Abstract
Objective
Many stroke patients make a partial recovery in function during the first 3 months, partially through promoting insulin‐like growth factor‐1 (IGF‐1) function. A prognostic biomarker that associates with IGF‐1 function may predict clinical outcome and recovery of stroke. This study evaluated plasma concentrations of IGF‐1, IGF binding protein (IGFBP)‐3 and cyclic‐glycine‐proline (cGP) and their associations with clinical outcome in stroke patients.
Methods
Thirty‐four patients were recruited within 3 days of stroke. Clinical assessments included the National Institutes of Health Stroke Scale (NIHSS) within 3 days (baseline), and at days 7 and 90; the modified Rankin Scale (mRS) and Fugl‐Meyer Upper‐Limb Assessment Scale (FM‐UL) at days 7 and 90. Plasma samples were collected from the patients at the baseline, days 7 and 90. Fifty age‐matched control participants with no history of stroke were also recruited and provided plasma samples. IGF‐1, IGFBP‐3, and cGP concentrations were analyzed using ELISA or HPLC‐MS.
Results
Baseline concentrations of IGFBP‐3, cGP, and cGP/IGF‐1 ratio were lower in stroke patients than the control group. The neurological scores of stroke patients were improved and plasma cGP and cGP/IGF‐1 ratio increased over time. Baseline cGP/IGF‐1 ratio was correlated with the NIHSS scores at day 90 and the changes in NIHSS scores from the baseline to 90 days.
Interpretation
Low cGP concentrations and cGP/IGF‐1 ratio in stroke patients suggest an impaired IGF‐1 function. The cGP/IGF‐1 ratio at admission maybe further developed as a prognostic biomarker for stroke recovery.
Introduction
Stroke remains to be a major cause of mortality and disability burden.1 Many surviving patients make at least a partial recovery in function over the first 3 months, a critical period for the recovery.2 A biomarker that is associated with recovery would be clinically useful for predicting outcome and assist clinical management.3 The expression of insulin growth factor‐1 (IGF‐1) increases in the brain tissues 1–5 days after hypoxic‐ischemic (HI) brain injury in rats,4 and this may contribute to a partial recovery of sensory‐motor function.5 Timely administration of human recombinant IGF‐1 within 6 h after HI injury reduces brain damage and improves long‐term sensory‐motor function.4, 5, 6, 7 Changes in plasma concentrations of IGF‐1 have been studied as a prognostic biomarker in stroke but the results have been inconsistent.8
The majority of circulating IGF‐1 is inactive due to high‐affinity binding, mainly to IGF binding protein (IGFBP)‐3, which prevents IGF‐1 to activate its functional receptors. Only unbound IGF‐1 is bioactive;9, 10, 11 however, it is either swiftly metabolized or internalized after activating IGF receptors.12, 13, 14 Thus, the plasma concentration of IGF‐1 do not represent IGF‐1 function. Binding of IGF‐1 to IGFBP‐3 is reversible and this regulates the amount of bioavailable IGF‐1 in plasma, a process namely autocrine regulation of IGF‐1. The molar ratio of IGF‐1 to IGFBP‐3 has been used to characterize IGF‐1 function, but this does not consistently represent IGF‐1 function because the majority of IGFBP‐3 is not associated with plasma IGF‐1.15
Our previous publication suggests that the changes in the plasma concentration of cGP and its molar ratio to plasma IGF‐1 may be a better representation of bioavailable IGF‐1.16, 17, 18 cGP naturally cleaves from the N‐terminus of unbound IGF‐119 by an acid enzyme.20, 21 The N‐terminal region of IGF‐1 is a major binding site to IGFBP‐322 and cGP retains the binding affinity to the IGFBP‐3, leading to a concentration‐dependent competitive binding between IGF‐1 and cGP.15 The relative concentration of cGP to IGF‐1 may be used to determine the bioavailability of IGF‐1 as a higher cGP/IGF‐1 ratio associates with a greater concentration of unbound, bioavailable IGF‐1.15, 16, 17 Administration of cGP has been shown to protect the brain from ischemic injury through promoting IGF‐1 function that in turn reduces vascular damage.15 The aim of this study was to evaluate whether the changes in plasma concentrations of cGP and cGP/IGF‐1 molar ratio would associate with the recovery during the first 3 months after stroke.
