Abstract
Objective
We studied the risk of dementia in patients with primary Sjögren's syndrome (pSS) using a nationwide, population‐based cohort in Taiwan.
Methods
Our study analyzed the medical data of the Taiwanese population from 2000 to 2014. We identified 17,072 patients with pSS and 68,270 controls. Dementia risk was analyzed using a Cox proportional hazards regression model stratified by sex, age, and comorbidities.
Results
A higher incidence of dementia development in the pSS group during the observation period (P = 0.0001). In multivariate analysis adjusted by age groups, gender, and the comorbidities, the adjusted hazard ratio (aHR) of developing dementia was 1.246 (95% CI 1.123–1.384) times greater in the pSS group than in the non‐pSS group. When stratified by sex, age, and comorbidities, the patients with pSS less than 60 years (aHR 1.67, 95% CI 1.16–2.41), and without any comorbidity (aHR 2.27, 95% CI 1.76–2.93) were particularly associated with a higher risk of dementia. Furthermore, the patients with pSS combined with any other comorbidity had an additionally higher risk of dementia (aHR: 3.978, 95% CI 3.309–4.782), also suggesting that pSS was an independent risk factor for the development of dementia.
Interpretation
Primary Sjogren's syndrome is associated with increased dementia risk and further study is needed to understand why and what the specific dementia phenotypes are.
Introduction
Sjögren's syndrome is one of the most prevalent autoimmune diseases. It is a slowly progressive inflammatory disease affecting the exocrine glands and the extraglandular organs. Primary Sjögren's syndrome (pSS) is defined as Sjögren's syndrome without underlying connective tissue diseases, and pSS is usually diagnosed between the ages of 40 and 60 years. Systemic involvement and extra‐glandular manifestations are common in patients with pSS, and a wide range of central nervous system and neurologic complications have been reported, including cognitive dysfunction and dementia.1, 2, 3, 4 Neurological involvement has been reported to range from 0% to 70%, and may present as central nervous system and/or peripheral nervous system involvement.5 Neurological manifestations have been linked with greater pSS activity.6 Cognitive deficits including mood disorder, and both cognitive and affective disorders can also result from immune‐mediated brain dysfunction.7 While psychological distress is frequent in patients with pSS, cognitive dysfunction is a commonly following symptom.7
Dementia is a neurological disease characterized by persistent intellectual deterioration that interferes with social and occupational functions, and it is a serious public health issue. Dementia is diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM‐IV‐TR).4 One previous study evaluated magnetic resonance imaging (MRI) and cognitive functioning in 15 patients with pSS, and the result revealed a larger ventricular volume than in control subjects with migraine. In addition, the severity of hyper‐intensities and ventricular volume were related to several cognitive and psychiatric variables.8 Another study reported that 15 of the 25 patients with pSS had cognitive impairment and five patients had dementia, and they suggested that the general practitioners and specialist should be informed about the potential for dementia in patients with pSS.4 Vascular brain involvement is considered to be the most likely cause of dementia development in patient with pSS.4
Hypothesis of pSS predisposing patients to the development of dementia is proposed. However, a link between pSS and dementia has not been fully confirmed due to an insufficient numbers of patients with dementia in study populations, short follow‐up periods, and also a lack of assessment of covariates influencing the risks of dementia. Therefore, the aim of this study was to study the risk of dementia by analyzing a large population‐based database to compare patients with pSS compared to the age‐ and sex‐matched controls without pSS selected from the general Taiwanese population.
Methods
Data sources
We used claims data from the Bureau of National Health Insurance (NHI) of Taiwan from 2000 to 2014 for analysis, and this retrospective case‐matched cohort study used clinical data from the National Health Insurance Research Database (NHIRD) which is maintained by the Department of Health and National Health Research Institute of Taiwan. Enrollment in the NHI program, a high‐performing single‐payer national health insurance system, is mandatory in Taiwan.9 This study was approved by the Institutional Review Board of Taipei Medical University Hospital (approval number of N201509007). The NHIRD contains electronic and personal information of all patients enrolled in the NHI program, and all data are encrypted to protect privacy. As the datasets used in this study was comprised of de‐identified secondary data for research purposes, patient consent was not required for this study. We used a frequency matching method to control the interference due to confounding factors in our study. In this frequency matching, we had used three matching variables – gender, age group, and comorbidities. This matching method requests the proportion of each matching variable in a comparison group similar to that in the pSS group. Therefore, in our matching process, we had found randomly target observations in a comparison group that was based on the proportion of each matching variable in the pSS group. Then 4:1 were being chosen randomly in comparison group and pSS group by matching variables with similarly frequencies. For each pSS patient, four non‐pSS patients were randomly selected from the same study period according to the same exclusion criteria and were frequency‐matched with the pSS patients according to age and sex to construct the non‐pSS cohort, which comprised 68,270 patients. A total of 17,072 patients with pSS and 68,270 matched controls were selected from the NHIRD database from 2000 to 2014. The flowchart of study population selection is also shown in Figure 1. The baseline characteristics and follow‐up status of both cohorts after propensity score matching were shown in Table 1.
