Abstract
Objective
To investigate the efficacy and safety of Tui Na for treating spasticity of the upper limbs of stroke patients.
Design
A prospective, multicenter, blinded, randomized controlled intervention study.
Subjects
Stroke patients with upper limb spasticity who were treated between December 2013 and February 2017 in 16 participating institutions in China were randomly assigned to receive either Tui Na plus conventional rehabilitation (Tui Na group, n = 222,) or conventional rehabilitation only (control group, n = 222).
Methods
Eligible adult patients (aged 18–75 years) were enrolled 1–12 months after stroke and randomly allocated in a 1:1 ratio to the two groups. Outcome assessors were blinded to treatment allocation. Muscle tone in the spastic muscles was evaluated using the Modified Ashworth Scale (MAS), and the primary endpoint was the change in MAS score over 4 weeks of treatment.
Results
Among patients who had experienced stroke 1–3 months before treatment, the Tui Na group experienced significantly greater reductions in MAS scores for three muscle groups than did the control group after 4 weeks of treatment. These improvements were sustained at the 3‐ and 6‐month follow‐ups. However, among patients who suffered from stroke 4–6 months and 7–12 months before treatment, the change in MAS with treatment did not differ significantly between those who did and those who did not receive Tui Na. No Tui Na‐related adverse events during treatment were reported the groups.
Conclusion
Tui Na was effective and safe for alleviating poststroke spasticity within 1–3 months after stroke onset.
Introduction
Muscle spasticity is a common poststroke complication, with an incidence ranging from 17% to 38%.1, 2, 3 This condition is disabling in 2–13% of these patients, and such cases most commonly involve the upper limbs.4 Given that upper limb spasticity can cause deformity, functional impairment and chronic pain, effective treatments are necessary for poststroke rehabilitation.3 However, few effective therapies have been developed for treating spasticity in the upper limbs.5
A systematic review supports the use of Tui Na for limb spasticity,6 and Tui Na has been found to reduce upper limb spasticity in patients after stroke.7, 8, 9 Trials to investigate its specific effects on upper limb spasticity were limited though by small sample size, poor study design, and high risk of bias.7, 8, 9 Additional research is particularly needed to determine the optimal timing of Tui Na treatment after stroke.
The present study aimed to assess the effectiveness and safety of Tui Na for reducing poststroke spasticity of the upper limbs over a 4‐week treatment period and to evaluate the long‐term effects of this treatment throughout 6 months of follow‐up. All patients in this study received conventional rehabilitation therapy, and outcomes were compared between those who did or did not also receive treatment with Tui Na. We also observed the antispasticity effect of Tui Na in different stages of poststroke recovery.
Materials and Methods
Study design
The multicenter, prospective, assessment‐blinded, randomized controlled study was conducted at 16 hospitals in nine cities in China: Nanjing, Zhengzhou, Wuhan, Shanghai, Harbin, Haikou, Tianjin, Xuzhou, and Lianyungang. The 16 neurology or rehabilitation clinics are all within hospitals that are affiliated with a university,and are esteemed comprehensive nonprofit public hospitals in China. The institutional review board at Jiangsu Provincial People's Hospital approved the study protocol, and all participants provided written informed consent before participation. The study was registered at ClincalTrials.gov (http://clinicaltrials.gov/;identifier:) with the registration number NCT03054974.
Study population
From December 2013 to February 2017, patients with poststroke upper limb spasticity were recruited through poster and newspaper advertisements in hospitals and surrounding communities. In each center, the investigators received the standard operating procedure and intensive training on the use of the assessments. Researchers screened candidate patients for study participation, and experienced physicians within the rehabilitation departments made the relevant diagnoses.
At baseline, each patient's demographic data, medical history, auxiliary examination results, imaging analysis findings, and medication use were recorded. Scores on the Mini‐Mental Status Examination (MMSE), Modified Ashworth Scale (MAS), Fugl‐Meyer Motor Assessment (FMA), and Modified Barthel Index (MBI) were obtained at baseline and later times as summarized in Table 1. Fugl‐Meyer total score (FMA‐T) and Fugl‐Meyer upper limb score (FMA‐U) were also applied. The study duration per patient was 29 weeks, including 1 week before randomization (baseline assessment), 4 weeks of treatment (conventional rehabilitation with or without Tui Na), and 24 weeks of follow‐up after the last antispasticity treatment (re‐examination follow‐up). During the following‐up period, rehabilitation therapy was carried out according to the patients' individual conditions.
Table 1.
Study schema
| Study assessment of stage | Day (approximate) | |||||
|---|---|---|---|---|---|---|
| −7 days | −1 day | 1 day | 28 days | 4 m | 7 m | |
| MMSE | X | |||||
| MAS | X | X | X | X | X | |
| FIM | X | X | X | X | X | |
| MBI | X | X | X | X | X | |
| treatments | X | X | ||||
| partial registration | X | |||||
| Full registration/random assignment | X | |||||
Participants were eligible for inclusion in the study if they were 18–75 years of age; had a definitive diagnosis of stroke, according to the World Health Organization definition10 and confirmed by computed tomography (CT) or magnetic resonance imaging (MRI) examination; had experienced stroke 1–12 months previously; had spasticity of at least one muscle group with a MAS score for the upper limbs of ≥ 1+; and had a MMSE score of ≥ 16.11
The exclusion criteria were: severe musculoskeletal condition (e.g., fracture, severe arthritis, joint contracture, amputation); severe medical condition (e.g., severe heart disease, liver dysfunction, renal insufficiency, cancer); pregnancy; previous surgery; treatment with antispasticity drugs in the previous 3 months; and participation in another clinical trial within the previous 6 months.
The purpose, nature, and potential risks of the trial were fully explained to all participants, and written informed consent was obtained before enrollment. Participants were free to drop out of the study at will and were not compensated for study participation.
