Abstract
Background:
Demographic shift leads to an increasing number of geriatric patients suffering from multimorbidity and resulting polypharmacy. Polypharmacy is shown to be associated with drug-related problems (DRPs) and increased morbidity. For Germany, a hospital-based intervention may be successful optimizing of polypharmacy. The aim of this study was to reduce DRPs in geriatric inpatients by a structured pharmacist’s intervention and to measure the acceptance rate of pharmaceutical recommendations.
Methods:
This study followed an open, prospective, quasi-randomized, controlled design and was conducted in a geriatric department in a teaching hospital in Germany. Patients of all sexes were included, with a minimum age of 70 years, a written informed consent and a regular intake of at least five drugs daily. Primary outcome was the percentage of patients having a DRP at admission and discharge. A DRP was defined as a prescription without indication or a relevant drug–drug interaction or prescription of a potentially inappropriate medication or presence of an adverse drug reaction. Recommendations were classified and discussed face to face. Statistical analyses were performed using a full-set analysis and a matched-pairs design.
Results:
Within 12 months, 411 patients were recruited with median age of 82 years (intervention: n = 209; control: n = 202). Median number of drugs at admission was 10 (range 5–24), at discharge 9 (range 3–21). In the intervention group, the percentage of patients with a DRP was reduced from 86.6% to 56.0%; in the control group, from 76.7% to 76.2% (p value < 0.001). Medication appropriateness index score was reduced by 56% in the intervention group and by 0.2% in the control group (p value < 0.001). Implementation rate of the pharmaceutical recommendation was 80%.
Conclusion:
This prospective controlled trial showed that a pharmacist’s intervention was successful in optimizing polypharmacy in geriatric inpatients.
Keywords: drug safety, geriatric inpatients, medication appropriateness index, pharmaceutical intervention, polypharmacy, potentially inappropriate medication
Introduction
Older adults represent a growing proportion of the population in most industrialized countries and suffer frequently from multimorbidity and resulting polypharmacy.1,2 Based on physiological changes occurring with advancing age, many drugs lead to more side effects in the older population when compared with younger adults,3,4 a problem which increases with the number of drugs prescribed. Polypharmacy is frequently and conveniently defined as the intake of five or more drugs per day on a regular basis;5 however, the uncontrolled use of many drugs without appropriate monitoring may also be understood as polypharmacy.6 Polypharmacy has been shown to be associated with drug–drug interactions (DDIs), a risk for potentially inappropriate medications (PIMs), adverse drug reactions (ADRs), drug omissions and finally increased morbidity, particularly falls, and mortality.7,8
Different tools and interventions have been developed and tested to optimize polypharmacy in diverse settings.9 Prospective studies were performed in the community-dwelling elderly,10 in nursing homes11 as well as in the hospital setting.9 Most interventions included training for doctors, involvement of pharmacists and medication reviews, as well as special education for nurses, and frequently applied a team-based approach.9 Many interventions focused on defined drug classes (e.g. psychotropic drugs); others targeted all prescriptions and over-the-counter drugs. The primary objective was, in many studies, the number of drugs. Very frequently, measures of ‘medication quality’ or appropriateness were documented applying validated instruments such as the medication appropriateness index (MAI).12,13 A few studies investigated the effect of their intervention on outcomes such as hospitalization or mortality.9,12 As a general trend, focused interventions (i.e. on psychotropic drugs) were more successful than broader approaches.11 On the contrary, using well-controlled measures, overall medication quality can be improved substantially.12
Depending on the healthcare system, different approaches may be effective and for Germany, a hospital-based intervention may have a good chance for a successful intervention. A minimum of 14 days’ duration of hospital stay in a specialized geriatric department is sufficiently long to assess the physical and psychosocial condition of a patient, allowing for medication changes and deprescribing with adequate monitoring.
The aim of our study was to reduce drug-related problems (DRPs) in geriatric inpatients by a structured pharmacist’s intervention and to measure the acceptance rate of the pharmacist’s suggestions.
Methods
Study design and setting
This study followed an open, prospective, quasi-randomized design including an intervention and a control group and was conducted in a geriatric department (56 beds) in a teaching hospital in Germany between January and December 2015. The study protocol was approved by the responsible ethics committee of the University of Witten/Herdecke, Germany, registered under number DRKS00014560 and can be retrieved on the World Health Organization (WHO) International Clinical Trials Registry Platform.14
Patients
Patients of all sexes were included with a minimum age of 70 years, regular intake of at least five drugs daily after written informed consent had been obtained. Patients with an estimated life expectancy of less than 1 week, cognitive impairment or previous participation in this study during the last 3 months in the same hospital were not included. However, rehospitalized patients after more than 3 months could be included again and were counted as a new case.
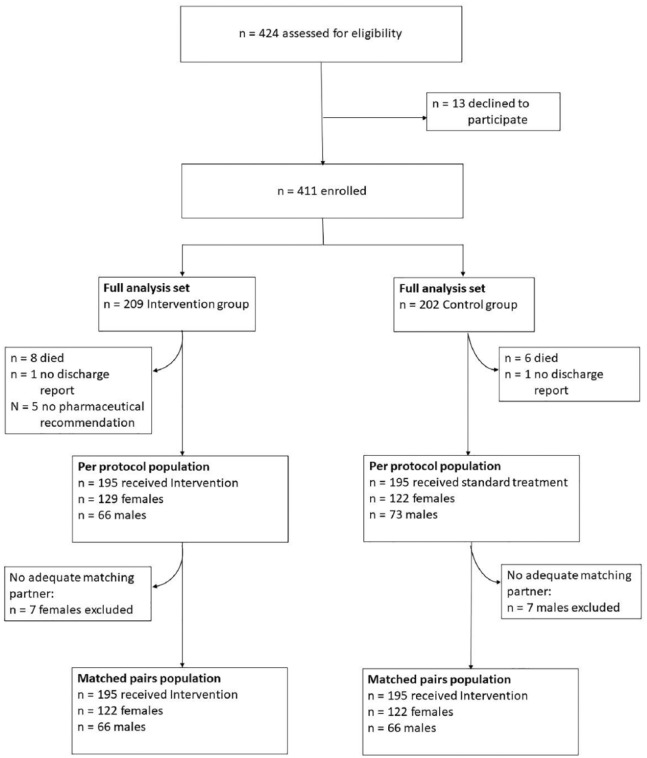
The geriatric department consists of two wards (26–30 beds) and all patients are admitted to one of them, depending on the availability of a bed. This procedure was unrelated to the study and was organized by administrative hospital staff without any knowledge of the study. Patients on ward A were defined as the intervention group, whereas patients on ward B served as the control group (see Figure 1). Physicians and nurses of both wards were informed about the study, but no formal training was given.
