Abstract
Feeding aversion in children may progress to severe feeding difficulties. While oral-motor and sensory issues are usually the leading causes, organic etiologies should be considered. This study aimed to assess the prevalence of gastrointestinal conditions in children with severe feeding difficulties. We conducted a retrospective study of 93 children requiring an intensive feeding program. The medical records, radiologic and diagnostic tests, use of gastric tube feedings, preexisting medical conditions, and medications were reviewed. Fifty-two percent (52%) had esophagitis, 26.2% gastritis, and 40.7% lactase deficiency in upper endoscopy. In those who underwent an upper endoscopy, 26% of patients that were also tested for small intestinal bacterial overgrowth were found to be positive. Allergy testing was abnormal in 56.6% of those tested, while 27.5% and 75% had abnormal gastric emptying times and pH impedance results, respectively. Constipation was present in 76.3%. Thirteen of 32 were weaned off tube feedings. We conclude that gastrointestinal conditions are common in children with feeding disorders and should be investigated prior to feeding therapy.
Keywords: feeding difficulties, intensive feeding program
Introduction
Feeding difficulties (FD) are common in childhood, presenting in up to 50% of children with normal development, up to 80% of children with developmental disabilities, and they are self-limited in most instances.1,2 However, in 3% to 10% of cases the problem persists.3 Children afflicted by FD may present with failure to thrive, nutritional and developmental deficits, and their families are often afflicted by altered family dynamics and parental concern.4 However, a FD does not result in failure to thrive in all cases, but rather manifests as an oral texture aversion with normal calorie intake, or as a sensory integrative difficulty with food selectivity.
Recent reviews of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) classification concluded that an early childhood FD should be grouped under the term “avoidant/restrictive food intake disorder,”1,5 with 3 abnormal feeding behaviors: (1) children eating too little, (2) eating a restricted number of foods, or (3) displaying a fear of eating. Furthermore, a FD has been described as a relational disorder between the feeder and the child,6 often presenting as learned maladaptive feeding behaviors with mechanical eating difficulties and altered mealtime dynamics.
A clinical examination may distinguish an oral-motor disorder from a sensory-based FD,7 while more in-depth studies are necessary to identify those resulting from organic gastrointestinal (GI) etiologies. The clinical evaluation of children with FD requires a multidisciplinary approach, including occupational and speech therapists, dietitians, social workers, and pediatric specialists. Red flags for underlying pathology in FDs are dysphagia, aspiration,8 odynophagia, coughing and choking during meals, recurrent pneumonia, and interruption of feedings by crying or pain, vomiting, diarrhea, failure to thrive, developmental anomalies, congenital abnormalities, and autism spectrum disorder.9 Evaluation by a pediatric gastroenterologist for gastroesophageal reflux (GER), constipation, eosinophilic esophagitis (EoE), neurodevelopmental conditions, food allergies, and other disorders should be conducted. Many children with feeding problems and neurodevelopmental disabilities have been found to have higher risk of GER,10-12 oropharyngeal dysphagia, food allergies,13 and vitamin deficiencies.2,14-16 Other FD comorbidities include EoE,17,18 genetic and metabolic syndromes,19-21 craniofacial and GI tract anatomical malformations,22-25 short bowel syndrome,26 and cystic fibrosis,27 among other.
The primary objective of this study is to report the prevalence of digestive problems identified prior to or during enrollment in the 4-week intensive feeding program (IFP). The hypothesis was that thorough clinical examination would reveal a high prevalence of underlying gastrointestinal (GI) conditions in children with severe FD requiring therapy. The secondary objective was to report the outcomes of patients with gastrostomy tubes (GT) on supplemental feedings.
Patients and Methods
Ethical Approval and Informed Consent
Approval was obtained from our institution’s Research Advisory Committee and the Institutional Review Board of the Arnold Palmer Medical Center (Assurance Number FWA00000384). Informed consent was not required because the study was based on retrospective data analysis.
We identified 93 children who completed the 4-week outpatient IFP at our institution between the dates of June 1, 2012, and October 1, 2017. Criteria for enrollment included being between 18 months and 18 years of age or having a minimum cognitive development of an 18-month-old child, being diagnosed with feeding difficulties, and having received outpatient occupational and/or speech therapies, which focused on feeding for at least 6 months without significant improvement.