Figure 1.
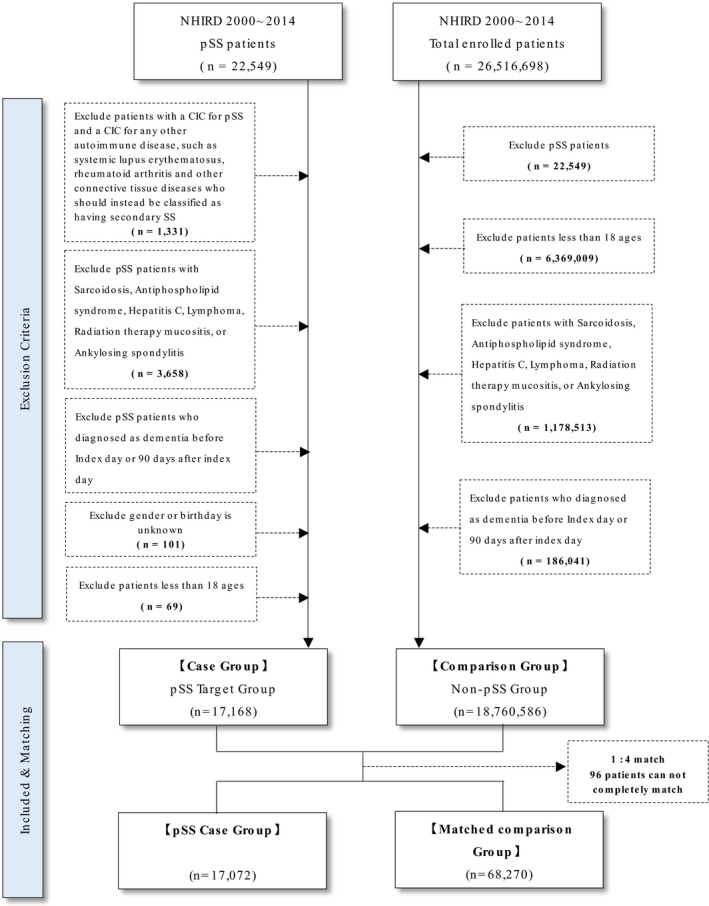
The flowchart of study population selection (pSS: Primary Sjögren's syndrome, CIC: catastrophic illness card).
Table 1.
Baseline of characteristic of the pSS cohort and age‐, sex‐, and comorbidities‐matched comparison cohort
| Variables | pSS cohort (N = 17,072) | Comparison cohort (N = 68,270) | P‐Value | ||
|---|---|---|---|---|---|
| N (%) | N (%) | ||||
| Gender | 0.4944 | ||||
| Male | 1751 | (10.26%) | 7124 | (10.44%) | |
| Female | 15,321 | (89.74%) | 61,146 | (89.56%) | |
| Age | 0.4963 | ||||
| 18–30 | 926 | (5.42%) | 3783 | (5.54%) | |
| 31–40 | 1994 | (11.68%) | 7850 | (11.50%) | |
| 41–50 | 3686 | (21.59%) | 14,751 | (21.61%) | |
| 51–60 | 4947 | (28.98%) | 19,659 | (28.80%) | |
| 61–70 | 3214 | (18.83%) | 12,760 | (18.69%) | |
| 71–80 | 1838 | (10.77%) | 7390 | (10.82%) | |
| >80 | 467 | (2.74%) | 2077 | (3.04%) | |
| Mean (SD) | 54.20 | (14.25%) | 54.02 | (14.02%) | 0.3875 |
| Median(IQR) | 54 | (19) | 54 | (19) | |
| Comorbidities | |||||
| Diabetes | 1560 | (9.2%) | 6284 | (9.14%) | 0.7868 |
| Hyperlipidemia | 2701 | (15.82%) | 10,758 | (15.76%) | 0.8394 |
| Hypertension | 4355 | (25.51%) | 17,345 | (25.41%) | 0.7820 |
| Heart failure | 330 | (1.93%) | 1326 | (1.94%) | 0.9372 |
| Cardiovascular disease | 4953 | (29.01%) | 19,803 | (29.01%) | 0.9886 |
| Stroke | 987 | (5.78%) | 3978 | (5.83%) | 0.8204 |
| Major psychosis or a substance‐related disorder | 824 | (4.83%) | 3268 | (4.79%) | 0.8279 |
| Traumatic brain injury | 145 | (0.85%) | 579 | (0.85%) | 0.9874 |
| Follow‐up time (days) | |||||
| Mean (SD) | 1897 | (1456) | 1855 | (1408) | 0.0005 |
| Median(IQR) | 1574 | (2118) | 1536 | (2074) | |
| Propensity score | |||||
| Mean (SD) | 0.0297 | (0.0477) | 0.0300 | (0.0491) | 0.4398 |
| Media(IQR) | 0.0115 | (0.0267) | 0.0115 | (0.0267) | |
| Disease | |||||
| Dementia | 503 | (2.95%) | 1467 | (2.15%) | <.0001 |
| Duration from index date to Dementia (days) | |||||
| Mean(SD) | 1752 | (1234) | 1520 | (1141) | 0.0001 |
| Median(IQR) | 1494 | 1983 | 1261 | 1654 | |
IQR, interquartile range; SD, standard deviation.
Criteria for patient selection
We used the registry of catastrophic illnesses to identify patients in the NHIRD diagnosed with Sjogren's syndrome according to an International Classification of Diseases, 9th revision, Clinical Modification (ICD‐9‐CM) diagnosis code of 710.2 from 2000 to 2014. Patients diagnosed with pSS were identified in the catastrophic illness registry in the NHIRD. Patients with a catastrophic illness certificate are exempted from the copayments in the NHI program. To obtain a catastrophic illness certificate for pSS, patients are required to meet the criteria of the American‐European Consensus Group for pSS, and are reviewed by a committee.10
