Abstract
Objectives:
This study was aimed at determining human T-lymphotropic virus 1/2 prevalence among apparently healthy, immunocompromised and haematologic malignant individuals in Nigeria’s capital, as well as meta-analysis of all Nigerian studies until date.
Methods:
A total of 200 participants were recruited into a cross-sectional study. In total, 1 mL each of sera and plasma were obtained from 5 mL blood of each participant and analysed for antibodies to human T-lymphotropic virus 1/2 using enzyme-linked immunosorbent assay; positive samples confirmed with qualitative real-time polymerase chain reaction, followed by statistical and meta-analysis. Sociodemographic characteristics and possible risk factors were assessed via questionnaires.
Results:
Enzyme-linked immunosorbent assay yielded 1% prevalence which was confirmed to be zero via polymerase chain reaction. A total of 119 (59.5%) of the participants were male, while the mean age was 35.28 ± 13.61 years. Apart from sex and blood reception/donation, there was generally a low rate of exposure to human T-lymphotropic virus–associated risk factors. Meta-analysis revealed pooled prevalence of human T-lymphotropic virus 1 and 2 to be 3% and 0%, respectively, from Nigerian studies.
Conclusion:
This study discovered zero prevalence of human T-lymphotropic virus 1/2 from five major hospitals in Nigeria’s capital, exposing the importance of confirmatory assays after positive antibody detection assay results. Meta-analysis highlighted the existence of very few reliable Nigerian studies compared to the demography of the nation. Large-scale epidemiological studies and routine screening of risk populations are therefore needed since Nigeria lies in the region of endemicity.
Keywords: HTLV, leukaemia, lymphoma, blood donors, lymphoproliferative disorders, haematologic malignancies
Background
An increase in the number of lymphocytes is part of a normal immune response; however, there are some diseases that result in an abnormal increase in the number of lymphocytes. These disorders are known as lymphoproliferative disorders (LPDs) and result from a defect within the immune system that would normally prevent uncontrolled production of lymphocytes. Human T-lymphotropic viruses 1/2 (HTLV-1/2) are primate T-lymphotropic viruses and members of the deltaretroviruses, which include simian T-cell leukaemia virus (STLV) and bovine leukaemia virus.1 In about 7% of the infected individuals, HTLV-1 is the aetiological agent of an aggressive lymphoproliferative malignancy known as adult T-cell leukaemia/lymphoma (ATLL), as well as HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), a neurological disorder associated with increased lymphoproliferation. It is also associated with a host of other diseases.2 Its Type 2 counterpart (HTLV-2), although lacking direct linkage with clinical manifestations and conclusive evidence as aetiologic agent in any specific disease,2 has been associated with resulting neurologic manifestations similar to the non-HAM complications of HTLV-1 infection. Due to their low replicating nature, there is little variation in the viruses’ genetic sequence; hence, most epidemiologic data are based on serologic studies rather than on molecular typing. HTLV-1 and -2 are endemic in certain geographical regions including West Africa,2,3 where Nigeria lies. HTLV has been reported in different regions of Nigeria, including South West,4,5 South East,6 and North Central.7 Olaleye et al.4 reported prevalence rates ranging from 0.5% to 5.50% in different South-Western studies in the 90s.8 In 2011, Terry Alli et al.9 found 3.6% seroprevalence of HTLV among blood donors in South-Western Nigeria. Durojaiye et al.5 found 0.5% prevalence among blood donors in 2014, while Yuguda et al.10 reported 3.2% in 2017 without confirmatory assays. Okoye et al.1,6 showed prevalence rates of 0.5% and 0%, respectively, in South-East. Zero prevalence has also been reported previously in Nigeria11 and cases of dual infection with both HTLV-1 and -2 have also been reported in Nigeria.4 There are limited data on HTLV-2 in Nigeria as compared to its Type 1 counterpart.8 Out of the four neighbouring countries of Nigeria, Cameroon seems to be the only country with relatively recent reports of HTLV incidence.2,8 Niger and Benin8 Republics have reports from 80s and 90s, while no reports of the virus has been found in Chad. The modes of transmission of HTLV-1/2, like human immunodeficiency virus (HIV), include vertical (prolonged breastfeeding, complications during delivery and transplacental (intrauterine) route), parenteral (blood transfusion–transmission, intravenous drug use, sharing of razor blades and/or other sharp objects, organs donation and transplantation from infected persons, iatrogenic and/or occupational hazards or accidents) and sexual transmission patterns. Sociodemographic factors that have been implicated in high prevalence of HTLV-1/2 infections include socioeconomic status, geographical location, gender (female), age (older), marital status, promiscuity, recurrent sexually transmitted diseases (STDs),2,6 transfusion history, family history of HTLV infection and intravenous drug use.