Exclusion criteria included developmental delay, cognitive age less than 18 months, nonadherence to medical and/or therapeutic recommendations, and aspiration diagnosed through oropharyngeal motility study or fiber-optic endoscopic evaluation of swallowing as appropriate.
We reviewed patient medical records and created a database with the date of birth, age, date of initial and final visit, anthropometric measurements at the initial evaluation and the completion of IFP, medical tests, and medications.
Our protocol in the IFP included an evaluation by a gastroenterologist and a multidisciplinary team, to identify behavioral and psychosocial issues, and potential underlying GI problems requiring management prior to enrollment. Based on each patient’s history and physical examination, pertinent tests were recommended that included esophago-gastro-duodenoscopy (EGD), flexible sigmoidoscopy, colonoscopy, small bowel disaccharidase levels, duodenal culture for small intestinal bacterial overgrowth (SIBO), pancreatic function test for exocrine pancreatic insufficiency, pH-impedance study for GER, gastric emptying scan, and immunologic panel for possible food allergies, among other. We extracted these results and included them in our study if they had been performed within 12 months prior to the initial therapy session. We also identified those treated medically with proton pump inhibitors, histamine-type 2 blockers, stool softeners, appetite stimulants, prokinetics, and inhaled or oral steroids. In addition, we assessed the cases of constipation at any point during the IFP.
Calorie, protein, and fluid intakes were calculated for each child at the beginning and end of the 4-week program. Meals were weighed before and after each therapy session that allowed monitoring their progress. For the outcome of this study, we focused in children with GT, who were on the more severe end of the feeding difficulties spectrum. We selected to report the in detail the changes in the GT feeding group and reported the difference in percentage of calorie, protein, and fluid requirements provided via GT at the beginning and at the end of the program.
With regard to the therapy protocols at the IFP, different treatment modalities were implemented to meet each patient and families individualized needs. Each therapy session included a combination of sensory, behavioral, oral motor, feeding therapies, and family education. The therapy setting provided the necessary repetitions to carry over the learned strategies to the daily life scenario with a higher success rate. Caregivers were able to not only observe the improvements in oral motor/feeding skills and behavior in their child, but also acknowledged the importance of carrying over the recommended feeding techniques when home for the evening meal and over the weekends. Caregivers were aware of their child’s oral motor skills level and learned how to redirect negative attention-seeking behaviors to make meal time an enjoyable experience for all members of the family.
Results
Demographics
This cohort was composed of 93 patients who completed the IFP. The male to female ratio was 4:1, with 73 males (80.2%) and 18 females (19.8%). The youngest child was 1.24 years old, and the oldest was 19.35 years old. The mean age was 4.86 ± 2.69 years. With regard to the referral basis, 69 patients (74.1%) were referred by GI physicians, while 23 of them (24.7%) were referred by their primary physician and 1 (1%) was self-referred. Many children had significant known preexisting medical conditions, while others were diagnosed with GI disorders during the pre-enrollment period (Table 1).
Table 1.
Medical Conditions in Patients Enrolled in the Program.
| Medical Conditions in Patients Enrolled in the Feeding Program | N | % (n/93) |
|---|---|---|
| ADHD | 5 | 5.4% |
| ASD or sensory hypersensitivity | 14 | 15% |
| Other behavioral or developmental problems | 7 | 7.5% |
| Neurologic, genetic, metabolic, or congenital problems with associated neurologic issues | 22 | 23.6% |
| Malformations of the upper GI tract | 2 | 2.2% |
| Endocrine disease | 4 | 4.3% |
| Pulmonary diseases | 18 | 19.4% |
| Prematurity | 12 | 13% |
| Cardiac problems | 9 | 9.7% |
| Eczema | 8 | 8.6% |
| Acute liver failure | 1 | 1% |
| Nephrologic/urologic conditions | 3 | 3.2% |
| Patients with abnormal GI evaluations prior to enrollment | 4 | 4.3% |
| Abnormal food allergy panel | 34 | 36.5% |
| Cystic fibrosis | 1 | 1% |
| Prenatal drug exposure | 1 | 1% |
| Tufting enteropathy | 1 | 1% |
| Gastroesophageal reflux or reflux esophagitis | 59 | 63.4% |
| Constipation | 71 | 76.3% |
| Esophageal eosinophilia | 14 | 15.0% |
| Positive duodenal culture | 16 | 17.2% |
| Gastroparesis | 8 | 8.6% |
| Anatomical abnormalities | 6 | 6.5% |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; GI, gastrointestinal.
