [DOI] [PubMed] [Google Scholar]
- 70.Molloy S.S., Kennedy M.B. Autophosphorylation of type II Ca2+/calmodulin-dependent protein kinase in cultures of postnatal rat hippocampal slices. Proc. Natl. Acad. Sci. USA. 1991;88:4756–4760. doi: 10.1073/pnas.88.11.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collins M.O., Yu L., Coba M.P., Husi H., Campuzano I., Blackstock W.P., Choudhary J.S., Grant S.G. Proteomic analysis of in vivo phosphorylated synaptic proteins. J. Biol. Chem. 2005;280:5972–5982. doi: 10.1074/jbc.M411220200. [DOI] [PubMed] [Google Scholar]
- 72.Migues P.V., Lehmann I.T., Fluechter L., Cammarota M., Gurd J.W., Sim A.T.R., Dickson P.W., Rostas J.A.P. Phosphorylation of CaMKII at Thr253 occurs in vivo and enhances binding to isolated postsynaptic densities. J. Neurochem. 2006;98:289–299. doi: 10.1111/j.1471-4159.2006.03876.x. [DOI] [PubMed] [Google Scholar]
- 73.Sakagami H., Kondo H. Cloning and sequencing of a gene encoding the beta polypeptide of Ca2+/calmodulin-dependent protein kinase IV and its expression confined to the mature cerebellar granule cells. Brain Res. Mol. Brain Res. 1993;19:215–218. doi: 10.1016/0169-328X(93)90029-O. [DOI] [PubMed] [Google Scholar]
- 74.Mosialos G., Hanissian S.H., Jawahar S., Vara L., Kieff E., Chatila T.A. A Ca2+/calmodulin-dependent protein kinase, CaM kinase-Gr, expressed after transformation of primary human B lymphocytes by Epstein-Barr virus (EBV) is induced by the EBV oncogene LMP1. J. Virol. 1994;68:1697–1705. doi: 10.1128/jvi.68.3.1697-1705.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu J.Y., Gonzalez-Robayana I.J., Richards J.S., Means A.R. Female fertility is reduced in mice lacking Ca2+/calmodulin-dependent protein kinase IV. Endocrinology. 2000;141:4777–4783. doi: 10.1210/endo.141.12.7826. [DOI] [PubMed] [Google Scholar]
- 76.Wu J.Y., Means A.R. Ca(2+)/calmodulin-dependent protein kinase IV is expressed in spermatids and targeted to chromatin and the nuclear matrix. J. Biol. Chem. 2000;275:7994–7999. doi: 10.1074/jbc.275.11.7994. [DOI] [PubMed] [Google Scholar]
- 77.Kimura Y., Corcoran E.E., Eto K., Gengyo-Ando K., Muramatsu M.A., Kobayashi R., Freedman J.H., Mitani S., Hagiwara M., Means A.R., et al. A CaMK cascade activates CRE-mediated transcription in neurons of Caenorhabditis elegans. EMBO Rep. 2002;3:962–966. doi: 10.1093/embo-reports/kvf191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bleier J., Toliver A. Exploring the Role of CaMKIV in Homeostatic Plasticity. J. Neurosci. 2017;37:11520–11522. doi: 10.1523/JNEUROSCI.2599-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takemura M., Mishima T., Wang Y., Kasahara J., Fukunaga K., Ohashi K., Mizuno K. Ca2+/Calmodulin-dependent Protein Kinase IV-mediated LIM Kinase Activation Is Critical for Calcium Signal-induced Neurite Outgrowth. J. Biol. Chem. 2009;284:28554–28562. doi: 10.1074/jbc.M109.006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei F., Qiu C.S., Liauw J., Robinson D.A., Ho N., Chatila T., Zhuo M. Calcium calmodulin-dependent protein kinase IV is required for fear memory. Nat. Neurosci. 2002;5:573–579. doi: 10.1038/nn0602-855. [DOI] [PubMed] [Google Scholar]
- 81.Racioppi L., Means A.R. Calcium/calmodulin-dependent kinase IV in immune and inflammatory responses: Novel routes for an ancient traveller. Trends Immunol. 2008;29:600–607. doi: 10.1016/j.it.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 82.Selbert M.A., Anderson K.A., Huang Q.H., Goldstein E.G., Means A.R., Edelman A.M. Phosphorylation and activation of Ca(2+)-calmodulin-dependent protein kinase IV by Ca(2+)-calmodulin-dependent protein kinase Ia kinase. Phosphorylation of threonine 196 is essential for activation. J. Biol. Chem. 1995;270:17616–17621. doi: 10.1074/jbc.270.29.17616. [DOI] [PubMed] [Google Scholar]
- 83.Chatila T., Anderson K.A., Ho N., Means A.R. A unique phosphorylation-dependent mechanism for the activation of Ca2+/calmodulin-dependent protein kinase type IV/GR. J. Biol. Chem. 1996;271:21542–21548. doi: 10.1074/jbc.271.35.21542. [DOI] [PubMed] [Google Scholar]
