Abstract
Objective
The objective of this study was to evaluate the thrombus characteristics affecting the extent of recanalization to identify patients with severe cerebral venous sinus thrombus (CVT) more likely to benefit from endovascular therapy.
Methods
Severe CVT patients scheduled for endovascular treatment were prospectively recruited into the study. Each thrombosed venous segment was evaluated regarding complete or partial recanalization based on digital subtraction angiography (DSA) after treatment. Magnetic resonance black‐blood thrombus imaging (MRBTI) was performed 1 day before endovascular treatment. The signal‐to‐noise ratio (SNR) of the clot, age of the clot, and baseline volume of the clot were compared between the complete and partial recanalization groups. A logistic regression analysis was performed to identify the association between these clot characteristics and recanalization status.
Results
Twenty‐one CVT patients undergoing endovascular therapy were prospectively included. There were 110 thrombosed segments, 54 of these segments were completely recanalized after endovascular treatment. An acute clot sign (ACS) was found in 39 segments and a subacute clot sign (SCS) was found in 71 segments. There was no significant difference on baseline volume of the clot (1638.8 ± 1432.2 mm3 vs. 1957.5 ± 2056.1 mm3, P > 0.05) between the complete and partial recanalization groups. Logistic regression analysis showed that ACS on MRBTI was associated with complete recanalization (P < 0.001, odds ratio = 3.937, 95% confidence interval, 1.6–9.5).
Interpretation
ACS can be used to predict complete recanalization in patients undergoing endovascular treatment. MRBTI provides a robust method to define clot composition and is potentially useful in selecting the most appropriate CVT patients for endovascular treatment.
Introduction
Cerebral venous thrombosis (CVT) is a relatively uncommon cause of stroke with various presentations,1 which accounts for nearly 1% of all strokes. The reported incidence of CVT is 1.32 per 100,000 person‐years.2 Systemic anticoagulation has been regarded as the first‐line treatment for CVT.3 However, anticoagulation alone does not always dissolve large and extensive CVT in some patients.4 Endovascular intervention, including mechanical thrombectomy, thromboaspiration, or balloon venoplasty with or without intrasinus thrombolysis,5, 6 is an alternative option for patients who deteriorate despite the use of anticoagulation.7 Since the thrombus itself is the primary target of endovascular therapy, understanding its composition is essential for complete resolution of the clot. Imaging techniques can be used to evaluate clot composition and measure thrombus burden.8 CT and MR venography are indirect luminal imaging methods based on the venous flow perturbation caused by thrombi, but these techniques cannot stage and quantify the thrombus.9, 10 Magnetic resonance black‐blood thrombus imaging (MRBTI) has recently been proposed as a noninvasive imaging tool for direct thrombus imaging.11, 12 In the current study, we aimed to investigate thrombus characteristics affecting the extent of recanalization to identify severe CVT patients more likely to benefit from endovascular treatment.
Methods
Patients
Between May 2014 and March 2018, acute CVT patients with severe symptoms scheduled for endovascular treatment were prospectively recruited into the study.13, 14 Patients were included based on the following criteria: intracerebral hemorrhagic lesion due to CVT, mental status impairment, coma (Glasgow coma scale<9), thrombosis in the deep cerebral venous system or cortical venous thrombosis, intracranial hypertension, and papilledema after anticoagulant treatment. Severe CVT patients presenting with one or more of the above characteristics were included. Exclusion criteria included recurrent CVT, not agreeing to have endovascular treatment and a contraindication to MRI. MRBTI was performed 1 day before endovascular treatment. Informed consent was obtained from all participants and all protocols were approved by the Institutional Review Board.
Recanalization extent on DSA
All DSA images were independently interpreted by two interventional neurologists to determine the extent of recanalization. Each treated segment was graded as partial (residual luminal narrowing >50%) or complete recanalization (residual luminal narrowing <50%). Discrepancies were resolved by two readers in the consensus reading.
MRBTI imaging protocol
All MR studies were conducted on a 3.0T system (MAGNETOM Verio, Siemens Healthcare, Erlangen, Germany) using a 32‐channel head coil for signal reception. Typical imaging parameters for MRBTI included: repetition time (TR) = 600 msec, echo time (TE) = 14 msec, spatial resolution = 0.8 × 0.8 × 0.8 mm3, scan time = 5 min.
