Short abstract
Primary cardiac tumors are typically benign, with myxomas being most common. We present a 32-year-old female with a chief complaint of dyspnea and a constant non-radiating chest pressure along the left sternal border. She was found to have a pulmonary embolism that was ultimately caused by embolization of a right atrial myxoma with remnants of a large, highly mobile mass attached to the right inter-atrial septum prolapsing through the tricuspid valve. The patient underwent a median sternotomy, right atrial mass resection, pulmonary embolectomy, and inter-atrial septum reconstruction using the patient’s pericardium. The importance of finding the etiology of initial diagnoses is stressed with long-term outcomes for patients.
Keywords: Myxoma, pulmonary embolism, transthoracic echocardiogram
Introduction
The incidence of primary cardiac tumors is very rare, with the frequency of primary cardiac tumors approximately 0.02% or 200 tumors in 1 million autopsies.1 Of the primary cardiac tumors, myxomas are by far the most common. About 75% of primary cardiac tumors are benign, and half of all benign cardiac tumors are atrial myxomas.2 Metastasis to the heart is significantly more common than primary cardiac tumors with the usual source being a carcinoma of the lung.2 Approximately 80% of myxomas are located in the left atrium, 7–10% are found in the right atrium, and up to 10% are found bi-atrial or in the left and right ventricles. Patients with right atrial myxomas can remain asymptomatic or more typically, present with nonspecific constitutional symptoms, pulmonary embolism (PE), pulmonary hypertension, and/or Budd-Chiari syndrome.3,4 We present a case that involves a 32-year-old female presenting to the emergency department with dyspnea and constant left chest pressure not relieved by rest after working out at the gym.
Case report
A 32-year-old female presented with dyspnea and constant, non-radiating chest pressure along the left sternal border associated with palpitations that started at the gym during her routine workout, with ongoing racing heartbeat after exercise. She had no medical history. She had been taking oral contraceptives for birth control since age 18 (ethinyl estradiol/desogestrel) with no recent changes, and had traveled one-week prior on a flight of 6 h with breaks.
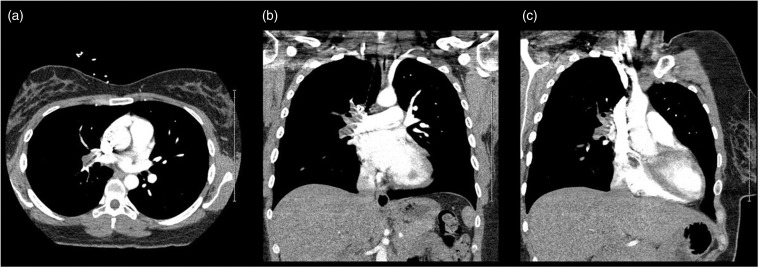
On presentation, she had tachypnea and decreased breath sounds in the right lower lobe. Laboratory results demonstrated a mildly elevated troponin I of 0.18 ng/mL (ref <0.03 ng/mL). CT angiogram chest showed a large embolus in the distal main right pulmonary artery straddling the right upper and lower lobe pulmonary artery divisions; almost completely occluding flow to the segmental arteries (Figure 1). The patient was started on a therapeutic unfractionated heparin. Lower extremity venous Doppler ultrasound was negative for deep vein thrombosis (DVT). Transthoracic echocardiogram (TTE) showed a large, highly mobile mass attached to the right inter-atrial septum (Figure 2) prolapsing through the tricuspid valve in diastole highly suggestive of a cardiac myxoma (Supplemental Video 1).
Figure 1.
Computed tomography (CT) scan of the chest with contrast. CT scan of the chest done using pulmonary embolism (PE) protocol that demonstrates extensive PE extending throughout the entire right pulmonary artery with sparing of the left pulmonary artery, seen in (a) axial, (b) coronal, and (c) sagittal views. Panel (c) also demonstrates the right atrial myxoma, which was identified as the source of the PE.