Medical Tests
EGDs were performed in 67 children of the entire cohort within 1 year prior to their enrollment. Of these, esophageal biopsies were taken in 59 patients, of which 31 (52.5%) were abnormal, mostly suggestive of mild reactive epithelial changes. Esophageal eosinophilia was found in 14 (23.7%). A pH-impedance probe study was abnormal in 19 out of the 25 children (76%) who received this study. Gastric biopsies were performed in 61 patients, of which 16 had chronic inactive gastritis (26.2%). Small bowel biopsies were performed in 61 patients and 4 (6.5%) were abnormal. Disaccharidase enzyme activities were measured in 54 children, and 22 had lactase deficiency (40.7%). Other disaccharidase levels tested were maltase, sucrase, palatinase, and glucoamylase, which were low in 7%, 7.1%, 3.7%, and 12.7%, respectively. Rectal biopsies were done in 10 patients and 2 had proctosigmoiditis. Duodenal samples with brushing were obtained in 61 patients and 16 (26.2%) showed SIBO. Pancreatic function testing in 14 patients revealed that 4 of them had selective or generalized pancreatic enzyme deficiencies. ImmunoCap food allergy panel was obtained in 60 children, out of which 34 (56.6%) tested positive for one or more foods with the common ones were milk, wheat, soy, eggs, fish, peanuts, beef, strawberries, and apples. With regard to gastric emptying, 40 children underwent gastric emptying scan. In 11 children (27.5%) the test was abnormal with delayed gastric emptying in 8 children, whereas 3 had rapid emptying consistent with dumping syndrome. Seventy-one (76.3%) were diagnosed with constipation and required either colonic clean-out and/or stool softener at some point during their enrollment. Many children received appetite stimulants, of which the most commonly used was cyproheptadine (32.2%; n = 30). Other medications were proton pump inhibitors (54.8%), histamine-type 2 blockers (28%), prokinetics (36.5%), and oral steroids (1%).
Changes in Supplemental Feedings
In this cohort, 32 children (34.4%) had a GT for nutritional support and 21(65.6%) received more than 50% of their daily calorie needs via the GT at their initial session. We calculated the daily intakes of calories, protein, and fluid for each patient. The percentage of calories given through GT was 80.6% at enrollment with decrease to 27.5% at the end of 4-week IFP. Similar results were observed for GT-provided protein and fluid percentages. Protein requirement from GT feeds dropped from 92.6% to 44.9%, and fluid needs from 75.8% to 26.1% by the end of the program (Table 2). Every child with a GT had improvement in their oral intake. By the end of the 4-week program, 13 children (39.4%) were complete weaned off the supplemental GT feedings. The mean body mass index (BMI) z score at enrollment was −0.65 (SD = ±1.05) and −0.87 (SD = ±1.14) at the final session. There was no statistically significant difference in BMI z score between the initial and final session for children with GT (Table 2).
Table 2.
GT Need Percentages and BMI Changes From Start to End of Programa.
| Start | End | Difference | |
|---|---|---|---|
| Calories (%) | 80.6 ± 4.6 | 27.5 ± 5.5 | 53.1 ± 6.2 |
| Protein (%) | 92.6 ± 3.8 | 44.9 ± 8.1 | 47.7 ± 8.6 |
| Fluid (%) | 75.8 ± 5.1 | 26.1 ± 5.5 | 51.6 ± 5.7 |
| BMI changes | −0.65 ± 1.05 | −0.87 ± 1.14 | −0.34 ± 0.85 |
Abbreviations: GT, gastrostomy tubes; BMI, body mass index.
Data are presented as mean ± SD.
