Abstract
Background:
We investigated whether early dynamic changes of circulating free (cfDNA) levels as well as the neutrophil to lymphocyte ratio (NLR) could predict nivolumab effectiveness in pretreated patients with advanced non-small cell lung cancer (NSCLC).
Methods:
A total of 45 patients receiving nivolumab 3 mg/kg every 2 weeks were enrolled. Patients underwent a computed tomography scan and responses were evaluated by the response evaluation criteria in solid tumors. Peripheral blood samples were obtained from the patients and the cfDNA level as well as the NLR were assessed. Time to progression (TTP) and overall survival (OS) were determined.
Results:
Patients with increased cfDNA >20% at the sixth week reported significantly worse survival outcomes (median OS: 5.7 versus 14.2 months, p < 0.001; median TTP: 3.3 versus 10.2 months, p < 0.001), as well as patients with increased NLR >20% (median OS: 8.7 versus 14.6 months, p = 0.035; median TTP: 5.2 versus 10.3 months, p = 0.039). The combined increase of cfDNA and NLR >20% was associated with significantly worse survival outcomes as compared with the remained population (median OS: 5.8 versus 15.5 months, p = 0.012; median TTP: 3.2 versus 11.9 months, p = 0.028). Multivariable analysis identified three significant factors associated with worse OS: combined cfDNA/NLR increase >20% [hazard ratio (HR): 5.16; 95% confidence interval (CI), 1.09–24.29; p = 0.038], liver metastasis (HR: 0.44; 95% CI, 0.20–0.96; p = 0.038), and extra-thoracic disease (HR: 0.33; 95% CI, 0.12–0.89; p = 0.029).
Conclusion:
An early combined increase of both cfDNA and NLR over the course of the first 6 weeks of nivolumab therapy predicted worse survival in pretreated patients with advanced NSCLC, suggesting a potential role in the real-time monitoring of immunotherapy resistance.
Keywords: blood-based biomarkers, Circulating free DNA (cfDNA), immunotherapy, neutrophil to lymphocyte ratio (NLR), non-small cell lung cancer (NSCLC)
Introduction
In the last few years we have witnessed a great step forward in the treatment of advanced non-small cell lung cancer (NSCLC), thanks to the advent of immune-checkpoint inhibitors (ICIs) in clinical practice.1 Overall, four randomized phase III trials2–5 have consistently demonstrated that single agent programmed cell death (PD)-1/programmed death ligand (PD-L)1 inhibitors significantly improved overall survival (OS) and quality of life (QoL) over docetaxel, representing the new standard of care for NSCLC patients who experienced disease progression after platinum combinations. Pembrolizumab showed a significant survival benefit as compared with first-line platinum-chemotherapy in nononcogene-addicted NSCLC patients with a PD-L1 tumor proportion score of 50% or greater.6 More recently pembrolizumab was also approved in combination with first-line carboplatin-pemetrexed based on an improved OS observed in the phase III Keynote 189 trial, regardless of tumor PD-L1 expression status. To date we have three ICIs, nivolumab, pembrolizumab and atezolizumab approved in pretreated patients, with pembrolizumab recommended also as a first-line option both as a single agent and in combination with platinum chemotherapy.7 Data emerging from both randomized trials and real-life experiences8,9 suggested that although immunotherapy worked very well in a significant subgroup of patients, about 50% of them did not gain any benefit from ICIs. Worth mentioning is also the recent evidence that 10–15% of pretreated NSCLC patients developed ‘hyperprogression’ to immunotherapy,10,11 defined as a rapid and dramatic increase of tumor burden during ICI administration. Thus, identifying predictive biomarkers of clinical response/resistance to ICIs remains a crucial issue for translational research. In addition to tumor PD-L1 expression, several other clinical and biological parameters have been investigated as biomarkers for clinical use. A post hoc exploratory analysis of the checkmate 057 trial showed that nivolumab-treated patients with lower or no tumor PD-L1 expression and poorer prognostic factors, including Eastern Cooperative Oncology Group (ECOG) performance status (PS) = 1, time since last treatment less than 3 months, and progression of disease as best response to prior therapy, were at higher risk of early death during drug administration.12 Data emerging from published studies and meta-analyses demonstrated that epidermal growth factor receptor (EGFR) activating mutations,13 inactivation of LKB1/STK11 tumor suppressor gene,14 low tumor mutational burden (TMB)15 and low tumor-infiltrating lymphocytes (TILs)16 were associated with poor responses to immunotherapy. Furthermore, a defective or absent pre-existing immune response was associated with innate/acquired resistance to ICIs,17,18 but the predictive role of immune-gene signatures is still under validation in clinical trials. All these biomarker analyses require high quality tumor tissue that in many cases is not available for patients with metastatic disease who receive ICIs in later lines of treatment. For this reason, several efforts are currently ongoing to identify easily detectable peripheral blood parameters which could guide clinical selection and real-time monitoring of lung cancer patients receiving immunotherapy.
