Short abstract
Background
Persons with multiple sclerosis may benefit from hospital-based multidisciplinary rehabilitation.
Objectives
To investigate the effects of hospital-based multidisciplinary rehabilitation and to identify their potential predictors in a large sample of persons with multiple sclerosis.
Methods
From the charts of 655 persons with multiple sclerosis consecutively admitted to our unit, disease profiles, modified Barthel index, Expanded Disability Status Scale (EDSS), pain numerical rating score and type of interventions were retrospectively collected. We defined an improvement at discharge as follows: modified Barthel index increase of at least 5 points, EDSS decrease of 1.0 if baseline score was 5.5 or less and of 0.5 if baseline score was greater than 5.5; any numerical rating score decrease.
Results
After a median admission period of 36 days, at discharge 65%, 22% and 89% of persons with multiple sclerosis improved for modified Barthel index, EDSS and numerical rating score, respectively. The modified Barthel index improvement was associated with shorter disease duration, lower EDSS at baseline and with access to psychological counselling. EDSS improvement was associated with shorter disease duration, relapsing–remitting course, female gender and longer duration of the admission period.
Conclusions
Inpatient multidisciplinary rehabilitation was associated with improved autonomy in activities of daily living in a relevant proportion of persons with multiple sclerosis. The effect seems to be more evident in individuals with shorter multiple sclerosis duration and relapsing–remitting disease course.
Keywords: Multidisciplinary rehabilitation, multiple sclerosis, inpatient setting
Introduction
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system, in which both inflammation and irreversible neuroaxonal damage can be present from the early stages.1 This may provoke moderate to severe physical, cognitive and psychological disabilities. Moreover, pain is a common symptom in MS by up to 75% of patients. It is, therefore, widely recognised that, beyond the management of disease-modifying therapies (DMTs), MS requires a multidisciplinary care, including rehabilitation. Rehabilitation can be defined as a process that helps a person to achieve and maintain maximal physical, psychological, social and vocational potential, and quality of life (QoL) consistent with impairment, environment and life goals. In principle, rehabilitation interventions for persons with multiple sclerosis (PwMS) may include exercise, functional training, equipment prescription, provision of assistive technology, orthotics prescription, teaching of compensatory strategies, caregiver/family support and education, counselling and referral to community resources. These can be targeted for a variety of impairments, such as mobility, fatigue, pain, dysphagia, bladder/bowel dysfunction, decreased independence in activities of daily living (ADL), communication, QoL, affective disorders and cognitive dysfunction.2 Several pieces of evidence confirm that rehabilitation is an effective intervention for PwMS,3 even though no consensus on a ‘best practice’ approach has been reached yet. It is worth remembering that generating evidence in rehabilitation research is more complex than in other medical fields, as the design and conduct of placebo controlled, double-blind studies is extremely difficult. Another problem is to find appropriate outcome measures, fully encompassing the actual disability of PwMS, to investigate the effectiveness of the interventions.4,5
According to the individual patient needs, rehabilitation can be administered with different protocols and settings. Among them, hospital-based multidisciplinary rehabilitation (MDR) has the advantage of allowing the administration of multiple interventions in a protected environment and, thereby, guaranteeing intensity, adherence and feasibility even for more disabled patients. Several studies have indicated that inpatient MDR can reduce disability and improve QoL in PwMS6–8 compared with no intervention. It remains to be established how long the effects of inpatient MDR last after discharge and what is the actual cost-effectiveness of this intervention, which requires relevant health service and community resources,9,10 thereby often limiting the access to a minority of PwMS.11,12
Against this background, profiling the best candidates for inpatient MDR can be useful to enhance the effectiveness of this intervention and to optimise the use of available resources. To the best of our knowledge, two studies13,14 have investigated whether the effectiveness of inpatient MDR can be predicted by the clinical characteristics of PwMS at treatment initiation. Those studies, which were conducted in relatively small cohorts and using different outcomes, achieved conflicting results, confirming that inpatient MDR leads to a significant improvement in functional status in the majority of treated PwMS, but identifying different possible predictors of such an effect. While Grasso and coworkers13 found that the effectiveness of MDR seems to be higher in patients with mild to moderate than in those with severe MS, Liberatore and colleagues14 reported that a more severe baseline impairment, a shorter MS duration and a relapsing–remitting (RR) disease course were predictive of rehabilitation effectiveness. With the present retrospective study we investigated, in a large single centre cohort of PwMS, whether clinical and demographic characteristics can be predictors of inpatient MDR effectiveness at discharge on limitations in ADL and on locomotor disability, with the aims to assess the impact of MDR in a real-life, hospital-based context and to understand better which individuals most benefit from this type of treatment.