MRBTI evaluation
All MRBTI images were blindly reviewed by two experienced neuroradiologists. The following segments were included: anterior (horizontal) superior sagittal sinus, posterior (vertical) superior sagittal sinus, inferior sagittal sinus, right transverse sinus, right sigmoid sinus, left transverse sinus, left sigmoid sinus, straight sinus, falcial sinus, veins of Galen, veins of Labbé, internal cerebral veins, Rosenthal vein, and cortical veins. Source images, multiplanar reformation, and minimum intensity projection images were used for evaluation.
The signal‐to‐noise ratio (SNR) of each thrombosed segment was measured and defined as the ratio of the thrombus signal intensity and the standard deviation of the background noise. Acute thrombi consisting of deoxyhemoglobin presented as an isointense signal on MRBTI which was defined as “acute clot sign (ACS)” and subacute thrombus which turns into methemoglobin (T1‐weighted is shorted) presented as hyperintense signal, defined as subacute clot sign (SCS) (Fig. 1). Baseline thrombus volume before endovascular treatment on MRBTI images was measured by commercial software (Vessel Analysis, China). Any discrepancies between the reviewers were resolved by consensus.
Figure 1.
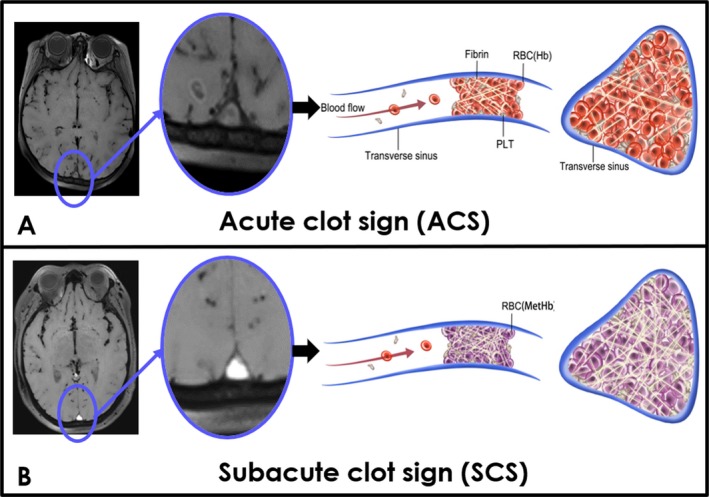
Schematic view of different stages of cerebral venous thrombus on MRBTI. MRBTI showed an acute clot sign (ACS) in the superior sagittal sinus (arrowhead). Acute thrombus with deoxyhemoglobin and less fibrin (A) and subacute thrombus with methemoglobin and more fibrin in superior sagittal sinus were demonstrated as a subacute clot sign (SCS) (B).
Outcome of endovascular therapy
The outcome of endovascular treatment was measured by the modified Rankin Score (mRS) 12 months after endovascular therapy. A good outcome was defined as mRS ≤ 2.
Statistical analysis
All statistical analyses were performed with SPSS 16.0 (IBM, USA). All continuous variables are presented as means ± SD. To compare the complete and partial recanalization groups, categorical variables were analyzed using a chi‐squared test and continuous variables were compared using an independent‐samples T test. A value of P < 0.05 was considered to indicate statistical significance. A stepwise logistic regression analysis was used to define independent factors to predict complete recanalization. The interobserver agreement in ACS and SCS quantification, and baseline thrombus volume measurement were evaluated by Cohen's kappa coefficient.
Results
Patient characteristics
From May 2014 to March 2018, 27 CVT patients scheduled for endovascular treatment were recruited. Three patients with recurrent CVT, two patients who rejected endovascular treatment, and one patient with claustrophobia were excluded. Twenty‐one CVT patients were finally included into the study (12 female, age 32.5 ± 9.8). The baseline characteristics of patients were summarized in Table 1.
Table 1.
