Abstract
Background:
Adjuvant chemotherapy (AC) is known to be beneficial for stage III colorectal cancer (CRC). In contrast, only a few studies have reported the survival benefits of AC for stage IV CRC after curative surgery.
Methods:
We identified 155 CRC patients with various organ metastases who underwent curative surgery in our hospital between 2003 and 2017. Clinicopathological parameters and postoperative AC were reviewed. Multivariate analyses were performed to identify prognostic factors. Moreover, the effects of AC on recurrence-free survival (RFS) and overall survival (OS) were analyzed using inverse probability of treatment weighting.
Results:
The cohort comprised 94 males and 61 females, with a mean age of 63 years. AC was administered to 57% of patients who underwent surgery between 2003 and 2010 and 76% between 2011 and 2017 (p = 0.015). AC was more likely administered to patients with a good performance status, high preoperative albumin level, regional node and peritoneal metastases, and no intraoperative blood transfusion. Multivariate analyses identified AC as a significant prognostic factors for RFS and OS [hazard ratio (HR): 1.86, p = 0.003, and 2.66, p = 0.002, respectively]. After adjusting for different backgrounds, 5-year RFS and OS rates were higher in patients receiving AC (27% and 67%) than in those without AC (14% and 46%, p < 0.0001 and p = 0.0005). Subgroup analyses showed that AC significantly improved RFS in node-negative patients (HR: 2.16, p = 0.029), and RFS and OS in node-positive patients (HR: 2.03, p < 0.0001, and 2.02, p = 0.001, respectively).
Conclusion:
AC can be discussed with resectable stage IV CRC patients because of its significant survival-improving effects.
Keywords: adjuvant chemotherapy, colorectal cancer, inverse probability of treatment weighting, prognosis, regional lymph node metastases, stage IV
Introduction
Multimodality treatment has been the basic tenet in the treatment of advanced colorectal cancer (CRC) with the aim of improving prognoses.1 In addition to complete surgical resection, adjuvant chemotherapy (AC) in resected CRC has been attracting increasing interest. Previous clinical trials showed that 5-fluorouracil (5-FU) based AC was effective for reducing recurrence and thereby contributed to longer overall survival (OS) in patients with stage III CRC.2–6 Moreover, oxaliplatin-including AC further improved the long-term prognosis of stage III colon patients.7–9 However, the efficacy of AC after curative resection for stage IV CRC has been debated, with conflicting reports of benefits. Several randomized trials on 5-FU-based AC failed to demonstrate any survival benefit in CRC patients who underwent the resection of liver metastases after curative resection.10–12 On the other hand, a recent phase III randomized controlled trial (RCT) showed that oral tegafur/uracil and leucovorin significantly prolonged recurrence-free survival (RFS) in CRC patients with synchronous or metachronous liver-limited metastases after primary resection and hepatectomy.13 However, the trial did not prove an OS benefit by oral AC in this patient cohort.13 Moreover, the efficacy of AC after curative resection for CRC with synchronous metastases in various organs remains unclear because of the paucity of systematic studies.
In stage II CRC, AC has only been recommended for selected patients with high-risk features, such as pathological T4, poorly differentiated histology, suboptimal lymph node retrieval, bowel perforation, bowel obstruction, lymphatic and venous invasion, perineural invasion, and positive resection margins.14–17 Subgroup analyses of controlled trials on stage II and III CRC have generally indicated that survival rates were increased by AC in patients with stage III disease, but not in those with stage II.3,7,18,19 These findings suggest that the benefit of adjuvant therapy partly depends on the nodal status,19 although DNA microsatellite instability (MSI) status was also reported to predict the efficacy of 5-FU-based AC.20,21 Furthermore, retrospective cohort studies recently demonstrated that the presence of regional lymph node metastases had a negative impact on survival in patients with resectable and/or unresectable stage IV CRC.22–24 The different efficacy of AC between stage II and stage III CRC motivated us to investigate whether the effectiveness of AC depends on regional spread of cancer or any cancer spread beyond the primary tumor in stage IV CRC.
Toward this end, the present study investigated the clinical significance of AC in CRC patients with various organ metastases after curative resection using a propensity score method. The associations between the effectiveness of AC and clinicopathological factors including regional nodal involvement were also analyzed.
Patients and methods
This study was conducted with the approval of the ethics committee of the University of Tokyo Hospital (reference number: 3252-6). For inclusion in this study, written informed consent was obtained from each patient, and the opportunity to opt out was also offered.