In almost four decades since the discovery of HTLV-1/2, HTLV-1 infection and its associated diseases have been extensively studied in developed countries, and many of their aspects have been clarified. However, this is not the case in developing and under-developed countries like Nigeria where, since the first report of HTLV-1-associated cancer,12 only a handful of studies have been carried out in various parts of the population.8 Furthermore, screening of blood and/or blood components prior to donation has hitherto not been mandated, as is the practice in the Western world.8 The country’s policy on blood transfusion is still restricted to other transfusion-transmitted infections such as hepatitis B virus (HBV), hepatitis C virus (HCV), HIV and Syphilis.1 Despite being the capital of Nigeria and a cosmopolitan city constantly visited en masse by individuals from all over the country, only one study7 (HTLV-1/HIV co-infection) has been carried out in Abuja, which found a prevalence of 6.5% among HIV-positive individuals. No report of HTLV-2 has been recorded.
With the aim of determining HTLV-1/2 prevalence among apparently healthy, immunocompromised and haematologic malignant individuals in Federal Capital Territory (FCT), Nigeria, this study is expected to serve as a baseline for further research, a comparative standpoint to findings from previous prevalence studies and also as a reference material for future comparative studies of the seroprevalence of HTLV infection in different geopolitical zones of Nigeria. The meta-analysis is expected to highlight the status of the viruses in Nigeria.
Materials and methods
Study area
This study was carried out among patients attending five hospitals in FCT, Abuja, Nigeria. The hospitals selected for this study include the National Hospital, Abuja; Garki Hospital, Abuja; Wuse General Hospital, Asokoro General Hospital and University of Abuja Teaching Hospital (UATH), Gwagwalada, all in FCT, Nigeria.
Study population and design
A hospital-based cross-sectional study was carried out among patients between the ages of 6–71 recruited from relevant sections of the hospital. The target population was grouped into three: haematological malignant patients (HMPs; lymphoma, leukaemia and multiple myeloma patients); HIV-positive patients (HIV) and apparently healthy individuals (qualified blood donors (BD)). Sampling was done using probability sampling technique for apparently healthy individuals and HIV-positive patients, while purposive sampling technique was used for malignant patients (due to the low number of patients who reported to the hospitals).
Ethical approval and informed consent
Informed consent was obtained from the study groups, and the participants were enrolled consecutively. Ethical consent for this study was sought from the Research Ethics Committees in charge of the selected hospitals. Reference numbers of approval are as follows: NHA/ADMIN/236/V.VII (National Hospital, Abuja), FCT/UATH/HREC/PR/538 (UATH) and FHREC/2016/01/52/20-07-16 (Garki Hospital Abuja, Wuse General Hospital and Asokoro General Hospital).
Inclusion and exclusion criteria
Individuals from the target population who gave informed consent and were using the services of the selected hospitals at the time of the study were included, while all individuals from the target population who did not give their consent, pregnant women, extremely anaemic patients (<70g/L) and any individual after the maximum number of samples were obtained were excluded.
Sample collection and processing
Anonymized sociodemographic data, laboratory data, clinical data, sera and plasma samples were obtained from 200 participants (51 HMPs, 86 HIV and 63 BD) between April 2016 and August 2017. Sociodemographic data were obtained from each participant using questionnaires. In total, 5 mL of blood was collected using sterile vacutainer plain tubes, while plasma samples were collected using ethylenediaminetetraacetic acid (EDTA) tubes. Sample collection was done with the aid of the hospital personnel. Sera and plasma samples were separated in two aliquots of 1 mL each. Healthy blood donors underwent prior HBV, HCV, HIV and Venereal Disease Research Laboratory (VDRL) screening. HMPs were those already enrolled in chemotherapy, as well as newly diagnosed ones showing clinical manifestations, while HIV-positive individuals were either newly diagnosed or undergoing highly active antiretroviral therapy (HAART). All samples were stored immediately at −70°C in 1.8-mL Thermo Scientific™ Nunc™ Biobanking and cell culture cryogenic tubes (Thermo Fisher Scientific, Massachusetts, United States), after which complete samples were transported to Translational Genomics Laboratory, Department of Biosciences, COMSATS University, Islamabad, Pakistan for further processing.