Baseline characteristics of patients
| n/N (%) | |
|---|---|
| Demographics | |
| Mean, age, y (SD) | 32.5±9.8 |
| Gender, female (%) | 12 (57.1) |
| Clinical characteristics (%) | |
| Headache | 20 (95.2) |
| Vomit | 11 (52.4) |
| Seizure | 7 (33.3) |
| Tinnitus | 1 (4.8) |
| Weakness | 2 (9.5) |
| Visual‐field defects | 4 (19.0) |
| Disturbance of consciousness | 8 (38.1) |
| Location of thrombus | |
| Superior sagittal sinus | 16 (76.2) |
| Transverse sinus | 19 (90.5) |
| Sigmoid sinus | 17 (81.0) |
| Cortical and deep cerebral venous system | 14 (66.1) |
Thrombosed segments and extent of recanalization
In total, 110 thrombosed segments were identified from 21 CVT patients. Five acute patients achieved complete recanalization (5/7, 71.4%) and nine subacute patient achieved partial recanalization (9/14, 64.3%). At a segment level, complete recanalization occurred in 54 segments and partial recanalization in 56 segments. There was a significant difference in the complete recanalization rates between acute clots and subacute clots (P < 0.001). Nineteen thrombosed segments were in the superior sagittal sinus, 22 in the transverse sinus, 19 in the sigmoid sinus, and 32 in the cortical or deep venous system. The rate of complete recanalization was 42.1% (8/19) in the superior sagittal sinus, 40.9% (9/22) in the transverse sinus, 31.6% (6/19) in the sigmoid sinus, and 40.6% (13/32) in the cortical and deep venous system.
Interobserver agreement on MRBTI characteristics evaluation
The interobserver reproducibility for the presence of ACS, SCS, and baseline thrombus volume measurement had К values of 0.93, 0.93, and 0.91, respectively.
Comparison of clot characteristics
There was a significant difference in the SNR of CVT clot between the complete and partial recanalization groups (120.5 ± 47.2 vs. 169.3 ± 80.5, P < 0.001). ACS was detected in 39 segments and SCS were found in 71 segments. There were no significant differences in baseline thrombus volume between the partial and complete recanalization groups (1638.8 ± 1432.2 vs. 1957.5 ± 2056.1 mm3, P > 0.05). Figures 2 and 3 showed representative CVT patients with complete recanalization and partial recanalization, respectively.
Figure 2.
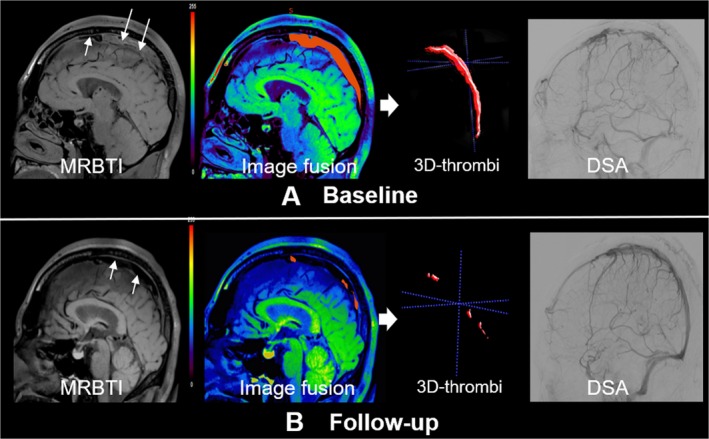
Complete recanalization of a 35 years old, male CVT patient underwent endovascular treatment. MRBTI images showed an acute clot sign (ACS) in the superior sagittal sinus (white arrows). Follow‐up MRBTI demonstrated complete recanalization (white arrows). Baseline and follow‐up thrombus volume was measured semi‐automatically by commercial software (Vessel Analysis). DSA confirmed recanalization of superior sagittal sinus.
Figure 3.