Patients and clinicopathological parameters
We retrospectively reviewed consecutive CRC patients diagnosed with synchronous distant organ metastases who underwent curative surgery in the University of Tokyo Hospital between January 2003 and December 2017. Patients who underwent chemotherapy and/or radiotherapy before radical surgery and those with multiple primary CRCs were excluded from the present study. Demographic data, such as the period of surgery, gender, age, the European Cooperative Oncology Group Performance Status (PS), and serum levels of hemoglobin, albumin, and the tumor markers carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9 at diagnosis and immediately before surgery were retrospectively retrieved from medical records. In our institute, the lower normal limit of serum hemoglobin is 13.7 g/dl (men) or 11.6 g/dl (women), and that of serum albumin is 4.1 g/dl, whereas the upper normal limits of serum CEA and CA 19-9 are 5.0 ng/ml and 37 U/ml, respectively. Information was obtained on the primary tumor, including location, size, obstruction, histology, tumor depth, regional lymph node metastasis, lymphatic and venous invasion, the KRAS exon 2 status, and MSI status judged based on two mononucleotide repeat markers, BAT25 and BAT26,25 if available; all resected specimens were histologically examined and documented according to the guidelines established by the eighth edition of the American Joint Committee on Cancer staging system26 and the guidelines of the Japanese Society for Cancer of the Colon and Rectum (JSCCR).27 Metastasized organs, intraoperative blood transfusions, and postoperative complications graded by the Clavien–Dindo classification28 were also reviewed.
Adjuvant chemotherapy and follow-up
Typical AC included oral 5-FU (+ folinate) and oral/infusional 5-FU and oxaliplatin for 6 months. The duration was chosen in accordance with the National Comprehensive Cancer Network guidelines.15,16 mFOLFOX6 (oxaliplatin 85 mg/m2 and folinate 200 mg/m2, followed by 5-FU as a 400 mg/m2 intravenous bolus and 2400 mg/m2 infusion over 46 h) was administered every 2 weeks (total: 12 planned cycles).29 CapeOX (oxaliplatin 130 mg/m2 over 2 hours on day 1 and oral capecitabine 1000 mg/m2 twice daily on days 1–14) was repeated every 3 weeks (total: eight planned cycles).9 SOX (oxaliplatin 130 mg/m2 over 2 hours on day 1 and oral S-1 40 mg/m2 twice daily on days 1–14) was repeated every 3 weeks (total: eight planned cycles).30 Some patients were recruited into RCTs; for example, a trial investigating tegafur/uracil after R0 resection for liver metastases from CRC conducted in our hospital between 2004 and 2010.13 In other patients, the implementation of AC depended on the patient’s preference and condition and/or the doctor’s discretion. The severity of adverse events and laboratory findings were graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.31
Patients were evaluated for tumor recurrence by a physical examination, serum tumor marker measurement, computed tomography scans every 6 months, and colonoscopy annually after curative surgery. When a new manifestation of symptoms occurred suggesting recurrent disease or serum tumor marker levels increased in a rapid manner, imaging studies including other modalities such as magnetic resonance imaging and positron emission tomography were additionally performed. RFS was defined as the time between the complete removal and diagnosis of any recurrence, and OS as the time between the first surgery and death from any cause. Data were collected up to the end of August 2018.
Statistical analysis
Categorized data in each group were compared by the Chi-squared or Fisher’s exact probability test, whereas continuous variables were compared by Student’s t test or Wilcoxon rank-sum test. Prognostic factors were estimated by univariate and multivariate analyses using Cox’s proportional hazard model, in which several variables were dichotomized by the mean value or normal limit (age, hemoglobin and albumin levels, tumor size, pT, and pN) or trichotomized (tumor location). The inverse probability of the treatment weighting (IPTW) method32,33 was used to reduce the selection bias between the two treatment groups. Covariates in the models for propensity scores included all clinicopathological factors, except for the KRAS exon 2 and MSI status because there were patients without the results of these genetic tests. In order to estimate RFS and OS, the adjusted Kaplan–Meier method with the log-rank test was performed by weighting each patient receiving AC by the inverse of the propensity score and each patient without AC by the inverse of (1 – the propensity score). The statistical analysis of data was performed using JMP Pro 14.0.0 software (SAS Institute Inc., Cary, NC, USA), and p values < 0.05 were considered to be significant.
Results
Patients and adjuvant chemotherapy
Among 155 patients (94 males and 61 females, mean age: 62.8 years old) with stage IV CRC during the study period, 103 (66%) received AC; 51 received 5-FU (and folinate), 30 mFOLFOX6, 15 CapeOX, and 7 SOX. When all patients were divided into two groups according to the period of surgery (2003–2010 and 2011–2017), the first period accounted for 63% of patients who did not receive AC whereas the second period accounted for 57% of patients treated with AC (p = 0.015). Fifteen patients were participants in previous RCTs, 11 of whom were administered AC (two tegafur/uracil plus folinate, two FOLFOX, and seven SOX). Table 1 summarizes the characteristics of stage IV CRC patients stratified by AC. Regional lymph node metastases were detected more frequently in patients treated with AC than in those not treated with AC (83% versus 69%, p = 0.041). Patients treated with AC were also characterized by a more frequent PS of 0 (98% versus 85%, p = 0.003), higher level of albumin (mean: 3.8 g/dl versus 3.6 g/dl, p = 0.011), more frequent peritoneal metastasis (27% versus 10%, p = 0.012), and less frequent blood transfusions (15% versus 35%, p = 0.004). No significant differences were observed in other parameters between these two groups. The characteristics of stage IV CRC patients who received AC were similar between 5-FU-based and oxaliplatin-including regimens, except for the period of surgery (Supplemental Table 1); 73% of patients who underwent surgery in 2003–2010 were treated by 5-FU-based AC, whereas 87% of those in 2011–2017 were treated by oxaliplatin-based AC (p < 0.0001).