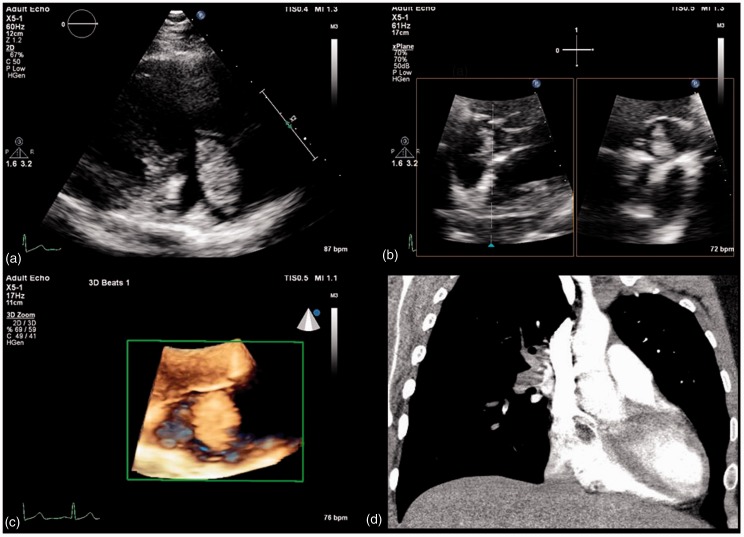
Figure 2.
Atrial myxoma on multi-modality imaging. (a) Transthoracic echocardiogram (TTE) (RA/RV view) showing mobile echo dense right atrial mass, tethered to the inter-atrial septum, consistent with right-sided atrial myxoma. (b) TTE xPlane of subcostal view depicting right atrial myxoma and origination from inter-atrial septum. (c) TTE with 3D reconstruction of right atrial myxoma seen through the tricuspid valve. (d) Right atrial myxoma with stalk tethered to inter-atrial septum on CT chest with contrast, also demonstrating complete opacification of right pulmonary artery with embolic myxoma.
The patient underwent median sternotomy, right atrial mass resection, repair of atrial septal defect with pericardium, and pulmonary embolectomy (Figure 3). Histologic evaluation of specimens confirmed benign myxoma. The patient was discharged home in stable condition after her surgical procedure and uneventful post-operative course.
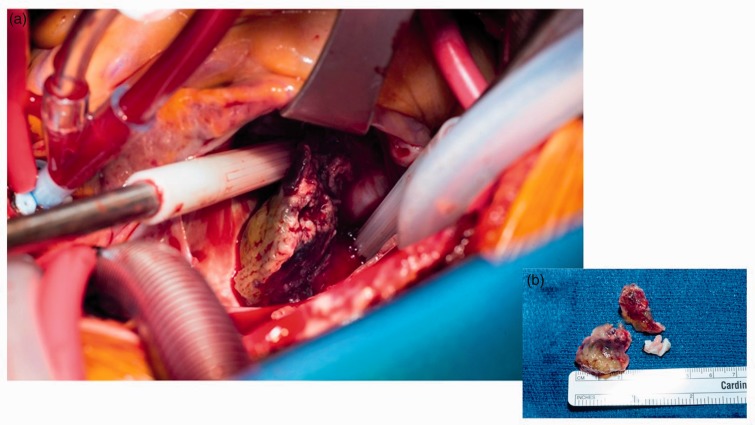
Figure 3.
(a) Atrial myxoma present in the right atrium. An intraoperative photograph of median sternotomy and right atrial mass resection with cardiac myxoma visible within the right atrium (centered in image). (b) Three papillary yellow and red soft tissue fragments from the right atrium, measuring 2.9 × 2.3 × 2.1 cm in aggregate.
Discussion
Our patient’s acute onset of dyspnea and left chest pressure was not preceded by constitutional symptoms—i.e., fever; weight loss; general arthralgia—that have been previously described with cardiac myxomas.3,4 It is hypothesized an immune response reaction to the neoplasm may result in an altered immunologic state, influencing interleukin 6, interleukin 2R, suppressor/helper cell ratio, tissue necrosis factor receptor, resulting in non-cardiac constitutional symptoms.5 Additionally, risk for embolism has been investigated without clear factors; however, theories have been postulated regarding platelet count influencing risk of embolism of myxomas.6 There was no characteristic tumor “plop” or extra heart sounds on physical exam, likely secondary to embolization of a large portion of the myxoma by the time of presentation. Indeed timing of her myxoma embolization was likely during her vigorous exercise regimen. However, accurate and complete diagnosis is of the utmost importance, especially in cases such as this, where incomplete diagnosis could have resulted in long-term unnecessary anticoagulation.