Methods
Study population and design
Forty‐four patients aged 18–90 years of age were screened within 3 days of stroke (Fig. 1). Thirteen patients withdrew, one patient died and two did not complete the follow‐up over the 90‐day course of the study (Fig. 1). Clinical assessments included the National Institutes of Health Stroke Scale (NIHSS) at baseline (within 3 days), then again at days 7 and 90. The modified Rankin Scale (mRS) and Fugl‐Meyer Upper Limb Assessment Scale (FM‐UL) were performed at days 7 and 90. Clinical assessments were performed by the members of the independent clinical team who were trained in their administration. All stroke patients have completed standard in‐patient rehabilitation.
Figure 1.
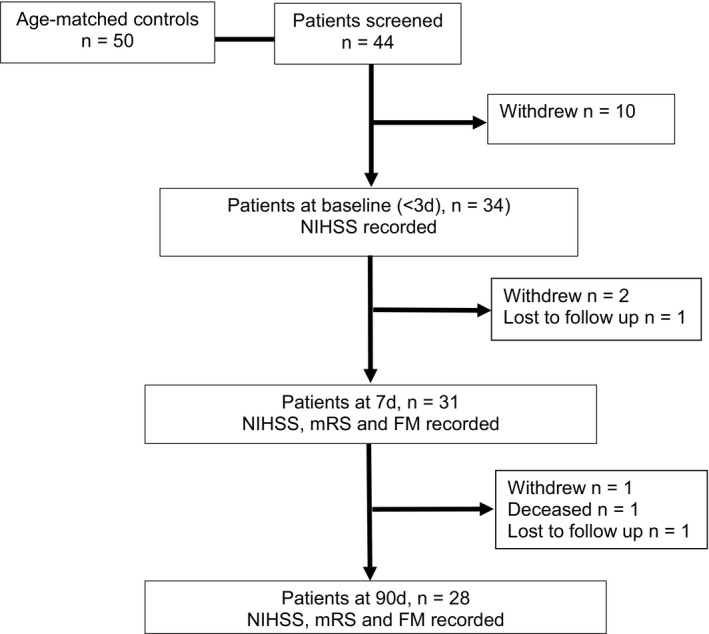
The flowchart shows the study population.
Plasma samples were collected from 34 stroke patients at baseline, from 21 patients at day 7 and from 26 patients at day 90 (Fig. 1). Twenty‐eight patients had completed the follow‐ups, 21 of whom provided plasma samples at day 7, and 26 who provided plasma samples at day 90. A total of 21 patients had completed clinical scores and provided plasma samples at all‐time points. Twenty‐eight patients provided plasma samples at baseline and had clinical assessments at day 90. Fifty age‐matched control participants (35 women and 15 men) with no history of stroke were also recruited and all provided plasma samples (Table S1 and S2).
Plasma sample collection
Plasma samples were collected and prepared by the Biomarkers and Recovery in Stroke (BARISTa), Brain Research New Zealand. Blood samples were collected in ethylene di‐amino tetra‐acetic acid (EDTA) tubes and immediately transferred to the processing laboratory. The samples were left for at least 1 h before being centrifuged at 1300g for 10 min at room temperature. The plasma samples were stored at −80°C until assayed.
Plasma extraction of cGP
The methodology of extraction of cGP from plasma has been previous described.16, 17 Briefly, cGP‐1,5,6,7,8‐13C, 4‐15N (cGP‐5x13C, 1x15N) provided an internal standard for cGP assay. cGP‐5x13C,1x15N (50 μL of 500 ng/mL) was added to 100 μL of plasma, vortex‐mixed. The solution was transferred to a 1 mL Phree phospholipid removal cartridge (Phenomenex, Auckland, New Zealand) contained in 4.5 mL tube; 500 μL of 1% formic acid in acetonitrile was added to the cartridge and centrifuged at 284 g for 5 min at 4°C to enable the collection of the filtrate. The filtrate was dried using a vacuum concentrator (1.5 mTor for an hour, then 0.7 mTor for 45 min, at room temperature). The dried samples were reconstituted in 100 μL 10% methanol/water (v/v) and transferred to an ultra‐pressure liquid chromatography vial for quantitation, then centrifuged at 500 rpm for minutes at 4°C to sediment any remaining particulates. Standards were prepared by spiking cGP into charcoal stripped human plasma, quality control samples, with cGP at two different concentrations, were utilized and then subjected to the same extraction procedure as the samples.