Sera samples were screened for anti-HTLV-1/2 IgG and IgM antibodies using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (Wantai anti-HTLV +2 Ab ELISA Kit, WH-9196; Beijing Wantai Biological Pharmacy Enterprise Co., Beijing, China) based on two-step incubation and double-antigen sandwich principle, according to the manufacturer’s instructions, indicating that sera samples that yielded an optical density (OD) or cut-off ⩾1.0 at 450 nm were considered positive for HTLV-1/2 antibodies, 0.2–0.9 were considered intermediate, while <0.2 were considered non-reactive. Samples were randomly selected for screening from each hospital in an equal ratio per gender, except blood donor samples which had more males; hence, more males were selected for screening. Reactive samples were subjected to qualitative real-time polymerase chain reaction (PCR), targeting HTLV-1/2 pol genes using primers, probes and conditions described by Andrade et al.13 after DNA extraction from plasma14 using GFX™ Genomic Blood DNA Purification Kit (GE Healthcare, Little Chalfont, UK) according to the manufacturer’s instructions. Detection of HTLV from plasma is relatively sparsely explored as very few studies14,15 have reported it before now. Therefore, purified DNA extracted from peripheral blood mononuclear cell (PBMC) using the same kit as in plasma samples were also subjected to real-time PCR using same primers, probes and conditions, to verify the results obtained using plasma. The PCR products were then separated by 2% agarose gel electrophoresis (with ethidium bromide contained in both gel and Tris/Borate/EDTA (TBE) running buffer) and examined under ultraviolet (UV).
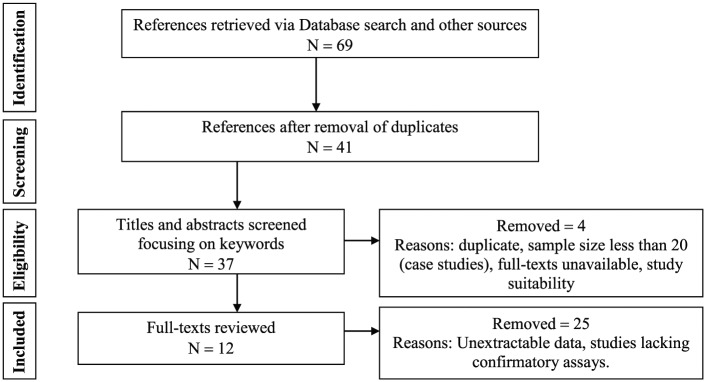
Statistical and meta-analysis
Data collected were subjected to descriptive and inferential analysis involving the generation of frequency and mean distribution of different variables using SPSS version 17 (SPSS Incorporated, Chicago, IL, USA). Generated data were presented in tables and figures. Systematic review of Nigerian studies was carried out as described by Anyanwu et al.8 for the purpose of meta-analysis. Pubmed, MEDLINE, Google scholar and CrossRef were searched, and references of all identified relevant publications describing the prevalence of HTLV in Nigeria were manually checked. Grey literatures and print-only publications were also sourced for from libraries and archives. In total, 12 (study) group data available for 10 studies published between 1990 and 2016 were collected (Table 1). All groups had sample size >20. Study groups of studies with more than one study group were treated as subgroup for purpose of meta-analysis and recorded as different studies in PRISMA chart (Figure 1). Meta-analysis was used to explore all included studies. The effect size (ES), lower and upper confidence intervals (LCI and UCI), as well as weight of the individual studies (with subgroups) were calculated using Comprehensive Meta-Analysis (CMA) software version 3 and repeated with STATA version 14.2. Point estimates (event rate proportions) were calculated from events and sample size. The combined estimate was calculated from the logit-transformed event rates (formula, , where p = prevalence proportion) using DerSimonian and Laird (D+L) Random-effects method. The lower and upper CI (formula, , where p = prevalence proportion, z at 5% = 1.96) were calculated and all rounded up to two decimal places. Zero outcomes were not adjusted because where no events are recorded, risk differences (RDs) and point estimates are still calculable, and studies are included in forest plots (Figure 2). I2 statistic (formula, , where Q = Cochrane’s heterogeneity statistic; df = degree of freedom) is the percentage of variation attributable to heterogeneity. Heterogeneity was thus auto generated via I2 and Cochrane’s Q statistic using D+L effect model; hence, studies received similar weight. Year of study, study groups and geopolitical zones were explored using subgroup analysis. Review Manager (RevMan) software version 5.2 was used to analyse the RD between HTLV-1 and -2 among studies, including this study. RD accounts for the pooled difference of point estimates (prevalence proportion) between HTLV-1 and -2 for different studies. This was presented in funnel plot. An asymmetrical plot indicates a difference between studies, while approximately symmetrical plot depicts lack of bias.