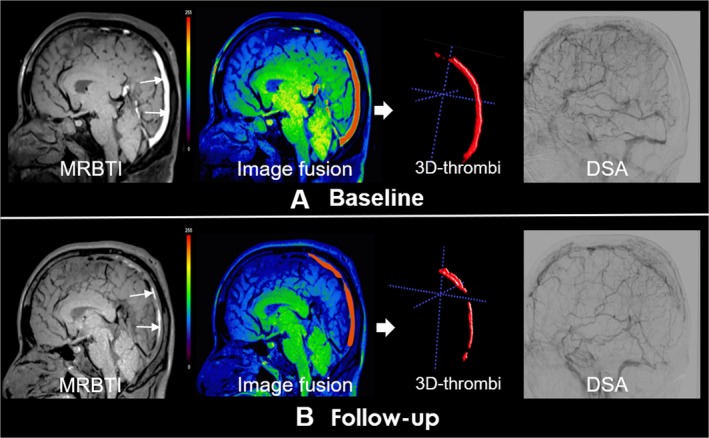
Partial recanalization of a 40 years old, male CVT patient underwent endovascular treatment. Subacute clot sign (SCS) in the superior sagittal sinus and straight sinus indicated subacute thrombus (white arrows). Follow‐up MRBTI image depicted partial recanalization (red arrows). DSA showed partial recanalization of superior sagittal sinus.
Multivariate analysis
Logistic regression analysis showed that ACS was associated with complete recanalization (P < 0.001), with an odds ratio of 3.937 (95% confidence interval, 1.6–9.5). SCS was associated with partial recanalization (P = 0.001), with an odds ratio of 4.237 (95% confidence interval, 1.7–10.3). The baseline volume of thrombus was not associated with complete recanalization (P = 0.363) (Table 2).
Table 2.
MRBTI characteristics of two groups
| MRBTI characteristics | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Complete recanalization | Partial recanalization | P value | OR | 95% CI | P value | |
| No. of thrombosed segments | 54 (49.1) | 56 (50.9) | ||||
| SNR | 120.5 ± 47.2 | 169.3 ± 80.5 | <0.001 | |||
| Clot age | ||||||
| ACS | 33 (64.1) | 6 (10.7) | <0.001 | 3.937 | 1.6–9.5 | 0.002 |
| SCS | 21 (38.9) | 50 (89.3) | <0.001 | 4.237 | 1.7–10.3 | 0.001 |
| Baseline thrombus volume | 1638.8 ± 1432.2 | 1957.5 ± 2056.1 | 0.055 | – | 0.363 | |
MRBTI, Magnetic resonance black‐blood imaging; CI, confidence interval; OR, odds ratio; ACS, acute clot sign; SCS, subacute clot sign.
Outcome of endovascular therapy
All twenty‐one patients underwent endovascular treatment had good prognosis at 1 year follow up (mRS, 2.15 ± 1.3 vs. 0.42 ± 0.84, P < 0.001).
Discussion
This is the first study investigating the association between clot age and recanalization using MRBTI in patients with severe CVT undergoing endovascular therapy. Our study showed that ACS on MRBTI was independently associated with complete recanalization. Understanding the age of the thrombus can help to determine the benefits and risks of treatment, as well as the likelihood of treatment success.
MRBTI, as a reasonably new technique, is a targeted‐thrombus imaging method.15 It has an inherent black‐blood effect and superior signal‐to‐noise ratio (SNR) performance. The black‐blood image contrast in MRBTI allows thrombi to be visually isolated within the dark lumen of venous sinuses. Configured as a T1‐weighted acquisition, BTI is particularly useful for the detection of subacute CVT that is rich in short‐T1 methemoglobins and thus appears hyperintense with respect to surrounding brain parenchyma and sinus lumen.
Although anticoagulant is the primary treatment, it may fail in some severe CVT patients. Endovascular treatment can be considered as an alternative therapeutic option in patients with worsening symptoms of CVT to potentially reduce mortality.16, 17, 18, 19 A recent study reported that the complete recanalization rate after endovascular treatment was 61% with or without systemic anticoagulant.20 In our study, the complete recanalization rate is lower than in that study (10/21, 47.6%). However, both on a segment level and patient level, the complete recanalization rate in the group with the acute clot sign (ACS) was higher than in the subacute group with a subacute clot sign (SCS) (84.6% vs. 29.6%, 71.4% vs. 35.7%, respectively). Selection of an effective therapy depends on many factors including the accurate determination of thrombus age and maturity.15 Thus, there is a growing need to develop better, novel minimally invasive imaging techniques, and diagnostic tools to accurately determine clot age in CVT and improve the recanalization rate.21 Hemoglobin in the thrombus changes from deoxyhemoglobin to methemoglobin in parallel to the duration of symptoms which can be used as imaging target.22, 23 MRBTI with hyper T1 weighting can differentiate acute clot and subacute clot thrombus based on their intrinsic MR signal intensity characteristics.