Materials and methods
All patients with MS consecutively admitted to the Neurorehabilitation Unit, MS Center, Scientific Institute Don Gnocchi (Milan) from July 2011 to June 2016 were selected for the present retrospective study. The unit is part of a scientific institute for rehabilitation which is not linked to an acute-care general hospital. The institute also hosts a centre for MS care and research, where PwMS can have access to multidisciplinary care including DMT if indicated. The criteria for admission to inpatient MDR were the presence of two or more moderate neurological disabilities at clinical evaluation and a recent (i.e. within 6 months) functional deterioration. For all patients fulfilling these criteria MDR programmes were publicly subsidised and fully reimbursed by the national health system. All admitted patients were enrolled in an intensive rehabilitation programme consisting of daily sessions from Monday to Saturday for a total of at least 500 minutes a week. The programme was based on a multidisciplinary evaluation assessing the patients’ needs and the possible goals, performed by a neurologist together with a physical medicine and rehabilitation specialist. When needed, other evaluations (cognitive, urological, ophthalmological, respiratory, etc.) were performed to define the programme. Interventions may include physiotherapy, occupational therapy (including assessment and eventually prescription of adequate aids), respiration therapy, cognitive rehabilitation, speech and swallowing rehabilitation, physical therapy for pain (e.g. massage therapy, transcutaneous electrical nerve stimulation, electrical stimulation, ionophoresis) and formal psychological counselling. The duration of the admission was established following an intermediate re-assessment of the programme and goals performed by the MDR team (physicians, therapists and nurses) after 2–3 weeks of admission. Our unit being part of a scientific institute, all subjects had provided informed consent to the use of clinical data collected during the admission for research purposes and no procedures other than the standard ones were performed for the present study.
From the clinical charts, we retrieved subjects’ demographics, disease duration, disease phenotype, ongoing therapies for MS, modified Barthel index (mBI),15 Expanded Disability Status Scale (EDSS),16 pain numerical rating score (NRS)17 and types of intervention administered. Clinical scales were rated at admission and discharge. When multiple admissions of the same subject occurred during the study time frame, only the first (i.e. the oldest) one was considered. All these pieces of information were collected by a single observer (EG) in a database and anonymised for data analysis. According to their functional scale scores, patients were classified into the following categories: for EDSS: A (EDSS ≤5.5) subjects able to walk without assistance; B (EDSS 6.0–7.5) subjects walking with support and increasing limitation; C (EDSS ≥8.0) wheelchair-bound subjects; for mBI: 1, subjects with complete dependency in ADL (mBI 0–24); 2, subjects with severe dependency in ADL (mBI 25–49); 3, subjects with moderate dependency in ADL (mBI 50–74); 4, subjects with mild dependency in ADL (mBI 75–90); 5, subjects with minimal dependency in ADL (mBI ≥91).
Statistical analysis
For collected variables, means and relative standard deviations (SDs) or medians and interquartile ranges (IQRs) were calculated. mBI changes between admission and discharge were analysed using the Student’s t-test for paired data, while the non-parametric Wilcoxon test for paired data was used for EDSS and NRS. A univariable and multivariable logistic regression analysis was used to assess whether the clinical and demographic characteristics of patients at baseline (i.e. age, gender, disease duration, disease phenotype, mBI score, EDSS score, pain NRS score), as well as the duration of the admission and the number of interventions, were predictors of MDR effects on mBI, EDSS and pain NRS scores. These latter outcomes were all considered as the binary dependent variable in each logistic model (yes/no improvement). Improvement at discharge was defined as follows: for mBI an increase of at least 5 points,13 for EDSS a decrease of at least 1 point if the score at baseline was 5.5 or less or a decrease of at least 0.5 point if EDSS at admission was greater than 5.5.14 For pain NRS any decrease was considered as an improvement.