In addition, we excluded pSS patients with other autoimmune diseases including systemic lupus erythematosus (SLE) (ICD‐9‐CM: 710.0), rheumatoid arthritis (RA) (ICD‐9‐CM: 714.0), systemic sclerosis (ICD‐9‐CM: 710.1), polymyositis and dermatomyositis (ICD‐9‐CM: 710.3), Behçet's disease (ICD‐9‐CM: 136.1), temporal arteritis (ICD‐9‐CM: 443.1), granulomatosis polyangiitis (ICD‐9‐CM: 446.4) [29], and Takayasu arteritis (ICD‐9‐CM: 446.7). Patients with secondary Sjogren's syndrome were excluded from this study. Patients with a history of sarcoidosis (ICD‐9‐CM: 135), anti‐phospholipid syndrome (ICD‐9‐CM: 286.53 and 289.81), hepatitis C (ICD‐9‐CM: 70.7, 070.41, 070.44, 070.51, 070.54, 571.5), lymphoma (ICD‐9‐CM: 201,202), radiation therapy related mucositis (ICD‐9‐CM: 528.1, 990), and ankylosing spondylitis (ICD‐9‐CM: 720) were also excluded. Patients younger than 18 years of age, and those with a history of dementia or Parkinson's disease identified before the enrollment date were also excluded. A total of 17,072 patients with pSS and 68,270 (a ratio of 1: 4) matched controls were recruited in this study.
Outcomes and relevant variables
The primary outcome was newly diagnosed dementia. Every patient was followed until a first diagnosis of any type of dementia made by neurologists in two outpatient visits or one hospital admission record. The types of dementia included Alzheimer's disease (ICD‐9‐CM: 331.0), arteriosclerotic dementia (ICD‐9‐CM: 290.4), and unspecified dementia (ICD‐9‐CM: 290.0–290.3, 294.1, 331.1–331.2, and 331.82). A patient was defined as being lost to follow‐up on death, withdrawal from the NHI program, or the end of 2014. In addition, major comorbidities such as diabetes (ICD‐9‐CM 250), hyperlipidemia (ICD‐9‐CM 272), hypertension (ICD‐9‐CM 401–405), heart failure (ICD‐9‐CM 428, I50.2, I50.3), cardiovascular disease (ICD‐9‐CM codes 393–398, 410–414, 420–429, 440–449, 451–459), stroke (ICD‐9‐CM 430–438), major psychosis or a substance‐related disorder (ICD‐9‐CM codes 291–299, 303–305), and traumatic brain injury (ICD‐9 CM codes 801–804, 850–854) were considered to be covariates in this study. Smoking and education were significant covariates, but not included in the analyses, because they were not available in the NHIRD database. The risk of more specific dementia clinical diagnosis like frontotemporal dementia or Alzheimer disease in patients with pSS were not included in this analyses, because they were not available in the NHIRD database using ICD‐9‐CM.