- 84.Watanabe S., Okuno S., Kitani T., Fujisawa H. Inactivation of calmodulin-dependent protein kinase IV by autophosphorylation of serine 332 within the putative calmodulin-binding domain. J. Biol. Chem. 1996;271:6903–6910. doi: 10.1074/jbc.271.12.6903. [DOI] [PubMed] [Google Scholar]
- 85.Subbannayya Y., Syed N., Barbhuiya M.A., Raja R., Marimuthu A., Sahasrabuddhe N., Pinto S.M., Manda S.S., Renuse S., Manju H.C., et al. Calcium calmodulin dependent kinase kinase 2—A novel therapeutic target for gastric adenocarcinoma. Cancer Biol. Ther. 2015;16:336–345. doi: 10.4161/15384047.2014.972264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin F., Marcelo K.L., Rajapakshe K., Coarfa C., Dean A., Wilganowski N., Robinson H., Sevick E., Bissig K.D., Goldie L.C., et al. The camKK2/camKIV relay is an essential regulator of hepatic cancer. Hepatology. 2015;62:505–520. doi: 10.1002/hep.27832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu D.M., Wang H.J., Han B., Meng X.Q., Chen M.H., Yang D.B., Sun Y., Li Y.L., Jiang C.L. CAMKK2, Regulated by Promoter Methylation, is a Prognostic Marker in Diffuse Gliomas. CNS Neurosci. Ther. 2016;22:518–524. doi: 10.1111/cns.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gocher A.M., Azabdaftari G., Euscher L.M., Dai S., Karacosta L.G., Franke T.F., Edelman A.M. Akt activation by Ca(2+)/calmodulin-dependent protein kinase kinase 2 (CaMKK2) in ovarian cancer cells. J. Biol. Chem. 2017;292:14188–14204. doi: 10.1074/jbc.M117.778464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karacosta L.G., Foster B.A., Azabdaftari G., Feliciano D.M., Edelman A.M. A regulatory feedback loop between Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2) and the androgen receptor in prostate cancer progression. J. Biol. Chem. 2012;287:24832–24843. doi: 10.1074/jbc.M112.370783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shima T., Mizokami A., Miyagi T., Kawai K., Izumi K., Kumaki M., Ofude M., Zhang J., Keller E.T., Namiki M. Down-regulation of calcium/calmodulin-dependent protein kinase kinase 2 by androgen deprivation induces castration-resistant prostate cancer. Prostate. 2012;72:1789–1801. doi: 10.1002/pros.22533. [DOI] [PubMed] [Google Scholar]
- 91.Massie C.E., Lynch A., Ramos-Montoya A., Boren J., Stark R., Fazli L., Warren A., Scott H., Madhu B., Sharma N., et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011;30:2719–2733. doi: 10.1038/emboj.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Frigo D.E., Howe M.K., Wittmann B.M., Brunner A.M., Cushman I., Wang Q., Brown M., Means A.R., McDonnell D.P. CaM kinase kinase beta-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. Cancer Res. 2011;71:528–537. doi: 10.1158/0008-5472.CAN-10-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kang X., Cui C., Wang C., Wu G., Chen H., Lu Z., Chen X., Wang L., Huang J., Geng H., et al. CAMKs support development of acute myeloid leukemia. J. Hematol. Oncol. 2018 doi: 10.1186/s13045-018-0574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takai N., Ueda T., Nasu K., Yamashita S., Toyofuku M., Narahara H. Targeting calcium/calmodulin-dependence kinase I and II as a potential anti-proliferation remedy for endometrial carcinomas. Cancer Lett. 2009;277:235–243. doi: 10.1016/j.canlet.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 95.Gardner H.P., Ha S.I., Reynolds C., Chodosh L.A. The caM kinase, Pnck, is spatially and temporally regulated during murine mammary gland development and may identify an epithelial cell subtype involved in breast cancer. Cancer Res. 2000;60:5571–5577. [PubMed] [Google Scholar]
- 96.Wu S., Lv Z.J., Wang Y., Sun L., Jiang Z.M., Xu C.J., Zhao J., Sun X.J., Li X.X., Hu L.J., et al. Increased Expression of Pregnancy Up-Regulated Non-Ubiquitous Calmodulin Kinase Is Associated with Poor Prognosis in Clear Cell Renal Cell Carcinoma. PLoS ONE. 2013 doi: 10.1371/journal.pone.0059936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gonzalez M., De Brasi C., Ferri C., Bengio R., Bianchini M., Larripa I. CAMKIIgamma, HSP70 and HSP90 transcripts are differentially expressed in chronic myeloid leukemia cells from patients with resistant mutated disease. Leuk. Lymphoma. 2014;55:2101–2108. doi: 10.3109/10428194.2013.861070. [DOI] [PubMed] [Google Scholar]