Multiple studies and meta-analyses revealed that elevated neutrophil to lymphocyte ratio (NLR) is associated with a poor prognosis in patients with lung cancer.19–21 Recently other studies investigated the association between pretreatment NLR and survival in patients with advanced NSCLC, overall showing controversial results.22–28 Circulating tumor DNA (ctDNA) is emerging as another promising biomarker, since monitoring its level changes in the peripheral blood of ICI-treated patients with lung cancer provide a real-time snapshot of active tumor cell death as well as a reliable measure of overall tumor burden.29 In the present study we investigated whether combined and dynamic assessment of cell-free DNA (cfDNA) and NLR variations over the course of the first 6 weeks of nivolumab therapy may predict nivolumab effectiveness in terms of time to progression (TTP) and overall survival (OS) in pretreated patients with advanced NSCLC.
Materials and methods
Patients
Patients were eligible if they were aged ⩾18 years, had histologically or cytologically confirmed diagnosis of NSCLC, stage IIIB–C/IV (according to version 8 of the International Association for the Study of Lung Cancer TNM Staging System), EGFR/Anaplastic lymphoma kinase (ALK): wildtype, ECOG-PS < 3, disease progression or recurrence after receiving at least one prior systemic therapy for advanced/metastatic disease, with blood cell count and blood samples available.
Patients were excluded if they had autoimmune disease, symptomatic interstitial lung disease, systemic immunosuppression, prior treatment with immune-stimulatory antitumor agents including checkpoint inhibitors. Patients with brain metastases were eligible if they had received prior locoregional treatment and were stable at the time of eligibility. Tumor PD-L1 status was not required.
The study was conducted in accordance with the International Conference on Harmonization Guidelines on Good Clinical Practice and the Declaration of Helsinki. The trial protocol was previously approved by the Independent Ethic Committee at University of Palermo (ethics approval number: 0006981) and all the patients provided a written informed consent before enrollment.
Study design and treatment
From September 2015 to January 2018 eligible patients were included and received nivolumab 3 mg/kg intravenously every 2 weeks until disease progression or unacceptable toxicity.
We retrospectively collected clinical data and routine blood tests from patient charts and medical records at two university hospitals.
Blood tests were obtained within 1 week prior to the first (baseline) and fourth infusion of nivolumab (sixth week) and included the white blood cell count with lymphocyte and neutrophil counts, from which the NLR was deduced.
Peripheral blood samples were collected from patients included in the study the day of the drug administration at baseline and at 6 weeks during the course of therapy. Each blood sample was immediately processed for plasma collection, according to a simple and standardized protocol, and stored frozen as 500 ml aliquots at −80°C. The cfDNA isolation was performed with the QIAamp Circulating Nucleic Acid Kit (Qiagen, 20100 Milan, Italy) starting from 2 ml of plasma according to the manufacture’s instruction. The quantification of cfDNA (ng/ml plasma) was performed by qubit dsDNA HS assay (Thermo Fisher Scientific, 20090 Rodano, Milan, Italy) and confirmed by qPCR on a 7900HT instrument (Applied Biosystems, 20900 Monza and Brianza - Italy) evaluating a 115 bp fragment of Arthrobacter luteus (ALU) repeats.
Radiological evaluation of treatment efficacy by computed tomography (CT) scan was performed at week 12 and every 12 weeks of therapy, thereafter until disease progression and responses were evaluated by the response evaluation criteria in solid tumors (RECIST) version 1.1.
Statistical analysis
The cfDNA plasma level was calculated in NSCLC patients at baseline and at the sixth week during the course of therapy with nivolumab, while the NLR was calculated from blood tests obtained from each patient at the same time points. Both median cfDNA and NLR were calculated as the ‘middle number’ separating the higher half from the lower half of a data sample represented by the sorted list of values derived from the patients included in the study. Median cfDNA and NLR increase were calculated as the ‘middle value’ of a sorted list including the differences between baseline and the 6-week measurements in each patient. A 20% increase from baseline was detected as the median increase for both cfDNA and NLR values and was used as the cut-off point for survival analysis.