Table 1.
Baseline characteristics of patients with stage IV colorectal cancer according to adjuvant chemotherapy.
| Variables | No AC |
AC |
p value | |
|---|---|---|---|---|
| (n = 52) | (n = 103) | |||
| Period of surgery | 2003–2010 | 33 (63%) | 44 (43%) | 0.015 |
| 2011–2017 | 19 (37%) | 59 (57%) | ||
| Age, year | Mean ± SD | 64.5 ± 12.3 | 62.0 ± 10.7 | 0.18 |
| Gender | Male | 35 (67%) | 59 (57%) | 0.23 |
| ECOG PS | 0 | 44 (85%) | 101 (98%) | 0.003 |
| 1 | 8 (15%) | 2 (2%) | ||
| Hemoglobin, g/dl | Mean ± SD | 11.7 ± 2.5 | 12.1 ± 1.8 | 0.29 |
| Albumin, g/dl | Mean ± SD | 3.6 ± 0.5 | 3.8 ± 0.4 | 0.011 |
| CEA | Elevated | 37 (71%) | 79 (77%) | 0.45 |
| CA 19-9 | Elevated | (44%) | 50 (49%) | 0.61 |
| Primary site | Right-sided colon | 11 (21%) | 27 (26%) | 0.19 |
| Left-sided colon | 15 (29%) | 40 (39%) | ||
| Rectum | 26 (50%) | 36 (35%) | ||
| Size of primary cancer, mm a | Mean ± SD | 58.0 ± 21.2 | 52.2 ± 20.7 | 0.11 |
| Obstruction | Yes | 24 (46%) | 52 (50%) | 0.61 |
| Histology | Differentiatedb | 48 (92%) | 91 (88%) | 0.63 |
| Others | 4 (8%) | 12 (12%) | ||
| Depth | -pT3 | 26 (50%) | 39 (38%) | 0.15 |
| pT4 | 26 (50%) | 64 (62%) | ||
| Regional lymph node metastasis | Yes | 36 (69%) | 86 (83%) | 0.041 |
| Lymphatic invasion | Yes | 23 (44%) | 62 (60%) | 0.059 |
| Venous invasion | Yes | 46 (88%) | 93 (90%) | 0.94 |
| KRAS exon 2 statusc | Wild-type | 13 (76%) | 26 (54%) | 0.19 |
| Mutant | 4 (24%) | 22 (46%) | ||
| DNA microsatellite instability c | Stable | 33 (100%) | 79 (100%) | 1.00 |
| Number of metastasized organs | 1 | 50 (96%) | 90 (87%) | 0.092 |
| 2 | 2 (4%) | 13 (13%) | ||
| Metastasized organs | Liver | 42 (81%) | 72 (70%) | 0.15 |
| Lung | 3 (6%) | 8 (8%) | 0.75 | |
| Distant lymph nodes | 3 (6%) | 4 (4%) | 0.69 | |
| Peritoneum | 5 (10%) | 28 (27%) | 0.012 | |
| Ovary | 1 (2%) | 2 (2%) | 1.00 | |
| Others | 0 (0%) | 2 (2%) | 0.55 | |
| Blood transfusions | Yes | 18 (35%) | 15 (15%) | 0.004 |
| Postoperative complications | Grade 2- | 24 (46%) | 36 (35%) | 0.18 |
AC, adjuvant chemotherapy; CA 19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; ECOG, Eastern Cooperative Oncology Group; PS, performance status; SD, standard deviation.
excluding one unavailable case; b differentiated adenocarcinoma; c excluding cases that were not evaluated.
The mean period of AC was 6.7 months. Adverse events were assessable in 101 out of the 103 patients who underwent AC. Neutropenia was the most common as a grade 3 or grade 4 event (16 patients, 16%), followed by neuropathy (three patients, 3%), infection (two patients, 2%), and diarrhea (two patients, 2%). No AC-related deaths occurred.
Prognostic factors in stage IV CRC
The median follow-up time was 54.3 months. Postoperative recurrence was observed in 43 (83%) of the 52 patients without AC and 71 (69%) of the 103 patients who received AC. Twenty-five patients without AC and 34 with AC died during the follow-up period. In order to confirm whether AC is a significant prognostic factor in stage IV CRC, we conducted univariate and multivariate analyses on RFS and OS. As shown in Table 2, surgery in 2003–2010, lymphatic invasion, and no AC were independent parameters associated with worse RFS. Table 3 shows the results of univariate and multivariate analyses for OS. Elevated CA 19-9, histology other than differentiated adenocarcinoma, lymphatic invasion, and no AC were independent factors for predicting worse OS.