High performance liquid chromatography mass spectrometry assay (HPLC‐MS)
The chromatography conditions consisted of a Synergy Hydro 2.5 μm column (Phenomenex) 100 × 2 mm with an initial mobile phase composition of 10% methanol/90% water at flow rate of 200 μL per minute and a column temperature of 35°C, as previously described.16, 17 The mass spectrometry conditions consisted of electrospray ionization in positive mode with a voltage of 4000 V, a sheath gas flow of 30 psi, an auxiliary gas flow of 2 psi, and a capillary temperature of 250°C. Fragmentation was achieved with argon at 1.2 mTorr as the collision gas and a dissociation voltage of 35 V. The mass spectrometer ran in selective reaction monitoring (SRM) mode with the following two transitions 155.1 → 70.2 m/z and 161 → 75.1 m/z utilized for cGP and cGP‐5x13C,1x15N, respectively. The retention time for both peaks was 3.6 min. Unknown samples were quantitated using the peak area ratio of cGP/cGP‐5x13C,1x15N compared with the standard curve of known concentrations.
Measurement of plasma concentrations of IGF‐1 and IGFBP‐3 using ELISA
Plasma concentration of IGF‐1 and IGFBP‐3 were measured using commercial ELISA kits (Crystal Chem, Chicago, IL) according to manufacturer's instructions.17 The absorbance values were obtained using a plate reader (BioTek® Synergy™ 2 Multi‐detection Microplate Reader, Gen5 software, Winooski, VT) set at 450 nm for the excitation wavelength and at 630 nm for the emission wavelength. Concentrations were reported as ng/mL.
Statistics
SPSS (IBM SPSS Statistics 24, Chicago, IL) was used for statistical analysis. The differences between the stroke (baseline) and the control groups were analyzed using unpaired t‐test, Chi‐Square tests, or Fisher's exact test as appropriate. Changes over time in stroke patients were analyzed using one‐way repeated ANOVA or Friedman test with post hoc tests using the Bonferroni correction. The association between biological changes and clinical scores were analyzed using linear regression model adjusted for age and baseline NIHSS. The significance level was set at P < 0.05. Data are presented as mean ± standard error of the mean (SEM) unless otherwise stated.
Results
Demographic details of the stroke patients and control participants are presented in Table 1. In stroke patients, NIHSS scores improved over time (F (2, 20) = 27.48, P < 0.001, n = 21, Fig. 2A). Compared to baseline, the NIHSS score reduced by day 7 (median: 2 vs. 4, P = 0.01) and day 90 (median: 1 vs. 4, P < 0.001). The mRS score also improved from (median: 3 vs. 2, P = 0.003, n = 21, Fig. 2B) as did the FM‐UL scores (median: 64 vs. 65, P = 0.001, n = 21, Fig. 2C). The control participants had no significant neurological deficits (NIHSS median (range): 0 (0–2)) nor global disability (mRS median (range) 0 (0–1)) with normal up limb functions (FM‐UL median (range): 66 (65–66)).
Table 1.
Baseline characteristics of acute stroke patients and controls
| Control | Patients | P value | |
|---|---|---|---|
| Number of participants | 50 | 34 | |
| Age, years (mean ± SD) | 64.8 ± 10.03 | 66.79 ± 14.64 | 0.49a |
| F/M | 35/15 | 15/19 | 0.018b |
| Diabetes (%) | 2 (4) | 6 (17.6) | 0.057c |
| Current smoker (%) | 1 (2) | 4 (11.8) | 0.153c |
| Ex‐smoker (%) | 16 (32) | 9 (26.5) | 0.586c |
| Hypertension (%) | 13 (26) | 15 (44.1) | 0.084b |
| Dyslipidemia (%) | 9 (18) | 12 (35.3) | 0.072b |
| Atrial fibrillation (%) | 4 (8) | 9 (26.5) | 0.022b |
a t‐test; bChi‐Square tests; cFisher's exact test.
Figure 2.