An index of MDR effectiveness on mBI was calculated as follows: MDR effectiveness = (discharge mBI score – admission mBI score) + (100 – admission mBI score) × 100%, following the formula proposed by Grasso et al.13 For pain NRS, the same formula was applied to compute percentage changes at discharge as follows: (discharge NRS score – admission NRS score) + (10 – admission NRS score) × 100%. For interpretation reasons the mathematical sign of this latter measure was changed into higher values corresponding to a greater improvement. Both mBI and NRS percentage changes were used as dependent variables in two linear regression models in which the role of baseline characteristics on these outcomes was assessed. For this analysis the disease duration was reported as a categorical variable with cut-off set at the first tertile (i.e. 15 years), as the improvement was consistently noted in patients with the shortest disease duration. Results were reported as odds ratios (ORs) with the corresponding 95% confidence interval (CI) for logistic regression and with linear regression coefficients (with 95% CI) for the linear regression. An OR greater than 1 corresponded to an increase in the probability of improvement for the investigated endpoint, while the opposite was true for ORs less than 1. Similarly, a regression coefficient greater than 0 corresponded to a greater improvement on the corresponding outcome.
To build both the logistic and the linear multivariable regression models, variables were selected among those reported in univariable analysis using lasso estimator with an information criteria approach.
As few patients showed missing data, no imputation or replacement of these data was considered. A P value less than 0.05 was considered statistically significant. The Stata software (v.14; Stata Corp) was used for the computations.
Results
The analysis included data from 655 PwMS, with a female gender predominance (Table 1). Among them, 70% had either a secondary progressive or a primary progressive and 30% had a RR disease course; the median disease duration was 19 years (range 0–58 years); most subjects (74.5%) were not taking any DMT. Of these patients, the vast majority (98.5%) was admitted from home. Most subjects (80.3%) reported pain at admission. The MDR programme included at least two types of interventions; these consisted of physiotherapy in all subjects, in most of whom it was associated with physical therapy for pain and occupational therapy (Table 1). The median duration of the admission period was 36 days (range 29–44 days). The median number of interventions was four (range one to six); in 484 (73.9%) subjects, the MDR programme consisted of three or more types of interventions.
Table 1.
Subject characteristics at baseline (n = 655) and types of intervention during the admission period.
| Gender (%) | |
| Female | 405 (61.8) |
| Male | 250 (38.2) |
| F/M | 1.6 |
| Mean age (years) (SD) | |
| Total | 51 (8.49) |
| Female | 49 (5.66) |
| Male | 51 (8.49) |
| MS phenotypes (%) | |
| Secondary progressive | 344 (52.5) |
| Primary progressive | 115 (17.5) |
| Relapsing–remitting | 196 (30) |
| Admission from (%) | |
| Home | 645 (98.5) |
| Hospital | 8 (1.2) |
| Residential unit | 2 (0.3) |
| NRS distribution (n=638) | |
| 0 No pain (%) | 129 (19.7) |
| 1–10 Any pain (%) | 526 (80.3) |
| Types of intervention (%) | |
| Physiotherapy | 655 (100) |
| Physical therapy for pain | 564 (86.1) |
| Occupational therapy | 524 (80.0) |
| Speech and swallowing rehabilitation | 374 (57.1) |
| Psychological counselling | 178 (27.2) |
| Cognitive rehabilitation | 150 (22.9) |
| Respiration therapy | 145 (22.2) |
MS: multiple sclerosis; NRS: numerical rating scale for pain.
At admission, the median EDSS was 6.5 and mBI was 63 (Table 2). These levels reflect a moderate to severe impairment of autonomy in ADL and a low level of mobility in our population, as confirmed by the categorical distribution of subjects for both mBI and EDSS scores.
Table 2.
Clinical outcomes following MDR.