Table 2.
Analyses of predictive factors for recurrence-free survival.
| Variables | Univariate |
Multivariate |
|
|---|---|---|---|
| p value | Hazard ratio (95% CI) | p value | |
| Period of surgery | 0.0006 | 1.52 (1.01–2.29) | 0.044 |
| Age (< 63 versus ⩾ 63 years old) | 0.042 | 1.42 (0.97–2.10) | 0.073 |
| Sex (male versus female) | 0.16 | ||
| ECOG PS (1 versus 0) | 0.45 | ||
| Hemoglobin (high versus normal) | 0.50 | ||
| Albumin (high versus normal) | 0.60 | ||
| CEA (high versus normal) | 0.62 | ||
| CA 19-9 (high versus normal) | 0.24 | ||
| Primary site (rectum versus left-sided colon/right-sided colon) | 0.059 | ||
| Size of primary cancer (< 55 versus ⩾ 55 mm) | 0.26 | ||
| Obstruction (yes versus no) | 0.79 | ||
| Histology (differentiated a versus others) | 0.10 | ||
| Depth (pT4 versus pT3) | 0.12 | ||
| Lymphatic invasion (yes versus no) | 0.031 | 1.54 (1.05–2.26) | 0.026 |
| Venous invasion (yes versus no) | 0.94 | ||
| KRAS exon 2 status (mutant versus wild-type) | 0.61 | ||
| Liver metastases (yes versus no) | 0.36 | ||
| Lung metastases (yes versus no) | 0.076 | ||
| Distant lymph node metastases (yes versus no) | 0.92 | ||
| Peritoneal metastases (yes versus no) | 0.14 | ||
| Ovarian metastases (yes versus no) | 0.87 | ||
| Blood transfusions (yes versus no) | 0.032 | 1.45 (0.92–2.24) | 0.11 |
| Postoperative complications (grade 2 versus grade 0/1) | 0.096 | ||
| Adjuvant chemotherapy (no versus yes) | 0.0007 | 1.86 (1.24–2.77) | 0.003 |
CA 19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
differentiated adenocarcinoma.
Table 3.
Analyses of predictive factors for overall survival.
| Variables | Univariate |
Multivariate |
|
|---|---|---|---|
| p value | Hazard ratio (95% CI) | p value | |
| Period of surgery | 0.18 | ||
| Age (< 63 versus ⩾ 63 years old) | 0.83 | ||
| Sex (male versus female) | 0.70 | ||
| ECOG PS (1 versus 0) | 0.020 | 2.55 (0.86–6.42) | 0.087 |
| Hemoglobin (high versus normal) | 0.67 | ||
| Albumin (high versus normal) | 0.82 | ||
| CEA (high versus normal) | 0.87 | ||
| CA 19-9 (high versus normal) | 0.037 | 2.29 (1.34–3.96) | 0.003 |
| Primary site (rectum versus left-sided colon/right-sided colon) | 0.31 | ||
| Size of primary cancer (< 55 versus ⩾ 55 mm) | 0.52 | ||
| Obstruction (yes versus no) | 0.090 | ||
| Histology (differentiated a versus others) | 0.002 | 0.40 (0.19–0.87) | 0.023 |
| Depth (pT4 versus pT3) | 0.080 | ||
| Lymphatic invasion (yes versus no) | 0.033 | 1.83 (1.03–3.33) | 0.039 |
| Venous invasion (yes versus no) | 0.78 | ||
| KRAS exon 2 status (mutant versus wild-type) | 0.15 | ||
| Liver metastases (yes versus no) | 0.002 | 0.82 (0.34–2.11) | 0.67 |
| Lung metastases (yes versus no) | 0.37 | ||
| Distant lymph node metastases (yes versus no) | 0.17 | ||
| Peritoneal metastases (yes versus no) | 0.007 | 2.15 (0.87–5.55) | 0.10 |
| Ovarian metastases (yes versus no) | 0.47 | ||
| Blood transfusions (yes versus no) | 0.29 | ||
| Postoperative complications (grade 2 versus grade 0/1) | 0.024 | 1.61 (0.95–2.76) | 0.079 |
| Adjuvant chemotherapy (no versus yes) | 0.007 | 2.66 (1.44–4.92) | 0.002 |
CA 19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Differentiated adenocarcinoma.
RFS and OS in propensity-matched cohorts
In order to mitigate clinicopathological backgrounds between patients receiving AC and those who did not, we performed propensity score matching using the IPTW method. We analyzed RFS according to AC in the adjusted cohorts of stage IV CRC patients. The 2-year and 5-year RFS rates were 28% and 25% for patients receiving AC, which were higher than those for patients without AC (19% and 16%, p < 0.0001, Figure 1). Similarly, OS curves were compared between patients receiving AC and those without AC in the same adjusted cohorts. The 2-year and 5-year OS rates were 88% and 66% for patients receiving AC, which were higher than those for patients without AC (76% and 46%, p = 0.0008, Figure 2).