- 98.Si J., Collins S.J. Activated Ca2+/calmodulin-dependent protein kinase IIgamma is a critical regulator of myeloid leukemia cell proliferation. Cancer Res. 2008;68:3733–3742. doi: 10.1158/0008-5472.CAN-07-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen W., An P., Quan X.J., Zhang J., Zhou Z.Y., Zou L.P., Luo H.S. Ca(2+)/calmodulin-dependent protein kinase II regulates colon cancer proliferation and migration via ERK1/2 and p38 pathways. World J. Gastroenterol. 2017;23:6111–6118. doi: 10.3748/wjg.v23.i33.6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chi M., Evans H., Gilchrist J., Mayhew J., Hoffman A., Pearsall E.A., Jankowski H., Brzozowski J.S., Skelding K.A. Phosphorylation of calcium/calmodulin-stimulated protein kinase II at T286 enhances invasion and migration of human breast cancer cells. Sci. Rep. 2016;6:33132. doi: 10.1038/srep33132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daft P.G., Yuan K., Warram J.M., Klein M.J., Siegal G.P., Zayzafoon M. Alpha-CaMKII plays a critical role in determining the aggressive behavior of human osteosarcoma. Mol. Cancer Res. 2013;11:349–359. doi: 10.1158/1541-7786.MCR-12-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yuan K., Chung L.W., Siegal G.P., Zayzafoon M. alpha-CaMKII controls the growth of human osteosarcoma by regulating cell cycle progression. Lab. Investig. 2007;87:938–950. doi: 10.1038/labinvest.3700658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chai S., Xu X., Wang Y., Zhou Y., Zhang C., Yang Y., Yang Y., Xu H., Xu R., Wang K. Ca2+/calmodulin-dependent protein kinase IIgamma enhances stem-like traits and tumorigenicity of lung cancer cells. Oncotarget. 2015;6:16069–16083. doi: 10.18632/oncotarget.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tang S., Pan Y., Wang Y., Hu L., Cao S., Chu M., Dai J., Shu Y., Xu L., Chen J., et al. Genome-wide association study of survival in early-stage non-small cell lung cancer. Ann. Surg. Oncol. 2015;22:630–635. doi: 10.1245/s10434-014-3983-0. [DOI] [PubMed] [Google Scholar]
- 105.Liu Z., Han G., Cao Y., Wang Y., Gong H. Calcium/calmodulindependent protein kinase II enhances metastasis of human gastric cancer by upregulating nuclear factorkappaB and Aktmediated matrix metalloproteinase9 production. Mol. Med. Rep. 2014;10:2459–2464. doi: 10.3892/mmr.2014.2525. [DOI] [PubMed] [Google Scholar]
- 106.Tamura N., Tai Y., Sugimoto K., Kobayashi R., Konishi R., Nishioka M., Masaki T., Nagahata S., Tokuda M. Enhanced expression and activation of Ca(2+)/calmodulin-dependent protein kinase IV in hepatocellular carcinoma. Cancer. 2000;89:1910–1916. doi: 10.1002/1097-0142(20001101)89:9<1910::AID-CNCR6>3.3.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 107.Fu H., He H.C., Han Z.D., Wan Y.P., Luo H.W., Huang Y.Q., Cai C., Liang Y.X., Dai Q.S., Jiang F.N., et al. MicroRNA-224 and its target CAMKK2 synergistically influence tumor progression and patient prognosis in prostate cancer. Tumour Biol. 2015;36:1983–1991. doi: 10.1007/s13277-014-2805-0. [DOI] [PubMed] [Google Scholar]
- 108.Ma Z., Wen D., Wang X., Yang L., Liu T., Liu J., Zhu J., Fang X. Growth inhibition of human gastric adenocarcinoma cells in vitro by STO-609 is independent of calcium/calmodulin-dependent protein kinase kinase-beta and adenosine monophosphate-activated protein kinase. Am. J. Transl. Res. 2016;8:1164–1171. [PMC free article] [PubMed] [Google Scholar]
- 109.Davare M.A., Saneyoshi T., Soderling T.R. Calmodulin-kinases regulate basal and estrogen stimulated medulloblastoma migration via Rac1. J. Neurooncol. 2011;104:65–82. doi: 10.1007/s11060-010-0472-6. [DOI] [PubMed] [Google Scholar]
- 110.Rodriguez-Mora O.G., LaHair M.M., McCubrey J.A., Franklin R.A. Calcium/calmodulin-dependent kinase I and calcium/calmodulin-dependent kinase kinase participate in the control of cell cycle progression in MCF-7 human breast cancer cells. Cancer Res. 2005;65:5408–5416. doi: 10.1158/0008-5472.CAN-05-0271. [DOI] [PubMed] [Google Scholar]