The Mann–Whitney U test was used for intergroup comparisons of two independent samples, while the Fisher’s exact test was used for categorical values. A paired Wilcoxon test was used to compare the median cfDNA plasma levels as well as median NLR before and after four cycles of therapy with nivolumab.
Survival analysis on the basis of the median cut-off values was performed using the Kaplan–Meier method, providing median and p values, with the use of the log-rank test for comparisons. Univariate and multivariate analyses were performed using the Cox proportional hazards and logistic regression models. All pretreatment parameters found to have a p value <0.05 at univariate analysis were included as covariates in the multivariable model.
Median TTP was defined as the time between the date of inclusion and the date of disease progression determined by RECIST version 1.1. Median OS was defined as the time between the date of inclusion and the date of death. A p value < 0.05 was used as threshold for statistical significance. All the statistical analyses were performed using SPSS statistics software, version 20 (IBM, Armonk, NY, USA).
Results
Patients’ characteristics
From September 2015 to January 2018 a total of 45 patients were considered eligible and were included in the study. Clinical characteristics of the patients are summarized in Table 1. The median age was 66 years (range 51–80 years); the majority of patients were men (71.1%), current or former smokers (84.4%) and exhibited an ECOG PS < 2 (82.2%). The bone was the most common metastatic site (44.4%) followed by liver (37.8%), adrenal gland (28.9%) and central nervous system (CNS; 15.6%). Patients received a median of 9 doses of nivolumab (range: 1–57), with a median follow up of 9.1 months (range: 0.1–29.7 months).
Table 1.
Baseline patient characteristics.
| Characteristic | Total (n = 45) | % |
|---|---|---|
| Median age (years - range) | 66 (51–80) | |
| Sex | ||
| Male | 32 | 71.1 |
| Female | 13 | 28.9 |
| Histology | ||
| Adenocarcinoma | 25 | 55.5 |
| Squamous cell carcinoma | 20 | 45.5 |
| Smoking history | ||
| Never | 7 | 15.6 |
| Current/former | 38 | 84.4 |
| Performance status | ||
| <2 | 37 | 82.2 |
| ⩾2 | 8 | 17.8 |
| Stage IV subgroup | ||
| M1a | 11 | 24.4 |
| M1b–c | 34 | 75.5 |
| Metastatic sites | ||
| Bone | 20 | 44.4 |
| Liver | 17 | 37.8 |
| Adrenal | 13 | 28.9 |
| Brain | 7 | 15.6 |
| Prior line of therapy | ||
| <2 | 22 | 48.9 |
| ⩾2 | 23 | 51.1 |
| Median NLR | ||
| Low (<3.3) | 21 | 46.6 |
| High (>3.3) | 24 | 53.4 |
| Median cfDNA | ||
| Low (<0.43 ng/ml) | 26 | 57.8 |
| High (>0.43 ng/ml) | 19 | 42.2 |
cfDNA: circulating free DNA; NLR, neutrophil to lymphocyte ratio.
Among 45 patients evaluable for cfDNA analysis at baseline, the median cfDNA level was 0.43 ng/ml, thus not significantly higher than that observed in 36 patients evaluated after four cycles of therapy with nivolumab (0.42 ng/ml, p = 0.81). The median NLR at baseline was 3.31, thus very similar to that observed after four cycles of nivolumab therapy (NLR: 3.2, p = 0.89).
Pretreatment parameters and patient survival
At the time of survival analysis (median follow up of 15.3 months, range: 2–39 months), disease progression occurred in 37 patients, while 31 patients died because of tumor progression, and 14 patients were still alive at the time of data analysis. Median TTP was 5.6 months and median OS was 8.8 months in the overall population.
To identify potential baseline biomarkers associated with nivolumab effectiveness we examined clinical-pathological characteristics, blood parameters and cfDNA levels before treatment initiation. Among clinical characteristics, ECOG-PS > 2 (OS, p = 0.032; TTP, p = 0.038), presence of extra-thoracic (M1b-c) disease (OS, p = 0.001; TTP, p = 0.004) and liver metastasis (OS, p = 0.001; TTP, p = 0.031) were significantly associated with worse survival outcomes in the overall population.
Elevated pretreatment cfDNA level was associated with inferior OS (7.2 versus 13.5 months, p = 0.04) while high NLR predicted inferior TTP (4.5 versus 9.7 months, p = 0.006) but not significant OS differences. Patients with elevated pretreatment cfDNA levels and NLR values showed a not significant trend toward worse survival outcomes as compared to patients reporting one or none of such elevated parameters (median OS: 9.4 versus 8.1 versus 9.6 versus 16.3 months, p = 0.088; median TTP: 6.2 versus 4.8 versus 7 versus 11.4 p = 0. 22; Table 2).