| Admission | Discharge | Difference (discharge vs. admission) | P value | |
|---|---|---|---|---|
| EDSS, median (range) | 6.5 (3–9.5) | 6.5 (1–9.5) | –0.18 (0.38; –3.5, 1)a | <0.001 |
| Category A, n (%) | 98 (15) | 129 (19.7) | ||
| Category B, n (%) | 384 (58.6) | 378 (57.7) | ||
| Category C, n (%) | 173 (26.4) | 146 (22.3) | ||
| EDSS improved, n (%) | 145 (22.2) | |||
| Changed category, n (%) | 57 (8.7) | |||
| From category B to A | 31 (4.7) | |||
| From category C to B | 26 (4.0) | |||
| mBI, mean (SD) | 54.7 (25.1) | 61.5 (26.9) | 6.8 (6.5) | <0.001 |
| mBI, median (IQR) | 63 (38–74) | 70 (45.5–82) | 5.5 (2–10) | |
| Category 1 (0–24), n (%) | 105 (16.6) | |||
| Category 2 (25–49) | 116 (18.3) | |||
| Category 3 (50–74) | 256 (40.4) | |||
| Category 4 (75–90) | 148 (23.3) | |||
| Category 5 (91–99) | 9 (1.4) | |||
| mBI improved, n (%) | 413 (65.1%) | |||
| Changed category, n (%) | ||||
| From category 1 to ≥2 | 15 (2.4) | |||
| From category 2 to ≥3 | 45 (7.0) | |||
| From category 3 to ≥4 | 112 (17.7) | |||
| From category 4 to 5 | 44 (6.9) | |||
| Rehabilitation effectiveness,b | 20.6 (21.2) | |||
| mean (SD) | ||||
| Rehabilitation effectiveness,b | 16.9 (3.9–30.4) | |||
| median (IQR) | ||||
| NRS, mean (SD) | 4.7 (2.9) | 2.5 (2.3) | –2.2 (2.1) | <0.001 |
| NRS, median (IQR) | 5 (3–7) | 2 (0–4) | –2 (–3–0) |
MDR: multidisciplinary rehabilitation; EDSS: Expanded Disability Status Scale; NRS: numerical rating scale for pain; mBI: modified Barthel index; IQR: interquartile range; SD: standard deviation.
EDSS categories: A (≤5.5) ambulation without assistance; B (6.0–7.5) ambulation with support and increasing limitation; C (≥8.0) wheelchair bound.
mBI categories (degree of dependency): 1, total; 2, severe; 3, moderate; 4, mild; 5, minimal.
aResults are reported as mean (SD; interquartile range).
bSee Materials and methods section for definition and formula.
At discharge, according to the predefined cut-offs, 435 of 636 patients (68.4%) showed an improvement of either mBI or EDSS score. In detail, 413 of 634 patients (65.1%) had an mBI increase of at least 5 points, and 145 of 653 (22.2%) had a significant EDSS decrease (see the Statistical analysis section for the cut-off definitions). Both mBI and EDSS showed an improvement in 123 of 636 (19.3%) patients. The NRS decreased in 468 of 526 (88.9%) patients reporting pain at admission. Interestingly, a significant mBI increase without a EDSS decrease was observed in 288 of 634 (45.4%) patients, while the reverse was true only for 20 of 653 (3.1%) patients. A significant mBI increase at discharge was observed in 60 of 98 (61.22%), 242 of 384 (63.02%) patients and 73 of 173 (42.2%) with EDSS scores at baseline of category A, B and C, respectively. Group comparisons between patients who showed mBI improvement at discharge and those who did not showed that the former had lower EDSS and higher mBI at baseline, were affected by RRMS in a higher proportion, and more frequently underwent psychological counselling during the admission period (Table 3). Patients who showed EDSS improvement at discharge had a shorter disease duration than those who did not (Table 3).
Table 3.
Group comparisons between patients who did and did not show mBI or EDSS improvement at discharge.