Statistical analysis
Baseline of characteristic in this cohort study included age, sex, and certain comorbidities. The baseline characteristics were matched between the pSS and non‐pSS comparison groups using the chi‐squared test for categorical variables, and a t‐test for continuous variables. The incidence rates of dementia were estimated during the follow‐up period in both cohorts. We used a multiple Cox proportional hazards model to explore the associations and estimate the corresponding hazard ratios (HRs) and 95% confidence intervals (CIs). The HRs were adjusted for sex, age, and comorbidities in the Cox regression model, and the adjusted HRs (aHRs) were presented after adjusting for covariates. Patient data were stratified by age, gender and with, or without other comorbidities, and then the incidence rates, incidence rate ratios (IRRs) and HRs of dementia in both cohorts were analyzed. These groups had a separate analysis where they were appropriately propensity matched. We then plotted the results in cumulative incidence plots using the Kaplan–Meier method, and the log‐rank test to examine the differences. SAS software (version 9.4, SAS Institute, Cary, NC, USA) was used for all data analyses, and a P < 0.05 was considered to be statistically significant.
Results
Demographic data and the flow chart of study population selection
Table 1 shows the baseline characteristics of both cohorts. The study population was predominantly female (89%), and the average ages of the patients with pSS and controls were 54.20 and 54.02 years, respectively. There were no significant differences between the two matched groups in sex, age and comorbidities including diabetes, hyperlipidemia, hypertension, heart failure, cardiovascular disease, stroke, major psychosis or a substance‐related disorder, and traumatic brain injury in the pSS and the non‐pSS groups. The pSS group had a significantly higher incidence of dementia development than the matched reference subjects during the observation period (2.95% vs. 2.15%, P < 0.0001).
Risk of dementia associated with pSS and other comorbidities
Table 2 shows the risk of dementia and comorbidities according to the Cox proportional hazard regression model. The overall HR for dementia during the study period was 1.338 (95% CI 1.215–1.472) in the pSS cohort compared to the non‐pSS cohort. In multivariate analysis adjusted by age groups, gender and the comorbidities, the HR of developing dementia was 1.246 (95% CI 1.123–1.384) times greater in the pSS group than in the non‐pSS group. This result suggested that pSS was an independent risk factor for the development of dementia.
Table 2.
Dementia risk due to pSS and other comorbidities
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR | 95% CI | Adjusted HR* | 95% CI | |
| Disease | ||||
| pSS | 1.338*** | (1.215–1.472) | 1.246*** | (1.123–1.384) |
| Gender | ||||
| Female (ref.) | 1.000 | 1.000 | ||
| Male | 2.130*** | (1.860–2.440) | 0.865 | (0.762–0.982) |
| Age group | ||||
| ≤60 (ref.) | 1.000 | 1.000 | ||
| 61–70 | 20.51*** | (16.98–24.78) | 17.06*** | (14.04–20.73) |
| 71–80 | 57.00*** | (47.42–68.54) | 42.30*** | (34.78–51.44) |
| >80 | 102.2*** | (82.89–126.1) | 77.73*** | (62.59–96.54) |
| Comorbidities | ||||
| Diabetes | 3.762*** | (3.319–4.263) | 1.608*** | (1.431–1.808) |
| Hyperlipidemia | 2.011*** | (1.781–2.270) | 0.958 | (0.853–1.075) |
| Hypertension | 5.198*** | (4.677–5.778) | 1.353*** | (1.216–1.505) |
| Heart failure | 3.650*** | (2.858–4.662) | 0.914 | (0.719–1.161) |
| Cardiovascular disease | 2.615*** | (2.351–2.908) | 1.380*** | (1.247–1.526) |
| Stroke | 5.159*** | (4.539–5.863) | 1.755*** | (1.549–1.990) |
| Major psychosis or a substance‐related disorder | 3.414*** | (2.860–4.076) | 3.296*** | (2.811–3.865) |
| Traumatic brain injury | 3.067*** | (2.150–4.376) | 1.556 | (1.145–2.114) |
Adjusted HR*: adjusted by age groups, gender, and the comorbidities.