Figure 1.
Study design and patient enrolment.
Study procedures
After admission to the respective wards, all patients were informed about the study by the pharmacist and inclusion/exclusion criteria were verified. After oral and written information was given, written informed consent was obtained from all study participants.
Baseline data such as age, sex, body size and weight, drugs (coded using the Anatomical Therapeutic Chemical Classification System code15) and allergies, as well as relevant laboratory data were entered pseudonymized into an Access (Microsoft® Access 2016, Microsoft Germany GmbH) database. Glomerular filtration rate was calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI)16 and Cockroft–Gault formulae.17 Results of several routine geriatric tests were documented: the Barthel Index18 of activities of daily living (IADL),19 the timed up-and-go test,20 the Tinetti test,21 the clock test22 and the Geriatric Depression Scale (GDS-15).23
Medication assessment
Medication of all patients in the intervention and control groups was assessed by the pharmacist at admission and discharge. In accordance with the Descriptive Elements of Pharmacist Interventions Characterization Tool (DEPICT) statement,24 medication was reviewed and documented using the hospital chart and by personal interview (see Table 1). Results of the medication analysis were documented in the Access database.
Table 1.
Criteria for medication assessment.
| Criterion | Example | Reference | |
|---|---|---|---|
| Potentially inadequate medication for older adults | PRISCUS list | Anticholinergic drugs, e.g. amitriptyline, oxybutynine | Holt et al.25 |
| Renal dosing | SPC | Duloxetine: GFR 30–80 ml/min: no dosage adjustment GFR < 30ml/min: contraindicated |
www.fachinfo.de
26,27
|
| No indication | SPC and statement of the physician (off-label use) | No indication if there was no documentation of symptoms of angina pectoris |
www.fachinfo.de
27,28
|
| Drug–drug interaction | ABDA database | Rivaroxaban and enoxaparin: increased risk for bleeding | ABDA database28 |
| Adverse drug effect | Either detected by physician or by pharmacist | Patient with hip fracture (reason for admission) says that she often needs to go to the toilet at night: she receives diuretics at bedtime | ABDA database28
www.fachinfo.de27 |
| Medication appropriateness index (MAI) | MAI | MAI modification for geriatric patients in nursing homes and geriatric inpatients (Joks29) | MAI,13 MAI modification after Joks29 |
| Further information about application of the drug | SPC | Patient did not use his medication correctly (e.g. inhalation of COPD drugs) | www.fachinfo.de 27 |
ABDA, Federal Union of German Associations of Pharmacists; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; PRISCUS, ; SPC, summary of product characteristics.
Detection and classification of adverse drug reactions
At admission, all patients were screened by the responsible physician and the pharmacist for ADRs, where all symptoms described by the patient, diagnoses at admission and pathological laboratory parameters where checked. At discharge, those ADRs still described by the patient, mentioned in the discharge report and detectable in the laboratory parameters were analysed.
All ADRs were coded according to the WHO System Organ Classification on the fifth level.30 Causality assessment was performed using the Naranjo algorithm.31 Severity of ADRs were classified using the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) grading system.32 Preventability of ADR was assessed applying the algorithm developed by Schumock and Thornton.33
Intervention
In both groups, medication analyses were performed (Table 1). Recommendations wereprepared for the treating physicians of the intervention-group patients only. These recommendations were classified using the ‘pharmacotherapy problem categories’ according to Hoth et al.34 with low, medium or high priority. This classification system is more detailed than our primary outcome criteria ‘drug-related problems’ and includes, for example, compliance issues and problems with the route of administration. A meeting with the physician was arranged by the pharmacist and all issues were discussed. Only in 20 cases, a face-to-face meeting was not feasible and a printout of the recommendations was placed into the patients’ charts, where it was found by the visiting physician. After discussion with the physician, the recommendations were accepted, modified or rejected. Discharge reports of all study patients were screened by the pharmcist and again, a medication assessment was performed.
Outcomes
Primary outcome parameter of the study was the percentage of patients with at least one DRP detected in the medication assessment. The following items were counted as DRPs and contributed to the combined primary outcome:
presence of at least one drug prescribed without indication,
presence of an ADR,
DDI with a severity grade of at least ‘concomitant use not recommended’ as listed in the DDI database applied,28
presence of a PIM according to the German PRISCUS list.25
Secondary outcome variable was the MAI, slightly adapted to the German healthcare system.13,29 This adaptation excludes price issues since (a) drug prices in German hospitals are lower than in ambulatory care, and (b) drugs available in the hospital are listed on the drug formulary, where cost issues have already been considered. In addition, the question for ‘missing indication’ receives a lower weighting, since information available from ambulatory care is frequently incomplete and comorbidities may exist without clear information in the documentation. We consider this question as highly relevant, but in many cases, it is too difficult for the pharmacist to obtain all information required to answer this question with a high level of certainty.
Statistical analyses
All data were documented into an Access database. For the sample size determination, the prevalence of a DRP was estimated with 50%, as described in the literature.35–39 After the pharmaceutical intervention, prevalence of DRPs should be reduced to 20%. However, during routine clinical practice, optimization of medication was expected in the DRP prevalence range of 30%, at discharge. Based on these assumptions and using McNemar’s test for comparison between groups, this 10% difference could be detected with a sample size of 170 patients per group with a power of 85% and a significance level of α = 0.05. To compensate for dropouts, we planned to include 200 patients per group.
Statistical analyses were performed using a full analysis set and applying a matched-pairs design. For the latter, all patients from the control and intervention groups who finished the study according to protocol were matched to achieve homogenous groups. Matching criteria were as follows: same sex, age ± 5 years and hospitalization during the same month.
To compare frequency distributions between two independent groups (full analysis set) two-sided Fisher’s exact test was applied for categorical data; for continuous variables, the Wilcoxon–Mann–Whitney test. In order to compare frequency distributions for the matched-pairs analysis, McNemar’s two-sided test was used.
A multivariate logistic-regression analysis was performed to assess the association between age, sex, estimated glomerular filtration rate (eGFR) and GDS (in categorial form with ⩽5 and >5) as independent variables and (a) the presence of an ADR at admission, and (b) the presence of a DRP at admission as dependent variables. Nagelkerke’s R2 has been chosen to describe the quality of the model.40
Medication was analysed descriptively between admission and discharge, as well as the percentage of acceptance and implementation of pharmacist’s recommendations.