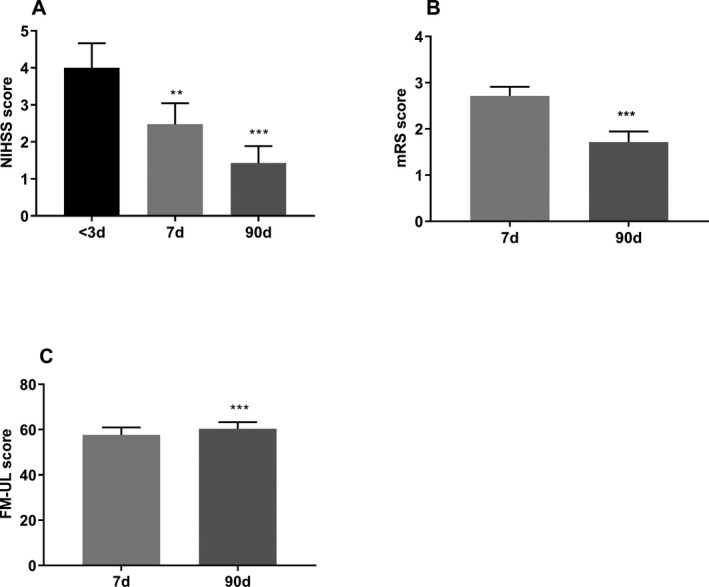
The changes in clinical scores over time after stroke. Compared to the baseline, NIHSS significantly decreased in 7 and 90 days after stroke (A). The modified Rankin Scale (mRS) score reduced from days 7 to 90 (B) and Fugl‐Meyer Upper Limb Assessment Scale (FM‐UL) score was increased from 7 to 90 days after stroke (C). Data are presented as mean ± SEM. **P < 0.01, ***P < 0.001, n = 21.
Biological changes
There was no difference in IGF‐1 concentration (Fig. 3A) between stoke and control groups. Compared to the control group, stroke patients had lower IGFBP‐3 concentrations (P = 0.002, Fig. 3B), cGP concentrations (P = 0.04, Fig. 3C), and cGP/IGF‐1 molar ratio (P = 0.04, Fig. 3B) at the baseline.
Figure 3.
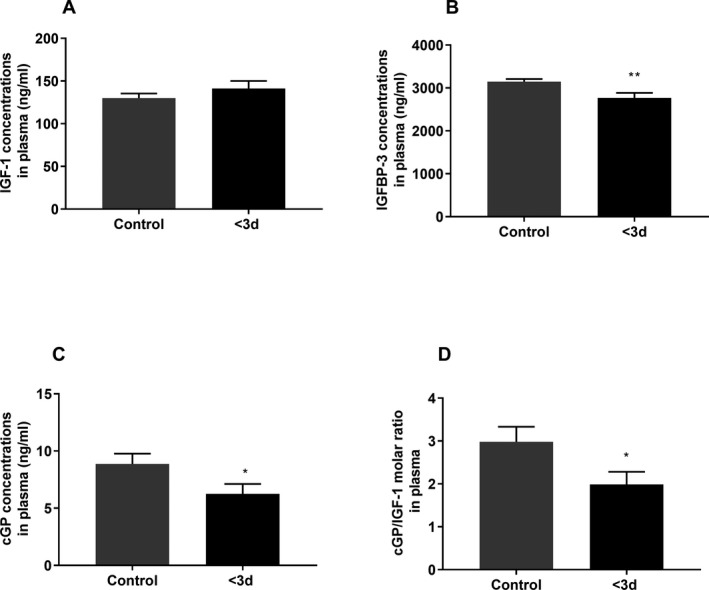
The differences in biological changes between the control group (n = 48–50) and stroke group at the baseline (<3 days after stroke, n = 34). IGF‐1 concentration did not change after stroke (A). Compared to the control group, the baseline concentrations of IGFBP‐3 (B), cGP (C), and cGP/IGF‐1 ratio (D) were lower in stroke patients. Data presented as Mean ± SEM, *P < 0.05, **P < 0.01.
The concentrations of IGF‐1 and IGFBP‐3 remained stable over time after stroke (Fig. 4A and B). There were significant increases in cGP concentrations (F (2, 20) = 5.34, P = 0.01, n = 21, Fig. 4C) and cGP/IGF‐1 molar ratio (F (2, 20) = 3.94, P = 0.02, n = 21, Fig. 4D) over time by ANOVA repeated analysis. Compare to the baseline, the concentration of cGP (P = 0.01) and cGP/IGF‐1 ratio (P = 0.03) was significantly increased by 90 days in the stroke patients.