Figure 1.

Adjusted recurrence-free survival curves in stage IV colorectal cancer patients according to adjuvant chemotherapy (AC) using the inverse probability of treatment weighted method. The bold line indicates the survival curve of patients receiving AC, and the dashed line that of those who did not receive AC.
Figure 2.

Adjusted overall survival curves in stage IV colorectal cancer patients according to adjuvant chemotherapy (AC) using the inverse probability of treatment weighted method. The bold line indicates the survival curve of patients receiving AC, and the dashed line that of those who did not receive AC.
Subgroup analyses for RFS and OS
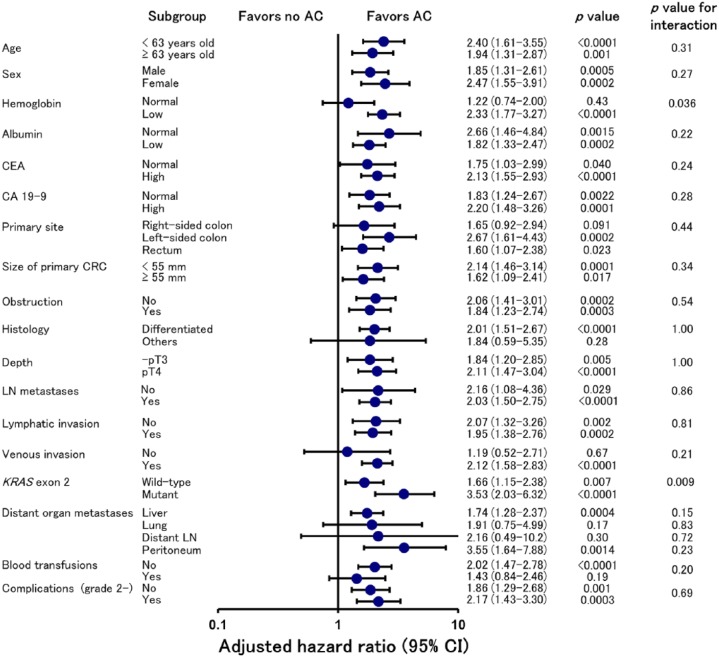
We performed subgroup analyses to further explore the advantages of AC in the adjusted cohorts stratified by clinicopathological factors. Figure 3 shows the results of subgroup analyses for RFS. Generally, the effect of AC on reducing the risk of recurrence was consistent across subgroups, with the exception of hemoglobin level, primary tumor location, histology, venous invasion, lung and distant lymph node metastases, and intraoperative blood transfusions. AC improved RFS in both patients with regional lymph node metastases (pN+ ) and those without nodal involvement (pN0) [hazard ratio: 2.16, 95% confidence interval (CI): 1.08–4.36, p = 0.029, and hazard ratio: 2.03, 95% CI: 1.50–2.75, p < 0.0001, respectively].
Figure 3.
Adjusted hazard ratio for recurrence-free survival with adjuvant chemotherapy (AC) versus no AC according to subgroups defined on the basis of baseline factors and perioperative variables.
CI, confidence interval.
In contrast, subgroups that showed a reduced risk of death were limited, as shown in Figure 4. AC markedly improved OS in patients with pN+ (hazard ratio: 2.02, 95% CI: 1.33–3.06, p = 0.001), whereas the effect of AC on OS was not significant in pN0 patients (hazard ratio: 2.02, 95% CI: 0.53–6.00, p = 0.36). However, the p value for interaction between AC and regional nodal metastases was 0.78.
Figure 4.
Adjusted hazard ratio for overall survival with adjuvant chemotherapy (AC) versus no AC according to subgroups defined on the basis of baseline factors and perioperative variables.
CI, confidence interval.
Discussion
Optimal treatments for patients with resectable stage IV CRC remain controversial. Standard treatments include complete resection of the primary tumor and all metastases when they were limited.15,16 In resectable stage IV CRC without any preoperative therapy, retrospective studies previously showed the positive impact of AC on postoperative survival.22,34,35 In addition, an RCT showed a significant increase in RFS by oral 5-FU and folinate, whereas OS was not affected by this AC regimen after the resection of synchronous or metachronous liver-limited metastases from CRC.13 Since the percentage of pN+ patients who received AC was significantly smaller than those who did not in that study (p = 0.041),13 the imbalance observed in the regional nodal status appears to have accounted for the improved RFS for the AC group. Therefore, we used the IPTW method in order to mitigate differences in pN and other clinicopathological factors that potentially affect long-term outcomes; we demonstrated that AC significantly prolonged RFS and OS in stage IV CRC patients. Moreover, the subgroup analyses showed that AC improved RFS in both pN0 and pN+ patients and OS in pN+ patients. Hence, the benefit of AC in stage IV CRC appeared independent of regional nodal involvement.