All statistical analyses were performed using SPSS® version 23 (IBM®, Ehningen, Germany). Descriptive analyses were performed with Microsoft® Excel 2016 and Microsoft® Access 2016.
Results
Over the 12-month study period, 411 patients were recruited (Figure 1). In the following, data of the full analysis set are presented. Matching resulted in 188 pairs (65% female pairs) with comparable baseline values for age (intervention versus controls: females 82.7 ± 6.2 years versus 81.4 ± 5.9 years; males 81.6 ± 5.9 years versus 80.9 ± 6.3 years) and GFR {intervention versus controls: 54.6 ml/min/1.73 m² [standard deviation (SD) 21.855; range 5–106 ml/min/1.73 m²]; 60.4 ml/min/1.73 m² (SD 21.977; range 2–118 ml/min/1.73 m²)}. Supporting data of the matched-pairs analysis are presented in the additional online version (online Table 5).
Patients
Population baseline data are depicted in Table 2. More than half (64%) of the study population was female and median age was 82 years (mean value ± SD: 81.91 ± 6.10; range 70–103 years). The higher percentage of female participants is in concordance with the prevalence of female patients on geriatric wards being 65%. Patients in the intervention group were slightly older and had a significantly lower eGFR. The median length of stay was 19 days (19.26 ± 7.69; 1–49 days). Out of the control group, six patients died during hospital stay and eight patients from the intervention group. Geriatric assessment variables are presented in Table 3 and are comparable between groups. As expected after a geriatric hospitalization, the Barthel IADL improved markedly.
Table 2.
Demographic data of study participants (full analysis set).
| Control group (n = 202) | Intervention group (n = 209) | Difference between groups (p value) | |
|---|---|---|---|
| Age, years, mean | 81.3 (SD 5.95; range: 70–96) | 82.5 (SD 6.18; range: 70–103) | p = 0.036 |
| Female % | 61.9 | 66.0 | p = 0.412 |
| Body weight, kg*, mean | 75.2 (SD 19.476; range: 39–170) | 75.9 (SD 16.895; range: 39–138) | p = 0.797 |
| Duration of hospital stay, days, mean | 19.47 (SD 7.33; range 3–48) | 19.05 (SD 8.03; range 1–49) | p = 0.584 |
| eGFR**
(ml/min/1,73m²), mean |
60.4 ml/min/1.73 m² (SD 21.977; range 2–118 ml/min/1.73 m²) | 54.6 ml/min/1.73 m² (SD 21.855; range 5–106 ml/min/1.73 m²) | p = 0.008 |
Body weight available for n = 114 patients in the control group, n = 120 patients in the intervention group.
eGFR available for n = 202 patients in the control group, n = 207 patients in the intervention group.
eGFR, estimated glomerular filtration rate; SD, standard deviation.
Table 3.
Clinical geriatric characteristics of the study population (full analysis set population).
| Admission |
Discharge |
|||||
|---|---|---|---|---|---|---|
| Control group | Intervention group | Comparison between groups | Control group | Intervention group | Comparison between groups | |
| Barthel Index (0–100)* | 42.75 (SD 22.48; range 0–100) (n = 191) | 39.13 (SD 20.04; range 0–100; n = 189) | p = 0.0984 | 67.71 (SD 25.32; 0–100; n = 176) | 61.94 (SD 22.16; 5–100; n = 165) | p = 0.001 |
| IADL** | 3.78 (SD 2.53; range 0–8; n = 181) | 3.65 (SD 2.53; range 0–8; n = 165) | p = 0.650 | – | – | – |
| Timed up-and-go test*** | 21.03 (SD 7.12; range 10–50; n = 93) | 27.28 (SD 11.06; range 9–65; n = 86) | p < 0.001 | 20.9 (SD 10.67; range 7–80; n = 114) | 26.17 (SD 10.68; range 9–65; n = 113) | p < 0.001 |
| Tinetti test$ | 12.40 (SD 8.39; range 0–27; n = 184) | 9.29 (SD 6.95; range 0–27; n = 178) | p < 0.001 | 16.73 (SD 7.17; range 0–27; n = 160) | 14.40 (SD 6.41; range 0–27; n = 146) | p < 0.001 |
| Clock completion test§ | 3.08 (SD 1.49; range 1–6; n = 156) | 3.30 (SD 1.28; range 1–6; n = 132) | p = 0.237 | – | – | – |
| GDS§§ | 4.87 (SD 3.39; range 0–14; n = 168) | 4.41 (SD 2.95; range 0–13; n = 147) | p = 0.285 | 5.20 (SD 3.54; range 0–15; n = 91) | 3.63 (SD 2.49; range 0–12; n = 97) | p = 0.003 |
Care dependency increases with falling Barthel Index.
IADL: 0: absolutely dependent; 8: independent.
Up-and-go test time (s): <10 s: normal mobility; <20 s: slightly limited mobility; <30 s: limited mobility; >30 s: definitive limited mobility.
Tinetti test: >20 points: normal mobility; 15–20 points: slightly limited mobility; 10–14 points: limited mobility; <10 points: definitive limited mobility.
Clock completion test scale, 1–6: low number indicates better cognitive skills.
GDS 0–15: 0–5: normal; >5: depression possible.
GDS, Geriatric Depression Scale; IADL, index of activities of daily living; SD, standard deviation.
Medication and drug-related problems
Patients in the intervention group received 10.85 ± 3.54 (median 10, range 5–24) drugs at admission, whereas patients in the control group received 10.26 ± 3.29 (median 10, range 5–24) drugs. At discharge, patients in the intervention group were prescribed 9.39 ± 2.93 (median 9, range 3–19) drugs, whereas those in the control group were prescribed 9.32 ± 3.20 (median 9, range 3–21) drugs (p < 0.039 between intervention and control groups).
At admission, at least one DRP could be detected in 86.6% of patients in the intervention group; at discharge this was reduced to 56.0%. DRP and their distribution are presented in Table 4. Out of the variables tested, only GDS > 5 showed a significant odds ratio with 2.183 (95% confidence interval 1.10–4.34, p = 0.026) in the bivariate analysis to experience a DRP at admission. The overall multivariate regression model failed to prove a significant influence of the tested variables on the chance to have a DRP (Nagelkerke’s R2 = 0.037).
Table 4.