Discussion
Feeding disorders often include more than one fundamental factor and involve a range of diagnostic inclusions.28 Rommel and colleagues29 analyzed 700 cases with feeding difficulties and categorized them as medical, oral, and behavioral FD. They reported that close to 50% of children referred for assessment had a combined medical and oral condition underlying their feeding problems. About half of children were evaluated for GI conditions, such as gastroesophageal reflux disease (GERD). We agree with their conclusion that a multidisciplinary team approach is essential in the assessment of feeding difficulties. In our cohort, 26 children had evident underlying behavioral problem, 89 had one or more GI findings, and the most frequent was constipation in 71 children. All children enrolled into the IFP had oral problems.
Our study cohort was composed of 80.2% males and 19.8% females. The male predominance was different from other reports, where males and female are affected equally. As other pediatric studies have reported that approximately half of the patients referred for feeding therapy due to severe feeding difficulties have combined medical and feeding disorders, we wanted to assess the prevalence of GI disorders in our cohort.
In this study, every child was evaluated by a gastroenterologist before and during the IFP. Based on the initial assessment, 72% of the children underwent an EGD and/or additional testing. It is important to rule out esophageal pathologies behind feeding difficulties, which may jeopardize therapy success if not recognized and treated appropriately. Of children that had an EGD, 52.5% had histologic evidence of esophagitis, mostly with mild reactive epithelial changes. Fourteen children (15%) had EoE as defined by >15 eosinophils per high-power field. Feeding aversion, dysphagia, esophageal food impactions, vomiting, chest pain, and heartburn not responding to acid suppression are common presenting symptoms of allergic esophagitis.30
A total of 25 children underwent a pH-impedance study and of these 19 (20.4% of the cohort) had significant reflux indexes. These finding are important because young children and those with developmental delay may not have other clinical manifestations other than oral aversion, which, if recognized and managed, may lead to improvement in oral intake. In those with positive ImmunoCap testing, a strict elimination diet was maintained before and during the intense program because we felt that any food challenge trial to verify the test results would have had a negative effect on the intense therapy. Further studies are necessary to understand the role of food allergies in feeding difficulties.
The direct effect of the other GI findings on the FD is less clear than the role of esophageal pathologies. Stomach biopsies were abnormal in 17.2% of tested children, mostly with mild to moderate chronic inactive gastritis and 1 patient with reactive gastropathy. None of the subjects in this cohort had evidence of Helicobacter pylori gastritis. Among the other findings, the prevalence of SIBO was high (26.2%) with over 100 000 colony forming units, usually oral flora. SIBO can lead to abdominal pain, eructation, bloating, and flatulence,28,31 which may result in decreased appetite. It is important to note that the majority of the patients (82.8%) received acid-suppressive medications during their enrollment, which may increase the risk of SIBO. Although we cannot affirm if SIBO played a direct role in these children’s feeding disorder, we believe it is imperative that managing clinicians consider SIBO in their differential diagnoses, as it is easily treatable and doing so may enhance patient response to intensive feeding therapy. Interestingly, SIBO has been found in up to 31% of children with autism spectrum disorder as compared to only 9% of children without this disorder.32 In our cohort, 3 out of 15 children with autism had SIBO.
Lactase deficiency was found in 40% of tested children (Figure 1). Lactase and other disaccharidase deficiencies may result in a variety of symptoms such as bloating, diarrhea, and abdominal pain, or may be asymptomatic. Interestingly, SIBO was found in 50% (n = 11) of children with lactase deficiency. Impaired gastric motility may manifest with symptoms such as postprandial fullness, early satiety, anorexia, GER, and chest pain.30,33 Nine patients had delayed gastric emptying based on >10% of residual food in the stomach after 4 hours. A prokinetic agent was used in 34 (36.5%) children for management of gastroparesis and/or reflux.
Figure 1.
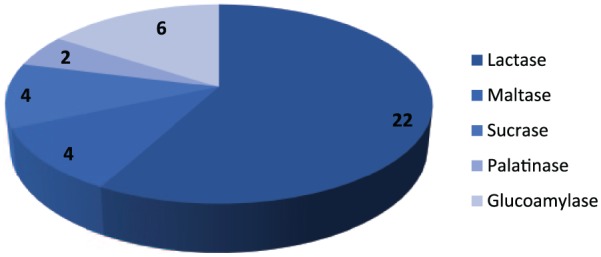
Disaccharidase enzyme deficiencies.