- 111.Williams C.L., Phelps S.H., Porter R.A. Expression of Ca2+/calmodulin-dependent protein kinase types II and IV, and reduced DNA synthesis due to the Ca2+/calmodulin-dependent protein kinase inhibitor KN-62 (1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenyl piperazine) in small cell lung carcinoma. Biochem. Pharmacol. 1996;51:707–715. doi: 10.1016/s0006-2952(95)02393-3. [DOI] [PubMed] [Google Scholar]
- 112.Russo E., Salzano M., De Falco V., Mian C., Barollo S., Secondo A., Bifulco M., Vitale M. Calcium/Calmodulin-dependent protein kinase II and its endogenous inhibitor alpha in medullary thyroid cancer. Clin. Cancer Res. 2014;20:1513–1520. doi: 10.1158/1078-0432.CCR-13-1683. [DOI] [PubMed] [Google Scholar]
- 113.Dai L., Zhuang L., Zhang B., Wang F., Chen X., Xia C., Zhang B. DAG/PKCdelta and IP3/Ca(2)(+)/CaMK IIbeta Operate in Parallel to Each Other in PLCgamma1-Driven Cell Proliferation and Migration of Human Gastric Adenocarcinoma Cells, through Akt/mTOR/S6 Pathway. Int. J. Mol. Sci. 2015;16:28510–28522. doi: 10.3390/ijms161226116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mamaeva O.A., Kim J., Feng G., McDonald J.M. Calcium/calmodulin-dependent kinase II regulates notch-1 signaling in prostate cancer cells. J. Cell. Biochem. 2009;106:25–32. doi: 10.1002/jcb.21973. [DOI] [PubMed] [Google Scholar]
- 115.Wang Q., Symes A.J., Kane C.A., Freeman A., Nariculam J., Munson P., Thrasivoulou C., Masters J.R., Ahmed A. A novel role for Wnt/Ca2+ signaling in actin cytoskeleton remodeling and cell motility in prostate cancer. PLoS ONE. 2010;5:e10456. doi: 10.1371/journal.pone.0010456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gu Y., Zhang J., Ma X., Kim B.W., Wang H., Li J., Pan Y., Xu Y., Ding L., Yang L., et al. Stabilization of the c-Myc Protein by CAMKIIgamma Promotes T Cell Lymphoma. Cancer Cell. 2017 doi: 10.1016/j.ccell.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoffman A., Carpenter H., Kahl R., Watt L.F., Dickson P.W., Rostas J.A.P., Verrills N.M., Skelding K.A. Dephosphorylation of CaMKII at T253 controls the metaphase-anaphase transition. Cell. Signal. 2014;26:748–756. doi: 10.1016/j.cellsig.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 118.Chen J.H., Huang S.M., Chen C.C., Tsai C.F., Yeh W.L., Chou S.J., Hsieh W.T., Lu D.Y. Ghrelin induces cell migration through GHS-R, CaMKII, AMPK, and NF-kappaB signaling pathway in glioma cells. J. Cell. Biochem. 2011;112:2931–2941. doi: 10.1002/jcb.23209. [DOI] [PubMed] [Google Scholar]
- 119.Cuddapah V.A., Sontheimer H. Molecular interaction and functional regulation of ClC-3 by Ca2+/calmodulin-dependent protein kinase II (CaMKII) in human malignant glioma. J. Biol. Chem. 2010;285:11188–11196. doi: 10.1074/jbc.M109.097675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shin H.J., Lee S., Jung H.J. A curcumin derivative hydrazinobenzoylcurcumin suppresses stem-like features of glioblastoma cells by targeting Ca(2+) /calmodulin-dependent protein kinase II. J. Cell. Biochem. 2018 doi: 10.1002/jcb.27972. [DOI] [PubMed] [Google Scholar]
- 121.Monaco S., Rusciano M.R., Maione A.S., Soprano M., Gomathinayagam R., Todd L.R., Campiglia P., Salzano S., Pastore L., Leggiero E., et al. A novel crosstalk between calcium/calmodulin kinases II and IV regulates cell proliferation in myeloid leukemia cells. Cell. Signal. 2015;27:204–214. doi: 10.1016/j.cellsig.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 122.Yamada T., Suzuki M., Satoh H., Kihara-Negishi F., Nakano H., Oikawa T. Effects of PU.1-induced mouse calcium-calmodulin-dependent kinase I-like kinase (CKLiK) on apoptosis of murine erythroleukemia cells. Exp. Cell Res. 2004;294:39–50. doi: 10.1016/j.yexcr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 123.Tombes R.M., Krystal G.W. Identification of novel human tumor cell-specific CaMK-II variants. Biochim. Biophys. Acta. 1997;1355:281–292. doi: 10.1016/S0167-4889(96)00141-3. [DOI] [PubMed] [Google Scholar]
- 124.Yang B.F., Xiao C., Roa W.H., Krammer P.H., Hao C. Calcium/calmodulin-dependent protein kinase II regulation of c-FLIP expression and phosphorylation in modulation of Fas-mediated signaling in malignant glioma cells. J. Biol. Chem. 2003;278:7043–7050. doi: 10.1074/jbc.M211278200. [DOI] [PubMed] [Google Scholar]