Primary outcome drug-related problems in the control and the intervention groups (full analysis of population).
| Variable | Time | Value | Intervention group
(n = 209) |
Control group (n =
202) |
p value (Fisher) | ||
|---|---|---|---|---|---|---|---|
| n | %* | n | %* | ||||
| PRISCUS PIM (⩾1) | Admission | No | 140 | 67.0% | 146 | 72.3% | |
| Yes | 69 | 33.0% | 56 | 27.7% | 0.284 | ||
| Discharge | No | 175 | 83.7% | 155 | 76.7% | ||
| Yes | 34 | 16.3% | 47 | 23.3% | 0.083 | ||
| Drug prescribed without indication (⩾1) | Admission | No | 45 | 21.5% | 62 | 30.7% | |
| Yes | 164 | 78.5% | 140 | 69.3% | 0.043 | ||
| Discharge | No | 125 | 59.8% | 68 | 33.7% | ||
| Yes | 84 | 40.2% | 134 | 66.3% | <0.001 | ||
| Adverse drug reaction (⩾1) | Admission | No | 171 | 81.8% | 170 | 84.2% | |
| Yes | 38 | 18.2% | 32 | 15.8% | 0.600 | ||
| Discharge | No | 202 | 96.7% | 162 | 80.2% | ||
| Yes | 7 | 3.4% | 40 | 19.8% | <0.001 | ||
| Drug–drug interaction (⩾1) | Admission | No | 177 | 84.7% | 172 | 85.2% | |
| Yes | 32 | 15.3% | 30 | 14.9% | 1 | ||
| Discharge | No | 179 | 85.7% | 170 | 84.2% | ||
| Yes | 30 | 14.4% | 32 | 15.8% | 0.682 | ||
| Combined primary outcome variable | Admission | No | 28 | 13.4% | 47 | 23.3% | |
| yes | 181 | 86.6% | 155 | 76.7% | 0.011 | ||
| Discharge | no | 92 | 44.0% | 48 | 23.8% | ||
| yes | 117 | 56.0% | 154 | 76.2% | <0.001 | ||
The percentages refer to the number of nonmissing values of the row variables within the groups and times (column sum per row variable = 100%).
PIM, potentially inappropriate medication; PRISCUS, .
In the control group, there were slightly less DRPs at admission (76.7%); however, at discharge, there was only a tendency towards an improvement (76.2%). This difference between groups was statistically significant (p < 0.001, see Table 4). Corresponding data for the matched-pairs comparison show a similar difference and can be found in online Table 5.
The MAI score in the intervention group could be reduced by 56%, from 56.35 ± 33.84 at admission to 24.92 ± 18.39 at discharge. On the contrary, in the control group, the MAI score remained almost unchanged with 43.21 ± 29.25 at admission and 43.13 ± 30.62 at discharge (p < 0.001). Detailed results of the MAI are presented in online Table 6.
A total of 32 ADRs were detected in the control group at admission and n = 40 at discharge. The corresponding figures for the intervention group were n = 38 and n = 7, respectively (p < 0.001). The most frequently ADR-associated drugs were diuretics, antidepressants (citalopram and amitriptyline), analgesics (opioids and nonsteroidal anti-inflammatory drugs) and antithrombotics. The leading presentations of ADR followed a typical geriatric pattern, with electrolyte disorders and exsiccosis, falls, gastrointestinal symptoms and cardiovascular problems (e.g. heart-rhythm disorder, hypotension). More than 80% of the ADRs were classified as type D (NCC MERP), that is, observation or monitoring required. Causality assessment revealed a possible ADR in approximately 80% and in 14%, the ADR was probable. In terms of preventability, 60% of ADRs were classified as preventable, 4% as ameliorable and 36% as nonpreventable.
In the bivariate analyses, women had a significantly higher odds ratio to experience an ADR at baseline with 2.187 (95% confidence interval 1.07–4.46, p = 0.032). The multivariate regression model showed no significant influence of the tested variables on the risk of experiencing an ADR at admission (Nagelkerke’s R2 = 0.039).
Pharmaceutical recommendations
The pharmacist detected 1657 pharmaceutical problems as described by Hoth et al.34 in the medication of 209 patients in the intervention group. For 1563 of those problems, pharmaceutical recommendations were written and discussed with the physician. Ninety-four of these problems and recommendations could not be discussed or applied, due to early discharge or patient death.
The largest proportion of recommendations referred to diuretics (9.5%), analgesics (7.6%), antithrombotic drugs (7.5%) and antacid drugs, usually proton-pump inhibitors (7.4%). Almost 25% of recommendations were given for pantoprazole, metoprolol, metamizole (available in Germany), furosemide/torasemide, ramipril and simvastatin. Overall, pharmaceutical recommendations were submitted for 243 different drugs. In all, 37% of pharmacist’s recommendation received a high priority, 55% moderate und 8% low priority.
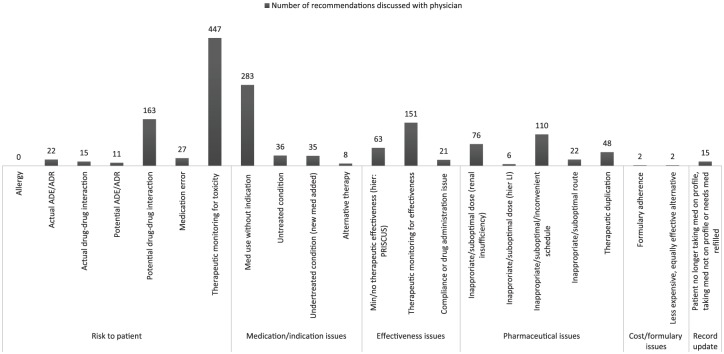
The most frequently stated reasons for a pharmaceutical recommendation were monitoring of side effects and toxicity (28.6%), drug prescribed without indication (18.1%) and DDI (10.4%). Almost half of recommendations (43.8%) fell into the category ‘risk to patient’, followed by problems with indication/medication (23.2%), pharmaceutical issues (16.8%) and problems regarding effectiveness (15.0%), as depicted in Figure 2.
Figure 2.
Pharmacy problem categories according to Hoth et al.34 detected by the pharmacist and discussed with the physician.
ADE, adverse drug event; ADR, adverse drug reaction; PRISCUS, .