Randomization and blinding
Therapists obtained each patient's random number and group assignment through a central randomization system developed by the Nan Jing Medical University teaching and Research Department of Statistics. Eligible patients were randomly assigned to receive either conventional rehabilitation and Tui Na (Tui Na group) or only conventional rehabilitation (control group) in a 1:1 ratio by a computer‐generated randomization list. All participants were treated separately to prevent communication. Before study participation, participants were informed that they had an equal chance of allocation to the experimental or control group. The outcome assessors and the statistical analyst were not involved in intervention administration and were blinded to the randomized allocations.
Intervention protocols
Study interventions were developed according to the consensus of rehabilitation and Chinese medicine experts and per the results of our pilot study. Rehabilitation and Tui Na treatments were conducted by certified therapists with 5–10 years of experience with stroke patients specifically. And the therapists were trained according to the investigator's brochure.
All participants received 4 weeks of regular inpatient rehabilitation, five times per week. Each session included physical therapy (PT) and occupational therapy (OT). In addition, the Tui Na group received the Tui Na intervention five times each week for the 4‐week study period.
Conventional rehabilitation included: (1) a 2‐h session of PT training involving muscle stretching, proper positioning and exercises to reduce spasticity, neuromuscular facilitation techniques therapy, and antagonistic muscle electrical stimulation therapy, 2 h per time; and (2) a 30‐min session of OT training according to the patients' individual conditions to carry out activities of daily living (ADL), including manual simulation operation, sports and entertainment training.
The Tui Na treatment included: (1) a 40‐min session of Tui Na according to specific methods established by experts with rich theoretical and practical experience in Chinese massage and used in a previous study12; and (2) anointing with Baimai‐ruangao (Z20043178, QiZheng Tibetan medicine, Data S1) as the massage medium, applied on the skin surface at a dose of 5 g/limb each time. During Tui Na treatment, patients should feel mild‐to‐moderate soreness and distension.12 The following manipulation methods were used: the operating hand of the therapist rolled on the shoulder of the paralyzed side for approximately 3 min; through hand rolling and thumb waving pressing, the arm flexors of the paralyzed limbs were manipulated from the shoulder to the wrist for approximately 5 min; through hand rolling and thumb waving pressing, the arm extensors of paralyzed limbs were manipulated from the shoulder to the wrist for approximately 5 min; finger pressure was applied on acupuncture points in the paralyzed limbs, including two points on the shoulder [Jianyu (LI15) and Jianliao (SJ14)], Quchi (LI11) on the lateral end of the elbow crease, three points on the forearm [Neiguan (PC6), Waiguan (SJ50), Shousanli (LI10)], and two points on the hand [Yangchi (SJ4), Hegu (LI4)]. Moreover, the Jiquan(HT1) acupoint on the paralyzed arm was manipulated by grasping; the fingers were manipulated by twisting; the elbow, wrist and fingers were manipulated by pulling, rotating, shaking, stretching and flexing; and the elbow and wrist were manipulated by rubbing.12
Outcome measurements
The primary outcome was the change from baseline to the end of the 4‐week treatment period in muscle tone as measured with the MAS in three spastic muscle groups (elbow flexors, wrist flexors, finger flexors). Muscle tone was selected as the primary outcome measure for easy comparison of the results to those of previous studies. The MAS, which was developed by Smith and Bohannon,13 is an ordinal, clinical assessment scale and the most commonly used clinical measurement for assessing the degree of muscle spasticity. With the MAS, the severity of spasticity is graded with a score from 0 to 4 and a grade 1+ is included between grades 1 and 2.13 The three selected muscle groups presented the greatest functional problems resulting from spasticity and were identified by both the clinicians and patients at the first visit with subsequent close follow‐up. Before MAS score assessment, each patient lay in the supine position and rested for at least 15 min to ensure accuracy. Given the influence of velocity on stretch responses, investigators were instructed to perform passive movements with an intermediate velocity, with each movement lasting about 1 sec.
Secondary endpoints included the changes from baseline to the end of the 4‐week treatment period in motor function of limbs as evaluated using the FMA and ADL as evaluated by the MBI as well as the changes from baseline to the 3‐month and 6‐month follow‐ups in limbs spasticity (MAS), motor function of limbs (FMA), and ADL (MBI). The FMA evaluates motor recovery after stroke,14 and is considered a valid indicator of motor recovery, and thus, widely used to assess stroke severity. The FMA scores motor functions, with maximum scores of 34 for lower extremities and 66 for upper extremities.15 A higher FMA score indicates better motor functional status. The MBI offers good sensitivity and reliability for evaluating patients' abilities to perform ADL16 and consists of 10 items, includes feeding, personal hygiene, dressing, bathing, etc. for the assessment of self‐care and mobility in ADL.17 Each item is scored from 0 to a maximum that varies from 5 to 15, and a higher score indicates more independence in the performance of ADL.
Because the effects of Tui Na on poststroke upper limb spasticity might vary depending on the disease course, a subgroup analysis for the primary outcome was preplanned based on the time elapsed between stroke and treatment, with patients divided into categories for a disease course of 1–3 months, 4–6 months, and 7–12 months.
Adverse events were recorded on a case report table for evaluation of their relationships with the study intervention.