The most striking finding was the high prevalence of constipation. Whenever a child unexpectedly did not do well during a therapy session the most frequent cause was fecal impaction. Seventy-one (76.3%) children suffered from constipation at some point during the IFP and required the use of a stool softener. Fecal impaction has been associated with delayed gastric emptying34,35 through a rectal inhibitory reflex.36 Constipation has also been associated with SIBO and reflux.37,38 Other symptoms include early satiety, loss of appetite, abdominal pain, and vomiting. Treatment of constipation has been associated with improved reflux symptom in children.37 Many of our patients already had a history of constipation or they developed it during the IFP. We observed that initiation of a stool softener led to improvement in their feeding progress. Therefore, we strongly recommend that clinicians managing children with FD assess and treat bowel habits and constipation. Overall, more than one abnormal GI finding was present in 71 out of 93 patients (76%). This strongly supports the role of a subspecialist’s in the evaluation and management of children with FD enrolled an IFP.
The most important outcome of a feeding program is the improvement in oral intake of children. We enrolled 32 children (34.4%) who were receiving GT feedings at the initial session. Of these, 15 received greater than 50% of their required daily calories via GT at the beginning of the program. Every child had improvement in their oral calorie intake by at least 20%. Thirteen of them were off GT by the end of the IFP as they were able to consume 100% of their calorie needs orally, which once again supports the efficacy of not only inpatient feeding programs but also outpatient ones. We stress the importance of addressing underlying GI conditions such as constipation, GER, EoE, and food allergies, and collaborating with a multidisciplinary team in order to obtain the best results in children with FD.
This study has several limitations due to its retrospective nature. Not all children had a full GI evaluation because it was based on the judgment of the physicians. A prospective study with 2 groups of children with the same severity of FD with and without gastroenterologist involvement would be able to assess the effect of the subspecialist’s involvement in an intense feeding program. The study population was small to assess the role of individual digestive problems in the feeding difficulties per se. Prospective studies designed to evaluate the impact of individual conditions in the development and progression of FD are needed to establish cause and effect.
In conclusion, the data presented here supports that underlying GI etiologies are common in children with feeding difficulties and should always be considered prior to enrollment into an intense feeding program.
Acknowledgments
We want to acknowledge the Feeding Difficulties Center staff at Arnold Palmer Hospital for Children in Orlando, Florida, for their incredible work with children with feeding difficulties, and whose help was essential to complete this study.
Footnotes
Author Contributions: DRN: Contributed to conception and design; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
AC: Contributed to design; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
KN: Contributed to conception; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
KS: Contributed to acquisition and interpretation; drafted manuscript; critically revised manuscript; gave final approval.
KH: Contributed to conception and design; contributed to acquisition and interpretation; drafted manuscript; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
DM: Contributed to conception; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Desirée Rivera-Nieves  https://orcid.org/0000-0001-6378-1981
https://orcid.org/0000-0001-6378-1981
References
- 1. Bryant-Waugh R, Markham L, Kreipe RE, Walsh BT. Feeding and eating disorders in childhood. Int J Eat Disord. 2010;43:98-111. [DOI] [PubMed] [Google Scholar]