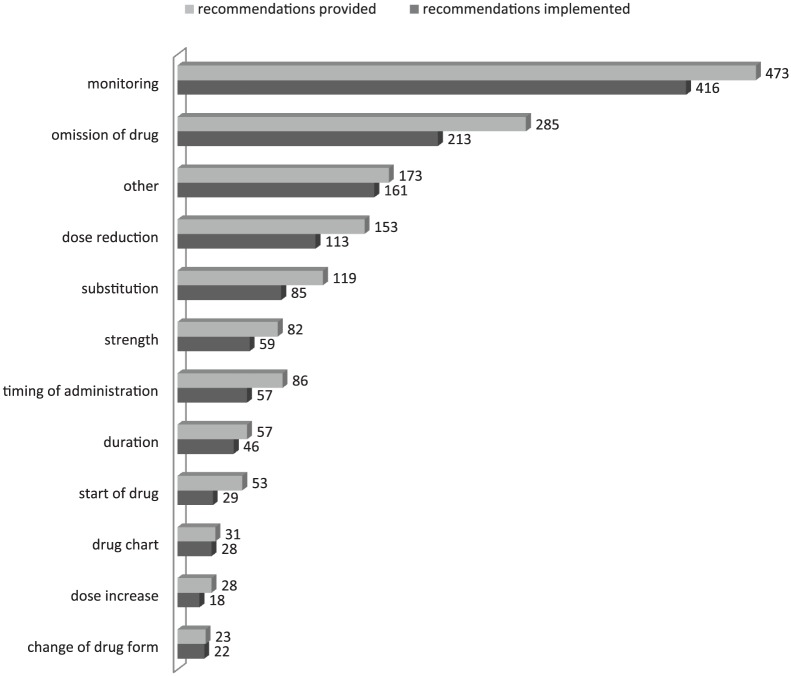
The types of pharmaceutical recommendations were classified as shown in Figure 3: in 30% of cases monitoring [laboratory, electrocardiogram (ECG), etc.] was advised. Second, in 18% of recommendations, drug withdrawal (intermittently or definitely) was stated. In 10%, a dose reduction was suggested and in 8%, replacement by a different drug. Figure 3 gives an overview of recommendations provided and shows the numbers of recommendations of each type accepted and implemented. Online Figure 4 demonstrates an example of a pharmaceutical recommendation. The overall acceptance rate of recommendations was very high, ranging from 96% (change of drug form) to 74% (dose reduction) and 55% (start of drug).
Figure 3.
Type of pharmaceutical recommendation according to Hoth et al.34 and rate of acceptance.
Finally, 80% of the recommendations were implemented in the discharge report, 73% without any change and 7% in a modified way; 20% were not implemented.
Discussion
In this pragmatic, controlled trial, we were able to demonstrate a significant influence of pharmacist recommendations on medication quality and safety for geriatric patients. This result is in agreement with other studies, where the number of PIMs or the MAI score had frequently been chosen as primary outcome variables.12,41–48 We chose a combined-outcome variable, as for these variables: unnecessary drug (without indication) and a major DDI, a substantial risk for ADRs and other adverse outcomes (e.g. falls, hospitalization) has been shown. In addition, we chose the existence of an ADR as one clinically relevant variable.
What concerned us with regard to the prevalence of these risk factors, was the prevalence of PIM according to the PRISCUS list was 30.8% in our cohort, which was markedly higher than expected. Following analyses of German healthcare insurance, a prevalence of 20–25% was assumed.49,50 However, Siebert and colleagues reported a PIM prevalence of 35% at admission to a geriatric department, which was reduced to 29% at discharge.35 In our study, a further reduction to 16.3% was achieved.
DDIs represent a major risk factor for ADRs, and they are frequently detected in observational studies. In our population, 15% of geriatric patients were exposed to a substantial risk, since these DDIs are characterized as ‘comedication not recommended’ or even ‘contraindicated’ according to a frequently used DDI software.28 Björkman and colleagues observed DDIs in 46% of elderly patients in six European countries.51 However, a review of 19 studies reported a prevalence of DDI ranging between 2.2% and 70.3% of which between 0.0% and 11.1% were of clinical relevance.52 This discrepancy between DDIs reported by software and clinical outcomes frequently results in an over-alert, with consequent unacceptability of suggested changes.53–56 We thus reported only DDIs with a certain severity and risk for clinical consequences. We cannot rule out that we still over-reported DDIs,57 since the acceptance rate came only to 73%. On the other hand, many DDI-related recommendations suggested monitoring (e.g. an ECG in case of two QT-prolonging drugs) and these ECGs were performed. Consequently, the DDI was still present in the discharge medication. Among other reasons, this may be one explanation for the fact that prevalence of DDIs was the only item of our primary outcome that could not be reduced and was similar between control and intervention groups.
ADRs were observed in 25% of patients at admission. This is in the range of previous studies describing the incidence of ADRs in geriatric patients admitted to hospital.58–61 As reported by others,62–65 electrolyte disorders and exsiccosis induced by diuretics, and falls associated with fall-risk-increasing drugs were the most common ADRs. A thorough medication review should result in a marked reduction of objectively measured and subjectively reported ADRs, as we prove in our intervention group.
The acceptance rate is higher or similar compared with other studies.39,66–68 Clinically relevant recommendations and the personal contact to the physicians probably supported the high acceptance rate. Viktil and colleagues found out that proactive communication leads to a higher acceptance rate of pharmaceutical interventions than an indirect method, such as written information.69 Weißenborn and colleagues came to the conclusion that in ambulatory care, a ‘successful cooperation between general practitioners and community pharmacist in daily routine care was often characterized by personal contact and long-time relationships’.70
Denneboom analysed the communication between pharmacists and physicians. They compared case conferences with written feedback and also came to the conclusion that with personal contact, the acceptance rate was higher.71 In our study, acceptance rate was high and in most cases, where recommendations were not followed, the physician had additional information, a consultant had to be involved or the issue was already resolved. It should be noted that duration of hospital stay seems to be long; however, this is due to the required minimum of 14 days stay in a geriatric department, including early rehabilitation in accordance with the German system for diagnosis-related groups (DRG).
Most of the recommendations addressed monitoring, deprescribing/stopping of a drug or ‘other’. Monitoring comprised checking laboratory values or an ECG, and ‘other’ includes missing indication (also indication not documented) and missing maximum dose for medication to be given only when needed (e.g. analgesics, neuroleptics). Treatment in a geriatric department can be seen as a chance to safely withhold medications and monitor for potential unwanted effects. The process of deprescribing is currently under debate in many publications,72,73 but was not the focus of the current investigation.