Figure 4.
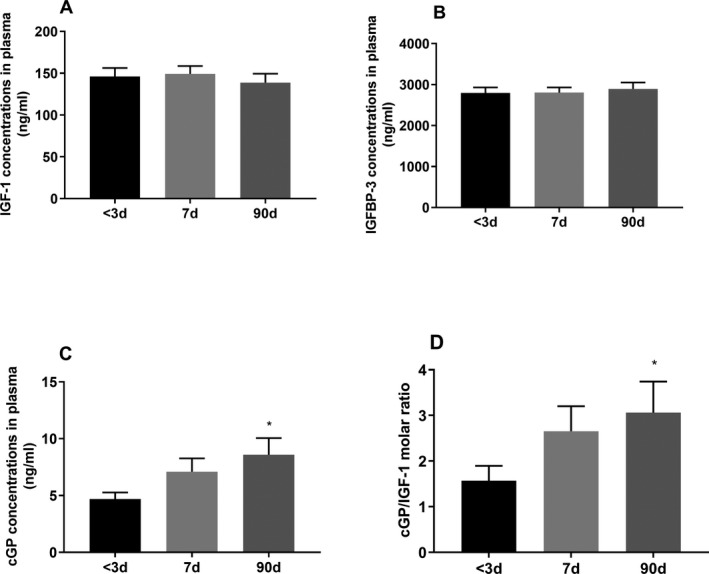
The biological changes over time in stroke patients. The plasma concentration of IGF‐1 (A) and IGFBP‐3 (B) did not change over time. cGP concentration (C) and cGP/IGF‐1 ratio (D) increased over time. Compare to the baseline, cGP concentration and cGP/IGF‐1 ratio were significantly increased 90 days after stroke. Data presented as Mean ± SEM, *P < 0.05, n = 21.
Correlation with biological and clinical changes of stroke patients
Table 2 shows the results from correlation analysis between biological changes at the baseline and clinical scores either at day 90 or the changes from the baseline to day 90 in the stroke patients. After adjustment for age and baseline NIHSS, we found that the cGP/IGF‐1 molar ratio was negatively correlated with the NIHSS scores at day 90 (B = −0.30; 95%CI = −0.59 — −0.02; P = 0.03; n = 28) and positively correlated with ∆NIHSS (B = 0.309; 95%CI = 0.02─0.59; P = 0.03; n = 28). The baseline cGP/IGF‐1 ratio showed a trend toward a positive correlation with FM‐UL scores of day 90 (B = 2.33, 95%CI = −0.07─4.73; P = 0.05; n = 27), but not with the mRS.
Table 2.
Relationships between clinical outcomes and recovery at 90 days and baseline biological concentrations in plasma
| IGF‐1 | IGFBP‐3 | cGP | cGP/IGF‐1 ratio | ||
|---|---|---|---|---|---|
| NIHSS (n = 28) | B | 0.006 | 0.00 | −0.06 | −0.30 |
| P | 0.11 | 0.09 | 0.36 | 0 .03 | |
| 95%CI | (−0.002, 0.01) | (0, 0.001) | (−0.21, 0.08) | (−0.59, −0.02) | |
| mRS (n = 28) | B | −0.001 | 0.00 | 0.09 | 0.19 |
| P | 0.72 | 0.52 | 0.10 | 0.08 | |
| 95%CI | (−0.008, 0.005) | (−0.001, 0) | (−0.02, 0.20) | (−0.03, 0.42) | |
| FM (n = 27) | B | −0.01 | −0.002 | 0.84 | 2.33 |
| p | 0.64 | 0.38 | 0.16 | 0.05 | |
| 95%CI | (−0.08, 0.05) | (−0.007, 0.003) | (−0.35, 2.04) | (−0.07, 4.73) | |
| ∆NIHSS (n = 28) | B | −0.006 | 0.00 | 0.06 | 0.30 |
| P | 0.11 | 0.09 | 0.36 | 0.03 | |
| 95%CI | (−0.01,0.002) | (−0.001,0.00) | (−0.08, 0.21) | (0.02,0.59) | |
NIHSS, the National Institutes of Health Stroke Scale; ∆NIHSS, the change between baseline and 90 days in the National Institutes of Health Stroke Scale; mRS, the modified Rankin Scale score; FM, The Fugl‐Meyer Upper Limb Assessment Scale score; B, beta coefficients; 95%CI, 95% confidence intervals. The analysis was adjusted for age and baseline NIHSS.