Thrombus composition is a factor in determining susceptibility to mechanical clot disruption and thus the degree of successful recanalization.24, 25 The acute clot is easier to completely recanalize because it primarily consists of red blood cells. Then, they become mechanically less compliant due to fibrin cross linking and collagen deposition over time. Subacute or chronic thrombi, which are rich in collagen and cross‐linked fibrin, harden and are more difficult to disrupt.26
Previous studies have reported that a thrombosed sinus will be quickly recanalized and CVT patients can obtain a good prognosis after endovascular treatment. Furthermore, it is very important to look for a characteristic to predict extensive recanalization. In this study, we found that patients with ACS on MRBTI can predict complete recanalization.
There were several limitations in the current study. First, with relatively low prevalence of CVT, the present study only recruited a limited number of patients who failed anticoagulation therapy and underwent endovascular treatment. Future large‐scale multicenter studies are needed to further validate our findings. Second, we need to investigate the association between MRBTI findings with different type of endovascular treatment techniques with multivariable regression analysis. Our classification of clot types is imperfect because precise interpretation of thrombus composition may need multi contrast MR sequences which were not performed in the current study due to scan time constrain. For example, the acute stage of thrombus formation on T2‐weighted images appears strongly hypointense and a subacute thrombus appears hyperintense which may help to better define the clot stage.27 Finally, we did not perform pathology on the retrieved clots. Collagen rich chronic thrombi have proven more difficult to treat and need to be investigated more completely in the future.
In conclusion, the ACS on MRBTI can be used to predict complete recanalization in CVT patients having endovascular treatment. MRBTI may improve efficacy of current endovascular treatments for CVT and help to reduce adverse outcomes.
Conflict of Interest
The authors report no disclosures relevant to the manuscript.
Funding Information
This work was partially supported by Beijing Natural Science Foundation (No. 17L20253), National Science Foundation of China (No. 91749127 and 81830056), and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201706).
Funding Statement
This work was funded by National Science Foundation of China grants 91749127 and 81830056; Natural Science Foundation of Beijing grant 17L20253; Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support grant ZYLX201706.
Contributor Information
Qi Yang, Email: yangyangqiqi@gmail.com.
Xunming Ji, Email: xunmingji2006@yeah.net.
References
- 1. Silvis SM, de Sousa DA, Ferro JM, Coutinho JM. Cerebral venous thrombosis. Nat Rev Neurol 2017;13:555–565. [DOI] [PubMed] [Google Scholar]
- 2. Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: a cross‐sectional study. Stroke 2012;43:3375–3377. [DOI] [PubMed] [Google Scholar]
- 3. Ferro JM, Bousser M‐G, Canhão P. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis ‐ endorsed by the European Academy of Neurology. Eur J Neurol 2017;24:1203–1213. [DOI] [PubMed] [Google Scholar]
- 4. Capecchi M, Abbattista M, Martinelli I. Cerebral venous sinus thrombosis. J Thromb Haemost 2018;16:1918–1931. [DOI] [PubMed] [Google Scholar]
- 5. Li G, Zeng X, Hussain M, et al. Safety and validity of mechanical thrombectomy and thrombolysis on severe cerebral venous sinus thrombosis. Neurosurgery 2013;72:730–738; discussion 730. [DOI] [PubMed] [Google Scholar]
- 6. Siddiqui FM, Dandapat S, Banerjee C, et al. Mechanical thrombectomy in cerebral venous thrombosis: systematic review of 185 cases. Stroke 2015;46:1263–1268. [DOI] [PubMed] [Google Scholar]
- 7. Einhäupl K, Stam J, Bousser MG, et al. EFNS guideline on the treatment of cerebral venous and sinus thrombosis in adult patients. Eur J Neurol 2010;17:1229–1235. [DOI] [PubMed] [Google Scholar]