Difficulties are associated with conducting prospective studies on AC for stage IV CRC with extrahepatic distant metastases because it is less common than hepatic metastases. Alternatively, retrospective studies shared the majority of publications regarding this issue. The clinical significance of AC after pulmonary metastasectomy in CRC remains controversial. A French multi-institutional study showed slightly reduced rates of recurrence in patients with AC,36 and a pooled analysis of published data did not reveal any change in OS by AC for resected lung metastases from CRC.37 On the other hand, a single-center Korean study reported significant increases in OS by AC in CRC patients with resectable lung metastases; however, more than half of these patients had also received preoperative chemotherapy.38 Furthermore, similar to the EPOC trial in CRC with resectable liver metastases,39 several groups attempted perioperative chemotherapy for patients with resectable lung metastases from CRC.40,41 Regarding peritoneal metastases, a multi-institutional database analysis of the JSCCR showed that AC was a significant factor for prolonged OS after the complete removal of peritoneal dissemination.23 The effects of AC after the removal of primary CRC with synchronous involvement in distant lymph nodes, such as those of the para-aortic region, have not yet been examined.
In the present study, elevated CA 19-9, undifferentiated histology, and lymphatic invasion were other independent prognostic factors in resectable stage IV CRC. Several groups, including ours, reported elevated CA 19-9 as a prognostic marker in resectable stage IV CRC.23,42 Shibutani and colleagues reported that tumor differentiation and lymphatic invasion together with preoperative serum C-reactive protein level were biomarkers for identifying patients with a poor cancer-specific survival for resectable and nonresectable stage IV CRC,43 whereas our group previously reported lymphatic invasion as an independent factor for reduced RFS in stage IV CRC after R0 resection by a multivariate analysis.42
Several pivotal studies demonstrated the benefits of oxaliplatin-based AC in addition to 5-FU for stage III CRC.6–8 More than 50% of stage IV CRC cases relapse even after curative resection;1 therefore, more intensive AC may be required to reduce recurrence. It remains unclear whether intensive regimens such as oxaliplatin-including therapy are more beneficial than 5-FU-based regimens for stage IV CRC. The aforementioned retrospective JSCCR study on R0 resection cases of peritoneal metastases showed that prognoses were better in patients receiving oxaliplatin-including AC.23 Nakayama and colleagues reported that oxaliplatin-based chemotherapy was a feasible AC option for stage IV CRC regardless of hepatic and extrahepatic metastases.44 mFOLFOX6 and SOX are currently being prospectively tested as AC regimens in stage IV CRC after curative hepatic resection.45,46
There were several limitations in the present study due to its retrospective nature. The distribution and number of metastatic lesions varied among patients. AC included several different regimens and durations. The choice of AC and its regimen depended on patients’ preferences and conditions and doctors’ discretion for most patients. In addition, newer patients were more likely to receive AC, which may affect the survival outcomes. Moreover, we could not address the impact of MSI status because there were no MSI-positive cases in our cohort; there might be false-negative MSI cases, although Jass and colleagues mentioned that MSI tumors can be most efficiently screened by the combination of BAT25 and BAT26.25 In addition, there was no available data on MSI in patients who underwent surgery in the early days.
In conclusion, we herein demonstrated that AC had markedly positive impacts on RFS and OS in patients with curatively resected stage IV CRC. Taking into account the limitations of our study just mentioned, AC can be discussed with all resectable stage IV CRC patients. Our results need to be confirmed by further investigation, such as an independent validation study using another set of stage IV CRC patients. It is also very important to find reliable biomarkers associated with the efficacy of AC in resectable stage IV CRC.
Supplemental Material
Supplemental material, AdjCTXM1_STablesTherAdvMedOncolrev_1 for Adjuvant chemotherapy improves prognosis of resectable stage IV colorectal cancer: a comparative study using inverse probability of treatment weighting by Hiroaki Nozawa, Hirotoshi Takiyama, Kiyoshi Hasegawa, Kazushige Kawai, Keisuke Hata, Toshiaki Tanaka, Takeshi Nishikawa, Kazuhito Sasaki, Manabu Kaneko, Koji Murono, Shigenobu Emoto, Hirofumi Sonoda and Jun Nakajima in Therapeutic Advances in Medical Oncology
Footnotes
Funding: This work was supported by Grants-in-Aid for Scientific Research (grant numbers: 16K07143, 16K07161, 17K10620, 17K10621, 17K10623, 18K07194) from the Japan Society for the promotion of Science, and by the Project for Cancer Research and Therapeutic Evolution (P-CREATE, grant number: JP18cm0106502h0003) from the Japan Agency for Medical Research and Development (AMED). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest statement: Hiroaki Nozawa reports endowments for research from Taiho Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd. and Yakult Pharmaceutical Industry Co. Ltd. Kiyoshi Hasegawa reports endowments for research and lecture fees from Taiho Pharmaceutical Co. Ltd., and endowments for research from Chugai Pharmaceutical Co. Ltd. and Yakult Pharmaceutical Industry Co. Ltd. Jun Nakajima reports lecture fees from Chugai Pharmaceutical Co. Ltd. and Kyowa Hakko Kirin Co. Ltd., and endowments for research from Taiho Pharmaceutical Co. Ltd. The remaining authors declare that there is no conflict of interest.