To optimise the intra- and inter-rater reliability of the MAI, a modified MAI was used,29 which additionally considered characteristic features of geriatric medicines. This slightly modified MAI13,29 was the secondary outcome. The MAI is a common tool for measuring medication quality and is used in several studies.43 A significant improvement of the MAI by pharmacist intervention was also investigated by others.67,74–77
There are several limitations of our study. First, the study was only quasi-randomized. However, an allocation bias to one of the two wards was ruled out, since admittance to a certain ward was organized independently from our study. For pragmatic reasons, it was easier to intervene on one ward only, since nurses and physicians on the intervention ward got used to the intervention and we had virtually no contamination to the other ward. In addition, we performed an additional matching approach, where comparable population characteristics were achieved, and statistical analysis revealed identical results for the outcome variables.
Second, we initiated a change in the medication without follow up into ambulatory care. Further studies should examine long-term effects, including a follow up to ambulatory care with clinically relevant endpoints (rehospitalization, quality of life). A randomized prospective trial by Gallagher and colleagues78 including 400 hospitalized geriatric patients showed an increased medication quality using medication review with START/STOPP criteria79 measured by MAI. Their results were sustained for over 6 months after discharge. Due to the experimental nature, we were not able to include patients with cognitive dysfunction who represent a vulnerable population, usually with a high number of drugs prescribed. However, when implemented into routine, their benefit from a pharmaceutical intervention should be at least as large as for the patients studied. Pharmaceutical interventions in nursing homes have been proven successful with regard to MAI and other outcomes describing the quality of the medication, particularly with the use of psychotropic drugs.11,80
This real-life prospective trial showed a high rate of ADRs and other medication-related problems in geriatric patients at hospital admission. The present data confirm the results of recent observations emphasizing the role of pharmaceutical care for hospitalized patients. However, personal contact seems to be relevant to achieve a high acceptance rate of pharmacist’s recommendations.
Supplemental Material
Supplemental material, Online_figure_5 for Influence of pharmacist intervention on drug safety of geriatric inpatients: a prospective, controlled trial by Angela Nachtigall, Hans J. Heppner and Petra A. Thürmann in Therapeutic Advances in Drug Safety
Supplemental Material
Supplemental material, Online_tables(5-6) for Influence of pharmacist intervention on drug safety of geriatric inpatients: a prospective, controlled trial by Angela Nachtigall, Hans J. Heppner and Petra A. Thürmann in Therapeutic Advances in Drug Safety
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by a grant of the Paul-Kuth Foundation, Wuppertal, Germany the Helios Research Fund grant ID 063614 and the Robert Bosch Foundation, Stuttgart, Germany
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Angela Nachtigall, Department of Human Medicine, University of Witten/Herdecke, Witten, Germany, Pharmacy, Helios Clinic Schwelm, Schwelm, Germany.
Hans J. Heppner, Department of Human Medicine, University of Witten/Herdecke, Witten, Germany Department of Geriatric Medicine, Helios Clinic Schwelm, Schwelm, Germany, Institute for Biomedicine of Ageing, FAU Erlangen-Nuremberg, Nuremberg, Germany
Petra A. Thürmann, Department of Human Medicine, University of Witten/Herdecke, Witten, Germany Department of Geriatric Medicine, Helios Clinic Schwelm, Schwelm, Germany, Institute for Biomedicine of Ageing, FAU Erlangen-Nuremberg, Nuremberg, Germany; Department of Human Medicine, University of Witten/Herdecke, Witten, Germany.
References
- 1. Lehnert T, Heider D, Leicht H, et al. Review: Health care utilization and costs of elderly persons with multiple chronic conditions. Med Care Res Rev 2011; 68: 387–420. [DOI] [PubMed] [Google Scholar]
- 2. Van den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity. Eur J Gen Pract 2009; 2: 65–70. [Google Scholar]
- 3. Reeve E, Wiese MD, Mangoni AA. Alterations in drug disposition in older adults. Expert Opin Drug Metab Toxicol 2015; 11: 491–508. [DOI] [PubMed] [Google Scholar]
- 4. Gnjidic D, Johnell K. Clinical implications from drug-drug and drug-disease interactions in older people. Clin Exp Pharmacol Physiol 2013; 40: 320–325. [DOI] [PubMed] [Google Scholar]
- 5. Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 2012; 65: 989–995. [DOI] [PubMed] [Google Scholar]
- 6. Viktil KK, Blix HS, Moger TA, et al. Polypharmacy as commonly defined is an indicator of limited value in the assessment of drug-related problems. Br J Clin Pharmacol 2007; 63: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc 2006; 54: 1516–1523. [DOI] [PubMed] [Google Scholar]
- 8. Fried TR, O’Leary J, Towle V, et al. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc 2014; 62: 2261–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johansson T, Abuzahra ME, Keller S, et al. Impact of strategies to reduce polypharmacy on clinically relevant endpoints: a systematic review and meta-analysis. Br J Clin Pharmacol 2016; 82: 532–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith SM, Wallace E, O’Dowd T, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2016; 3: CD006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alldred DP, Kennedy M-C, Hughes C, et al. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev 2016; 2: CD009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cooper JA, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open 2015; 5: e009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol 1992; 45: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization. ICTRP Search Portal: International Clinical Trials Registry Platform, http://apps.who.int/trialsearch/ (accessed 3 June 2018).
- 15. WIdO/DIMDI. Amtlicher ATC-Index mit DDD-Angaben für Deutschland im Jahre http://www.dimdi.de/dynamic/de/klassi/downloadcenter/atcddd/version2016/atc-ddd-amtlich-2016.pdf (accessed 7 August 2016).