Statistical analysis
The needed sample size was calculated according to the primary endpoint (change in MAS score). With the two‐sided comparison type 1 error rate controlled at 0.05, a power of 90%, expected mean changes in MAS scores over the treatment period of –0.6 and –0.3 in the active and control groups, respectively, a common standard deviation (SD) of 0.9, and a 15% drop‐out rate by week 4, we calculated that a total of 224 patients per treatment group was needed to show a significant treatment effect.18, 19, 20
SPSS17.0 software (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses, and an alpha level of 5% was chosen. Statistical description and comparability analysis were conducted for the demographic data (gender, age, height, weight), general condition, and baseline condition. In addition, the number of included cases from each center, the number of excluded cases, and the number of lost cases were counted, and the full analysis set (FAS) and per‐protocol set (PPS) were defined. The conformance set included cases that conformed to the inclusion criteria, did not conform to the exclusion criteria, and completed the treatment scheme, allowing analysis of cases that conformed to the test plan, had good compliance, and correctly completed the requested contents. If the primary endpoint was missing, the first result was carried forward according to intention‐to‐treat (ITT) analysis. Comparability analysis and missing values for secondary endpoints were not carried forward, and cases were analyzed based on the actual data obtained.
We used the paired t test to compare quantitative data between groups. Single factor variance analysis was adopted for the comparison of normally distributed quantitative data. Dunnett t test was used for multiple comparisons of the experimental group and the control group. Categorical data were compared using the Pearson χ 2 test, and the Fisher exact probabilities method was adopted when the data did not meet the requirement of the χ 2 test. Kruskal–Wallis rank sum test was used to compare quantitative or ranked data that did not follow a normal distribution.
For practicality in the analysis of MAS scores, the MAS scores were redistributed on a 6‐point scale from 0 to 5 (with 1+ transformed into 2, 2 into 3, 3 into 4, and 4 into 5).
Results
Between December 2013 and February 2017, 558 patients were screened at 16 sites. From these, 444 patients were included in the study, with 222 randomly allocated to the Tui Na group and 222 to the control group. In total, 438 stroke patients received conventional rehabilitation with or without Tui Na, with 193 patients in the Tui Na group and 204 in the control group completing the 4‐week intervention. At the end of the 6‐month follow‐up, the Tui Na group included 152 patients and the control group included 155 patients. Patients withdrew from the study or were lost to follow‐up for a variety of reasons (e.g., distance to the hospital, change in telephone number, lack of family support). Figure 1 summarizes the cases that withdrew or were lost to follow‐up. Notably, no cases of drop‐out or loss of follow‐up were known to be directly linked to the study intervention, and no adverse events were reported in this study.
Figure 1.
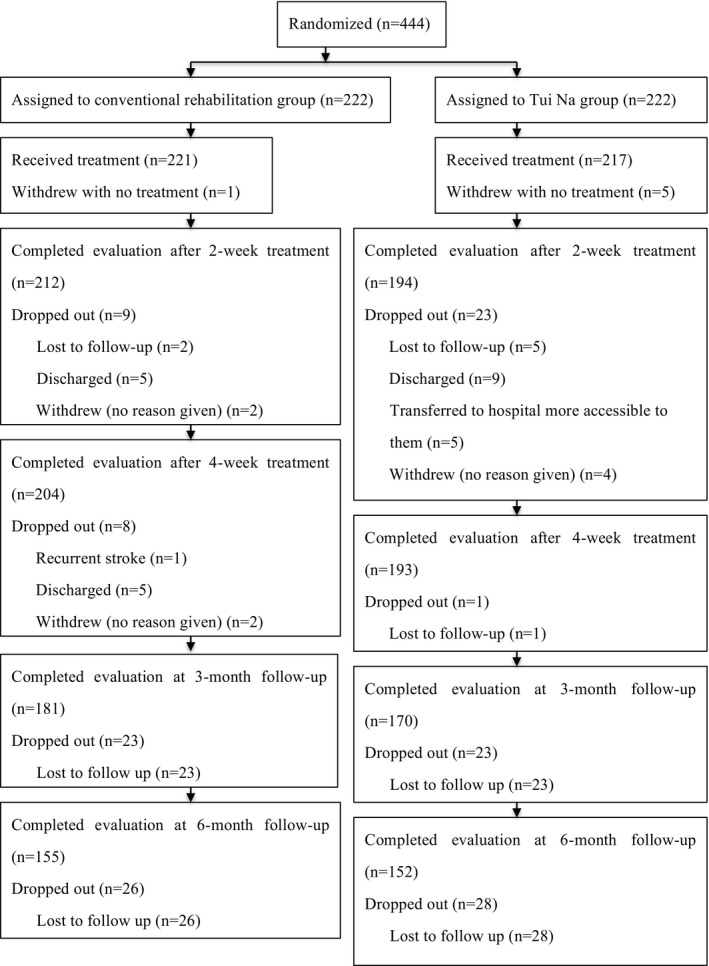
Trial flow diagram.
With regard to the disease course among the patients, 270 patients were treated within 1–3 months after the onset of stroke, 101 patients within 4–6 months, and 67 patients within 7–12 months. The primary and secondary outcomes were compared between those who received Tui Na and those who did not within these groups.
Among the patients treated within 1–3 months poststroke, no significant differences in demographic characteristics were observed between the Tui Na and control groups. Also at baseline, the FMA, MBI, and MAS scores were balanced between the Tui Na and control groups (Table S1). After the 4‐week treatment period through, the mean changes in the MAS scores for the wrist flexors and finger flexors from baseline to week 4 were significantly greater in the Tui Na group than in the control group (P = 0.0042 and P = 0.0234, respectively). The relief effect on spasticity in the Tui Na group was better than that experienced in the conventional rehabilitation group, and the benefit persisted at the 3‐ and 6‐month follow‐ups for both the wrist flexors (P = 0.0002 and P = 0.0006, respectively) and finger flexors (P = 0.0002 and P = 0.0002, respectively). No significant difference was noted between the two groups in the change in MAS score for the elbow flexors from baseline to week 4 (P = 0.3107), but at 3 and 6 months after the last intervention, the change in the MAS score for the elbow flexors was greater in the Tui Na group than in the control group (P = 0.0009 and P = 0.0099, respectively; Table 2). With regard to the motor function of the upper extremities, greater improvements in the scores on the FMA‐T and FMA‐U from baseline to week 4 were observed in the Tui Na group compared with the control group (FMA‐T, P = 0.0006; FMA‐U, P < 0.0001). The superiority of Tui Na with conventional rehabilitation over conventional rehabilitation alone for motor function of the upper extremities was maintained at the 3‐ and 6‐month follow‐ups (FMA‐T, 3 months P = 0.0001, 6 months P = 0.0028; FMA‐U, 3 months P < 0.0001, 6 months P = 0.0005; Table 2). In terms of patients' abilities to perform ADL, the mean change in the MBI score showed no significant difference between the Tui Na and control groups from baseline to week 4 (P = 0.1124) or at the 3‐month follow‐up (P = 0.1795), but was greater in the Tui Na group than in the control group at the 6‐month follow‐up (P = 0.0099; Table 2).