- 125.Xiao C., Yang B.F., Song J.H., Schulman H., Li L., Hao C. Inhibition of CaMKII-mediated c-FLIP expression sensitizes malignant melanoma cells to TRAIL-induced apoptosis. Exp. Cell Res. 2005;304:244–255. doi: 10.1016/j.yexcr.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 126.Gu Y., Chen T., Meng Z.P., Gan Y.C., Xu X.H., Lou G.Y., Li H.Z., Gan X.X., Zhou H., Tang J.F., et al. CaMKII gamma, a critical regulator of CML stem/progenitor cells, is a target of the natural product berbamine. Blood. 2012;120:4829–4839. doi: 10.1182/blood-2012-06-434894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.House S.J., Ginnan R.G., Armstrong S.E., Singer H.A. Calcium/calmodulin-dependent protein kinase II-delta isoform regulation of vascular smooth muscle cell proliferation. Am. J. Physiol. Cell Physiol. 2007;292:C2276–C2287. doi: 10.1152/ajpcell.00606.2006. [DOI] [PubMed] [Google Scholar]
- 128.Scott J.A., Xie L., Li H., Li W., He J.B., Sanders P.N., Carter A.B., Backs J., Anderson M.E., Grumbach I.M. The multifunctional Ca2+/calmodulin-dependent kinase II regulates vascular smooth muscle migration through matrix metalloproteinase 9. Am. J. Physiol. Heart Circ. Physiol. 2012;302:H1953–H1964. doi: 10.1152/ajpheart.00978.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mercure M.Z., Ginnan R., Singer H.A. CaM kinase II delta2-dependent regulation of vascular smooth muscle cell polarization and migration. Am. J. Physiol. Cell Physiol. 2008;294:C1465–C1475. doi: 10.1152/ajpcell.90638.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang M., Kahn A.M. Insulin-inhibited and stimulated cultured vascular smooth muscle cell migration are related to divergent effects on protein phosphatase-2A and autonomous calcium/calmodulin-dependent protein kinase II. Atherosclerosis. 2008;196:227–233. doi: 10.1016/j.atherosclerosis.2007.04.050. [DOI] [PubMed] [Google Scholar]
- 131.Daft P.G., Yang Y., Napierala D., Zayzafoon M. The Growth and Aggressive Behavior of Human Osteosarcoma Is Regulated by a CaMKII-Controlled Autocrine VEGF Signaling Mechanism. PLoS ONE. 2015;10:e0121568. doi: 10.1371/journal.pone.0121568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ma X., Meng Z., Jin L., Xiao Z., Wang X., Tsark W.M., Ding L., Gu Y., Zhang J., Kim B., et al. CAMK2gamma in intestinal epithelial cells modulates colitis-associated colorectal carcinogenesis via enhancing STAT3 activation. Oncogene. 2017;36:4060–4071. doi: 10.1038/onc.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meng Z., Ma X., Du J., Wang X., He M., Gu Y., Zhang J., Han W., Fang Z., Gan X., et al. CAMK2gamma antagonizes mTORC1 activation during hepatocarcinogenesis. Oncogene. 2017;36:2446–2456. doi: 10.1038/onc.2016.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shang S., Takai N., Nishida M., Miyazaki T., Nasu K., Miyakawa I. CaMKIV expression is associated with clinical stage and PCNA-labeling index in endometrial carcinoma. Int. J. Mol. Med. 2003;11:181–186. doi: 10.3892/ijmm.11.2.181. [DOI] [PubMed] [Google Scholar]
- 135.Takai N., Ueda T., Nishida M., Nasu K., Miyakawa I. The relationship between oncogene expression and clinical outcome in endometrial carcinoma. Curr. Cancer Drug Targets. 2004;4:511–520. doi: 10.2174/1568009043332871. [DOI] [PubMed] [Google Scholar]
- 136.Tokumitsu H., Inuzuka H., Ishikawa Y., Ikeda M., Saji I., Kobayashi R. STO-609, a specific inhibitor of the Ca(2+)/calmodulin-dependent protein kinase kinase. J. Biol. Chem. 2002;277:15813–15818. doi: 10.1074/jbc.M201075200. [DOI] [PubMed] [Google Scholar]
- 137.Monteiro P., Gilot D., Langouet S., Fardel O. Activation of the aryl hydrocarbon receptor by the calcium/calmodulin-dependent protein kinase kinase inhibitor 7-oxo-7H-benzimidazo[2,1-a]benz[de]isoquinoline-3-carboxylic acid (STO-609) Drug Metab. Dispos. 2008;36:2556–2563. doi: 10.1124/dmd.108.023333. [DOI] [PubMed] [Google Scholar]