- 16. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31–41. [DOI] [PubMed] [Google Scholar]
- 18. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J 1965; 14: 61–65. [PubMed] [Google Scholar]
- 19. Juchli L. Ganzheitliche Pflege: Vision oder Wirklichkeit. 3. Aufl. Basel, Eberswalde: RECOM-Verl, 1993. [Google Scholar]
- 20. Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 21. Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc 1986; 34: 119–126. [DOI] [PubMed] [Google Scholar]
- 22. Shulman KI, Pushkar Gold D, Cohen CA, et al. Clock-drawing and dementia in the community: a longitudinal study. Int J Geriat Psychiatry 1993; 8: 487–496. [Google Scholar]
- 23. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982; 17: 37–49. [DOI] [PubMed] [Google Scholar]
- 24. Rotta I, Salgado TM, Felix DC, et al. Ensuring consistent reporting of clinical pharmacy services to enhance reproducibility in practice: an improved version of DEPICT. J Eval Clin Pract 2015; 21: 584–590. [DOI] [PubMed] [Google Scholar]
- 25. Holt S, Schmiedl S, Thurmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int 2010; 107: 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klinische Pharmakologie & Pharmakoepidemiologie - Universitätsklinikum Heidelberg. DOSING: Wirkstoffliste Dosisanpassung bei Niereninsuffizienz. Hilfsmittel zur Arzneimittel-Anwendung & -Sicherheit, http://www.dosing.de/nierelst.php (1998. –2017, accessed 3 July 2017). [Google Scholar]
- 27. Fachinfo-Service, Rote Liste Service GmbH. Fachinfo: Fachinformation, https://www.fachinfo.de/ (accessed 7 August 2018). [Google Scholar]
- 28. ABDATA. Interaktionsmodul der ABDA-Datenbank: ABDA - Interaktionen, http://abdata.de/datenangebot/abda-datenbank/interaktionen/ (accessed 7 August 2018).
- 29. Joks G. Evaluation und Optimierung der Arzneimitteltherapiesicherheit in einer Einrichtung der Altenpflege. Inaugural-Dissertation, Universität Witten/Herdecke; Witten/Herdecke, 2014. [Google Scholar]
- 30. International Council on Harmonization (ICH) of Technical Requirements of Pharmaceuticals for Human Use. Medizinisches Wörterbuch für Aktivitäten im Rahmen der Arzneimittelzulassung (MedDRA®): Leitfaden MedDRA Version 19.0. (2016) MSSO-DI-6003-19.0.0, http://www.meddra.org/sites/default/files/guidance/file/intguide_19_0_german.pdf (accessed 9 August 2016).
- 31. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–245. [DOI] [PubMed] [Google Scholar]
- 32. National Coordinating Council for Medication Error Reporting and Prevention (2001). NCC MERP index for categorizing medication errors, http://www.nccmerp.org/sites/default/files/indexColor2001-06-12.pdf (accessed 9 August 2016). [DOI] [PubMed]
- 33. Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm 1992; 27: 538. [PubMed] [Google Scholar]
- 34. Hoth AB, Carter BL, Ness J, et al. Development and reliability testing of the clinical pharmacist recommendation taxonomy. Pharmacotherapy 2007; 27: 639–646. [DOI] [PubMed] [Google Scholar]
- 35. Siebert S, Elkeles B, Hempel G, et al. The PRISCUS list in clinical routine. Practicability and comparison to international PIM lists. Z Gerontol Geriatr 2013; 46: 35–47. [DOI] [PubMed] [Google Scholar]
- 36. Schmader K, Hanlon JT, Weinberger M, et al. Appropriateness of medication prescribing in ambulatory elderly patients. J Am Geriatr Soc 1994; 42: 1241–1247. [DOI] [PubMed] [Google Scholar]
- 37. Hohmann C, Neumann-Haefelin T, Klotz JM, et al. Drug-related problems in patients with ischemic stroke in hospital. Int J Clin Pharm 2012; 34: 828–831. [DOI] [PubMed] [Google Scholar]
- 38. Somers A, Robays H, Vander Stichele R, et al. Contribution of drug related problems to hospital admission in the elderly. J Nutr Health Aging 2010; 14: 477–482. [DOI] [PubMed] [Google Scholar]
- 39. Taegtmeyer AB, Curkovic I, Corti N, et al. Drug-related problems and factors influencing acceptance of clinical pharmacologists’ alerts in a large cohort of neurology inpatients. Swiss Med Wkly 2012; 142: w13615. [DOI] [PubMed] [Google Scholar]
- 40. Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika 1991; 78: 691–692. [Google Scholar]
- 41. Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and hospital mortality rates. Pharmacotherapy 2007; 27: 481–493. [DOI] [PubMed] [Google Scholar]
- 42. Brown BK, Earnhart J. Pharmacists and their effectiveness in ensuring the appropriateness of the chronic medication regimens of geriatric inpatients. Consult Pharm 2004; 19: 432–436. [DOI] [PubMed] [Google Scholar]
- 43. Castelino RL, Bajorek BV, Chen TF. Targeting suboptimal prescribing in the elderly: a review of the impact of pharmacy services. Ann Pharmacother 2009; 43: 1096–1106. [DOI] [PubMed] [Google Scholar]
- 44. Bladh L, Ottosson E, Karlsson J, et al. Effects of a clinical pharmacist service on health-related quality of life and prescribing of drugs: a randomised controlled trial. BMJ Qual Saf 2011; 20: 738–746. [DOI] [PubMed] [Google Scholar]
- 45. Fertleman M, Barnett N, Patel T. Improving medication management for patients: the effect of a pharmacist on post-admission ward rounds. Qual Saf Health Care 2005; 14: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mortimer C, Emmerton L, Lum E. The impact of an aged care pharmacist in a department of emergency medicine. J Eval Clin Pract 2011; 17: 478–485. [DOI] [PubMed] [Google Scholar]
- 47. Hanlon JT, Lindblad CI, Gray SL. Can clinical pharmacy services have a positive impact on drug-related problems and health outcomes in community-based older adults? Am J Geriatr Pharmacother 2004; 2: 3–13. [DOI] [PubMed] [Google Scholar]
- 48. Patterson SM, Cadogan CA, Kerse N, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2014: CD008165. [DOI] [PubMed] [Google Scholar]
- 49. Schubert I, Kupper-Nybelen J, Ihle P, et al. Prescribing potentially inappropriate medication (PIM) in Germany’s elderly as indicated by the PRISCUS list. An analysis based on regional claims data. Pharmacoepidemiol Drug Saf 2013; 22: 719–727. [DOI] [PubMed] [Google Scholar]
- 50. Amann U, Schmedt N, Garbe E. Prescribing of potentially inappropriate medications for the elderly: an analysis based on the PRISCUS list. Dtsch Arztebl Int 2012; 109: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Björkman IK, Fastbom J, Schmidt IK, et al. Drug-drug interactions in the elderly. Ann Pharmacother 2002; 36: 1675–1681. [DOI] [PubMed] [Google Scholar]