*P value for HR <0.05, **P value for HR <0.01, ***P value for HR <0.001.
The risk of dementia increased proportionally with increasing age (using aHR in the patients less than 60 as reference, Table 2). Comorbidities including diabetes (aHR 1.608, 95% CI 1.431–1.808), hypertension (aHR 1.353, 95% CI 1.216–1.505), cardiovascular disease (aHR 1.380, 95% CI 1.247–1.526), stroke (aHR 1.755, 95% CI 1.549–1.990), and major psychosis or a substance‐related disorder (aHR 3.296, 95% CI 2.811–3.865) also increased the risk of dementia (Table 2).
Increased incidence rate and incidence rate ratio of dementia in the patients with pSS
Table 3 shows the incidence rates (IR), incidence rate ratio (IRR) and aHR of dementia during the follow‐up period. Comparing the pSS cohort and non‐pSS cohort (as reference), a higher IRR and aHR of dementia were found in pSS cohort (IRR: 1.37, 95% CI 1.24–1.52 and aHR: 1.25, 95% CI 1.12–1.38, respectively) was found. When stratified by sex, age, and comorbidities, the patients with pSS less than 60 years (IRR 1.87, 95% CI 1.30–2.67; aHR 1.67, 95% CI 1.16–2.41), and without comorbidities (IRR 2.36, 95% CI 1.85–3.02; aHR 2.27, 95% CI 1.76–2.93) were particularly associated with a higher risk of dementia.
Table 3.
Incidence rate, incidence rate ratio, and hazard ratios of dementia for the patient with pSS and non‐pSS cohorts and in different subgroups
| Variables | Non‐pSS cohort | pSS cohort | IRR | Crude HR | Adj. HR# | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event | PY† | IR‡ | Event | PY† | IR‡ | |||||||
| Disease | ||||||||||||
| Dementia | 1467 | 346,746 | 423.08 | 503 | 88,669 | 567.28 | 1.37*** | (1.24–1.52) | 1.34*** | (1.22–1.47) | 1.25*** | (1.12–1.38) |
| Gender | ||||||||||||
| Female | 1195 | 312,845 | 381.98 | 406 | 79,917 | 508.03 | 1.36*** | (1.21–1.52) | 1.33*** | (1.19–1.48) | 1.24** | (1.10–1.39) |
| Male | 272 | 33,900 | 802.36 | 97 | 8752 | 1108.32 | 1.45** | (1.15–1.83) | 1.37** | (1.11–1.70) | 1.26 | (0.99–1.60) |
| Age group | ||||||||||||
| 18–60 | 94 | 251,729 | 37.34 | 44 | 63,795 | 68.97 | 1.87** | (1.30–2.67) | 1.78** | (1.24–2.54) | 1.67** | (1.16–2.41) |
| 61–70 | 478 | 58,277 | 820.22 | 155 | 15,240 | 1017.06 | 1.29** | (1.07–1.54) | 1.22* | (1.02–1.46) | 1.17 | (0.97–1.40) |
| 71–80 | 656 | 30,214 | 2171.18 | 231 | 8104 | 2850.44 | 1.42*** | (1.22–1.65) | 1.30** | (1.12–1.50) | 1.27* | (1.09–1.47) |
| >80 | 239 | 6525 | 3662.84 | 73 | 1529 | 4774.36 | 1.36* | (1.05–1.77) | 1.30 | (0.99–1.70) | 1.19 | (0.91–1.56) |
| With other comorbidities | ||||||||||||
| No | 171 | 177,258 | 96.47 | 101 | 44,776 | 225.57 | 2.36*** | (1.85–3.02) | 2.30*** | (1.81–2.93) | 2.27*** | (1.76–2.93) |
| Yes | 1296 | 169,487 | 764.66 | 402 | 43,894 | 915.84 | 1.24** | (1.11–1.39) | 1.19** | (1.07–1.33) | 1.14* | (1.02–1.27) |
†PY, person‐years; ‡IR, incidence rate; #Adj. HR, adjusted hazard ratio; IRR, incidence rate ratio.