Table 2.
Comparison of ΔMAS,ΔFMA and ΔMBI scores between the two groups (duration of the disease 1–3 months)
| Outcomes | CR | TN | Between 2 Groups | |
|---|---|---|---|---|
| z | P‐value | |||
| ΔMAS‐Elbow flexors muscle | ||||
| Baseline‐WK2 | 0.10 ± 0.36 (0.04, 0.17) | 0.18 ± 0.53 (0.08, 0.27) | 2.99 | 0.0836 |
| Baseline‐WK4 | 0.30 ± 0.57 (0.19, 0.40) | 0.37 ± 0.68 (0.25, 0.50) | 1.03 | 0.3107 |
| Baseline‐MTH3 | 0.25 ± 0.66 (0.12, 0.38) | 0.61 ± 0.75 (0.46, 0.75) | 10.95 | 0.0009 |
| Baseline‐MTH6 | 0.38 ± 0.68 (0.24, 0.52) | 0.65 ± 0.76 (0.49, 0.80) | 6.65 | 0.0099 |
| ΔMAS‐Wrist flexors muscle | ||||
| Baseline‐WK2 | 0.06 ± 0.53 (−0.04, 0.16) | 0.15 ± 0.56 (0.05, 0.25) | 2.79 | 0.0951 |
| Baseline‐WK4 | 0.06 ± 0.65 (−0.06, 0.19) | 0.31 ± 0.74 (0.18, 0.44) | 8.19 | 0.0042 |
| Baseline‐MTH3 | 0.05 ± 0.81 (−0.11, 0.20) | 0.47 ± 0.82 (0.31, 0.62) | 13.60 | 0.0002 |
| Baseline‐MTH6 | 0.16 ± 0.89 (−0.03, 0.34) | 0.53 ± 0.75 (0.37, 0.68) | 11.63 | 0.0006 |
| ΔMAS‐Finger flexors muscle | ||||
| Baseline‐WK2 | 0.03 ± 0.35 (−0.03, 0.10) | 0.10 ± 0.56 (0.00, 0.20) | 3.06 | 0.0804 |
| Baseline‐WK4 | 0.10 ± 0.56 (−0.01, 0.20) | 0.24 ± 0.71 (0.11, 0.36) | 5.14 | 0.0234 |
| Baseline‐MTH3 | 0.07 ± 0.65 (−0.06, 0.19) | 0.39 ± 0.72 (0.25, 0.53) | 13.55 | 0.0002 |
| Baseline‐MTH6 | 0.03 ± 0.77 (−0.13, 0.19) | 0.43 ± 0.65 (0.29, 0.56) | 13.96 | 0.0002 |
| ΔFMA‐T | ||||
| Baseline‐WK2 | −3.19 ± 4.48 (−4.01, −2.37) | −5.65 ± 7.36 (−6.95, −4.34) | 9.59 | 0.0022 |
| Baseline‐WK4 | −7.39 ± 8.54 (−8.99, −5.78) | −11.73 ± 10.42 (−13.59, −9.87) | 12.01 | 0.0006 |
| Baseline‐MTH3 | −12.83 ± 12.78 (−15.30, −10.35) | −20.03 ± 13.89 (−22.69, −17.36) | 15.40 | 0.0001 |
| Baseline‐MTH6 | −17.61 ± 16.78 (−21.13, −14.10) | −25.10 ± 16.76 (−28.53, −21.66) | 9.16 | 0.0028 |
| ΔFMA‐U | ||||
| Baseline‐WK2 | −1.67 ± 2.89 (−2.20, −1.14) | −3.87 ± 5.41 (−4.83, −2.91) | 15.14 | 0.0001 |
| Baseline‐WK4 | −3.99 ± 5.38 (−5.00, −2.98) | −7.75 ± 7.58 (−9.10, −6.40) | 18.75 | <0.0001 |
| Baseline‐MTH3 | −7.65 ± 8.27 (−9.25, −6.05) | −13.48 ± 10.06 (−15.41, −11.55) | 21.19 | <0.0001 |
| Baseline‐MTH6 | −10.89 ± 11.40 (−13.28, −8.50) | −17.10 ± 12.33 (−19.62, −14.57) | 12.54 | 0.0005 |
| ΔMBI | ||||
| Baseline‐WK2 | −3.83 ± 6.63 (−5.05, −2.61) | −5.27 ± 6.75 (−6.47, −4.08) | 2.80 | 0.0954 |
| Baseline‐WK4 | −9.50 ± 10.10 (−11.40, −7.60) | −11.67 ± 10.75 (−13.59, −9.76) | 2.54 | 0.1124 |
| Baseline‐MTH3 | −14.60 ± 13.50 (−17.21, −11.99) | −16.99 ± 12.33 (−19.35, −14.63) | 1.81 | 0.1795 |
| Baseline‐MTH6 | −18.78 ± 17.77 (−22.50, −15.06) | −26.12 ± 20.21 (−30.28, −21.96) | 6.79 | 0.0099 |
The data are presented as the mean ± standard deviation (SD), 95%CI. ΔChange in value before versus after treatment.