- 2. Phalen JA. Managing feeding problems and feeding disorders. Pediatr Rev. 2013;34:549-557. [DOI] [PubMed] [Google Scholar]
- 3. Bernard-Bonnin AC. Feeding problems of infants and toddlers. Can Fam Physician. 2006;52:1247-1251. [PMC free article] [PubMed] [Google Scholar]
- 4. Estrem HH, Pados BF, Thoyre S, Knafl K, McComish C, Park J. Concept of pediatric feeding problems from the parent perspective. MCN Am J Matern Child Nurs. 2016;41:212-220. [DOI] [PubMed] [Google Scholar]
- 5. Kreipe RE, Palomaki A. Beyond picky eating: avoidant/restrictive food intake disorder. Curr Psychiatry Rep. 2012;14:421-431. [DOI] [PubMed] [Google Scholar]
- 6. Davies WH, Satter E, Berlin KS, et al. Reconceptualizing feeding and feeding disorders in interpersonal context: the case for a relational disorder. J Fam Psychol. 2006;20:409-417. [DOI] [PubMed] [Google Scholar]
- 7. Palmer MM, Heyman MB. Assessment and treatment of sensory versus motor-based feeding problems in very young children. Inf Yng Child. 1993;6:67-73. [Google Scholar]
- 8. Kerzner B, Milano K, MacLean WC, Jr, Berall G, Stuart S, Chatoor I. A practical approach to classifying and managing feeding difficulties. Pediatrics. 2015;135:344-353. [DOI] [PubMed] [Google Scholar]
- 9. Kerzner B. Clinical investigation of feeding difficulties in young children: a practical approach. Clin Pediatr (Phila). 2009;48:960-965. [DOI] [PubMed] [Google Scholar]
- 10. Glassman LW, Grocott OR, Kunz PA, et al. Prevalence of gastrointestinal symptoms in Angelman syndrome. Am J Med Genet A. 2017;173:2703-2709. [DOI] [PubMed] [Google Scholar]
- 11. Heine RG, Jaquiery A, Lubitz L, Cameron DJ, Catto-Smith AG. Role of gastro-oesophageal reflux in infant irritability. Arch Dis Child. 1995;73:121-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heine RG, Jordan B, Lubitz L, Meehan M, Catto-Smith AG. Clinical predictors of pathological gastro-oesophageal reflux in infants with persistent distress. J Paediatr Child Health. 2006;42:134-139. [DOI] [PubMed] [Google Scholar]
- 13. Mehta H, Groetch M, Wang J. Growth and nutritional concerns in children with food allergy. Curr Opin Allergy Clin Immunol. 2013;13:275-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ide E, Van Biervliet S, Thijs J, Velde SV, De Bruyne R, Van Winckel M. Solid food refusal as the presenting sign of vitamin B12 deficiency in a breastfed infant. Eur J Pediatr. 2011;170:1453-1455. [DOI] [PubMed] [Google Scholar]
- 15. Schwarz SM, Corredor J, Fisher-Medina J, Cohen J, Rabinowitz S. Diagnosis and treatment of feeding disorders in children with developmental disabilities. Pediatrics. 2001;108:671-676. [DOI] [PubMed] [Google Scholar]
- 16. Yeung KA, Taylor T, Scheimann A, et al. The prevalence of food allergies in children referred to a multidisciplinary feeding program. Clin Pediatr (Phila). 2015;54:1081-1086. [DOI] [PubMed] [Google Scholar]
- 17. Iwanczak B, Janczyk W, Ryzko J, et al. Eosinophilic esophagitis in children: frequency, clinical manifestations, endoscopic findings, and seasonal distribution. Adv Med Sci. 2011;56:151-157. [DOI] [PubMed] [Google Scholar]
- 18. Sorser SA, Barawi M, Hagglund K, Almojaned M, Lyons H. Eosinophilic esophagitis in children and adolescents: epidemiology, clinical presentation and seasonal variation. J Gastroenterol. 2013;48:81-85. [DOI] [PubMed] [Google Scholar]
- 19. Al-Shanafey S, Alkhudhur H. Food aversion among patients with persistent hyperinsulinemic hypoglycemia of infancy. J Pediatr Surg. 2012;47:895-897. [DOI] [PubMed] [Google Scholar]
- 20. Cooper-Brown L, Copeland S, Dailey S, et al. Feeding and swallowing dysfunction in genetic syndromes. Dev Disabil Res Rev. 2008;14:147-157. [DOI] [PubMed] [Google Scholar]
- 21. Dobbelsteyn C, Marche DM, Blake K, Rashid M. Early oral sensory experiences and feeding development in children with CHARGE syndrome: a report of five cases. Dysphagia. 2005;20:89-100. [DOI] [PubMed] [Google Scholar]