Table 2.
Univariate analysis for survival outcomes.
| Parameter | OS |
TTP |
||
|---|---|---|---|---|
| Median, months (95% CI) |
Log-rank p value |
Median, months (95% CI) |
Log-rank p value |
|
| cfDNA/NLR increase (>20% versus <20%) |
5.8 (4.1–7.5) 15.4 (11.3–19.6) |
p = 0.012 | 3.2 (1.7–4.6) 11.9 (7.7–16.1) |
p = 0.028 |
| cfDNA increase (>20% versus <20%) |
5.7 (3.1–8.3) 14.2 (10.8–17.7) |
p < 0.001 | 3.3 (1.8–4.8) 10.2 (6.9–13.5) |
p < 0.001 |
| NLR increase (>20% versus <20%) |
8.7 (5.7–11.8) 14.6 (11.3–17.8) |
p = 0.035 | 5.2 (2.3–8.2) 10.3 (10.3–16.4) |
p = 0.039 |
| NLR baseline (>3.3 versus <3.3) |
8.6 (5.5–11.8) 13 (9.6–13.3) |
p = 0.084 | 4.5 (2.6–6.4) 9.8 (6.3–13.1) |
p = 0.006 |
| cfDNA baseline (>0.43 versus <0.43) |
7.2 (4.4–10.04) 13.5 (10.1–17) |
p = 0.033 | 4.7 (1.9–7.4) 8.9 (6.02–11.8) |
p = 0.069 |
| cfDNA/NLR baseline (high versus low) |
9.4 (4.6–14.1) 16.2 (12.5–20.06) |
p = 0.088 | 6.2 (0.14–12.25) 11.4 (7.6–15.3) |
p = 0.22 |
| Age (years) (>70 versus <70) |
12.2 (6.8–17.6) 9.5 (6.7–12.4) |
p = 0.273 | 10 (5.6–14.4) 5.5 (3.6–7.3) |
p = 0.064 |
| Sex (male versus female) |
10.07 (7.05–13.08) 11.1 (6.01–16.08) |
p = 0.557 | 7.1 (4.5–9.7) 6.7 (3.4–10.09) |
p = 0.8 |
| ECOG PS (2 versus 0–1) |
5.3 (2.4–8.3) 11.9 (8.8–14.9) |
p = 0.016 | 3 (1.7–4.2) 8 (5.5–10.3) |
p = 0.038 |
| Smoking
habits (current/former versus never) |
9.8 (7.1–12.5) 14.5 (6.9–22.1) |
p = 0.317 | 6.03 (4.1–7.9) 12.6 (6.3–18.8) |
p = 0.088 |
| Histology (squamous versus nonsquamous) |
7.6 (3.7–11.5) 11.6 (8.5–14.8) |
p = 0.182 | 5.7 (2.6–8.8) 7.5 (5.03–10.1) |
p = 0.49 |
| Stage IV subgroup (M1b–c versus M1a) |
7.9 (5.4–10.3) 19.3 (15.04–23.7) |
p = 0.001 | 5.4 (3.2–7.5) 12.2 (8.8–15.6) |
p = 0.017 |
| Previous lines (>1 versus 1) |
9.7 (6.1–13.4) 11.06 (7.3–14.8) |
p = 0.62 | 6.5 (3.9–9) 7.6 (4.4–10.8) |
p = 0.63 |
| Brain metastasis (yes versus no) |
9.5 (2.8–16.2) 10.6 (7.7–13.5) |
p = 0.8 | 5.2 (1.5–9) 7.3 (4.9–9.6) |
p = 0.68 |
| Liver metastasis (yes versus no) |
5.1 (2.5–7.7) 13.2 (10.04–16.5) |
p = 0.001 | 3.9 (2.2–5.6) 8.6 (5.8–11.4) |
p= 0.018 |
| Adrenal metastasis (yes versus no) |
7.2 (4.04–10.6) 11.8 (8.5–15.2) |
p = 0.09 | 3.08 (1.8–4.3) 8.5 (5.9–11.09) |
p = 0.056 |
| Bone metastasis (yes versus no) |
8.7 (5.6–11.8) 12.2 (8.8–16.1) |
p = 0.23 | 6.3 (3–9.5) 7.6 (5.1–10.2) |
p = 0.57 |
CI, confidence interval; cfDNA, circulating free DNA; ECOG-PS, Eastern Cooperative Oncology Group performance status; NLR, neutrophil to lymphocyte ratio; OS, overall survival; TTP, time to progression.
cfDNA/NLR dynamic changes and patient survival
Among 36 out of 45 patients evaluable for cfDNA or NLR analysis after four cycles of therapy with nivolumab, a 20% median cfDNA and NLR increase has been detected and was used as the cut-off point for survival analysis.