Discussion
This study has shown the lower plasma concentrations of cGP, IGFBP‐3, and cGP/IGF‐1 ratio in stroke patients within 3 days of stroke, suggesting an impairment of autocrine regulation by cGP. Over next 90 days, the stroke patients showed an improvement in neurological function and global disability, which occurred in parallel with the gradual increases in plasma cGP and cGP/IGF‐1 ratio. The stroke patients with higher baseline cGP/IGF‐1 ratio had less neurological deficits at day 90 of stroke and made a better recovery by 90 days. The results of this study may suggest a role for autocrine regulation of IGF‐1 in stroke recovery and that the molar ratio of cGP/IGF‐1, if further confirmed through larger studies, may be a potential prognostic biomarker for predicting the ability of recovery in stroke patients.
The amount of bioavailable IGF‐1 in plasma is collectively regulated by IGFBP‐3 and cGP. The less IGFBP‐3 and more cGP can increase the amount of bioavailable IGF‐1 in plasma.15, 16, 17, 18 Compared to the age‐matched controls, the concentrations of IGFBP‐3 and cGP were lower in stroke patients at the baseline. We have recently showed that hypertensive women also have lower plasma IGFBP‐3 and cGP compared to the normotensive women.16 These changes in hypertension and stroke patients suggest a role for autocrine regulation in cardiovascular functions. While the reduction in plasma IGFBP‐3 is an autocrine response to increase bioavailable IGF‐1 in plasma, the lower plasma cGP may suggest an impairment of autocrine regulation by cGP in hypertension and stroke patients.16 This interpretation is also supported by the observations that cGP administration prevents neuronal and vascular damage after ischemic brain injury in rats.15 The function of cGP in vascular protection is mediated through improving IGF‐1 function which has been described by in vivo and in vitro studies.15 Hypertension is a major risk factor of stroke23 and the impairment of autocrine regulation of IGF‐1 may be a pathophysiology shared by hypertension and stroke.
Most patients in our study made a partial recovery over the 90‐days of the study. These clinical improvements were in parallel with an increase in cGP concentrations and cGP/IGF‐1 ratio over time. We speculate that the improvement of plasma IGF‐1 bioavailability18, 22 may contribute to the functional recovery, but a correlation analysis failed to show a significant association (data not shown), possibly due to the limited sample size in this study. While there was no change in IGF‐1 concentration during stroke recovery, the increase in plasma cGP concentration over time is more likely a result of promoting enzymatic formation of cGP in plasma. However, the mechanism that modulates the enzymatic activity is unknown and needs to be investigated.
Age and baseline neurological deficit are crucial factors that influence stroke recovery.24 The correlation analysis with the adjustment for age and baseline NIHSS scores showed that the patients with a higher molar ratio of cGP/IGF‐1 at the baseline had less neurological deficits at day 90 of stroke and made a better recovery by 90 days. This hypothesis‐generating study is limited by a small sample size but the results merit further investigation.
Previous studies have reported either lower25 or higher plasma IGF‐1 concentrations26 3 days after stroke compared to control groups, and changes in plasma IGF‐1 concentration has been associated with mortality but not functional recovery.27 This study has shown no changes in IGF‐1 concentration during the first 90 days following stroke. This supports the suggestion that changes in IGF‐1 plasma concentration are not a reliable measure of IGF‐1 function during stroke recovery.
Sensitivity and specificity are two critical elements for identifying potential clinical biomarkers. The cGP‐related changes are specific to IGF‐1 function, but not stroke. Their association with stroke was the IGF‐1 function‐mediated recovery. Thus, the biomarker may also have potential clinical applications for other medical conditions with IGF‐1 deficiency. The cGP‐related changes in plasma appeared to be sensitive, which has been demonstrated by other small clinical observations in obesity, hypertension, and Parkinson diseases.16, 17
Neuroprotective function of IGF‐1 has been well‐demonstrated in stroke models.28 cGP protects the brain from ischemic injury by improving phosphorylation of IGF‐1 receptors, with a well‐defined mechanism.15, 16, 18 Given the oral availability and dynamic central uptake of cGP in human,17 further development of this biomarker may provide a guidance to assist their future clinical trial in stroke.