| Characteristics | mBI improvement (n=413) | No mBI improvement (n=221) | P valuea |
|---|---|---|---|
| Age (years), mean (SD) | 53.4 (11.6) | 55.2 (11.4) | 0.059 |
| Female gender, n (%) | 263 (63.7) | 129 (58.4) | 0.20 |
| Disease duration (years), median (IQR) | 18 (12–27) | 21 (15–29) | 0.008 |
| Baseline EDSS, median (IQR) | 6.5 (6–7) | 7 (6.5–8) | <0.001 |
| Baseline mBI, mean (SD); median (IQR) | 59.6 (19.9); 64 (49–74) | 51.8 (27.7); 60 (26–76) | 0.017 |
| MS phenotype, n (%) | 0.007 | ||
| RR | 142 (34.7) | 53 (24.1) | |
| Progressive | 267 (65.3) | 167 (75.9) | |
| Number of interventions, mean; median (IQR) | 2.95; 3 (2–3) | 2.79; 3 (2–3) | 0.066 |
| Access to psychological counselling, n (%) | 127 (31.5) | 47 (21.7) | 0.011 |
| Length of admission (days), mean (SD); median (IQR) | 35.7 (8.8); 35 (29–41) | 35.7 (9.9); 35 (29–41) | 0.51 |
| EDSS improvement (n=145) | No EDSS improvement (n=508) | P valuea | |
| Age (years), mean (SD) | 52.4 (13.0) | 54.4 (11.1) | 0.062 |
| Females, n (%) | 100 (69) | 305 (60) | 0.053 |
| Disease duration (years), median (IQR) | 18 (11–25) | 20 (14–28) | 0.008 |
| Baseline EDSS, median (IQR) | 7 (6–7.5) | 6.5 (6–8) | 0.17 |
| MS Phenotype, n (%) | 0.051 | ||
| RR | 53/144 (36.8) | 142/504 (28.2) | |
| Progressive | 91 (63.2) | 362/504 (71.8) | |
| Number of interventions, mean; median (IQR) | 2.87; 3 (2–4) | 2.80; 3 (2–4) | 0.45 |
| Access to psychological counselling, n (%) | 47 (33.1) | 131 (26.3) | 0.11 |
| Length of admission (days), mean (SD); median (IQR) | 37.6 (10.4); 36 (29–44) | 35.2 (9.4); 35 (29–41) | 0.059 |
aCalculated from univariable logistic regression with mBI and EDSS improvement status as dependent variables.
mBI: modified Barthel index; EDSS: Expanded Disability Status Scale; SD: standard deviation; IQR: interquartile range, RR: relapsing–remitting.
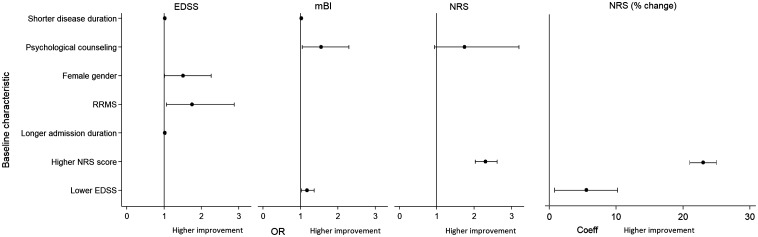
The multivariate analysis of MDR effect predictors (Table 4, Figure 1) showed that mBI improvement was significantly associated with access to psychological counselling during the admission period, a shorter disease duration and a lower EDSS score. EDSS improvement was significantly more frequent in patients with a shorter disease duration, female gender, RRMS course and longer admission duration. Pain NRS improvement was significantly associated with higher NRS scores at admission and with access to psychological counselling during the admission period.
Table 4.
Predictors of mBI, EDSS and NRS changes at discharge.
| Predictors | OR and regression coefficients (95% CI) | P value |
|---|---|---|
| mBI improvement | ||
| Psychological counselling (yes vs. no) | 1.55 (1.05–2.30) | 0.028 |
| Lower disease duration (1 year) | 1.02 (1.01–1.04) | 0.014 |
| Lower baseline EDSS (1 point) | 1.18 (1.02–1.37) | 0.031 |
| EDSS improvement | ||
| Lower disease duration (1 year) | 1.02 (1.00–1.04) | 0.021 |
| MS phenotype | ||
| Relapsing–remitting vs. progressive | 1.75 (1.07–2.88) | 0.027 |
| Gender | ||
| Female vs. male | 1.51 (1.01–2.26) | 0.048 |
| Length of admission (days) | 1.02 (1.00–1.04) | 0.049 |
| NRS decrease (binary) | ||
| Baseline NRS (1 point) | 2.30 (2.03–2.61) | <0.001 |
| Psychological counselling (yes vs. no) | 1.74 (0.94–3.20) | 0.077 |
| NRS decrease (% change) | ||
| Baseline NRS (1 point) | 23 (21–25) | <0.001 |
| Lower baseline EDSS (1 point) | 5.48 (0.79–10.18) | 0.022 |
| MDR effectiveness | ||
| Baseline mBI (1 point) | 0.2 (0.12, 0.28) | <0.001 |
| Disease duration (<15 vs. ≥15 years) | 4 (0.7, 7.2) | 0.017 |
| MS phenotype (relapsing–remitting vs. progressive) | 5 (0.4, 9.6) | 0.032 |
OR: odds ratio; CI: confidence interval; EDSS: Expanded Disability Status Scale; NRS: numerical rating scale for pain; mBI: modified Barthel index.