Clinical suspicion was raised for an intra-cardiac mass as the source of embolization due to lack of comorbidities, low pre-test probability for PE, and negative lower extremity duplex for DVT. While left atrial myxomas are more common than the right-sided counterpart and give suspicion for their presence via their distant manifestations despite other risk factors for distal embolic phenomenon, right atrial myxomas still need attention especially in the setting of PE without any or significant risk factors.5–9 Furthermore, evidence of myocardial injury suggested an alternative diagnosis was reasonable. However, there seemed to be a disconnect between the patient’s clinical evaluation and her radiographic thrombus burden. Typically, a TTE is utilized in the setting of PE and hemodynamic instability to assess right ventricular dilatation. In the case of our patient, with evidence of PE without a source, elevated troponin suggestive of massive PE in the setting of relative hemodynamic stability other than hypoxia, a TTE remains useful in assessment for right ventricular assessment, pulmonary artery pressure, and other abnormalities, including the rare diagnosis of myxoma. A TTE has sensitivity up to 95% for detecting atrial myxomas, and in our patient revealed a right atrial myxoma as the source of embolization.3 Furthermore, the remnant of the myxoma still attached to its stalk originating from the inter-atrial septum, was seen prolapsing through the tricuspid valve.
Identification of this was key in solidifying the decision for open-heart surgery for our patient. Embolization of intra-cardiac tumors is a common manifestation occurring more often with left sided than right-sided tumors secondary to the high flow state of the left heart.5 Surgical intervention with complete tumor removal including wide tissue margins is the gold-standard therapeutic approach for right atrial myxomas and has excellent clinical results.3,4 Additionally, in our patient, identification of the cause of her PE as myxoma rather than thrombotic was of the utmost importance. Her young age would have subjected her to years of unnecessary anticoagulation.
Conclusion
Consideration for right atrial myxoma, while rare, should be when the current diagnosis seems incomplete or out of place. Right atrial myxoma was identified in our patient after TTE was performed. However, she had few risk factors for PE that exploration of alternative diagnoses to explain the PE were essential. Although CT scan demonstrated PE, until the right atrial myxoma was identified, her clinical findings were divergent. Echocardiogram was able to assist in both evaluation of the right ventricle, due to the troponin leak, and help to identify the definite and complete diagnosis. A TTE was originally ordered to evaluate for a cardiac source of embolization, and to evaluate for right ventricular dilatation in the setting of mildly elevated troponin I and evidence of massive right PE, for improved risk/prognostic stratification.10 Using clinical judgment, a TTE in this case proved invaluable to discovering the etiology of PE and directing appropriate surgical intervention.
Key message
Several studies have illustrated the utility of echocardiogram in evaluation. The role TTE can play in diagnosis and prognostic stratification in a non-invasive manner, allows it to be an important tool in the armamentarium of physicians.
Acknowledgements
We acknowledge Dr. Brandon T. Larsen for providing interpretation of the pathology for this case. Written informed consent and verbal informed consent were obtained from the patient for her anonymized information to be published in this article.
Contributorship
ACP conceived the article. ACP, JJC and JLT were involved in direct patient care pre- and post-operatively. JLT performed the surgical procedure. ACP and JJC wrote the article. ACP, JJC and JLT revised the article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval
Our institution does not require ethical approval for reporting individual cases or case series.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Reynen K. Frequency of primary tumors of the heart. Am J Cardiol 1996; 77: 107. [DOI] [PubMed] [Google Scholar]
- 2.Butany J, Nair V, Naseemuddin A, et al. Cardiac tumours: diagnosis and management. Lancet Oncol 2005; 6: 219–228. [DOI] [PubMed] [Google Scholar]
- 3.Kuon E, Kreplin M, Weiss W, et al. The challenge presented by right atrial myxoma. Herz 2004; 29: 702–709. [DOI] [PubMed] [Google Scholar]
- 4.Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore) 2001; 80: 159–172. [DOI] [PubMed] [Google Scholar]
- 5.Keeling IM, Oberwalder P, Anelli-Monti M, et al. Cardiac myxomas: 24 years of experience in 49 patients. Eur J Cardio-Thoracic Surg 2002; 22: 971–977. [DOI] [PubMed] [Google Scholar]
- 6.He DK, Zhang YF, Liang Y, et al. Risk factors for embolism in cardiac myxoma: a retrospective analysis. Med Sci Monit 2015; 21: 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obrenovic-Kircanski B, Mikic A, Parapid B, et al. A 30-year-single-center experience in atrial myxomas: from presentation to treatment and prognosis. Thorac Cardiovasc Surg 2013; 61: 530–536. [DOI] [PubMed] [Google Scholar]
- 8.Perez Diaz P, Munoz Ruiz N, Jurado Roman A. Left ventricular myxoma: recurrence and risk of embolism. Ann Thorac Surg 2017; 103: e553. [DOI] [PubMed] [Google Scholar]
- 9.Zhang RD, Zeng ZH, Zheng JY, et al. Left atrial myxoma complicated with multi-system embolization. J Cardiothorac Surg 2017; 12: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saric M, Armour AC, Arnaout MS, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr 2016; 29: 1–42. [DOI] [PubMed] [Google Scholar]