MAS, Modified Ashworth Scale; FMA‐U, Fugl‐Meyer upper limb score; FMA‐T, Fugl‐Meyer total score; MBI, Modified Barthel Index; WK, week; MTH, month. CR, Conventional rehabilitation group TN, Tui Na group.
Among the patients treated within 4–6 months poststroke, no significant differences were observed in the demographic characteristics of the Tui Na and control groups, and at baseline, the FMA, MBI, and MAS scores were balanced between the two groups (Table S2). At the end of the 4‐week treatment period and at the 3‐ and 6‐month follow‐ups, only the change in the MAS score for the elbow flexors was greater in the Tui Na group compared with the control group (Table 3), with no significant differences noted in the changes in MAS scores for the wrist flexors and finger flexors (Table 3). Overall, most of the changes in the FMA‐T, FMA‐U, and MBI scores at the observed time points also did not differ significantly between the Tui Na and control groups (Table 3).
Table 3.
Comparison of ΔMAS,ΔFMA and ΔMBI scores between the two groups (duration of the disease 4–6 months)
| Outcomes | CR | TN | Between 2 Groups | |
|---|---|---|---|---|
| z | P‐value | |||
| ΔMAS‐Elbow flexors muscle | ||||
| Baseline‐WK2 | 0.13 ± 0.34 (0.03, 0.23) | 0.23 ± 0.43 (0.10, 0.36) | 1.67 | 0.1960 |
| Baseline‐WK4 | 0.28 ± 0.54 (0.12, 0.44) | 0.55 ± 0.59 (0.36, 0.73) | 4.70 | 0.0301 |
| Baseline‐MTH3 | 0.29 ± 0.55 (0.11, 0.46) | 0.68 ± 0.77 (0.43, 0.94) | 7.24 | 0.0071 |
| Baseline‐MTH6 | 0.38 ± 0.59 (0.18, 0.58) | 0.88 ± 0.84 (0.59, 1.18) | 8.93 | 0.0028 |
| ΔMAS‐Wrist flexors muscle | ||||
| Baseline‐WK2 | 0.06 ± 0.25 (−0.01, 0.14) | 0.07 ± 0.26 (−0.01, 0.15) | 0.01 | 0.9107 |
| Baseline‐WK4 | 0.26 ± 0.61 (0.08, 0.44) | 0.33 ± 0.61 (0.14, 0.52) | 0.35 | 0.5537 |
| Baseline‐MTH3 | 0.31 ± 0.60 (0.12, 0.50) | 0.53 ± 0.80 (0.26, 0.79) | 1.20 | 0.2724 |
| Baseline‐MTH6 | 0.30 ± 0.78 (0.04, 0.56) | 0.56 ± 0.86 (0.26, 0.86) | 1.43 | 0.2325 |
| ΔMAS‐Finger flexors muscle | ||||
| Baseline‐WK2 | 0.09 ± 0.41 (−0.03, 0.20) | 0.14 ± 0.35 (0.03, 0.25) | 1.00 | 0.3161 |
| Baseline‐WK4 | 0.33 ± 0.60 (0.15, 0.50) | 0.29 ± 0.51 (0.13, 0.44) | 0.01 | 0.9231 |
| Baseline‐MTH3 | 0.29 ± 0.74 (0.05, 0.52) | 0.37 ± 0.54 (0.19, 0.55) | 0.50 | 0.4789 |
| Baseline‐MTH6 | 0.24 ± 0.88 (−0.02, 0.51) | 0.44 ± 0.66 (0.21, 0.67) | 0.92 | 0.3371 |
| ΔFMA‐T | ||||
| Baseline‐WK2 | −1.96 ± 4.19 (−3.19, −0.73) | −1.93 ± 3.92 (−3.14, −0.72) | 0.00 | 0.9748 |
| Baseline‐WK4 | −7.20 ± 7.09 (−9.30, −5.09) | −5.76 ± 7.57 (−8.12, −3.40) | 0.84 | 0.3617 |
| Baseline‐MTH3 | −12.74 ± 10.77 (−16.10, −9.38) | −13.00 ± 10.88 (−16.58, −9.42) | 0.01 | 0.9142 |
| Baseline‐MTH6 | −15.32 ± 14.80 (−20.26, −10.39) | −16.15 ± 14.49 (−21.20, −11.09) | 0.06 | 0.8138 |
| ΔFMA‐U | ||||
| Baseline‐WK2 | −1.43 ± 2.44 (−2.14, −0.71) | −1.12 ± 2.71 (−1.95, −0.28) | 0.32 | 0.5702 |
| Baseline‐WK4 | −4.48 ± 4.59 (−5.84, −3.12) | −3.71 ± 5.72 (−5.50, −1.93) | 0.48 | 0.4896 |
| Baseline‐MTH3 | −7.98 ± 6.84 (−10.11, −5.85) | −8.50 ± 8.58 (−11.32, −5.68) | 0.09 | 0.7625 |
| Baseline‐MTH6 | −9.76 ± 9.46 (−12.91, −6.60) | −10.85 ± 10.93 (−14.67, −7.04) | 0.20 | 0.6522 |
| ΔMBI | ||||
| Baseline‐WK2 | −2.34 ± 5.23 (−3.87, −0.81) | −2.14 ± 3.69 (−3.27, −1.00) | 0.04 | 0.8350 |
| Baseline‐WK4 | −6.37 ± 7.04 (−8.46, −4.28) | −6.17 ± 6.07 (−8.06, −4.28) | 0.02 | 0.8857 |
| Baseline‐MTH3 | −12.26 ± 10.64 (−15.58, −8.95) | −12.63 ± 10.17 (−15.98, −9.29) | 0.03 | 0.8745 |
| Baseline‐MTH6 | −14.73 ± 14.45 (−19.55, −9.91) | −16.32 ± 11.92 (−20.48, −12.17) | 0.25 | 0.6155 |
The data are presented as the mean ± standard deviation (SD), 95%CI. ΔChange in value before versus after treatment.
