Abstract
Background
To evaluate the time trends of colorectal cancer (CRC) affected by a Nationwide Colorectal Cancer Screening (NCCS) programme with biennial faecal immunochemical testing (FIT) and Nationwide Healthcare Insurance (NHI).
Methods
Data from the national registries on cancer and death in Taiwan were separated into years 1984–1993, 1994–2003 and 2004–2013 based on the implementations of NHI (starting 1995) and NCCS (starting 2004). The adult population was divided into three age groups (young, 30–49; middle-aged, 50–69; and old, 70–84 years); only the middle-aged were eligible for NCCS. Crude and adjusted effects of NCCS and NHI were quantified by percentage change and 95% confidence interval (CI) with respect to CRC mortality, according to the attributions from incidence and survival.
Results
Within 335 million person-years of follow-up, 204 362 incident CRCs and 80 771 CRC-related deaths were identified. Increasing mortality trends were noted for 1994–2003 (post-NHI) vs 1984–1993 due to remarkable increasing incidence trends that could not be offset by improved survival as a result of NHI. During 2004–13 (post-NCCS), mortality continued to increase by 15% (95% CI: 10–21%) in young adults (30–49 years) and 8% (95% CI: 6–11%) in older adults (70–84 years), whereas middle-aged adults (50–69 years) had a reduction of 7% (95% CI: 5–9%) due to a remarkable stage shift and subsequent improvement in survival. In the middle-aged adults, increased incidence was less but survival improvement was more compared with other age groups.
Conclusions
Whereas universal healthcare insurance led to improvement in CRC survival, FIT-based screening has made an even greater contribution to reducing CRC mortality.
Keywords: cancer prevention, screening, colorectal cancer, colonoscopy
Key Messages
We used an age-period-cohort analysis to investigate the impacts of secondary prevention with faecal immunochemical testing (FIT) and tertiary prevention with universal healthcare on the time trends of colorectal cancer (CRC) mortality.
We also separated the attributions of CRC incidence and survival on the observed mortality changes.
A significant reduction in CRC mortality occurred only 10 years after the implementation of a FIT-based screening programme in the eligible subpopulation, even though the equal access to the best healthcare through universal insurance coverage has continuously improved the survival of CRC patients.
By considering the common global phenomenon of increased CRC incidence but improved survival over time, the present study particularly proves that active screening with FIT as a healthcare policy can lead to a substantial shift in CRC stages at diagnosis and efficiently reduce the number of deaths from CRC on a nationwide scale.
Introduction
Colorectal cancer (CRC) is a global health threat; among the new cases and deaths from CRC, 42.6 and 45.6%, respectively, occurred in Asia in parallel with economic growth, changes in lifestyle and increased life expectancy.1 To reduce the burden of CRC, global populations require an effective strategy by which to control modifiable risk factors (primary prevention), implementation of a screening programme for early detection (secondary prevention) and provision of universal healthcare and better access to the best healthcare available (tertiary prevention).
Compared with the long time before the benefits of new behaviours become observable and high cost and limited survival after aggressive therapy for advanced diseases, screening is the most effective method to modify risk.2 Colonoscopy is the main tool used to reduce the incidence and mortality of CRC via the removal of colon polyps and early detection of cancer;3–8 nonetheless, low population adherence and limited availability of endoscopists have hindered the widespread use of colonoscopy as the primary tool for population-based screening programmes. The use of faecal occult blood testing is an alternative approach to identifying patients with the highest risk so endoscopic resources can be allocated efficiently.9 This is especially true because the traditional guaiac-based test has been replaced increasingly by the faecal immunochemical test (FIT),10–12 which has higher specificity and a more patient-friendly sampling design.13
Research evidence is needed to support the effectiveness of interventions so that adjustments can be made to maximize these benefits. Reduction of CRC mortality is the uppermost goal, whereas mortality is the final sequelae of the counterbalance between incidence and survival,14 which, in turn, is associated with a complex causal interaction between risk-factor exposure, screening and treatment. In the absence of data from a large cohort with chronological changes in the disease burden after different interventions, it remains challenging to clarify the benefits generated from an ongoing screening programme (secondary prevention) and universal healthcare (tertiary prevention).
In Taiwan, cancer epidemiology has gradually shifted towards the Western pattern.15 The Nationwide Colorectal Cancer Screening (NCCS) programme was launched in 2004 to control the soaring burden of CRC.16 In addition, universal healthcare insurance, starting in 1995, has given all residents of Taiwan equal access to medical care.17 Given data collected one decade before and after each intervention, our primary objective was to answer whether or not a FIT-based screening programme and universal healthcare could reduce CRC mortality, and to do this by evaluating the common temporal trends of an increased incidence of CRC along with improved survival over time. Our secondary objective was to explore the cohort effect, which may serve as a measure of risk-factor exposure and provide a basis for primary prevention.