Table 1.
Reports of HTLV prevalence in Nigeria from discovery till 2018.
| Author | Publication year | Region | Study group | Sample size | Confirmatory assay used | HTLV-1 | HTLV-2 | Overall |
|---|---|---|---|---|---|---|---|---|
| Olusanya et al.16 | 1990 | SW | AHI | 385 | WB | 0.0052 | 0 | 0.0052 |
| Williams et al.17 | 1993 | SW | C | 46 | WB | 6.52 | 0 | 6.52 |
| Williams et al.17 | 1993 | SW | BD | 123 | WB | 11 | 0 | 11 |
| Olaleye et al.4 | 1995 | SW | ANC | 364 | WB, SPA | 5.5 | 3.85 | 11.54 |
| Olaleye et al.18 | 1996 | SW | STD | 40 | PCR | 15 | 0 | 15 |
| Olaleye et al.18 | 1996 | SW | MI | 65 | PCR | 1.5 | 0 | 1.5 |
| Terry Alli et al.9 | 2011 | SW | BD | 372 | WB | 1.88 | 1.88 | 3.76 |
| Akinbami et al.19 | 2014 | SW | MI | 39 | WB | 5.1 | 0 | 5.1 |
| Durojaiye et al.5 | 2014 | SW | BD | 210 | WB | 0.5 | 0 | 0.5 |
| Okoye et al.6 | 2014 | SE | ANC | 200 | WB | 0.5 | 0 | 0.5 |
| Nasir et al.7 | 2015 | NC | HIV | 184 | PCR | 4.9 | 0 | 4.9 |
| Manga et al.11 | 2016 | NE | BD | 355 | WB | 0 | 0 | 0 |
SW: South West; SE: South East; NE: North East; NC: North Central; BD: blood donors; C: children; AHI: apparently healthy individuals; MI: malignant individuals; ANC: antenatal care (pregnant) women; STD: sexually transmitted disease–infected individuals; WB: western blotting; PCR: polymerase chain reaction; SPA: synthetic peptide assay; HIV: human immunodeficiency virus; HTLV: human T-lymphotropic virus.
Figure 1.
PRISMA flow chart of study selection.
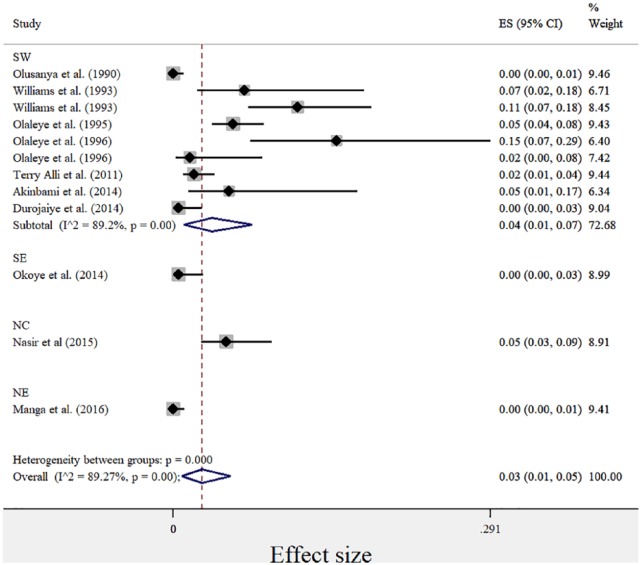
Figure 2.
Forest plot showing prevalence of HTLV-1 in different geopolitical zones of Nigeria.
SW: South West; NC: North Central; SE: South East; NE: North East.
Results
Two samples (UH4 and UH16) were reactive to ELISA, yielding 1% prevalence which was confirmed to be zero via confirmatory assays (Table 2). The age range (in years) for blood donors was 18–60, while the other participants fell between 6–71 years. The mean age was 35.28 (±13.61) years. There were more male participants (119, 59.5%) than female participants (81, 40.5%) in this study, with the ratio of 1.47:1. The number of male donors was higher across ages. HMPs were only found in National Hospital, UATH and Garki Hospital.