MAS, Modified Ashworth Scale; FMA‐U, Fugl‐Meyer upper limb score; FMA‐T, Fugl‐Meyer total score; MBI, Modified Barthel Index; WK, week; MTH, month. CR, Conventional rehabilitation group TN, Tui Na group.
Among the patients treated within 7–12 months poststroke, no significant differences were observed in the demographic characteristics of the Tui Na and control groups, and at baseline, the FMA, MBI, and MAS scores were balanced between the two groups (Table S3). At the end of the 4‐week treatment period as well as at the 3‐ and 6‐month follow‐ups, most of the mean changes in the MAS scores for the elbow flexors, wrist flexors, and finger flexors showed no significant differences between the Tui Na and control groups (Table 4). Moreover, most of the changes in the FMA‐T, FMA‐U, and MBI scores at the observed time points also did not differ significantly between the Tui Na and control groups (Table 4).
Table 4.
Comparison of ΔMAS,ΔFMA and ΔMBI scores between the two groups (duration of the disease 7–12 months)
| Outcomes | CR | TN | Between 2 groups | |
|---|---|---|---|---|
| z | P‐value | |||
| ΔMAS‐Elbow flexors muscle | ||||
| Baseline‐WK2 | 0.13 ± 0.47 (−0.02, 0.28) | 0.20 ± 0.41 (0.03, 0.37) | 0.80 | 0.3721 |
| Baseline‐WK4 | 0.42 ± 0.55 (0.24, 0.60) | 0.60 ± 0.71 (0.31, 0.89) | 0.84 | 0.3598 |
| Baseline‐MTH3 | 0.30 ± 0.77 (0.03, 0.58) | 0.56 ± 0.58 (0.32, 0.80) | 2.00 | 0.1577 |
| Baseline‐MTH6 | 0.18 ± 0.66 (0.09, 0.28) | 0.27 ± 0.57 (0.19, 0.35) | 3.63 | 0.0567 |
| ΔMAS‐Wrist flexors muscle | ||||
| Baseline‐WK2 | 0.13 ± 0.34 (0.02, 0.24) | 0.20 ± 0.50 (−0.01, 0.41) | 0.17 | 0.6812 |
| Baseline‐WK4 | 0.32 ± 0.66 (0.10, 0.53) | 0.60 ± 0.65 (0.33, 0.87) | 3.76 | 0.0525 |
| Baseline‐MTH3 | 0.27 ± 0.67 (0.03, 0.51) | 0.56 ± 0.65 (0.29, 0.83) | 2.25 | 0.1335 |
| Baseline‐MTH6 | 0.39 ± 0.74 (0.11, 0.68) | 0.64 ± 0.70 (0.35, 0.93) | 1.97 | 0.1606 |
| ΔMAS‐Finger flexors muscle | ||||
| Baseline‐WK2 | 0.21 ± 4.54 (−0.42, 0.84) | 0.16 ± 4.08 (−0.41, 0.73) | 0.01 | 0.9038 |
| Baseline‐WK4 | 0.39 ± 4.49 (−0.24, 1.02) | 0.11 ± 4.65 (−0.55, 0.76) | 0.37 | 0.5428 |
| Baseline‐MTH3 | 0.68 ± 5.38 (−0.11, 1.47) | 0.63 ± 4.76 (−0.08, 1.33) | 0.01 | 0.9220 |
| Baseline‐MTH6 | 0.79 ± 5.48 (−0.08, 1.66) | 0.99 ± 5.22 (0.16, 1.83) | 0.11 | 0.7354 |
| ΔFMA‐T | ||||
| Baseline‐WK2 | −1.38 ± 6.20 (−3.39, 0.63) | −3.92 ± 5.93 (−6.37, −1.47) | 2.63 | 0.1097 |
| Baseline‐WK4 | −5.21 ± 9.78 (−8.43, −2.00) | −8.40 ± 8.50 (−11.91, −4.89) | 1.77 | 0.1877 |
| Baseline‐MTH3 | −10.24 ± 12.91 (−14.82, −5.67) | −13.96 ± 10.60 (−18.33, −9.59) | 1.37 | 0.2465 |
| Baseline‐MTH6 | −12.18 ± 17.00 (−18.77, −5.59) | −17.96 ± 13.34 (−23.47, −12.45) | 1.86 | 0.1781 |
| ΔFMA‐U | ||||
| Baseline‐WK2 | −0.64 ± 4.93 (−2.24, 0.96) | −2.96 ± 4.92 (−4.99, −0.93) | 3.38 | 0.0709 |
| Baseline‐WK4 | −3.21 ± 6.96 (−5.50, −0.92) | −6.04 ± 7.04 (−8.95, −3.13) | 2.47 | 0.1213 |
| Baseline‐MTH3 | −6.58 ± 9.25 (−9.86, −3.29) | −10.04 ± 7.79 (−13.25, −6.83) | 2.28 | 0.1368 |
| Baseline‐MTH6 | −7.64 ± 11.97 (−12.28, −3.00) | −12.80 ± 9.18 (−16.59, −9.01) | 3.04 | 0.0872 |
| ΔMBI | ||||
| Baseline‐WK2 | −1.46 ± 7.12 (−3.77, 0.84) | −1.16 ± 2.01 (−1.99, −0.33) | 0.04 | 0.8374 |
| Baseline‐WK4 | −4.50 ± 9.27 (−7.55, −1.45) | −4.76 ± 3.84 (−6.35, −3.17) | 0.02 | 0.8949 |
| Baseline‐MTH3 | −8.97 ± 11.76 (−13.14, −4.80) | −9.16 ± 8.07 (−12.49, −5.83) | 0.00 | 0.9449 |
| Baseline‐MTH6 | −9.75 ± 13.31 (−14.91, −4.59) | −10.44 ± 8.82 (−14.08, −6.80) | 0.05 | 0.8271 |
The data are presented as the mean ± standard deviation (SD), 95% CI. ΔChange in value before versus after treatment.