*P value for HR <0.05, **P value for HR <0.01, ***P value for HR <0.001.
pSS was an independent risk factor for the development of dementia
Table 4 shows the aHR in the non‐pSS and pSS groups, and groups with or without comorbidities during the study period. A potential independent effect of pSS affecting the development of dementia was assessed. The matched reference subjects with any comorbidity associated with the development of dementia (including diabetes, hyperlipidemia, hypetension, heart failure, cardiovascular disease, stroke, traumatic brain injury, psychosis and a substance‐related disorder) had a higher risk of dementia (aHR: 3.502, 95% CI 2.966–4.135) compared to those without any comorbidity. Moreover, the patients with pSS had a higher risk of dementia (aHR: 2.357, 95% CI 1.834–3.028) as those with any comorbidities associated with the development of dementia. Furthermore, the patients with pSS combined with any other comorbidity had an additionally higher risk of dementia (aHR: 3.978, 95% CI 3.309–4.782), also suggesting that pSS was an independent risk factor for the development of dementia. Figure 2 shows the cumulative incidence of dementia for the pSS and non‐pSS matched reference groups, compared generally (Fig. 2A), and between different age subgroups (Fig. 2B–E). Overall, the pSS patients had poorer consequences from developing dementia than the matched reference subjects (log‐rank test, P < 0.001, Fig. 2A), which was also seen in different age subgroups (log‐rank test, P < 0.01, Fig. 2B and D).
Table 4.
Adjusted hazard ratio in pSS and non‐pSS cohorts with or without comorbidity
| Variables | Number | Number of event | Adj. hazard ratio# | |
|---|---|---|---|---|
| Non‐pSS × No‐comorbidity (ref.) | 32,664 | 171 | 1.000 | – |
| Non‐pSS × comorbidity | 35,606 | 1296 | 3.502*** | (2.966–4.135) |
| pSS × No‐comorbidity | 8167 | 101 | 2.357*** | (1.834–3.028) |
| pSS × comorbidity | 8905 | 402 | 3.978*** | (3.309–4.782) |
Adj. hazard ratio#, adjusted by age groups, gender, and the comorbidities; ***P value for HR < 0.001.
Figure 2.
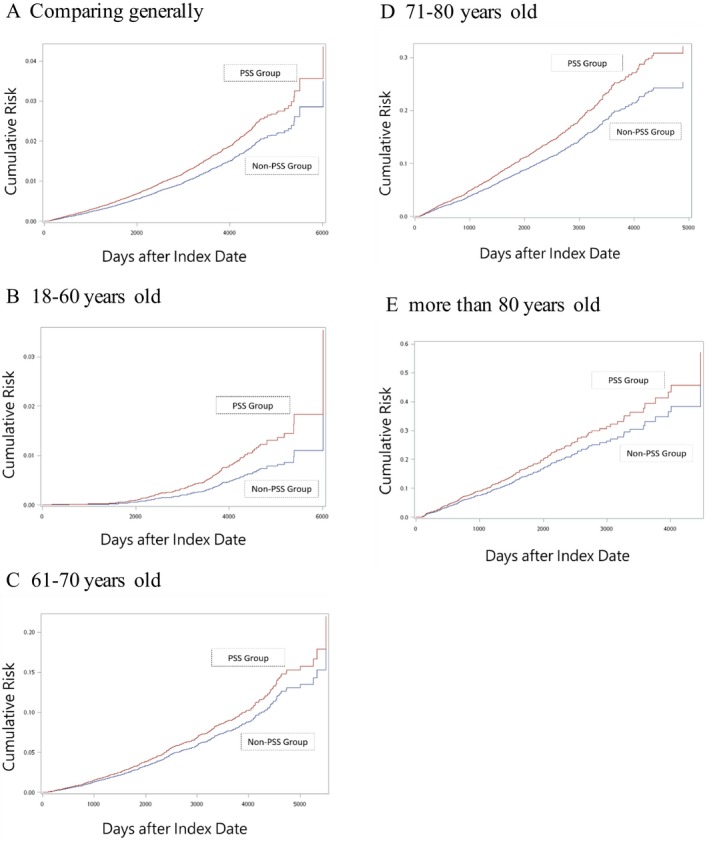
Cumulative incidence plot for the pSS cohort and non‐pSS matched reference subjects, comparing generally (A), and between different age subgroups (B: 18–60, C: 61–70, D: 71–80, and E: more than 80 years old).