Table 2.
Prevalence of HTLV-1/2 among the study population.
| Assay | Result | Frequency (%) |
|---|---|---|
| HTLV-1/2 ELISA | Positive | 2 (1.0) |
| Negative | 198 (99.0) | |
| PCR (plasma and PBMC) | Positive | 0 (0.0) |
| Negative | 200 (100.0) |
ELISA: enzyme-linked immunosorbent assay; PCR: polymerase chain reaction; PBMC: peripheral blood mononuclear cell; HTLV: human T-lymphotropic virus.
More than half of the study participants (52.5% and 56.0%) were married and had tertiary education. While 46.0% of the study population was gainfully employed, 26.5% and 27.5% were unemployed and self-employed, respectively. Most of the individuals (72.5%) lived in urban areas (Table 3). Apart from sex and blood reception/donation, there was generally low rate of exposure to HTLV-associated risk factors in the population studied (Table 4).
Table 3.
Sociodemographic characteristics of study participants.
| Variable | Category | Frequency | Percentage (%) |
|---|---|---|---|
| Marital status | Single | 81 | 40.5 |
| Married | 105 | 52.5 | |
| Divorced | 8 | 4.0 | |
| Widowed | 6 | 3.0 | |
| Total | 200 | 100.0 | |
| Ethnicity | Yoruba | 24 | 12.0 |
| Hausa | 85 | 42.5 | |
| Igbo | 36 | 18.0 | |
| Others | 55 | 27.5 | |
| Total | 200 | 100.0 | |
| Level of education | None | 19 | 9.5 |
| Informal | 12 | 6.0 | |
| Primary | 11 | 5.5 | |
| Secondary | 46 | 23.0 | |
| Tertiary | 112 | 56.0 | |
| Total | 200 | 100.0 | |
| Employment status | Unemployed | 53 | 26.5 |
| Self-employed | 55 | 27.5 | |
| Employed | 92 | 46.0 | |
| Total | 200 | 100.0 | |
| Locality | Rural | 55 | 27.5 |
| Urban | 145 | 72.5 | |
| Total | 200 | 100.0 |
Table 4.
Frequencies of dichotomous response to risk factors.
| Risk factor | Yes (%) | No (%) |
|---|---|---|
| History of blood donation or reception | 116 (58.0) | 84 (42.0) |
| Sharing of sharp objects | 52 (26) | 148 (74.0) |
| History of drug use | 26 (13.0) | 174 (87.0) |
| History of STD | 47 (23.5) | 153 (76.5) |
| Engagement in protected sex | 117 (58.5) | 83 (41.5) |
| History of unprotected sex | 161 (80.5) | 39 (19.5) |
| Family history of cancer | 50 (25.0) | 150 (75.0) |
| History of exposure to radiotherapy | 30 (15.0) | 170 (85.0) |
STD: sexually transmitted disease.
For the meta-analysis, the pooled sample size for explored studies was 2383. The sample size for explored studies ranged from 40 to 385. Using the D+L effect model, the pooled prevalence of HTLV-1 and -2 were 3% and 0%, respectively. The pooled prevalence for HTLV-1 according to geopolitical zones is presented in Figure 2. The black diamonds represent the point estimate of each study, the grey boxes represent the study weights, while the black lines represent the CI (at 95%). The blue diamonds represent the combined or pooled estimates. A value of 0% indicates heterogeneity and larger values show increasing heterogeneity. Hence, 25%–50% = low, 50%–75% = moderate, while >75% = high heterogeneity. For HTLV-1, there was significantly high heterogeneity observed between studies included in this study, explaining the effect estimates being relatively far from each other (I2 = 89.27%, p = 0.00, Q = 102.48). Test for publication bias is presented in Figure 3. The asymmetrical plot seen is due to small study effect rather than publication bias. HTLV-2 prevalence was found in only two studies, and hence, there was no need to generate forest and funnel plots. The risk of HTLV-1 from included studies is significantly higher than the risk of HTLV-2 without bias shown between studies (I2 = 87%, p = 0.00, Q = 110.99%; Figure 4).
Figure 3.