In conclusion, these hypothesis‐generating observations suggest that the increase in cGP/IGF‐1 ratio, but not plasma IGF‐1 within 3 days after stroke may be further developed as a prognostic biomarker for predicting the ability of functional recovery in stroke patients. The hypothesis that the progressive decline of cGP concentration in hypertensive patients can be a biomarker for stroke risk merits further investigation.
Conflict of Interest
The authors declaimed that there is no conflict of interest.
Ethics Compliances
The study was approved by the regional Ethics Committees (A+: 6627; UAHPEC: 015655 and HDEC: 15STH73) and written informed consent was obtained from all enrolled patients and control participants. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee.
Author Contributions
D. F., analyzing data and wrote manuscript; R. K. and P. H. editing manuscript; A. B., input to study design and editing manuscript; JG, conception, study design, and wrote manuscript.
Supporting information
Table S1. Clinical information of controls.
Table S2. Clinical information of the patients at admission.
Acknowledgment
The authors would like to thank the platform of the Biomarkers and Recovery in Stroke (BARISTa), Brain Research New Zealand‐A Centre of Research Excellence for providing plasma samples and clinical information. We also want to thank Professor Cathy Stinear, the Department of Medicine, University of Auckland for her advice on data analysis.
Funding Information
The research is mainly funded by the Brain Research New Zealand ‐ A Centre of Research Excellence, New Zealand (BRNZ 371526 and BRNZ 3709482) with co‐funding from the Vitality New Zealand, Christchurch, New Zealand (UniServices 36423).
Funding Statement
This work was funded by Brain Research New Zealand ‐ A Centre of Research Excellence, New Zealand grants BRNZ 371526 and BRNZ 3709482; Vitality New Zealand, Christchurch, New Zealand grant UniServices 36423.
References
- 1. Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee KB, Lim SH, Kim KH, et al. Six‐month functional recovery of stroke patients: a multi‐time‐point study. Int J Rehabil Res 2015;38:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biomarkers Definitions Working Group . Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95. [DOI] [PubMed] [Google Scholar]
- 4. Gluckman P, Klempt N, Guan J, et al. A role for IGF‐1 in the rescue of CNS neurons following hypoxic‐ischemic injury. Biochem Biophys Res Commun 1992;182:593–599. [DOI] [PubMed] [Google Scholar]
- 5. Guan J, Miller OT, Waugh KM, et al. Insulin‐like growth factor‐1 improves somatosensory function and reduces the extent of cortical infarction and ongoing neuronal loss after hypoxia‐ischemia in rats. Neuroscience 2001;105:299–306. [DOI] [PubMed] [Google Scholar]
- 6. Guan J, Williams C, Gunning M, et al. The effects of IGF‐1 treatment after hypoxic‐ischemic brain injury in adult rats. J Cereb Blood Flow Metab 1993;13:609–616. [DOI] [PubMed] [Google Scholar]
- 7. Guan J, Gunn AJ, Sirimanne ES, et al. The window of opportunity for neuronal rescue with insulin‐like growth factor‐1 after hypoxia‐ischemia in rats is critically modulated by cerebral temperature during recovery. J Cereb Blood Flow Metab 2000;20:513–519. [DOI] [PubMed] [Google Scholar]
- 8. Gandolfi M, Smania N, Vella A, et al. Assessed and emerging biomarkers in stroke and training‐mediated stroke recovery: state of the art. Neural Plast 2017;2017:1389475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duan C, Xu Q. Roles of insulin‐like growth factor (IGF) binding proteins in regulating IGF actions. Gen Comp Endocrinol 2005;142:44–52. [DOI] [PubMed] [Google Scholar]
- 10. Kelley KM, Schmidt KE, Berg L, et al. Comparative endocrinology of the insulin‐like growth factor‐binding protein. J Endocrinol 2002;175:3–18. [DOI] [PubMed] [Google Scholar]