Patients with an increased cfDNA level >20% at the sixth week reported significantly worse OS and TTP as compared with other patients (median OS: 5.7 versus 14.2 months, p < 0.001; median TTP: 3.3 versus 10.2 months, p < 0.001; Figure 1 and Table 2).
Figure 1.
Kaplan–Meier analysis of OS and TTP in NSCLC patients according to the cfDNA increase.
cfDNA, circulating free DNA; NSCLC, non-small cell lung cancer; OS, overall survival; TTP, time to progression.
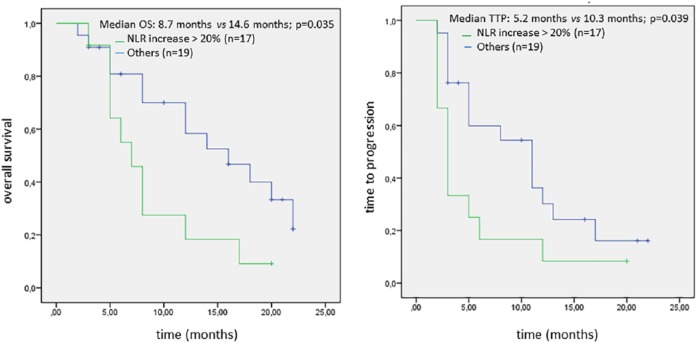
Patients with increased NLR > 20% at the sixth week showed significantly worse median OS and TTP as compared with the remaining population (median OS: 8.7 versus 14.6 months, p = 0.035; median TTP: 5.2 versus 10.3 months, p = 0.039; Figure 2 and Table 2).
Figure 2.
Kaplan–Meier analysis of OS and TTP in NSCLC patients according to the NLR increase.
NLR, neutrophil to lymphocyte ratio; NSCLC, non-small cell lung cancer; OS, overall survival; TTP, time to progression.
The combined increase >20% of both cfDNA and NLR values between baseline and the sixth week of nivolumab therapy was associated with significantly worse survival outcomes as compared with patients with no increase of both such parameters (median OS: 5.8 versus 15.5 months, p = 0.012; median TTP: 3.2 versus 11.9 months, p = 0.028; Figure 3 and Table 2).
Figure 3.
Kaplan–Meier analysis of OS and TTP in NSCLC patients according to the combined cfDNA/NLR increase.
cfDNA, circulating free DNA; NLR, neutrophil to lymphocyte ratio; NSCLC, non-small cell lung cancer; OS, overall survival; TTP, time to progression.
Multivariate analysis for survival outcomes
Multivariable Cox proportional regression analysis was performed to assess whether cfDNA or NLR increases over the course of the first 6 weeks of therapy were independent factors related to nivolumab effectiveness in terms of TTP and OS. All pretreatment parameters found to have a p value <0.05 at univariate analysis were included as covariates in the multivariable model.
Multivariable analysis identified three significant factors associated with a worse OS: combined cfDNA/NLR increase >20% [hazard ratio (HR): 5.16; 95% confidence interval (CI), 1.09–24.29; p = 0.038], liver metastasis (HR: 0.44; 95% CI, 0.20–0.96; p = 0.038), extra-thoracic disease (HR: 0.33; 95% CI, 0.12–0.89; p = 0.029; Table 3).
Table 3.
Multivariate analysis for survival outcomes.
| Parameter | OS |
TTP |
||
|---|---|---|---|---|
| HR (95% CI); | p value | HR (95% CI) | p value | |
| cfDNA/NLR increase (>20% versus <20%) |
5.16 (1.09–24.29) | p = 0.038 | 0.61 (0.41–0.9) | p = 0.013 |
| cfDNA increase (>20% versus <20%) |
0.95 (0.24–3.76) | p= 0.94 | 2.37 (0.86–6.52) | p = 0.09 |
| NLR increase (>20% versus <20%) |
1.90 (0.71–5.11) | P = 0.202 | 1.89 (0.83–4.3) | p = 0.13 |
| NLR baseline (low versus high) |
— | — | 0.56 (0.24–1.25) | p = 0.16 |
| cfDNA baseline (low versus high) |
0.63 (0.29–1.36) | p = 0.24 | — | — |
| ECOG PS (<2 versus >2) |
0.51 (0.21–1.24) | p = 0.140 | 0.48 (0.2–1.17) | p = 0.11 |
| Stage IV subgroup (M1a versus M1b–c) |
0.33 (0.12–0.89) | p = 0.029 | 0.43 (0.18–1.02) | p = 0.057 |
| Liver metastasis (no versus yes) |
0.44 (0.20–0.96) | p = 0.038 | 0.60 (0.26–1.39) | p = 0.24 |
CI, confidence interval; cfDNA, circulating free DNA; ECOG-PS, Eastern Cooperative Oncology Group performance status; HR, hazard ratio; NLR, neutrophil to lymphocyte ratio; OS, overall survival; TTP, time to progression.