Discussion
To the best of our knowledge, this is the first large‐scale population‐based study to assess the association between pSS and dementia. This association has been suggested in a previous study involving 25 patients with pSS,4 and a brain magnetic resonance imaging study including a few patients with pSS.8 Our findings in this retrospective study confirm and extend the findings of an independent risk of dementia in the patients with pSS (n = 17,072) relative to the reference‐matched subjects (n = 68,270). In our study, patients with pSS were selected from the NHIRD catastrophic illness card (CIC) registry from 2000 to 2014, and excluding patients with conditions such as systemic lupus erythematosus, rheumatoid arthritis and sarcoidosis, anti‐phospholipid syndrome, hepatitis C, lymphoma, radiation therapy mucositis, ankylosing spondylitis, diagnosed as dementia before Index day or 90 days after index day, unknown gender or birthday and aged less than 18 years old. Therefore, the prevalence and incidence in this study was lower than the overall SS prevalence/incidence from other epidemiologic studies.
The overall IR, IRR, and aHR were significantly higher in the patients with pSS than in the reference‐matched subjects. Younger patients with pSS (less than 60 years old) were especially associated with a higher risk of dementia. After multivariate adjustments, the hazard of developing dementia was still greater in the pSS than in the non‐pSS group. Furthermore, the patients with pSS combined with any other comorbidity had an additionally higher risk of dementia than those without any comorbidity. Taken together, the results suggest that pSS is an independent risk factor for dementia. Potential theories of the mechanism by which pSS may increase the risk of dementia include vascular damage such as vasculitis, autoantibodies and immune complex deposition, occlusion of vessels, and cellular inflammation leading to neuro‐destructive effects resulting in reduction of cognitive reserve and initiation of incipient dementia as a neurodegenerative process.5 However, further basic and translational research is required to elucidate the precise pathophysiological mechanism.
National databases of health administration registries allow for epidemiological research due to the large cohort size and long‐term follow‐up period. The validity of this study is strengthened by the stringent design and careful ICD‐9‐CM coding. Hence, we used NHIRD catastrophic illness registry data, in which patients are exempted from copayments of all medical costs in the NHI system, and in which confirmation of pSS disease requires a committee peer‐review of the fulfillment of the AECG criteria for pSS. In addition, we also excluded patients with other autoimmune diseases. Thus, we believe our cohort study is highly accurate and dependable. However, there are still limitations in this study, including the lack of data on clinical parameters such as disease severity, laboratory data including autoantibodies, body mass index, smoking exposure, intelligence, education level and whether major psychosis and substance disorder are primary or secondary to a dementia syndrome, which are not recorded in the NHIRD, all of which have also been associated with pSS and dementia. Another limitation of this study was that we did not evaluate sleep‐disordered breathing (SDB) as a risk factor for dementia, and patients with pSS may have SDB more frequently than normal people. Another potential limitation may arise due to the likely differences in the clinical evaluation frequency, which may lead to bias in the analysis, as the diagnosis of dementia could be identified earlier in patients with pSS due to more frequent clinical evaluations including cognitive testing. In addition, the interpretation of our results is limited to pSS, because this study did not include patients with secondary Sjogen's syndrome).
Our results highlight the greater risk of dementia in the younger patients with pSS, and especially those without comorbidities. Alzheimer's disease, vascular disease, frontotemporal lobar degeneration, and dementia with Lewy bodies are the most common diseases that cause dementia. A clinical concept of “dementia plus syndromes” has recently been proposed, in which the dementia syndromes involving cognitive impairment are accompanied by additional neurological or systemic features, and the pattern of cognitive impairment can be relatively specific to the underlying molecular pathology.11 Cognitive manifestations have been frequently observed in patients with pSS,1, 2, 3, 4, 8 and several studies have suggested that the diagnosis of pSS should be considered in patients with unexplained neuro‐psychiatric illnesses, and memory or executive dysfunctions detected on neuropsychological tests.2, 12 One study used brain functional SPECT imaging to evaluate 10 patients with pSS and abnormalities were found in 100% of the patients with pSS, and in 20% of the age‐ and sex‐matched controls.13 The burden of young‐onset dementia (with onset before 65 years of age) have been reported to be higher than in late‐onset dementia,11 and more importantly, many of the cases of young‐onset dementias may be treatable based on specific treatment of the underlying conditions.11 Sjogren's syndrome occurs worldwide without notable regional differences and the annual incidence of pSS ranges from 5.3 to 6.0 patients per 100,000 inhabitants.10, 14 In Taiwan, the estimated mean annual incidence of pSS was 6.0 patients per 100,000 inhabitants (95% CI 5.8–6.2), and the incidence has been shown to increase with age, peaking at 55–64 years of age in women and 65–74 years in men.10 Given the number of patients who do not seek medical attention, underestimation of the actual prevalence of pSS is likely. Because pSS is a common autoimmune disease, thus, even a small increase in the risk of dementia in young patients with pSS could have significant implications on public health. Therefore, we emphasize the need to consider the possibility of pSS as a differential diagnosis, with a clinical approach to young‐onset dementia, and the potential benefits of early treatment with immunomodulatory or immunosuppressive drugs.