- 11. Sara VR, Hall K. Insulin‐like growth factors and their binding proteins. Physiol Rev 1990;70:591–614. [DOI] [PubMed] [Google Scholar]
- 12. Fernandez AM, Torres‐Alemán I. The many faces of insulin‐like peptide signalling in the brain. Nat Rev Neurosci 2012;13:225–239. [DOI] [PubMed] [Google Scholar]
- 13. Fowlkes JL, Serra DM, Bunn RC, et al. Regulation of insulin‐like growth factor (igf)‐i action by matrix metalloproteinase‐3 involves selective disruption of IGF‐I/IGF‐binding protein‐3 complexes. Endocrinology 2004;145:620–626. [DOI] [PubMed] [Google Scholar]
- 14. Nishijima T, Piriz J, Duflot S, et al. Neuronal activity drives localized blood‐brain‐barrier transport of serum insulin‐like growth factor‐I into the CNS. Neuron 2010;67:834–846. [DOI] [PubMed] [Google Scholar]
- 15. Guan J, Gluckman P, Yang P, et al. Cyclic glycine‐proline regulates IGF‐1 homeostasis by altering the binding of IGFBP‐3 to IGF‐1. Sci Rep 2014;4:4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guan J, Singh‐Mallah G, Liu K, et al. The role for cyclic glycine‐proline, a biological regulator of insulin‐like growth factor‐1 in pregnancy‐related obesity and weight changes. J Biol Regul Homeost Agents 2018;32:465–478. [PubMed] [Google Scholar]
- 17. Fan D, Alamri Y, Liu K, et al. Supplementation of blackcurrant anthocyanins increased cyclic glycine‐proline in the cerebrospinal fluid of parkinson patients: potential treatment to improve insulin‐like growth factor‐1 function. Nutrients 2018;10:714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh‐Mallah G, Singh K, McMahon CD, et al. Maternally administered cyclic glycine‐proline increases insulin‐like growth factor‐1 bioavailability and novelty recognition in developing offspring. Endocrinology 2016;157:3130–3139. [DOI] [PubMed] [Google Scholar]
- 19. Guan J, Harris P, Brimble M, et al. The role for IGF‐1‐derived small neuropeptides as a therapeutic target for neurological disorders. Expert Opin Ther Targets 2015;19:785–793. [DOI] [PubMed] [Google Scholar]
- 20. Yamamoto H, Murphy LJ. Generation of des‐(1‐3) insulin‐like growth factor‐I in serum by an acid protease. Endocrinology 1994;135:2432–2439. [DOI] [PubMed] [Google Scholar]
- 21. Yamamoto H, Murphy LJ. Enzymatic conversion of IGF‐I to des(1–3)IGF‐I in rat serum and tissues: a further potential site of growth hormone regulation of IGF‐I action. J Endocrinol 1995;146:141–148. [DOI] [PubMed] [Google Scholar]
- 22. Sara VR, Carlsson‐Skwirut C, Drakenberg K, et al. The biological role of truncated insulin‐like growth factor‐1 and the tripeptide GPE in the central nervous systema. Ann N Y Acad Sci 1993;692:183–191. [DOI] [PubMed] [Google Scholar]
- 23. Owolabi MO, Agunloye AM. Risk factors for stroke among patients with hypertension: a case‐control study. J Neurol Sci 2013;325:51–56. [DOI] [PubMed] [Google Scholar]
- 24. Rost NS, Bottle A, Lee J‐M, et al. Stroke severity is a crucial predictor of outcome: an international prospective validation study. J Am Heart Assoc 2016;5:e002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwab S, Spranger M, Krempien S, et al. Plasma insulin‐like growth factor I and IGF binding protein 3 levels in patients with acute cerebral ischemic injury. Stroke J Cereb Circ 1997;28:1744–1748. [DOI] [PubMed] [Google Scholar]
- 26. Åberg D, Jood K, Blomstrand C, et al. Serum IGF‐I levels correlate to improvement of functional outcome after ischemic stroke. J Clin Endocrinol Metab 2011;96:E1055–E1064. [DOI] [PubMed] [Google Scholar]
- 27. Dong X, Chang G, Ji X‐F, et al. The relationship between serum insulin‐like growth factor I levels and ischemic stroke risk. PLoS ONE 2014;9:e94845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28. Guan J, Bennet L, Gluckman PD, Gunn AJ. Insulin‐like growth factor‐1 and post‐ischemic brain injury. Prog Neurobiol 2003;70:443–462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical information of controls.
Table S2. Clinical information of the patients at admission.