Discussion
This study represents a retrospective analysis with nivolumab in patients with previously treated, EGFR/ALK wildtype, advanced NSCLC. The results of this work demonstrated that an early combined increase of both cfDNA and NLR over the course of the first 6 weeks of nivolumab therapy is associated with worse survival outcomes, suggesting a potential role in the realtime monitoring of immunotherapy resistance. In line with other evidences,30–32 the presence of extra-thoracic disease as well as liver metastases also remain independent predictors of poor survival in our series of nivolumab-treated patients with NSCLC.
Interest in circulating biomarkers associated with immunotherapy efficacy is rapidly growing, with many potential candidates, such as soluble PD-L1,33 blood-based TMB,34 serum chemokines/cytokines,35 ctDNA,29,36 microRNA, and circulating immune cell subsets,37 currently under investigation in clinical studies.
In this scenario the NLR, a reliable index of systemic inflammation, represents an easy and accessible biomarker with potential application in the clinical context. Although its prognostic role in lung cancer is well established, the clinical ability of NLR in predicting ICI efficacy remains far from clear. The majority of available evidence suggests that high pretreatment NLR is associated with poor response and survival in advanced NSCLC patients treated with ICIs.23–26 Conversely other trials did not find any significant correlation between pretreatment NLR and the clinical response to nivolumab, revealing that only a lower value of NLR at the sixth week was significantly associated with better patient survival.27,28 Overall these studies have several limitations, including retrospective design, small number of patients, different drugs as well as different NLR cut-off points, thus questioning the reliability of this biomarker for clinical practice. Other groups included pretreatment NLR values along with other clinical-pathological features in the context of more complex prognostic scores aiming at identifying patients unlikely to benefit from immunotherapy.38 Considering that the systemic balance between neutrophil-related inflammation and lymphocyte-mediated anti-tumor immunity may influence clinical response to immunotherapy, it is plausible that the real-time monitoring of dynamic variations in the relative proportions of circulating immune cells under ICI therapy could more accurately reflect the therapeutic efficacy of these agents compared with baseline evaluation.
As mentioned before, the cfDNA level in the peripheral blood of patients with NSCLC provides a real-time snapshot of active tumor cell death as well as an indirect measure of overall tumor burden, thus emerging as a reliable surrogate of tumor response to immunotherapy. Recent evidence has shown that the serial monitoring of ctDNA level changes predicted tumor response and survival outcomes in patients with advanced NSCLC receiving ICI therapy.29,36 Another paper39 demonstrated that a higher tumor burden may be associated with a blunted CD8+ T-cell immune response, suggesting that a clinical response to PD-1 blockade is correlated with reinvigoration of exhausted CD8+ T-cells, which is inversely associated with a pretreatment tumor burden, thus providing a biological explanation for our findings.
The complementary effects of these two different approaches suggest an intriguing potential for the combined use of NLR and cfDNA in clinical practice. Indeed, the deterioration of tumor-associated inflammatory response reflected by an NLR increase along with the cfDNA level increment produced by tumor growth predicted a poor benefit to immunotherapy in pretreated patients with advanced NSCLC. Monitoring an individual patient’s immune repertoire in the peripheral blood provides insights into their clinical response to immunotherapies. Thus, the early assessment of such biomarkers during the course of ICI therapy could play a critical role in treatment decision, especially when the synchronous radiological evaluation is difficult or equivocal.
This study has some limitations, including the retrospective design, the small number of patients, and the heterogeneity of clinical-pathological characteristics (e.g. high percentage of men, smokers, and PS < 2) which may have produced longer survival compared with those reported in randomized trials. Different to circulating tumor-specific DNA, cfDNA may also include nontumor DNA that is elevated as a result of cancer-related systemic inflammation, thus leading to potential detection bias in our cohort. Nevertheless, we performed a thorough evaluation of a real-world series providing preliminary evidence on the potential role of a circulating biomarker and generating a study hypothesis which should be adequately addressed by prospective trials in order to identify the most appropriate cut-offs for clinical use.