In conclusion, our results disclosed that the patients with pSS had a higher risk of dementia. Sjogren's syndrome should be considered as the underlying disease in patients with young‐onset dementia, and especially in those without any preceding comorbidities. Rheumatologists, neurologists and general practitioners should be aware about the risk of dementia due to pSS.
Conflict of Interest
None declared.
Acknowledgment
This study received support from Wan Fang Hospital and Taipei Medical University (108‐wf‐eva‐02, 104‐WF‐IIT‐05).
Funding Information
This study received support from Wan Fang Hospital and Taipei Medical University (108‐wf‐eva‐02, 104‐WF‐IIT‐05).
Funding Statement
This work was funded by Wan Fang Hospital grant ; Taipei Medical University grants 108‐wf‐eva‐02 and 104‐WF‐IIT‐05.
Contributor Information
Chi‐Ching Chang, Email: ccchang@tmu.edu.tw.
Jin‐Hua Chen, Email: jh_chen@tmu.edu.tw.
References
- 1. Creange A, Laplane D, Habib K, et al. Dementia disclosing primary Gougerot‐Sjogren syndrome. Rev Neurol (Paris) 1992;148:376–380. [PubMed] [Google Scholar]
- 2. Teixeira F, Moreira I, Silva AM, et al. Neurological involvement in Primary Sjogren Syndrome. Acta Reumatol Port 2013;38:29–36. [PubMed] [Google Scholar]
- 3. Delalande S, de Seze J, Fauchais AL, et al. Neurologic manifestations in primary Sjogren syndrome: a study of 82 patients. Medicine (Baltimore) 2004;83:280–291. [DOI] [PubMed] [Google Scholar]
- 4. Blanc F, Longato N, Jung B, et al. Cognitive Dysfunction and Dementia in Primary Sjogren's Syndrome. ISRN Neurol 2013;2013:501327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tobon GJ, Pers JO, Devauchelle‐Pensec V, Youinou P. Neurological Disorders in Primary Sjogren's Syndrome. Autoimmune Dis 2012;2012:645967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carvajal Alegria G, Guellec D, Mariette X, et al. Epidemiology of neurological manifestations in Sjogren's syndrome: data from the French ASSESS Cohort. RMD Open 2016;2:e000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Segal BM, Pogatchnik B, Holker E, et al. Primary Sjogren's syndrome: cognitive symptoms, mood, and cognitive performance. Acta Neurol Scand 2012;125:272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mataro M, Escudero D, Ariza M, et al. Magnetic resonance abnormalities associated with cognitive dysfunction in primary Sjogren syndrome. J Neurol 2003;250:1070–1076. [DOI] [PubMed] [Google Scholar]
- 9. Cheng TM. Reflections on the 20th anniversary of Taiwan's single‐payer National Health Insurance System. Health Aff (Millwood) 2015;34:502–510. [DOI] [PubMed] [Google Scholar]
- 10. Weng MY, Huang YT, Liu MF, Lu TH. Incidence and mortality of treated primary Sjogren's syndrome in Taiwan: a population‐based study. J Rheumatol 2011;38:706–708. [DOI] [PubMed] [Google Scholar]
- 11. Rossor MN, Fox NC, Mummery CJ, et al. The diagnosis of young‐onset dementia. Lancet Neurol 2010;9:793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Malinow KL, Molina R, Gordon B, et al. Neuropsychiatric dysfunction in primary Sjogren's syndrome. Ann Intern Med 1985;103:344–350. [DOI] [PubMed] [Google Scholar]
- 13. Le Guern V, Belin C, Henegar C, et al. Cognitive function and 99mTc‐ECD brain SPECT are significantly correlated in patients with primary Sjogren syndrome: a case‐control study. Ann Rheum Dis 2010;69:132–137. [DOI] [PubMed] [Google Scholar]
- 14. Alamanos Y, Tsifetaki N, Voulgari PV, et al. Epidemiology of primary Sjogren's syndrome in north‐west Greece, 1982–2003. Rheumatology (Oxford) 2006;45:187–191. [DOI] [PubMed] [Google Scholar]