- 138.York B., Li F., Lin F., Marcelo K.L., Mao J., Dean A., Gonzales N., Gooden D., Maity S., Coarfa C., et al. Pharmacological inhibition of CaMKK2 with the selective antagonist STO-609 regresses NAFLD. Sci. Rep. 2017;7:11793. doi: 10.1038/s41598-017-12139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meng Z., Li T., Ma X., Wang X., Van Ness C., Gan Y., Zhou H., Tang J., Lou G., Wang Y., et al. Berbamine inhibits the growth of liver cancer cells and cancer-initiating cells by targeting Ca(2)(+)/calmodulin-dependent protein kinase II. Mol. Cancer Ther. 2013;12:2067–2077. doi: 10.1158/1535-7163.MCT-13-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ishida A., Kameshita I., Okuno S., Kitani T., Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem. Biophys. Res. Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- 141.Tokumitsu H., Chijiwa T., Hagiwara M., Mizutani A., Terasawa M., Hidaka H. KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-l-tyrosyl]-4-phenylpiperazi ne, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 1990;265:4315–4320. [PubMed] [Google Scholar]
- 142.Hidaka H., Ishikawa T. Molecular pharmacology of calmodulin pathways in the cell functions. Cell Calcium. 1992;13:465–472. doi: 10.1016/0143-4160(92)90059-2. [DOI] [PubMed] [Google Scholar]
- 143.Kahl C.R., Means A.R. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr. Rev. 2003;24:719–736. doi: 10.1210/er.2003-0008. [DOI] [PubMed] [Google Scholar]
- 144.Ledoux J., Chartier D., Leblanc N. Inhibitors of calmodulin-dependent protein kinase are nonspecific blockers of voltage-dependent K+ channels in vascular myocytes. J. Pharmacol. Exp. Ther. 1999;290:1165–1174. [PubMed] [Google Scholar]
- 145.Rezazadeh S., Claydon T.W., Fedida D. KN-93 (2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine), a calcium/calmodulin-dependent protein kinase II inhibitor, is a direct extracellular blocker of voltage-gated potassium channels. J. Pharmacol. Exp. Ther. 2006;317:292–299. doi: 10.1124/jpet.105.097618. [DOI] [PubMed] [Google Scholar]
- 146.Gao L., Blair L.A., Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: Reversible inhibition of L-type calcium channels. Biochem. Biophys. Res. Commun. 2006;345:1606–1610. doi: 10.1016/j.bbrc.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 147.Rokhlin O.W., Taghiyev A.F., Bayer K.U., Bumcrot D., Koteliansk V.E., Glover R.A., Cohen M.B. Calcium/calmodulin-dependent kinase II plays an important role in prostate cancer cell survival. Cancer Biol. Ther. 2007;6:732–742. doi: 10.4161/cbt.6.5.3975. [DOI] [PubMed] [Google Scholar]
- 148.Rokhlin O.W., Guseva N.V., Taghiyev A.F., Glover R.A., Cohen M.B. KN-93 inhibits androgen receptor activity and induces cell death irrespective of p53 and Akt status in prostate cancer. Cancer Biol. Ther. 2010;9:224–235. doi: 10.4161/cbt.9.3.10747. [DOI] [PubMed] [Google Scholar]
- 149.Yang B.F., Xiao C., Li H., Yang S.J. Resistance to Fas-mediated apoptosis in malignant tumours is rescued by KN-93 and cisplatin via downregulation of c-FLIP expression and phosphorylation. Clin. Exp. Pharmacol. Physiol. 2007;34:1245–1251. doi: 10.1111/j.1440-1681.2007.04711.x. [DOI] [PubMed] [Google Scholar]
- 150.Si J.T., Mueller L., Collins S.J. CaMKII regulates retinoic acid receptor transcriptional activity and the differentiation of myeloid leukemia cells. J. Clin. Investig. 2007;117:1412–1421. doi: 10.1172/JCI30779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee K.H. CaMKII Inhibitor KN-62 Blunts Tumor Response to Hypoxia by Inhibiting HIF-1alpha in Hepatoma Cells. Korean J. Physiol. Pharmacol. 2010;14:331–336. doi: 10.4196/kjpp.2010.14.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang S.J., Peyrollier K., Bourguignon L.Y. The influence of hyaluronan-CD44 interaction on topoisomerase II activity and etoposide cytotoxicity in head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2007;133:281–288. doi: 10.1001/archotol.133.3.281. [DOI] [PubMed] [Google Scholar]
- 153.Aoyama M., Grabowski D.R., Holmes K.A., Rybicki L.A., Bukowski R.M., Ganapathi M.K., Ganapathi R. Cell cycle phase specificity in the potentiation of etoposide-induced DNA damage and apoptosis by KN-62, an inhibitor of calcium-calmodulin-dependent enzymes. Biochem. Pharmacol. 2001;61:49–54. doi: 10.1016/S0006-2952(00)00539-6. [DOI] [PubMed] [Google Scholar]
- 154.Obata N.H., Okazaki K., Maeda O., Kikkawa F., Tomoda Y., Hidaka H. Effect of KN-62, Ca2+/calmodulin-dependent protein kinase II inhibitor, on adriamycin resistance of human ovarian cancer cells. Biochem. Biophys. Res. Commun. 1995;215:566–571. doi: 10.1006/bbrc.1995.2502. [DOI] [PubMed] [Google Scholar]