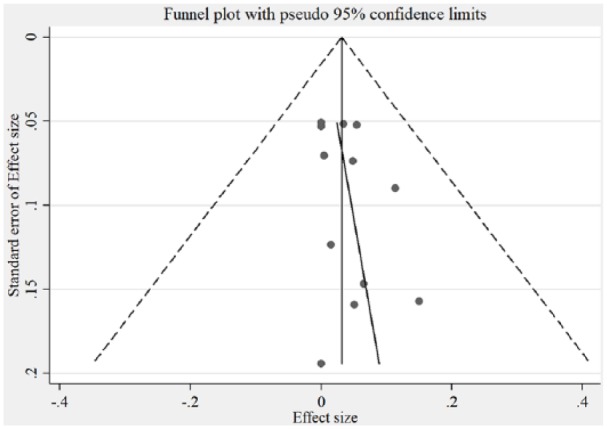
Funnel plot showing bias between HTLV-1 studies.
Figure 4.
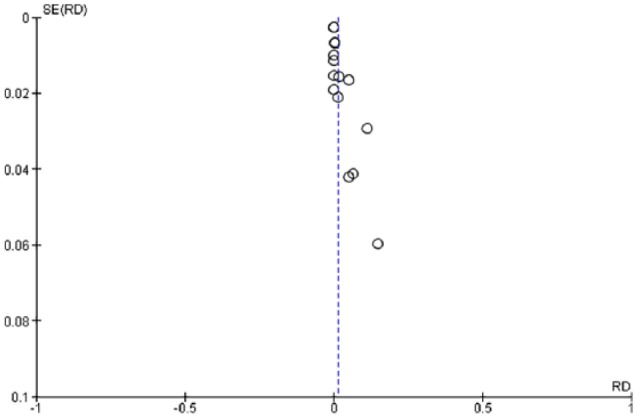
Funnel plot of risk differences between HTLV-1 and -2 studies.
RD: risk difference; SE: standard error.
Discussion
HTLV-1 and -2 have experienced gradual, but considerably consistent, increase in prevalence since their discovery.8 HTLV-1 subtypes are associated with specific regions of the globe, while HTLV-2 subtypes are related to highly specific subpopulations and behaviour like injection drug use.8 This study seems to be the first report of HTLV-1/2 prevalence in both healthy and diseased study groups in FCT, Nigeria. A previous study7 was limited to HIV-positive individuals.
The zero prevalence obtained from this study is similar to those obtained from a different region of the country.11 Zero prevalence rates have also been reported in other parts of Africa like Mali and Benin Republic.20,21
Low prevalence rates have been obtained from different populations in other studies5,6 as well as moderate4,9 and high prevalence.4
Abuja is a cosmopolitan city and the capital of Nigeria, characterized by high level of ethno-cultural diversity; this could therefore be the reason for the zero prevalence obtained from the study as ethno-cultural diversity and environmental differences have been found to be linked to the geographical variation in HTLV prevalence.2,8 The limited number of study participants analysed could be the cause of zero prevalence obtained. The study participants also may not have been exposed to the viruses. However, the possibility of uncaptured cases of infection should not be completely ruled out as this study was solely a hospital-based study. Exposure to risk factors and behaviour seemed not to play any role in infection with, and/or, spread of the retroviruses in this study. The high-risk groups enlisted were also not infected.
Findings from this study are dissimilar to a previous finding7 which reported 4.9% seroprevalence of HTLV among HIV-positive individuals in the UATH. In the same study, 6.5% prevalence was obtained using confirmatory PCR, indicating a possible false-negative ELISA result. ELISA is not the gold standard for the confirmation of the presence of HTLV, as there could be false-negative results as obtained in the previous study, or false-positive results as obtained in this study. Serological screening methods for HTLV-1 and -2 are generally far from specific, especially in sera/plasma from individuals living in tropical areas, including Africa. Hence, it is essential to have a specific serological confirmation test such as a western blot or INNO-LIA™ or PCR confirmation. Seroprevalence studies based on screening tests alone overestimate the reality of infection with these viruses and are therefore unreliable, as seen in this study. It is therefore impossible to compare data if there is no confirmatory test. However, it should be noted that the detection of HTLV from the serum/plasma of infected individuals may be dependent on the viral load. Hence, it is advised that other sensitive and specific confirmatory assays which include DNA extraction from PBMC be performed alongside to verify the HTLV detected from plasma where present.