Methods
Study population
Data on the burden of CRC in Taiwan were retrieved from the National Cancer Registry, with indication of high coverage (99%; each hospital mandated to report all cases of CRC) and high accuracy (percentage of death-certificate-only cases of less than 1% for CRC).18 Patient data included gender, number of patients with a specific type of cancer, age at cancer diagnosis and age at cancer-related death for each calendar year since 1979.19 Incidence and mortality rates were calculated by dividing the numbers of incident cases of CRC and deaths from CRC by the number of persons at risk. The definition of CRC was based on the International Statistical Classification of Diseases (ICD) and Related Health Problems, 10th revision, in which CRCs were coded as C18–21.20 Cancer staging was based on the American Joint Committee on Cancer (AJCC) system, which has been available since 2004.21
NCCS programme
In 2004, a nationwide screening programme for CRC was launched in Taiwan; details regarding the accuracy of FIT,11 colonoscopic referral22 and preliminary effectiveness16 have been reported elsewhere. In brief, residents aged 50–69 years were invited to have biennial FIT screening. The cut-off concentration for a positive test was 20 μg haemoglobin per gram of faeces (using kits from Eiken Chemical Co, Tokyo, Japan or Kyowa Medex Co Ltd, Tokyo, Japan). Colonoscopy was recommended within 3 months of a positive faecal test.23 The histopathology of colonic lesions was classified according to the World Health Organization criteria.24
In the inaugural period between 2004 and 2009, the delivery of FIT was based on a community-based outreach to community residents who did not use or who underused medical services, encouraging them to participate in the free screening service at public health centres. The annual number of persons screened was about 250 000 and, with a target population of about 5 million, the coverage rate reached 21.4% in 2009.
To increase the accessibility of screening, starting in 2010, the previous approach was combined with an in-reach, hospital-based programme that invited subjects who were already engaged in the healthcare system for treatment unrelated to CRC.25 During this full-implementation period, the annual number of persons screened increased substantially to about 1 million, and the coverage rate reached 44.6% in 2011 and 63.8% in 2013.
Nationwide Healthcare Insurance (NHI)
Before the implementation of NHI, public healthcare insurance in Taiwan included only certain subpopulations such as government employees, labourers, farmers, fishermen and low-income households. To expand the coverage, starting in 1995, NHI consolidated all small insurance plans into a universal insurance system that aimed to provide equal access and standard quality of healthcare for all citizens at affordable costs.17 A family of four typically paid a premium equivalent to about US $100 per month, which accounted for about 2% of the average household income in Taiwan. In 1995, about 92% of the whole population was covered by NHI and the coverage rate increased to 96% in 2000 and reached 99.9% in 2016.
In addition to achieving good accessibility to treatment, comprehensive population coverage and relatively low costs, NHI quality control was ensured by providing a nationwide databank to generate studies to monitor and evaluate healthcare services with the purpose of narrowing healthcare disparities.26,27
Study design
This study followed the principle of age-period-cohort analysis to evaluate the effects of NCCS and NHI on chronological changes in the CRC burden in Taiwan. To allow sufficient follow-up time for evaluation, data from 1984 to 2013 were retrieved and separated into three 10-year periods, including Period 1, 1984–1993; Period 2, 1994–2003 (post-NHI); and Period 3, 2004–2013 (post-NCCS), according to the implementation dates of NHI and NCCS. Because CRC is rare in patients aged <30 years and the average lifespan of a Taiwanese is about 80 years (83.2 years for women and 76.7 years for men), analyses were limited to the population aged 30–84 years.
Statistical analysis
For descriptive analyses, data were first stratified into 11 5-year age bands: 35–39, 40–44 … to 80–84. Next, participants were divided into six time periods: 1984–1988, 1989–1993 … to 2009–2013 for every 5 years. Together, these stratifications generated 16 successive birth cohorts: 1902–1906, 1907–1911 … to 1977–1981 with equal lengths of time. An age-and-period cross-table was generated that allowed evaluation of the changes in CRC. Different outcomes were also evaluated according to gender because of its important role in policymaking.
Second, time-trend analyses were performed by presenting CRC incidence, survival (case-fatality rates: the mortality/incidence ratio, an indicator for cancer survival; a lower case-fatality rate indicates better survival) and mortality in graphs, giving special attention to the changes before and after the implementation of NCCS and NHI. Because only subjects aged 50–69 years were eligible for NCCS, the population was divided into three age groups (young adults, 30–49; middle-aged, 50–69; and older adults, 70–84 years) in order to identify differences between them.