- 155.Okazaki K., Ishikawa T., Inui M., Tada M., Goshima K., Okamoto T., Hidaka H. KN-62, a specific Ca++/calmodulin-dependent protein kinase inhibitor, reversibly depresses the rate of beating of cultured fetal mouse cardiac myocytes. J. Pharmacol. Exp. Ther. 1994;270:1319–1324. [PubMed] [Google Scholar]
- 156.Anderson M.E., Braun A.P., Wu Y., Lu T., Wu Y., Schulman H., Sung R.J. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J. Pharmacol. Exp Ther. 1998;287:996–1006. [PubMed] [Google Scholar]
- 157.Ito I., Hidaka H., Sugiyama H. Effects of KN-62, a specific inhibitor of calcium/calmodulin-dependent protein kinase II, on long-term potentiation in the rat hippocampus. Neurosci. Lett. 1991;121:119–121. doi: 10.1016/0304-3940(91)90663-E. [DOI] [PubMed] [Google Scholar]
- 158.Hanson P.I., Kapiloff M.S., Lou L.L., Rosenfeld M.G., Schulman H. Expression of a multifunctional Ca2+/calmodulin-dependent protein kinase and mutational analysis of its autoregulation. Neuron. 1989;3:59–70. doi: 10.1016/0896-6273(89)90115-3. [DOI] [PubMed] [Google Scholar]
- 159.Braun A.P., Schulman H. A non-selective cation current activated via the multifunctional Ca(2+)-calmodulin-dependent protein kinase in human epithelial cells. Pt 1J. Physiol. 1995;488:37–55. doi: 10.1113/jphysiol.1995.sp020944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu Y., Gao Z., Chen B., Koval O.M., Singh M.V., Guan X., Hund T.J., Kutschke W., Sarma S., Grumbach I.M., et al. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc. Natl. Acad. Sci. USA. 2009;106:5972–5977. doi: 10.1073/pnas.0806422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Backs J., Backs T., Neef S., Kreusser M.M., Lehmann L.H., Patrick D.M., Grueter C.E., Qi X., Richardson J.A., Hill J.A., et al. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc. Natl. Acad. Sci. USA. 2009;106:2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sun X., Zhao D., Li Y.L., Sun Y., Lei X.H., Zhang J.N., Wu M.M., Li R.Y., Zhao Z.F., Zhang Z.R., et al. Regulation of ASIC1 by Ca2+/calmodulin-dependent protein kinase II in human glioblastoma multiforme. Oncol. Rep. 2013;30:2852–2858. doi: 10.3892/or.2013.2777. [DOI] [PubMed] [Google Scholar]
- 163.Patel R., Holt M., Philipova R., Moss S., Schulman H., Hidaka H., Whitaker M. Calcium/calmodulin-dependent phosphorylation and activation of human Cdc25-C at the G2/M phase transition in HeLa cells. J. Biol. Chem. 1999;274:7958–7968. doi: 10.1074/jbc.274.12.7958. [DOI] [PubMed] [Google Scholar]
- 164.Chang B.H., Mukherji S., Soderling T.R. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc. Natl. Acad. Sci. USA. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chang B.H., Mukherji S., Soderling T.R. Calcium/calmodulin-dependent protein kinase II inhibitor protein: Localization of isoforms in rat brain. Neuroscience. 2001;102:767–777. doi: 10.1016/S0306-4522(00)00520-0. [DOI] [PubMed] [Google Scholar]
- 166.Zhang J., Li N., Yu J., Zhang W., Cao X. Molecular cloning and characterization of a novel calcium/calmodulin-dependent protein kinase II inhibitor from human dendritic cells. Biochem. Biophys. Res. Commun. 2001;285:229–234. doi: 10.1006/bbrc.2001.5175. [DOI] [PubMed] [Google Scholar]
- 167.Wang C., Li N., Liu X., Zheng Y., Cao X. A novel endogenous human CaMKII inhibitory protein suppresses tumor growth by inducing cell cycle arrest via p27 stabilization. J. Biol. Chem. 2008;283:11565–11574. doi: 10.1074/jbc.M800436200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 168.Ma S., Yang Y., Wang C., Hui N., Gu L., Zhong H., Cai Z., Wang Q., Zhang Q., Li N., et al. Endogenous human CaMKII inhibitory protein suppresses tumor growth by inducing cell cycle arrest and apoptosis through down-regulation of the phosphatidylinositide 3-kinase/Akt/HDM2 pathway. J. Biol. Chem. 2009;284:24773–24782. doi: 10.1074/jbc.M109.028621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 169.Heinze K., Kritsch D., Mosig A.S., Durst M., Hafner N., Runnebaum I.B. Functional Analyses of RUNX3 and CaMKIINalpha in Ovarian Cancer Cell Lines Reveal Tumor-Suppressive Functions for CaMKIINalpha and