See the text for definitions and statistical analysis methods.
Figure 1.
Forest plot graphs showing significant baseline predictors of modified Barthel index (mBI), Expanded Disability Status Scale (EDSS) and numerical rating score (NRS) improvement at discharge (see the text for abbreviations and definitions). Odds ratios (ORs) are shown as mean values with the corresponding 95% confidence intervals.
A greater NRS improvement, when computed as a percentage change, was significantly associated with higher NRS and lower EDSS scores at admission (Table 4); association coefficients indicate that, on average, NRS percentage improvement at discharge was 23% greater for one more NRS point and 5.5% greater for one less EDSS point scored at admission. A greater MDR effectiveness on mBI was significantly associated with higher baseline mBI, shorter disease duration and RRMS course (Table 4); on average, patients with disease duration longer than 15 years had a 4% lower MDR effectiveness than those with shorter disease duration, and patients with progressive MS a 5% lower effectiveness than those with RRMS.
Discussion
MS requires a multimodal care, including both pharmacological therapies and rehabilitation. Hospital-based rehabilitation, performed by a coordinated multidisciplinary group, has the advantage of addressing multiple needs and guaranteeing intensity, adherence and feasibility in a protected environment. Its effectiveness in reducing disability and improving QoL has been repeatedly demonstrated in PwMS.7,8,10,13,14 With the present study we aimed at generating additional pieces of evidence about the effects of inpatient MDR by analysing data from a large cohort treated in a single centre in a real-life setting. In addition, we investigated whether subjects’ clinical and demographic characteristics may help to identify predictive factors of treatment effects.
The study design was observational and retrospective; source data had been generated prospectively in a large population of PwMS who were consecutively admitted to our hospital unit. Most of them were affected by the progressive form of the disease, with moderate to high disability (more than 80% requiring walking support) and limitation in ADL. The lack of exclusion criteria corroborates the notion that the population is well representative of the real-life scenario. The multidisciplinary intervention was individualised and goal-oriented, thus this study does not allow the identification of the effectiveness of a specific treatment programme. Furthermore, we selected as outcome measures of MDR effectiveness both a widely applied scale for ADL (i.e. mBI) and the commonest disease-specific scale used to quantify neurological disability in MS (i.e. EDSS).
Following MDR about two-thirds of these patients reported a significant improvement of mBI, while a EDSS decrease was observed in 22% of them. Our findings are consistent with those from previous studies and trials in showing the positive effects of inpatient MDR in MS.7,8,10,13,14 This intervention lead to a short-term functional improvement in the majority of admitted PwMS, even if they were severely limited on a motor ground. Interestingly, a EDSS decrease without mBI improvement was reported in very few (about 3%) patients. This suggests that the observed reduction of dependency and limitations in ADL may not be fully reflected by changes of a scale which is heavily weighted towards locomotor impairment and poorly sensitive to other functional changes. The EDSS score can, therefore, be viewed as a disease-specific descriptive scale rather than a complementary outcome measure of improvement following MDR, especially in patients with a more severe and long-lasting MS. On the other hand, mBI captured a significant functional improvement in the majority of our patients, as did the motor sub-items of the functional independence measure (FIM) in the study by Liberatore et al.,14 in which patients had, on average, less disability at baseline. The latter difference may, however, explain why, in that population,14 EDSS was more sensitive to the observed changes than in our study. mBI is widely used to measure the level of autonomy in ADL and our findings confirm that it can be considered a valuable tool for monitoring functional recovery due to rehabilitation in PwMS.18
We were not able to identify strong clinical predictors of the MDR effectiveness reflected by mBI or EDSS improvement at discharge. The multivariable analysis revealed that only a shorter disease duration was associated with a greater chance of improvement for both these outcomes; for EDSS decrease, the model retained RR phenotype, female gender and longer admission period too. Our findings are, at least partially, consistent with those obtained by previous studies,13,14 which were, however, characterised by the use of different or additional outcome measures (i.e. Rivermead mobility index (RMI) and motor FIM, respectively) and conducted in smaller samples of subjects. It is also worth noting that, in both those studies,13,14 the average levels of patients’ disability and impairment at baseline were lower than in ours and the treatment duration was longer (about 2 months). Our findings and those from the other studies13,14 seem, however, to suggest that functional recovery following inpatient MDR can be more pronounced in MS subjects with less severe and long-lasting disease, who may, therefore, warrant priority of access to this setting. Interestingly, we found that psychological counselling was associated with enhanced MDR effects on mBI and this confirms the importance of patients’ motivation and mood in achieving functional improvement.