Contrary to the findings of this study, prevalence of HTLV has previously been reported in healthy blood donors, STD/HIV positive individuals and haematologic malignant individuals (Table 1).8 The absence of the viruses in this study could be attributed to the smaller sample size screened, as increase in sample size increases the chances of obtaining positive outcomes.
The meta-analysis of HTLV-1 and -2 in the included Nigerian studies confirmed the distribution of HTLV-1 and HTLV-2 in four and one geopolitical zones, respectively, out of the six zones found in the country. South-Western part of Nigeria seems to have the highest awareness cum reports on the re-emerging viruses compared to the other parts of the country. This could be because the region is the most educationally exposed, with the highest number of intellectuals in the country. Other regions had reports form only one author each. All studies that explored multiple regions were carried out more than a decade and half ago, highlighting the need for a current population–based study.
Using the D+L (Random) effect model, the prevalence of HTLV-1 and -2 in Nigeria were observed to be average (3%) and nil (0%), respectively. The low prevalence could be attributed to limited available data on the viruses. Possible increase in prevalence should not be overlooked, as Nigeria has Cameroon – an endemic country – as her neighbouring country. Preventive measures particularly channelled towards high-risk populations in countries like Nigeria, which is prone to high endemicity, will significantly curb the transmission and/or spread of the viruses.
Conclusion
This study discovered zero prevalence of HTLV-1/2 from five major hospitals in Nigeria’s capital, exposing the importance of confirmatory assays after positive antibody detection assay results. Meta-analysis highlighted the existence of very few reliable Nigerian studies compared to the demography of the nation.
Recommendation
Cases of HTLV infection in the general population which were not reported or brought to the hospitals might have been missed in this study; hence, future studies should be carried out on/or extended to general population and other high-risk groups, including injecting drug users (IDUs), commercial sex workers, homosexuals, former paid blood donors as well as patients attending clinics for other sexually transmitted infections. This is imperative to capture probable restricted and/or heterogenous infection cases.
Acknowledgments
The authors would like to acknowledge the support and contributions from Dr A.B. Suleiman who contributed towards the study design, as well as Mr Steve Oricha, Dr (Mrs) Udoh, Mr Faith Oche, Mr Abdullahi, Dr Adeniyi, Dr (Mrs) Badejo, Dr Atodo, Dr (Mrs) Ibrahim, Dr Ajobiewe, Dr Y. Tanko, Dr Olomukoro, Dr Rotimi, Dr Ejike, Mr Tunde, Ms Mercy, Mrs Chika, Mr Michael and other members of staff of the five hospitals captured in this study.
Footnotes
Author contributions: N.C.J.A., E.E.E., M.A. and H.M.K. designed the study. N.C.J.A. performed the experiments with the guidance of E.E.E., M.A., H.M.K., M.A. and M.A. N.C.J.A analysed the data and drafted the manuscript. E.E.E., M.A. N.C.J.A H.M.K. revised the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from the Research Ethics Committees in charge of the selected hospitals. Reference numbers of approval are as follows: NHA/ADMIN/236/V.VII (National Hospital, Abuja), FCT/UATH/HREC/PR/538 (University of Abuja Teaching Hospital) and FHREC/2016/01/52/20-07-16 (Garki Hospital Abuja, Wuse General Hospital and Asokoro General Hospital).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from all subjects before the study.