- 52. Jankel CA, Speedie SM. Detecting drug interactions: a review of the literature. DICP 1990; 24: 982–989. [DOI] [PubMed] [Google Scholar]
- 53. Vogel G. Management von Arzneimittelinteraktionen in der öffentlichen Apotheke. Inaugural-Dissertation, Rheinische Friedrich-Wilhelms-Universität Bonn; Bonn, 2012. [Google Scholar]
- 54. Becker ML, Kallewaard M, Caspers PWJ, et al. Potential determinants of drug-drug interaction associated dispensing in community pharmacies. Drug Saf 2005; 28: 371–378. [DOI] [PubMed] [Google Scholar]
- 55. Horn JR, Hansten PD, Osborn JD, et al. Customizing clinical decision support to prevent excessive drug-drug interaction alerts. Am J Health Syst Pharm 2011; 68: 662–664. [DOI] [PubMed] [Google Scholar]
- 56. Seidling HM, Paterno MD, Haefeli WE, et al. Coded entry versus free-text and alert overrides: what you get depends on how you ask. Int J Med Inform 2010; 79: 792–796. [DOI] [PubMed] [Google Scholar]
- 57. Barth M, Holzmüller G, Fritsch H, et al. Vergleich verschiedener Interaktionsdatenbanken: Poster - 3. Kongress für Arzneimittelinformation, Köln, Januar 2013, http://www.adka-arznei.info/aminfo2013/downloads/Poster_2013/06_Sturm.pdf (2013, accessed 13 December 2016). [Google Scholar]
- 58. O’Sullivan D, O’Mahony D, O’Connor MN, et al. Prevention of adverse drug reactions in hospitalised older patients using a software-supported structured pharmacist intervention: a cluster randomised controlled trial. Drugs Aging 2016; 33: 63–73. [DOI] [PubMed] [Google Scholar]
- 59. O’Connor MN, Gallagher P, Byrne S, et al. Adverse drug reactions in older patients during hospitalisation: are they predictable? Age Ageing 2012; 41: 771–776. [DOI] [PubMed] [Google Scholar]
- 60. Lavan A, Eustace J, Dahly D, et al. Incident adverse drug reactions in geriatric inpatients: a multicentred observational study. Ther Adv Drug Saf 2018; 9: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kanagaratnam L, Mahmoudi R, Novella J-L, et al. Adverse drug reactions in elderly subjects hospitalized in a specialized dementia management unit. Drugs Aging 2014; 31: 769–776. [DOI] [PubMed] [Google Scholar]
- 62. Beltrami GC, Menegolli GP, Corrà L, et al. Effetti collaterali da farmaci in anziani ospedalizzati per patologie acute. Clin Ter 2000; 151: 19–23. [PubMed] [Google Scholar]
- 63. Liamis G, Milionis H, Elisaf M. A review of drug-induced hyponatremia. Am J Kidney Dis 2008; 52: 144–153. [DOI] [PubMed] [Google Scholar]
- 64. Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc 1999; 47: 40–50. [DOI] [PubMed] [Google Scholar]
- 65. Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc 1999; 47: 30–39. [DOI] [PubMed] [Google Scholar]
- 66. Blix HS, Viktil KK, Moger TA, et al. Characteristics of drug-related problems discussed by hospital pharmacists in multidisciplinary teams. Pharm World Sci 2006; 28: 152–158. [DOI] [PubMed] [Google Scholar]
- 67. O’Sullivan D, O’Mahony D, O’Connor MN, et al. The impact of a structured pharmacist intervention on the appropriateness of prescribing in older hospitalized patients. Drugs Aging 2014; 31: 471–481. [DOI] [PubMed] [Google Scholar]
- 68. Bondesson A, Holmdahl L, Midlov P, et al. Acceptance and importance of clinical pharmacists’ LIMM-based recommendations. Int J Clin Pharm 2012; 34: 272–276. [DOI] [PubMed] [Google Scholar]
- 69. Viktil KK, Blix HS. The impact of clinical pharmacists on drug-related problems and clinical outcomes. Basic Clin Pharmacol Toxicol 2008; 102: 275–280. [DOI] [PubMed] [Google Scholar]
- 70. Weißenborn M, Schulz M, Kraft M, et al. Potential benchmarks for successful interdisciplinary collaboration projects in Germany: A systematic review. Gesundheitswesen 2018. June 21. doi: 10.1055/a-0592-7184. [DOI] [PubMed] [Google Scholar]
- 71. Denneboom W, Dautzenberg MGH, Grol R, et al. Comparison of two methods for performing treatment reviews by pharmacists and general practitioners for home-dwelling elderly people. J Eval Clin Pract 2008; 14: 446–452. [DOI] [PubMed] [Google Scholar]
- 72. Garfinkel D. Poly-de-prescribing to treat polypharmacy: efficacy and safety. Ther Adv Drug Saf 2018; 9: 25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reeve E, Moriarty F, Nahas R, et al. A narrative review of the safety concerns of deprescribing in older adults and strategies to mitigate potential harms. Expert Opin Drug Saf 2018; 17: 39–49. [DOI] [PubMed] [Google Scholar]
- 74. Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med 2009; 169: 894–900. [DOI] [PubMed] [Google Scholar]
- 75. Somers A, Robays H, de Paepe P, et al. Evaluation of clinical pharmacist recommendations in the geriatric ward of a Belgian university hospital. Clin Interv Aging 2013; 8: 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Spinewine A, Swine C, Dhillon S, et al. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized, controlled trial. J Am Geriatr Soc 2007; 55: 658–665. [DOI] [PubMed] [Google Scholar]
- 77. Köberlein-Neu J, Mennemann H, Hamacher S, et al. [Interprofessional medication management in patients with multiple morbidities—a cluster-randomized trial (the WestGem study).] Dtsch Arztebl Int 2016; 113: 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther 2011; 89: 845–854. [DOI] [PubMed] [Google Scholar]
- 79. Gallagher P, Ryan C, Byrne S, et al. STOPP (screening tool of older person’s prescriptions) and START (screening tool to alert doctors to right treatment). Consensus validation. Int J Clin Pharmacol Ther 2008; 46: 72–83. [DOI] [PubMed] [Google Scholar]
- 80. McGrattan M, Ryan C, Barry HE, et al. Interventions to improve medicines management for people with dementia: a systematic review. Drugs Aging 2017; 34: 907–916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Online_figure_5 for Influence of pharmacist intervention on drug safety of geriatric inpatients: a prospective, controlled trial by Angela Nachtigall, Hans J. Heppner and Petra A. Thürmann in Therapeutic Advances in Drug Safety
Supplemental material, Online_tables(5-6) for Influence of pharmacist intervention on drug safety of geriatric inpatients: a prospective, controlled trial by Angela Nachtigall, Hans J. Heppner and Petra A. Thürmann in Therapeutic Advances in Drug Safety