Even though systematic reviews provide strong evidence of the short-term effectiveness of multi-disciplinary packages of care in improving activities and participations in PwMS,6 determining the actual benefit of inpatient MDR remains challenging, also because its effectiveness is a complex variable to define and quantify. First of all, MDR programmes must be individualised and can include a wide range of interventions depending on the goals and on the patient needs and priorities. Therefore, it is not surprising that, having limited our evaluation to a comprehensive and non-specific functional scale such as the mBI, we may not have fully encompassed the impact of MDR on the multiple dysfunctions caused by MS. Recently, Barin and colleagues19 assessed the concurrent validity and relative responsiveness of a multidimensional outcome measure core set, including FIM, RMI, the 2-Minute Walk Test, Timed 25-Foot Walk test and Nine-Hole Peg Test, in a population of inpatients with progressive MS admitted to a neurorehabilitation centre. In this study, for PwMS with EDSS of 7 or greater the only responsive measures were RMI and FIM motor, indicating that high EDSS scores may be associated with a lesser sensitivity to changes for scales mainly reflecting motor abilities.19 Other researchers used outcome measures of inpatient MDR focused on health-related QoL rather than on functional/motor impairment.10 They reported a significant effectiveness of inpatient MDR on two measures of QoL, which was persistent 6 months after discharge.10 All those studies10,13,14,19 consistently show the effectiveness of inpatient MDR on different outcomes and seem to suggest that a composite measure reflecting both functioning (e.g. mBI, FIM and other mobility scales) and QoL might fully encompass and assess the ‘global’ impact of this intervention. Perhaps only the integration between the International Classification of Functioning, Disability and Health (ICF) and outcome measure scales18 might enable us to obtain reliable and comprehensive measures and to facilitate the conduct of multicentre studies to address this issue better.
Admittedly, our study had several limitations. These include the retrospective design, the high variability of interventions and the use of ‘generic’ scales rated by different observers, who were, however, skilled physicians in MS care and functional scale administration. In particular, the assessment of changes in EDSS may have also been limited by the well-known subjectivity of this scale.20 As regards the described effects of MDR on pain intensity, it is worth remembering that NRS remains a subjective scale, which was not specifically set and validated for MS and that PwMS may simultaneously experience pain of heterogeneous causes and types, including neuropathic, musculoskeletal and ‘mixed’ pain in both continuous and intermittent ways.21,22 All of this may, on the one hand, explain the very high prevalence of pain in our population of aged and severely disabled PwMS, which remains, however, consistent with the frequency of pain complaints described in MS studies.21 On the other hand, these considerations make it difficult to interpret the actual impact of inpatient MDR programmes on pain in the absence of more specific measures. Moreover, we do not have data about the post-discharge effects of MDR (i.e. duration and impact on ADL at home). Nevertheless, to the best of our knowledge, it reports findings from the largest sample of PwMS consecutively admitted to a single rehabilitation unit, thereby providing us with ‘real-life’ data without selection or effectiveness bias. Beyond the confirmation that inpatient MDR seems to be able to improve the autonomy in most PwMS, our results seem to indicate that such an effect, albeit stronger in individuals with shorter disease duration, lesser neurological disability and RR course, is maintained in those with a long-lasting and severely disabling disease. This suggests that inpatient MDR, despite its well-known costs, must be available to ensure the best disease management to all PwMS, once its objectives and goals are defined. Additional studies are warranted to clarify what could be the best outcomes to measure reliably the inpatient MDR effectiveness size and its duration over time, as well as to compare it with that of rehabilitation administered in other settings.
Conflict of Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the Italian Ministry of Health (‘Ricerca Corrente’ programme, years 2016–2017).
References
- 1.Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet Neurol 2018; 21: 1622–1636. [DOI] [PubMed] [Google Scholar]
- 2.National Medical Advisory Board of the National Multiple Sclerosis Society. Treatment recommendations for clinicians. National Multiple Sclerosis Society, New York, 2004; pp. 1–9.