ORCID iD: Nneoma Confidence JeanStephanie Anyanwu  https://orcid.org/0000-0003-1280-7605
https://orcid.org/0000-0003-1280-7605
References
- 1. Okoye AE, Ibegbulam OG, Onoh RC, et al. Seroprevalence of human T-cell lymphoma/leukemia virus type-1 (HTLV-1) antibodies among blood donors at Enugu, Nigeria. J Blood Med 2015; 6: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 2012; 3: 388–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goubau P, Desmyter J, Ghesquiere J, et al. HTLV-II among pygmies. Nature 1992; 359(6392): 201–201. [DOI] [PubMed] [Google Scholar]
- 4. Olaleye DO, Ekweozor CC, Sheng Z, et al. Evidence of serological cross-reactivities with human immunodeficiency virus types 1 and 2 and human T-lymphotropic virus types I and II in sera of pregnant women in Ibadan, Nigeria. Int J Epidemiol 1995; 24: 198–203. [DOI] [PubMed] [Google Scholar]
- 5. Durojaiye I, Akinbami A, Dosunmu A, et al. Seroprevalence of human T lymphotropic virus antibodies among healthy blood donors at a tertiary centre in Lagos, Nigeria. Pan Afr Med J 2014; 17: 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okoye AE, Ibegbulam OG, Onoh RC, et al. Seroprevalence and correlates of human T-cell lymphoma/leukemia virus type 1 antibodies among pregnant women at the university of nigeria teaching hospital, Enugu, Nigeria. Int J Womens Health 2014; 6: 849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nasir IA, Ahmad AE, Emeribe AU, et al. Molecular detection and clinical implications of HTLV-1 infections among antiretroviral therapy-Naïve HIV-1-infected individuals in Abuja, Nigeria. Virol Res Treat 2015; 6: VRTS35331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anyanwu NCJ, Ella EE, Ohwofasa A, et al. Re-emergence of human T-lymphotropic viruses in West Africa. Braz J Infect Dis 2018; 22: 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Terry Alli O, Olusoga O, Oluremi A, et al. Seroprevalence HTLV-I/II amongst blood donors Osogbo, Nigeria. Sudan J Med Sci 2011; 6(3): 177–182. [Google Scholar]
- 10. Yuguda S, Manga M, Fowotade A, et al. Seroprevalence of human T-cell lymphoma/Leukemia virus type-1 (HTLV-1) antibodies among blood donors at Ibadan, Nigeria. J Human Virol Retrovirol 2017; 1(5): 1–5. [Google Scholar]
- 11. Manga MM, Fowotade A, Yuguda S, et al. Serosurvey of human T cell lymphotropic virus I / II among blood donors in Gombe (Nigeria). Edorium J 2016; 6: 12–19. [Google Scholar]
- 12. Williams CK, Alabi GO, Junaid TA, et al. Human T cell leukaemia virus associated lymphoproliferative disease: report of two cases in Nigeria. Br Med J (Clin Res Ed) 1984; 288(6429): 1495–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andrade RG, Ribeiro MA, Namen-Lopes MSS, et al. Evaluation of the use of real-time PCR for human T cell lymphotropic virus 1 and 2 as a confirmatory test in screening for blood donors Análise do uso da PCR em tempo real para HTLV-1 e 2 como teste confirmatório na triagem de doadores de sangue. Open Access J 2010; 43(2): 111–115. [DOI] [PubMed] [Google Scholar]
- 14. Cabral F, Arruda LB, deAraujo ML, et al. Detection of human T-cell lymphotropic virus type 1 in plasma samples. Virus Res 2012; 163(1): 87–90. [DOI] [PubMed] [Google Scholar]
- 15. Suryanarayana K, Miley W, Lifson J, et al. Viral load determination in plasma and CSF from HTLV-I-infected patients. Real-time quantitation using the Prism7700. JAIDS 1999; 20(4): pA20. [Google Scholar]
- 16. Olusanya O, Lawoko A, Blomberg J. Seroepidemiology of human retroviruses in Ogun State of Nigeria. Scand J Infect Dis 1990; 22(2): 155–160. [DOI] [PubMed] [Google Scholar]
- 17. Williams CKO, Alexander SS, Bodner A, et al. Frequency of adult T-cell leukaemia/lymphoma and HTLV-I in Ibadan, Nigeria. Br J Cancer 1993; 67(4): 783–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Olaleye OD, Ekweozor CC, Li ZL, et al. Human T-cell lymphotropic virus types I and II infections in patients with leukaemia/lymphoma and in subjects with sexually transmitted diseases in Nigeria. Arch Virol 1996; 141(2): 345–355. [DOI] [PubMed] [Google Scholar]
- 19. Akinbami A, Durojaiye I, Dosunmu A, et al. Seroprevalence of human T-lymphotropic virus antibodies among patients with lymphoid malignancies at a tertiary center in Lagos, Nigeria. J Blood Med 2014; 5: 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Denis F, Verdier M, Chout R, et al. Prevalence of HTLV-1 virus in pregnant women in Black Africa, Martinique, and foreigners living in France. Bull Acad Natl Med 1988; 172(5): 717–722. [PubMed] [Google Scholar]
- 21. Houinato D, Verdier M, Josse R, et al. Seroepidemiological study of retroviruses (HTLV-I/II, HIV-1, HIV-2) in the Department of Atacora, Northern Benin. Trop Med Int Heal 2007; 1(2): 205–209. [DOI] [PubMed] [Google Scholar]