- 22. de Vries IA, Breugem CC, van der Heul AM, Eijkemans MJ, Kon M, van der Molen ABM. Prevalence of feeding disorders in children with cleft palate only: a retrospective study. Clin Oral Investig. 2014;18:1507-1515. [DOI] [PubMed] [Google Scholar]
- 23. Krishnan U, Mousa H, Dall’Oglio L, et al. ESPGHAN-NASPGHAN guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with esophageal atresia-tracheoesophageal fistula. J Pediatr Gastroenterol Nutr. 2016;63:550-570. [DOI] [PubMed] [Google Scholar]
- 24. Mahoney L, Rosen R. Feeding problems and their underlying mechanisms in the esophageal atresia-tracheoesophageal fistula patient. Front Pediatr. 2017;5:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paes EC, de Vries IAC, Penris WM, et al. Growth and prevalence of feeding difficulties in children with Robin sequence: a retrospective cohort study. Clin Oral Investig. 2017;21:2063-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hopkins J, Cermak SA, Merritt RJ. Oral feeding difficulties in children with short bowel syndrome: a narrative review. Nutr Clin Pract. 2017;33:99-106. [DOI] [PubMed] [Google Scholar]
- 27. Debray D, Mas E, Munck A, Gerardin M, Clouzeau H. Liver disease, gastrointestinal complications, nutritional management and feeding disorders in pediatric cystic fibrosis [in French]. Arch Pediatr. 2016;23:12S15-12S20. [DOI] [PubMed] [Google Scholar]
- 28. Sharp WG, Volkert VM, Scahill L, McCracken CE, McElhanon B. A systematic review and meta-analysis of intensive multidisciplinary intervention for pediatric feeding disorders: how standard is the standard of care? J Pediatr. 2017;181:116-124.e4. [DOI] [PubMed] [Google Scholar]
- 29. Rommel N, De Meyer AM, Feenstra L, Veereman-Wauters G. The complexity of feeding problems in 700 infants and young children presenting to a tertiary care institution. J Pediatr Gastroenterol Nutr. 2003;37:75-84. [DOI] [PubMed] [Google Scholar]
- 30. Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198-1206. [DOI] [PubMed] [Google Scholar]
- 31. Sieczkowska A, Landowski P, Zagozdzon P, Kaminska B, Lifschitz C. The association of proton pump inhibitor therapy and small bowel bacterial overgrowth in children. Eur J Gastroenterol Hepatol. 2017;29:1190-1191. [DOI] [PubMed] [Google Scholar]
- 32. Wang L, Yu YM, Zhang YQ, Zhang J, Lu N, Liu N. Hydrogen breath test to detect small intestinal bacterial overgrowth: a prevalence case-control study in autism. Eur Child Adolesc Psychiatry. 2018;27:233-240. [DOI] [PubMed] [Google Scholar]
- 33. Islam S, McLaughlin J, Pierson J, Jolley C, Kedar A, Abell T. Long-term outcomes of gastric electrical stimulation in children with gastroparesis. J Pediatr Surg. 2016;51:67-71. [DOI] [PubMed] [Google Scholar]
- 34. Coremans G, Geypens B, Vos R, et al. Influence of continuous isobaric rectal distension on gastric emptying and small bowel transit in young healthy women. Neurogastroenterol Motil. 2004;16:107-111. [DOI] [PubMed] [Google Scholar]
- 35. Fernandes VP, Lima MC, Camargo EE, Collares EF, Bustorff-Silva JM, Lomazi EA. Gastric emptying of water in children with severe functional fecal retention. Braz J Med Biol Res. 2013;46:293-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bjornsson ES, Chey WD, Hooper F, Woods ML, Owyang C, Hasler WL. Impaired gastrocolonic response and peristaltic reflex in slow-transit constipation: role of 5-HT(3) pathways. Am J Physiol Gastrointest Liver Physiol. 2002;283:G400-G407. [DOI] [PubMed] [Google Scholar]
- 37. Baran M, Appak YC, Karakoyun M, Yalcinkaya S, Eliacik K, Dundar BN. The overlap of gastroesophageal reflux disease and functional constipation in children: the efficacy of constipation treatment. Eur J Gastroenterol Hepatol. 2017;29:1264-1268. [DOI] [PubMed] [Google Scholar]
- 38. Lee KM, Paik CN, Chung WC, Yang JM, Choi MG. Breath methane positivity is more common and higher in patients with objectively proven delayed transit constipation. Eur J Gastroenterol Hepatol. 2013;25:726-732. [DOI] [PubMed] [Google Scholar]