- 3.Khan F, Amatya B. Rehabilitation in multiple sclerosis: a systematic review of systematic reviews. Arch Phys Med Rehabil 2017; 98: 353–367. [DOI] [PubMed] [Google Scholar]
- 4.Kraft GH, Johnson KL, Yorkston K, et al. Setting the agenda for multiple sclerosis rehabilitation research. Mult Scler 2008; 14: 1292–1297. [DOI] [PubMed] [Google Scholar]
- 5.Green R, Kalina J, Ford R, et al. SymptoMScreen: a tool for rapid assessment of symptom severity in MS across multiple domains. Appl Neuropsychol Adult 2017; 24: 183–189. [DOI] [PubMed] [Google Scholar]
- 6.Khan F, Turner-Stokes L, Ng L, et al. Multidisciplinary rehabilitation for adults with multiple sclerosis. Cochrane Database Syst Rev 2007; 2: CD006036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salhofer-Polanyi S, Windt J, Sumper H, et al. Benefits of inpatient multidisciplinary rehabilitation in multiple sclerosis. NeuroRehabilitation 2013; 33: 285–292. [DOI] [PubMed] [Google Scholar]
- 8.Drulovic J, Bursac LO, Milojkovic D, et al. MSQoL-54 predicts change in fatigue after inpatient rehabilitation for people with multiple sclerosis. Disabil Rehabil 2013; 35: 362–366. [DOI] [PubMed] [Google Scholar]
- 9.Sutliff MH, Bennett SE, Bobryk P, et al. Rehabilitation in multiple sclerosis: commentary on the recent AAN systematic review. Neurol Clin Pract 2016; 6: 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boesen F, Nørgaard M, Trénel P, et al. Longer term effectiveness of inpatient multidisciplinary rehabilitation on health-related quality of life in MS patients: a pragmatic randomized controlled trial – The Danish MS Hospitals Rehabilitation Study. Mult Scler 2018; 24: 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponzio M, Gerzeli S, Brichetto G, et al. Economic impact of multiple sclerosis in Italy: focus on rehabilitation costs. Neurol Sci 2015; 36: 227–234. [DOI] [PubMed] [Google Scholar]
- 12.Tacchino A, Brichetto G, Zaratin P, et al. Multiple sclerosis and rehabilitation: an overview of the different rehabilitation settings. Neurol Sci 2017; 38: 2131–2138. [DOI] [PubMed] [Google Scholar]
- 13.Grasso MG, Pace L, Troisi E, et al. Prognostic factors in multiple sclerosis rehabilitation. Eur J Phys Rehabil Med 2009; 45: 47–51. [PubMed] [Google Scholar]
- 14.Liberatore G, Clarelli F, Nuara A, et al. Predictors of effectiveness of multidisciplinary rehabilitation treatment on motor dysfunction in multiple sclerosis. Mult Scler 2014; 20: 862–870. [DOI] [PubMed] [Google Scholar]
- 15.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965; 14: 61–65. [PubMed] [Google Scholar]
- 16.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain 2011; 152: 2399–2404. [DOI] [PubMed] [Google Scholar]
- 18.Prodinger B, O’Connor RJ, Stucki G, et al. ; on behalf of the ICF INFO Network. Establishing score equivalence of the Functional Independence Measure motor scale and the Barthel Index, utilizing the International Classification of Functioning, Disability and Health and Rasch measurement theory. J Rehabil Med 2017; 49: 416–422. [DOI] [PubMed] [Google Scholar]
- 19.Barin L, Vaney C, Puhan MA, et al. Recommended outcome measures for inpatient rehabilitation of multiple sclerosis are not appropriate for the patients with substantially impaired mobility. Mult Scler Relat Disord 2018; 22: 108–114. [DOI] [PubMed] [Google Scholar]
- 20.Meyer-Moock S, Feng YS, Maeurer M, et al. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurology 2014; 14: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connor AB, Schwid SR, Herrmann DN, et al. Pain associated with multiple sclerosis: systematic review and proposed classification. Pain 2008; 137: 96–111. [DOI] [PubMed] [Google Scholar]
- 22.Solaro C, Trabucco E, Messmer Uccelli M. . Pain and multiple sclerosis: pathophysiology and treatment. Curr Neurol Neurosci Rep 2013; 13: 320. [DOI] [PubMed] [Google Scholar]