Supplemental material: Supplemental material is available for this article online.
ORCID iD: Hiroaki Nozawa  https://orcid.org/0000-0002-7891-5986
https://orcid.org/0000-0002-7891-5986
Contributor Information
Hiroaki Nozawa, Department of Surgical Oncology, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8655, Japan.
Hirotoshi Takiyama, Department of Surgical Oncology, The University of Tokyo, Tokyo, Japan.
Kiyoshi Hasegawa, Department of Hepatobiliary and Pancreatic Surgery, The University of Tokyo, Tokyo, Japan.
Kazushige Kawai, Department of Surgical Oncology, The University of Tokyo, Tokyo, Japan.
Keisuke Hata, Department of Surgical Oncology, The University of Tokyo, Tokyo, Japan.
Toshiaki Tanaka, Department of Surgical Oncology, The University of Tokyo, Tokyo, Japan.
Takeshi Nishikawa, Department of Surgical Oncology, The University of Tokyo, Tokyo, Japan.
Kazuhito Sasaki, Department of Surgical Oncology, The University of Tokyo, Tokyo, Japan.
Manabu Kaneko, Department of Surgical Oncology, The University of Tokyo, Tokyo, Japan.
Koji Murono, Department of Surgical Oncology, The University of Tokyo, Tokyo, Japan.
Shigenobu Emoto, Department of Surgical Oncology, The University of Tokyo, Tokyo, Japan.
Hirofumi Sonoda, Department of Surgical Oncology, The University of Tokyo, Tokyo, Japan.
Jun Nakajima, Department of Thoracic Surgery, The University of Tokyo, Tokyo, Japan.
References
- 1. Ahmed S, Johnson K, Ahmed O, et al. Advances in the management of colorectal cancer: from biology to treatment. Int J Colorectal Dis 2014; 29: 1031–1042. [DOI] [PubMed] [Google Scholar]
- 2. Moertel CG, Fleming TR, Macdonald JS, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med 1995; 122: 321–326. [DOI] [PubMed] [Google Scholar]
- 3. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet 1995; 345: 939–944. [PubMed] [Google Scholar]
- 4. O’Connell MJ, Mailliard JA, Kahn MJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 1997; 15: 246–250. [DOI] [PubMed] [Google Scholar]
- 5. Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005: 352: 2696–2704. [DOI] [PubMed] [Google Scholar]
- 6. Yoshida M, Ishiguro M, Ikejiri K, et al. S-1 as adjuvant chemotherapy for stage III colon cancer: a randomized phase III study (ACTS-CC trial). Ann Oncol 2014; 25: 1743–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 27: 3109–3116. [DOI] [PubMed] [Google Scholar]
- 8. Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011; 29: 3768–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011; 29: 1465–1471. [DOI] [PubMed] [Google Scholar]
- 10. Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol 2006; 24: 4976–4982. [DOI] [PubMed] [Google Scholar]
- 11. Mitry E, Fields AL, Bleiberg H, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol 2008; 26: 4906–4911. [DOI] [PubMed] [Google Scholar]
- 12. Ychou M, Hohenberger W, Thezenas S, et al. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol 2009; 20: 1964–1970. [DOI] [PubMed] [Google Scholar]
- 13. Hasegawa K, Saiura A, Takayama T, et al. Oral uracil-tegafur with leucovorin for colorectal cancer liver metastases: a randomized controlled trial. PLoS One 2016; 11: e0162400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benson AB, 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 2004; 22: 3408–3419. [DOI] [PubMed] [Google Scholar]
- 15. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Colon Cancer, version 3, 2018, https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (2018, accessed 10 September 2018).
- 16. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Rectal Cancer, version 3 2018, https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (2018, accessed 10 September 2018).
- 17. Labianca R, Nordlinger B, Beretta GD, et al. Early colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24: vi64–vi72. [DOI] [PubMed] [Google Scholar]
- 18. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. J Clin Oncol 1999; 17: 1356–1363. [PubMed] [Google Scholar]
- 19. Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004; 22: 1797–1806. [DOI] [PubMed] [Google Scholar]
- 20. Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003; 349: 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010; 28: 3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kobayashi H, Kotake K, Sugihara K, et al. Impact of adjuvant chemotherapy in patients with curatively resected stage IV colorectal cancer. Medicine (Baltimore) 2015; 94: e696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sato H, Kotake K, Sugihara K, et al. Clinicopathological factors associated with recurrence and prognosis after R0 resection for stage IV colorectal cancer with peritoneal metastasis. Dig Surg 2016; 33: 382–391. [DOI] [PubMed] [Google Scholar]
- 24. Ahmed S, Leis A, Chandra-Kanthan S, et al. Regional lymph nodes status and ratio of metastatic to examined lymph nodes correlate with survival in stage IV colorectal cancer. Ann Surg Oncol 2016; 23: 2287–2294. [DOI] [PubMed] [Google Scholar]
- 25. Jass JR, Walsh MD, Barker M, et al. Distinction between familial and sporadic forms of colorectal cancer showing DNA microsatellite instability. Eur J Cancer 2002; 38: 858–866. [DOI] [PubMed] [Google Scholar]
- 26. Amin MB, Edge S, Greene F, et al. AJCC cancer staging manual. 8th ed. New York: Springer, 2017. [Google Scholar]
- 27. Japanese Society for Cancer of the Colon and Rectum. JSCCR guidelines 2014 for the treatment of colorectal cancer. Tokyo: Kanehara and Co. Ltd, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004; 22: 229–237. [DOI] [PubMed] [Google Scholar]
- 30. Hong YS, Park YS, Lim HY, et al. S-1 plus oxaliplatin versus capecitabine plus oxaliplatin for first-line treatment of patients with metastatic colorectal cancer: a randomised, non-inferiority phase 3 trial. Lancet Oncol 2012; 13: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 31. National Cancer Institute, Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE) v5.0, https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 (2017, accessed 10 September 2018).