Dichotomous Roles for RUNX3 Transcript Variants. Int. J. Mol. Sci. 2018 doi: 10.3390/ijms19010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Coultrap S.J., Bayer K.U. Improving a natural CaMKII inhibitor by random and rational design. PLoS ONE. 2011;6:e25245. doi: 10.1371/journal.pone.0025245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gomez-Monterrey I., Sala M., Rusciano M.R., Monaco S., Maione A.S., Iaccarino G., Tortorella P., D’Ursi A.M., Scrima M., Carotenuto A., et al. Characterization of a selective CaMKII peptide inhibitor. Eur. J. Med. Chem. 2013;62:425–434. doi: 10.1016/j.ejmech.2012.12.053. [DOI] [PubMed] [Google Scholar]
- 172.Nam S., Xie J., Perkins A., Ma Y., Yang F., Wu J., Wang Y., Xu R.Z., Huang W., Horne D.A., et al. Novel synthetic derivatives of the natural product berbamine inhibit Jak2/Stat3 signaling and induce apoptosis of human melanoma cells. Mol. Oncol. 2012;6:484–493. doi: 10.1016/j.molonc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhao Y., Tan Y., Wu G., Liu L., Wang Y., Luo Y., Shi J., Huang H. Berbamine overcomes imatinib-induced neutropenia and permits cytogenetic responses in Chinese patients with chronic-phase chronic myeloid leukemia. Int. J. Hematol. 2011;94:156–162. doi: 10.1007/s12185-011-0887-7. [DOI] [PubMed] [Google Scholar]
- 174.Wang S., Liu Q., Zhang Y., Liu K., Yu P., Liu K., Luan J., Duan H., Lu Z., Wang F., et al. Suppression of growth, migration and invasion of highly-metastatic human breast cancer cells by berbamine and its molecular mechanisms of action. Mol. Cancer. 2009;8:81. doi: 10.1186/1476-4598-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xu R., Dong Q., Yu Y., Zhao X., Gan X., Wu D., Lu Q., Xu X., Yu X.F. Berbamine: A novel inhibitor of bcr/abl fusion gene with potent anti-leukemia activity. Leuk. Res. 2006;30:17–23. doi: 10.1016/j.leukres.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 176.Wei Y.L., Liang Y., Xu L., Zhao X.Y. The antiproliferation effect of berbamine on k562 resistant cells by inhibiting NF-kappaB pathway. Anat. Rec. 2009;292:945–950. doi: 10.1002/ar.20924. [DOI] [PubMed] [Google Scholar]
- 177.Hou Z.B., Lu K.J., Wu X.L., Chen C., Huang X.E., Yin H.T. In vitro and in vivo antitumor evaluation of berbamine for lung cancer treatment. Asian Pac. J. Cancer Prev. 2014;15:1767–1769. doi: 10.7314/APJCP.2014.15.4.1767. [DOI] [PubMed] [Google Scholar]
- 178.Wang G.Y., Lv Q.H., Dong Q., Xu R.Z., Dong Q.H. Berbamine induces Fas-mediated apoptosis in human hepatocellular carcinoma HepG2 cells and inhibits its tumor growth in nude mice. J. Asian Nat. Prod. Res. 2009;11:219–228. doi: 10.1080/10286020802675076. [DOI] [PubMed] [Google Scholar]
- 179.Kirkwood N.K., O’Reilly M., Derudas M., Kenyon E.J., Huckvale R., van Netten S.M., Ward S.E., Richardson G.P., Kros C.J. d-Tubocurarine and Berbamine: Alkaloids That Are Permeant Blockers of the Hair Cell’s Mechano-Electrical Transducer Channel and Protect from Aminoglycoside Toxicity. Front. Cell. Neurosci. 2017;11:262. doi: 10.3389/fncel.2017.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liang Y., Xu R.Z., Zhang L., Zhao X.Y. Berbamine, a novel nuclear factor kappaB inhibitor, inhibits growth and induces apoptosis in human myeloma cells. Acta Pharmacol. Sin. 2009;30:1659–1665. doi: 10.1038/aps.2009.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Parhi P., Suklabaidya S., Kumar Sahoo S. Enhanced anti-metastatic and anti-tumorigenic efficacy of Berbamine loaded lipid nanoparticles in vivo. Sci. Rep. 2017;7:5806. doi: 10.1038/s41598-017-05296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Morris A.S., Sebag S.C., Paschke J.D., Wongrakpanich A., Ebeid K., Anderson M.E., Grumbach I.M., Salem A.K. Cationic CaMKII Inhibiting Nanoparticles Prevent Allergic Asthma. Mol. Pharm. 2017;14:2166–2175. doi: 10.1021/acs.molpharmaceut.7b00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wongrakpanich A., Morris A.S., Geary S.M., Joiner M.A., Salem A.K. Surface-modified particles loaded with CaMKII inhibitor protect cardiac cells against mitochondrial injury. Int. J. Pharm. 2017;520:275–283. doi: 10.1016/j.ijpharm.2017.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]