MAS, Modified Ashworth Scale; FMA‐U, Fugl‐Meyer upper limb score; FMA‐T, Fugl‐Meyer total score; MBI, Modified Barthel Index; WK, week; MTH, month; MTH, month. CR, Conventional rehabilitation group TN, Tui Na group.
Discussion
In this multicenter, prospective, randomized controlled study to investigate the effectiveness of Tui Na for treating upper limb spasticity in stroke patients, the addition of Tui Na to conventional rehabilitation provided a significantly greater reduction in upper limb spasticity than did conventional rehabilitation alone among patients who were treated within 1–3 months poststroke, and the observed benefits were sustained at the end of the 6‐month follow‐up. In addition, the addition of Tui Na to the treatment strategy resulted in a greater improvement in motor function as assessed by the FMA. Together our results indicated that the observed reduction in spasticity after 4 weeks of Tui Na may be beneficial for hand function and upper limb control, and the effect was maintained for 3 and 6 months. Furthermore, no adverse events were reported, and no cases of drop‐out were known to be related to the study intervention. Thus, Tui Na is likely a safe and effective treatment with early (4 weeks) and extended (6 month) curative effects for reducing upper limb spasticity in stroke patients.
Previous studies have demonstrated that Tui Na has a positive effect on upper limb spasticity in stroke patients.21, 22 However, the follow‐up periods were rarely longer than 3 months in published studies.21, 22 In addition, the muscle groups of the upper extremities are relatively small and complicated and thus, may not be as easily affected via Tui Na.12 The present findings that Tui Na did decrease spasticity in the small flexor muscles of the wrist and hand in stroke patients treated within 1–3 months confirm the results of previous studies and then extend these findings by showing the effect was sustained at least 6 months after the 4‐week treatment period.
However, the addition of Tui Na to conventional rehabilitation for patients treated beyond 3 months (4–6 months and 7–12 months) after stroke did not have a greater antispasticity effect than conventional rehabilitation alone. These findings suggested that the effect of Tui Na on poststroke upper limb spasticity was different at different stages of poststroke recovery, and Tui Na should be used only in early stage stroke patients.
Studies investigating the effect of Tui Na on spasticity seldom measure motor functional outcomes. Chen et al.21 showed a significant important in FAM of the upper limb in patients with stroke after Tui Na treatment. However, the number of patients enrolled in the study was small and the study was of high risk of bias with flawed study design and poor methodological quality. Our results provide strong evidence that Tui Na has beneficial effects on motor function recovery among patients who were treated within 1–3 months poststroke. In our findings, a significant improvement in FMA scores for upper limb motor function after 4‐week Tui Na treatment was maintained for 3 and 6 months, compared with those of conventional rehabilitation group. In addition, Tui Na has been practiced in China for thousands of year and one of the most popular complementary therapies in China. Although acupuncture as a complementary therapy method for spasticity in stroke patients is widely recognized in the west,it is still unsatisfactory in improving motor function.23 Compared with these complementary therapy methods, Tui Na is a safe, effective, easy to operate, noninvasive method for relieving spasticity and improving motor function.
Standard operating procedures for Tui Na on the upper limbs of stroke patients were established by experts from the authoritative Chinese Medicine Research Institute in China who have extensive theoretical and practical experience in Chinese massage. A standard operation video which explains how Tui Na should be applied was sent to each center to ensure homogeneity of treatment among centers and therapists, who were trained before they carried out any treatment. All of Tui Na therapists were licensed and had 5–10 years of experience. Although we had taken those measures to minimize the practical variation, the Tui Na performance among the centers in our study may still be affected by the practical variation among physical therapists. Our study does have several other limitations though. Due to the nature of the treatment, the therapists and patients could not be blinded to treatment allocation. The evaluation measures were all rather subjective as well, and additional research with more objective evaluation indicators would be beneficial. Patients underwent rehabilitation therapy during the follow‐up period, but in this study, interventions of rehabilitation therapy were not documented in detail over a 6‐month follow‐up period. However, we speculate that Tui Na has a long‐term effect on poststroke upper limb spasticity. Further investigations are needed to clarify this. In addition, Baimai‐ruangao was used during Tui Na in this study, but it could lead to some uncertain bias. The mechanism of this drug remains unexplained.
Conclusions
The results of the present study showed that Tui Na is safe and effective for alleviating poststroke upper limb spasticity when applied within 1–3 months after stroke, and the effect of a 4‐week Tui Na treatment for alleviating poststroke upper limb spasticity persisted for 6 months after treatment.
Conflict of Interest
The authors declare that no conflict of interest exists.
Supporting information
Table S1. Patients' characteristics of the study participants at baseline (duration of the disease 1–3 months).
Table S2. Patients' characteristics of the study participants at baseline (duration of the disease 4–6 months).
Table S3. Patients' characteristics of the study participants at baseline (duration of the disease 7–12 months).