- 32. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004; 75: 45–49. [DOI] [PubMed] [Google Scholar]
- 34. Nozawa H, Kitayama J, Sunami E, et al. FOLFOX as adjuvant chemotherapy after curative resection of distant metastases in patients with colorectal cancer. Oncology 2011; 80: 84–91. [DOI] [PubMed] [Google Scholar]
- 35. Kim HR, Min BS, Kim JS, et al. Efficacy of oxaliplatin-based chemotherapy in curatively resected colorectal cancer with liver metastasis. Oncology 2011; 81: 175–183. [DOI] [PubMed] [Google Scholar]
- 36. Pagès PB, Serayssol C, Brioude G, et al. Risk factors for survival and recurrence after lung metastasectomy. J Surg Res 2016; 203: 293–300. [DOI] [PubMed] [Google Scholar]
- 37. Salah S, Ardissone F, Gonzalez M, et al. Pulmonary metastasectomy in colorectal cancer patients with previously resected liver metastasis: pooled analysis. Ann Surg Oncol 2015; 22: 1844–1850. [DOI] [PubMed] [Google Scholar]
- 38. Cho JH, Kim S, Namgung M, et al. The prognostic importance of the number of metastases in pulmonary metastasectomy of colorectal cancer. World J Surg Oncol 2015; 13: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008; 371: 1007–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hawkes EA, Ladas G, Cunningham D, et al. Peri-operative chemotherapy in the management of resectable colorectal cancer pulmonary metastases. BMC Cancer 2012; 12: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karim S, Nanji S, Brennan K, et al. Chemotherapy for resected colorectal cancer pulmonary metastases: utilization and outcomes in routine clinical practice. Eur J Surg Oncol 2017; 43: 1481–1487. [DOI] [PubMed] [Google Scholar]
- 42. Abe S, Kawai K, Ishihara S, et al. Prognostic impact of carcinoembryonic antigen and carbohydrate antigen 19-9 in stage IV colorectal cancer patients after R0 resection. J Surg Res 2016; 205: 384–392. [DOI] [PubMed] [Google Scholar]
- 43. Shibutani M, Maeda K, Nagahara H, et al. Prognostic significance of the preoperative serum C-reactive protein level in patients with stage IV colorectal cancer. Surg Today 2015; 45: 315–321. [DOI] [PubMed] [Google Scholar]
- 44. Nakayama I, Suenaga M, Wakatsuki T, et al. Safety, tolerability, and efficacy of oxaliplatin-based adjuvant chemotherapy after curative resection of hepatic or extrahepatic metastases of stage IV colorectal cancer. Cancer Chemother Pharmacol 2015; 76: 133–139. [DOI] [PubMed] [Google Scholar]
- 45. Kanemitsu Y, Kato T, Shimizu Y, et al. A randomized phase II/III trial comparing hepatectomy followed by mFOLFOX6 with hepatectomy alone as treatment for liver metastasis from colorectal cancer: Japan Clinical Oncology Group Study JCOG0603. Jpn J Clin Oncol 2009; 39: 406–409. [DOI] [PubMed] [Google Scholar]
- 46. Takahashi M, Hasegawa K, Oba M, et al. Phase 1 study on S-1 and oxaliplatin therapy as an adjuvant after hepatectomy for colorectal liver metastases. Invest New Drugs 2016; 34: 468–473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, AdjCTXM1_STablesTherAdvMedOncolrev_1 for Adjuvant chemotherapy improves prognosis of resectable stage IV colorectal cancer: a comparative study using inverse probability of treatment weighting by Hiroaki Nozawa, Hirotoshi Takiyama, Kiyoshi Hasegawa, Kazushige Kawai, Keisuke Hata, Toshiaki Tanaka, Takeshi Nishikawa, Kazuhito Sasaki, Manabu Kaneko, Koji Murono, Shigenobu Emoto, Hirofumi Sonoda and Jun Nakajima in Therapeutic Advances in Medical Oncology