Third, to quantify the effects of interventions, the Poisson regression model was applied, with Periods 1–3 as the explanatory indicators, after adjusting for age and sex. Periods 1 and 2 were compared with assess the effect of NHI and between Periods 2 and 3 to assess the effect of NCCS. The main objective was to evaluate the changes in the middle-aged subgroup during Period 3 because they had been exposed to NCCS; hence, within this period, other age groups could also serve as comparators. The results are presented with the relative risk or the percentage of change expressed by (relative risk – 1) × 100% and the corresponding 95% confidence interval (CI). Interactions of period-by-age and period-by-sex were also evaluated.
To verify the benefit of early detection of CRC by the NCCS programme, cancer-stage data of the middle-aged subgroup and the other age groups were compared by hypothesizing that, in the middle-aged group, early detection would lead to a lower incidence rate of advanced-stage CRC. The results are presented with the proportion of advanced-stage (defined as stage II–IV) to overall CRCs over time.
Since cancer mortality is determined by incidence and survival, we estimated the attributable proportion of CRC mortality related to these two determinants (detailed in Supplementary Material, available as Supplementary Data at IJE online), assuming that a substantial increase in screening volume for the middle-aged group would lead to widespread early detection of CRC and improvement in CRC survival may outweigh the increase in new incident cases.
Finally, knowing that patients in the same birth cohort may share exposure to similar socio-environmental factors, a cohort analysis was conducted to quantify the effect of risk-factor exposures. Most subjects in the 16 birth cohorts had been exposed to both NHI and NCCS, so this analysis does not address the effects of these two interventions. A Bayesian approach was applied to deal with the potential confounding effects and linear dependencies of age, period and cohort (Supplementary Figure 1, available as Supplementary Data at IJE online).28,29 The results are presented as the annual percentage change of CRC incidence.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and WinBUGS version 1.4 (MRC Biostatistics Unit, Cambridge, UK).
Results
Descriptive analysis
Data for the present study were derived from approximately 11 million persons per year, a follow-up time of three decades and approximately 335 million person-years of follow-up. Overall, there were 204 362 incident CRCs and 80 771 CRC-related deaths. As shown in Tables 1 and 2, the incidence and mortality rates of CRC increased with both age and period. When data were stratified by sex (Supplementary Tables 1 and 2, available as Supplementary Data at IJE online), the increasing trends were similar but their magnitudes were different. For the younger-age subgroup, the incidence and mortality rates were similar between sexes; during middle age, the increasing trends became more substantial in men. Until the period of 2009–13, women and men reached parity in CRC incidence and mortality rates only when the women were about 10 and 5 years older than men, respectively.
Table 1.
Age-specific incidence rates of CRC cross-tabulated by period in Taiwan from 1984 to 2013 to derive the birth-cohort-specific rates in the diagonal matrix
| Period |
|||||||
|---|---|---|---|---|---|---|---|
| 1984–1988 | 1989–1993 | 1994–1998 | 1999–2003 | 2004–2008 | 2009–2013 | ||
| 30–34 | 3.7 | 4.5 | 5.2 | 6.0 | 6.9 | 7.9 | |
| 35–49 | 6.0 | 7.5 | 8.1 | 9.7 | 11.5 | 14.6 | |
| 40–44 | 9.1 | 12.0 | 14.8 | 16.9 | 19.1 | 23.2 | |
| 45–49 | 16.4 | 19.9 | 23.9 | 29.8 | 33.6 | 40.8 | |
| 50–54 | 25.8 | 33.3 | 41.9 | 47.8 | 59.1 | 72.2 | |
| Age | 55–59 | 39.2 | 50.9 | 67.1 | 77.0 | 91.9 | 111.5 |
| 60–64 | 55.9 | 76.6 | 101.2 | 120.5 | 138.8 | 161.5 | |
| 65–69 | 72.1 | 99.4 | 141.2 | 174.1 | 194.7 | 224.1 | |
| 70–74 | 88.9 | 135.6 | 178.0 | 226.7 | 255.9 | 274.5 | |
| 75–79 | 77.6 | 152.0 | 216.8 | 276.0 | 318.4 | 357.6 | |
| 80–84 | 74.8 | 111.6 | 224.1 | 299.0 | 358.3 | 406.4 | |
Per 100 000 person-years, crude rate.
The numbers on the diagonal indicate the incidence rates with age for a specific birth cohort; e.g. the cells in shadow indicate the incidence rates of the population bore during the period of 1947–51.
Table 2.