Data S1. Supplementary Materials and Methods
Acknowledgments
The authors wish to thank all patients for their participation in this study and its follow‐up. The authors would like to acknowledge the following persons for providing support for this study: Zhihang Peng, Nan Jing Medical University teaching and research section of statistics; Xiaorong Hu, Binbing Yu, Rong Cao,Yan Jiang, Yuxia Wu, Yongqiang Li, Rong Bian, and Shaohua Gu, First Affiliated Hospital of Nanjing Medical University. In addition, the authors acknowledge the support of the assistants: Yuemei Lu, Jingjing Shao, Xiao tan, and Yang Yang, Nanjing University of Chinese Medicine.
Funding Information
Ministry of Science and Technology of the People's Republic of China through the Twelfth Five‐Year National Science and Technology Pillar Program (2013BAI10B04).
Contributor Information
Xiao Lu, Email: luxiao1972@163.com.
Jianan Li, Email: jiananli77@126.com.
References
- 1. Lundström E, Terént A, Borg J. Prevalence of disabling spasticity 1 year after first‐ever stroke. Eur J Neurol 2008;15:533–539. [DOI] [PubMed] [Google Scholar]
- 2. Watkins CL, Leathley MJ, Gregson JM, et al. Prevalence of spasticity post stroke. Clin Rehabil 2002;16:515–522. [DOI] [PubMed] [Google Scholar]
- 3. Sommerfeld DK, Eek EU, Svensson AK, et al. Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke 2004;35:134–139. [DOI] [PubMed] [Google Scholar]
- 4. Wissel J, Manack A, Brainin M. Toward an epidemiology of poststroke spasticity. Neurology 2013;80:S13–S19. [DOI] [PubMed] [Google Scholar]
- 5. Francisco GE, McGuire JR. Poststroke spasticity management. Stroke 2012;43:3132–3136. [DOI] [PubMed] [Google Scholar]
- 6. Wang KL, Huang Y, Fan JH. Tui Na for spasticity after stroke: meta‐analysis. Chin J Ethnomed Ethnopharm 2016;3:24–27. [Google Scholar]
- 7. He J, Yi XJ. Effect of Tui Na combined with rehabilitation training on post‐stroke spasticity: a pilot study. J Emerg Tradit Chin Med 2009;18:27–28. [Google Scholar]
- 8. Ding ZL. Clinical research of effect of Tui Na on post‐stroke upper limb spasticity. Hebei J Tradit Chin Med 2010;32:1522–1523. [Google Scholar]
- 9. Di HY, Han SK, Du XL, et al. Applying Tui Na to exterior‐interiorly connected meridians for post‐stroke upper limb spasticity. J Acupunct Tui Na Sci 2017;15:27–30. [Google Scholar]
- 10. Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 1976;54:541–553. [PMC free article] [PubMed] [Google Scholar]
- 11. Tombaugh TN, McIntyre NJ. The mini‐mental state examination: a comprehensive review. J Am Geriatr Soc 1992;40:922–935. [DOI] [PubMed] [Google Scholar]
- 12. Yang YJ, Zhang J, Hou Y, et al. Effectiveness and safety of Chinese massage therapy (Tui Na) on post‐stroke spasticity: a prospective multicenter randomized controlled trial. Clin Rehabil 2016;31:1–9. [DOI] [PubMed] [Google Scholar]
- 13. Sloan RL, Sinclair E, Thompson J, et al. Inter‐rater reliability of the modified Ashworth Scale for spasticity in hemiplegic patients. Int J Rehabil Res 1992;15:158–161. [DOI] [PubMed] [Google Scholar]
- 14. Fu TS, Wu CY, Lin KC, et al. Psychometric comparison of the shortened fugl‐meyer assessment and the streamlined wolf motor function test in stroke rehabilitation. Clin Rehabil 2012;26:1043–1047. [DOI] [PubMed] [Google Scholar]
- 15. Duncan PW, Propst M, Nelson SG. Reliability of the fugl‐meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther 1983;63:1606–1646. [DOI] [PubMed] [Google Scholar]
- 16. Bennett M, Ryall N. Using the modified barthel index to estimate survival in cancer patients in hospice: observational study. BMJ 2000;321:1381–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah S, Vanclay F, Cooper B. Improving the sensitivity of the barthel index for stroke rehabilitation. J Clin Epidemiol 1989;42:703–709. [DOI] [PubMed] [Google Scholar]
- 18. Gracies JM, Brashear A, Jech R, et al. Safety and efficacy of abobotulinumtoxinA for hemiparesis in adults with upper limb spasticity after stroke or traumatic brain injury: a double‐blind randomised controlled trial. Lancet Neurol 2015;14:992–1001. [DOI] [PubMed] [Google Scholar]
- 19. Lim SM, Yoo J, Lee E, et al. Acupuncture for spasticity after stroke: a systematic review and meta‐analysis of randomized controlled trials. Evid Based Complement Alternat Med 2015;2015:870398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park SW, Yi SH, Lee JA, et al. Acupuncture for the treatment of spasticity after stroke: a meta‐analysis of randomized controlled trials. J Altern Comp Med 2014;20:672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen F, Su QJ. Tui Na and anti‐spasticity techniques on treatment of post stroke upper limb spasticity. Chin J Pract Nervous Dis 2011;14:74–75. [Google Scholar]
- 22. Yan LF, Jin HZ. Massage treatment on spasm after stroke. J Changchun Uni Chinese Med 2015;31:311–312. [Google Scholar]
- 23. Sze FK‐H, Wong E, Kevin KH, et al. Does acupuncture improve motor recovery after stroke? A meta‐analysis of randomized controlled trials. Stroke 2002;33:2604–2619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patients' characteristics of the study participants at baseline (duration of the disease 1–3 months).
Table S2. Patients' characteristics of the study participants at baseline (duration of the disease 4–6 months).
Table S3. Patients' characteristics of the study participants at baseline (duration of the disease 7–12 months).
Data S1. Supplementary Materials and Methods