- 8. Bonneville F. Imaging of cerebral venous thrombosis. Diagn Interv Imaging 2014;95:1145–1150. [DOI] [PubMed] [Google Scholar]
- 9. Renard D, Le Bars E, Arquizan C, et al. Time‐of‐flight MR angiography in cerebral venous sinus thrombosis. Acta Neurol Belg 2017;117:837–840. [DOI] [PubMed] [Google Scholar]
- 10. Khandelwal N, Agarwal A, Kochhar R, et al. Comparison of CT venography with MR venography in cerebral sinovenous thrombosis. AJR Am J Roentgenol 2006;187:1637–1643. [DOI] [PubMed] [Google Scholar]
- 11. Yang Q, Duan J, Fan Z, et al. Early detection and quantification of cerebral venous thrombosis by magnetic resonance black‐blood thrombus imaging. Stroke 2016;47:404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie G, Chen H, He X, et al. Black‐blood thrombus imaging (BTI): a contrast‐free cardiovascular magnetic resonance approach for the diagnosis of non‐acute deep vein thrombosis. J Cardiovasc Magn Reson 2017;19:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee SK, Mokin M. Current endovascular strategies for cerebral venous thrombosis: report of the SNIS Standards and Guidelines Committee. J Neurointerv Surg 2018;10:803–810. [DOI] [PubMed] [Google Scholar]
- 14. Leach JL, Fortuna RB, Jones BV, Gaskill‐Shipley MF. Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radiographics 2006;26(Suppl 1):S19–S43. [DOI] [PubMed] [Google Scholar]
- 15. Guo XB, Song LJ, Guan S. Endovascular treatment of chronic, recurrent headache secondary to chronic cerebral venous sinus thrombosis. J Stroke Cerebrovasc Dis 2014;23:560–563. [DOI] [PubMed] [Google Scholar]
- 16. Konakondla S, Schirmer CM, Li F, et al. New developments in the pathophysiology, workup, and diagnosis of dural venous sinus thrombosis (DVST) and a systematic review of endovascular treatments. Aging Dis 2017;8:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rahman M, Velat GJ, Hoh BL, Mocco J. Direct thrombolysis for cerebral venous sinus thrombosis. Neurosurg Focus 2009;27:E7. [DOI] [PubMed] [Google Scholar]
- 18. Ilyas A, Chen CJ, Raper DM, et al. Endovascular mechanical thrombectomy for cerebral venous sinus thrombosis: a systematic review. J Neurointerv Surg 2017;9:1086–1092. [DOI] [PubMed] [Google Scholar]
- 19. Luo Y, Tian X, Wang X. Diagnosis and treatment of cerebral venous thrombosis: a review. Front Aging Neurosci 2018;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dmytriw AA, Song JS, Yu E, Poon CS. Cerebral venous thrombosis: state of the art diagnosis and management. Neuroradiology 2018;60:669–685. [DOI] [PubMed] [Google Scholar]
- 21. Schuchardt F, Hennemuth A, Schroeder L, et al. Acute cerebral venous thrombosis: three‐dimensional visualization and quantification of hemodynamic alterations using 4‐dimensional flow magnetic resonance imaging. Stroke 2017;48:671–677. [DOI] [PubMed] [Google Scholar]
- 22. Diaz JA, Obi AT, Myers DD Jr, et al. Critical review of mouse models of venous thrombosis. Arterioscler Thromb Vasc Biol 2012;32:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garg A. Vascular brain pathologies. Neuroimaging Clin 2011;21:897–926. [DOI] [PubMed] [Google Scholar]
- 24. Farrag A, Irfan M, Guliani GK, et al. Occurrence of post‐acute recanalization and collateral formation in patients with cerebral venous and sinus thrombosis. A serial venographic study. Neurocrit Care 2010;13:373–379. [DOI] [PubMed] [Google Scholar]
- 25. Stolz E, Trittmacher S, Rahimi A, et al. Influence of recanalization on outcome in dural sinus thrombosis: a prospective study. Stroke 2004;35:544–547. [DOI] [PubMed] [Google Scholar]
- 26. Chen H, He X, Xie G, et al. Cardiovascular magnetic resonance black‐blood thrombus imaging for the diagnosis of acute deep vein thrombosis at 1.5 Tesla. J Cardiovasc Magn Reson 2018;20:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Steven A, Raghavan P, Altmeyer W, Gandhi D. Venous thrombosis: causes and imaging appearance. Hematol/Oncol Clin 2016;30:867–885. [DOI] [PubMed] [Google Scholar]