In conclusion, the results of this study showed that the early combined increase of both cfDNA and NLR over the course of the first 6 weeks of nivolumab therapy predict worse survival outcomes in pretreated patients with advanced NSCLC, suggesting a potential role in the real-time monitoring of immunotherapy resistance.
Acknowledgments
F.P. and A.R. contributed to the design of the study; S.R. and H.S.P. contributed to the clinical management of the patients. F.P., L.I., A.L., and S.M. contributed to the database, providing clinical, pathological and molecular data; A.P., M.C., V.C. and V.B. contributed to the experiments; F.P., A.G., and G.G. performed data analysis and interpretation; F.P. wrote the manuscript. All authors read and approved the final version of the manuscript.
F.P., A.G. and M.C. equally contributed to this work. V.B. and A.R. are both the last authors of this work.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Antonio Russo  https://orcid.org/0000-0002-4370-2008
https://orcid.org/0000-0002-4370-2008
Contributor Information
Francesco Passiglia, Department of Surgical, Oncological and Stomatological Disciplines, University of Palermo, Palermo, Italy.
Antonio Galvano, Department of Surgical, Oncological and Stomatological Disciplines, University of Palermo, Palermo, Italy.
Marta Castiglia, Department of Surgical, Oncological and Stomatological Disciplines, University of Palermo, Palermo, Italy.
Lorena Incorvaia, Department of Surgical, Oncological and Stomatological Disciplines, University of Palermo, Palermo, Italy.
Valentina Calò, Department of Surgical, Oncological and Stomatological Disciplines, University of Palermo, Palermo, Italy.
Angela Listì, Department of Surgical, Oncological and Stomatological Disciplines, University of Palermo, Palermo, Italy.
Salvatore Mazzarisi, Medical Oncology Unit, AOU Policlinico Vittorio Emanuele, Catania, Italy.
Alessandro Perez, Department of Surgical, Oncological and Stomatological Disciplines, University of Palermo, Palermo, Italy.
Giuseppe Gallina, Department of Surgical, Oncological and Stomatological Disciplines, University of Palermo, Palermo, Italy.
Sergio Rizzo, Department of Surgical, Oncological and Stomatological Disciplines, University of Palermo, Palermo, Italy.
Hector Soto Parra, Medical Oncology Unit, AOU Policlinico Vittorio Emanuele, Catania, Italy.
Viviana Bazan, Department of Surgical, Oncological and Stomatological Disciplines, University of Palermo, Palermo, Italy.
Antonio Russo, Department of Oncology, A.O.U.P. ‘P. Giaccone’ University Hospital, 2013 ESMO Designated Centres of Integrated Oncology and Palliative Care, Via del Vespro 129, 90127 Palermo, Italy.
References
- 1. Brahmer JR. Harnessing the immune system for the treatment of non-small-cell lung cancer. J Clin Oncol 2013; 31: 1021–1028. [DOI] [PubMed] [Google Scholar]
- 2. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 5. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 7. Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27(Suppl. 5): v1–v27. [DOI] [PubMed] [Google Scholar]
- 8. Dudnik E, Moskovitz M, Daher S, et al. Effectiveness and safety of nivolumab in advanced non-small cell lung cancer: the real-life data. Lung Cancer. Epub ahead of print 23 November 2017. DOI: 10.1016/j.lungcan.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 9. Schouten RD, Muller M, de Gooijer CJ, et al. Real-life experience with nivolumab for the treatment of non-small cell lung carcinoma: data from the expanded access program and routine clinical care in a tertiary cancer centre-The Netherlands Cancer Institute. Lung Cancer. Epub ahead of print 17 November 2017. DOI: 10.1016/j.lungcan.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 10. Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017; 23: 1920–1928. [DOI] [PubMed] [Google Scholar]
- 11. Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017; 23: 4242–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peters S, Cappuzzo F, Horn L, et al. Analysis of early survival in patients with advanced non-squamous NSCLC treated with nivolumab vs docetaxel in checkmate 057. J Thorac Oncol 2017; 12: S253. [Google Scholar]
- 13. Lee CK, Man J, Lord S, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol 2017; 12: 403–407. [DOI] [PubMed] [Google Scholar]
- 14. Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-Mutant lung adenocarcinoma. Cancer Discov 2018; 8: 822–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carbone DP, Reck M, Paz-Ares L, et al. First-Line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017; 376: 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lizotte PH, Ivanova EV, Awad MM, et al. Multiparametric profiling of non-small-cell lung cancers reveals distinct immunophenotypes. JCI Insight 2016; 1: e89014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgs BW, Morehouse CA, Streicher K, et al. A baseline IFNG gene expression signature correlates with clinical outcomes in durvalumab-treated advanced NSCLC cancer patients. Proceedings of the American Association for Cancer Research Annual Meeting 2017; 2017 Apr 1–5; Washington, DC Philadelphia (PA): AACR; Cancer Res 2017;77(13 Suppl.): Abstract nr 1773. [Google Scholar]
- 18. Karachaliou N, Gonzalez-Cao M, Crespo G, et al. Interferon gamma, an important marker of response to immune checkpoint blockade in non-small cell lung cancer and melanoma patients. Ther Adv Med Oncol 2018; 10: 1758834017749748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mei Z, Shi L, Wang B, et al. Prognostic role of pretreatment blood neutrophil-to-lymphocyte ratio in advanced cancer survivors: a systematic review and meta-analysis of 66 cohort studies. Cancer Treat Rev 2017; 58: 1–13. [DOI] [PubMed] [Google Scholar]
- 20. Yu Y, Qian L, Cui J. Value of neutrophil-to-lymphocyte ratio for predicting lung cancer prognosis: a meta-analysis of 7,219 patients. Mol Clin Oncol 2017; 7: 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Derman BA, Macklis JN, Azeem MS, et al. Relationships between longitudinal neutrophil to lymphocyte ratios, body weight changes, and overall survival in patients with non-small cell lung cancer. BMC Cancer 2017; 17: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte Ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017; 111: 176–181. [DOI] [PubMed] [Google Scholar]
- 23. Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017; 106: 1–7. [DOI] [PubMed] [Google Scholar]
- 24. Tanizaki J, Haratani K, Hayashi H, et al. Peripheral blood biomarkers associated with clinical outcome in non-small cell lung cancer patients treated with nivolumab. J Thorac Oncol 2018; 13: 97–105. [DOI] [PubMed] [Google Scholar]
- 25. Zer A, Sung MR, Walia P, et al. Correlation of Neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD-1 axis inhibitors in patients with advanced non-small-cell lung cancer. Clin Lung Cancer 2018; 19: 426–434.e1. [DOI] [PubMed] [Google Scholar]
- 26. Fukui T, Okuma Y, Nakahara Y, et al. Activity of nivolumab and utility of neutrophil-to-lymphocyte ratio as a predictive biomarker for advanced non-small-cell lung cancer: a prospective observational study. Clin Lung Cancer 2018; pii: S1525–7304(18)30103-7. [DOI] [PubMed] [Google Scholar]
- 27. Suh KJ, Kim SH, Kim YJ, et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother 2018; 67: 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Putzu C, Cortinovis DL, Colonese F, et al. Blood cell count indexes as predictors of outcomes in advanced non-small-cell lung cancer patients treated with Nivolumab. Cancer Immunol Immunother 2018; 67: 1349–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cabel L, Riva F, Servois V, et al. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann Oncol 2017; 28: 1996–2001. [DOI] [PubMed] [Google Scholar]
- 30. Tamiya M, Tamiya A, Inoue T, et al. Metastatic site as a predictor of nivolumab efficacy in patients with advanced non-small cell lung cancer: a retrospective multicenter trial. PLoS One 2018; 13: e0192227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Funazo T, Nomizo T, Kim YH. Liver metastasis is associated with poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. J Thorac Oncol 2017; 12: e140–e141. [DOI] [PubMed] [Google Scholar]
- 32. Dias M, Antunes A, Campainha S, et al. Prognostic impact of M descriptors of the 8th edition of TNM classification of lung cancer. J Thorac Dis 2017; 9: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vecchiarelli S, Passiglia F, D’Incecco A, et al. Circulating programmed death ligand-1 (cPD-L1) in non-small-cell lung cancer (NSCLC). Oncotarget 2018; 9: 17554–17563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 2018; 24: 1441–1448. [DOI] [PubMed] [Google Scholar]
- 35. Borghaei H, Brahmer J, Horn L, et al. Nivolumab (nivo) vs docetaxel (doc) in patients (pts) with advanced NSCLC: checkMate 017/057 2-y update and exploratory cytokine profile analyses. J Clin Oncol 2016; 34: 9025–9025. [Google Scholar]
- 36. Goldberg SB, Narayan A, Kole AJ, et al. Early assessment of lung cancer immunotherapy response via circulating tumor DNA. Clin Cancer Res 2018; 24: 1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kamphorst AO, Pillai RN, Yang S, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci USA 2017; 114: 4993–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol 2018; 4: 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017; 545: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]