Age-specific mortality rates of CRC cross-tabulated by period in Taiwan from 1984 to 2013 to derive the birth-cohort-specific rates in the diagonal matrix
| Period |
|||||||
|---|---|---|---|---|---|---|---|
| 1984–1988 | 1989–1993 | 1994–1998 | 1999–2003 | 2004-2008 | 2009–2013 | ||
| 30–34 | 1.9 | 2.0 | 2.1 | 2.2 | 2.3 | 2.1 | |
| 35–49 | 3.0 | 3.1 | 3.2 | 3.3 | 3.4 | 3.9 | |
| 40–44 | 4.4 | 4.9 | 5.3 | 5.7 | 5.5 | 6.0 | |
| 45–49 | 7.3 | 7.6 | 8.4 | 9.2 | 10.0 | 9.7 | |
| 50–54 | 11.7 | 13.2 | 13.3 | 15.6 | 16.3 | 16.4 | |
| Age | 55–59 | 19.2 | 20.6 | 24.3 | 25.7 | 25.4 | 27.5 |
| 60–64 | 30.2 | 32.2 | 41.5 | 40.8 | 41.6 | 39.8 | |
| 65–69 | 45.6 | 49.6 | 62.0 | 65.3 | 62.6 | 61.7 | |
| 70–74 | 68.4 | 73.1 | 89.3 | 100.9 | 101.2 | 90.2 | |
| 75–79 | 98.2 | 102.6 | 135.2 | 153.0 | 149.3 | 145.9 | |
| 80–84 | 120.3 | 112.8 | 173.2 | 207.7 | 216.7 | 213.9 | |
Per 100 000 person-years, crude rate.
The numbers on the diagonal indicate the mortality rates with age for a specific birth cohort; e.g. the cells in shadow indicate the mortality rates of the population bore during the period of 1947–51 (Table 2).
Time-trend analysis
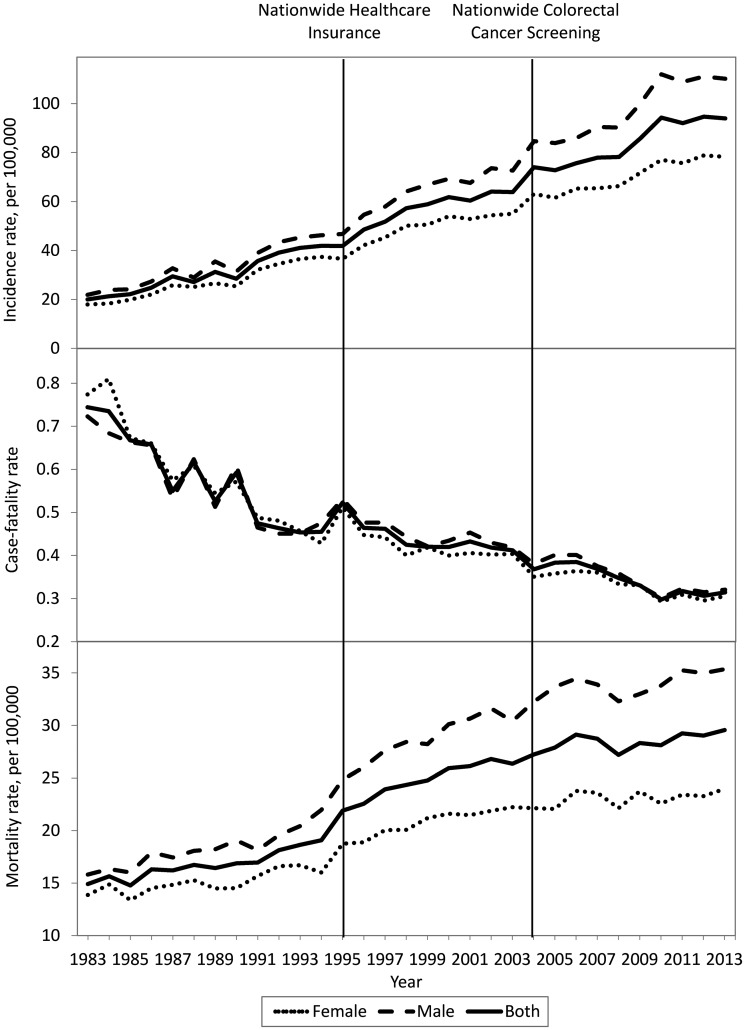
As shown in Figure 1, the overall CRC incidence rate increased with time. Men had a more dramatic increase in CRC incidence than women. During Period 3, although it had a similar increasing trend in mortality, the slope decreased slightly. Using Period 1 as the reference group, 1.83- and 2.78-fold increases were observed in incidence, and 1.45- and 1.70-fold increases in mortality, respectively, during Periods 2 and 3.
Figure 1.
Chronological trends regarding CRC incidence (upper), survival (case-fatality, middle) and mortality rates (lower) in Taiwan during 1983–2013 for the overall population, stratified by sex.
Age-specific findings
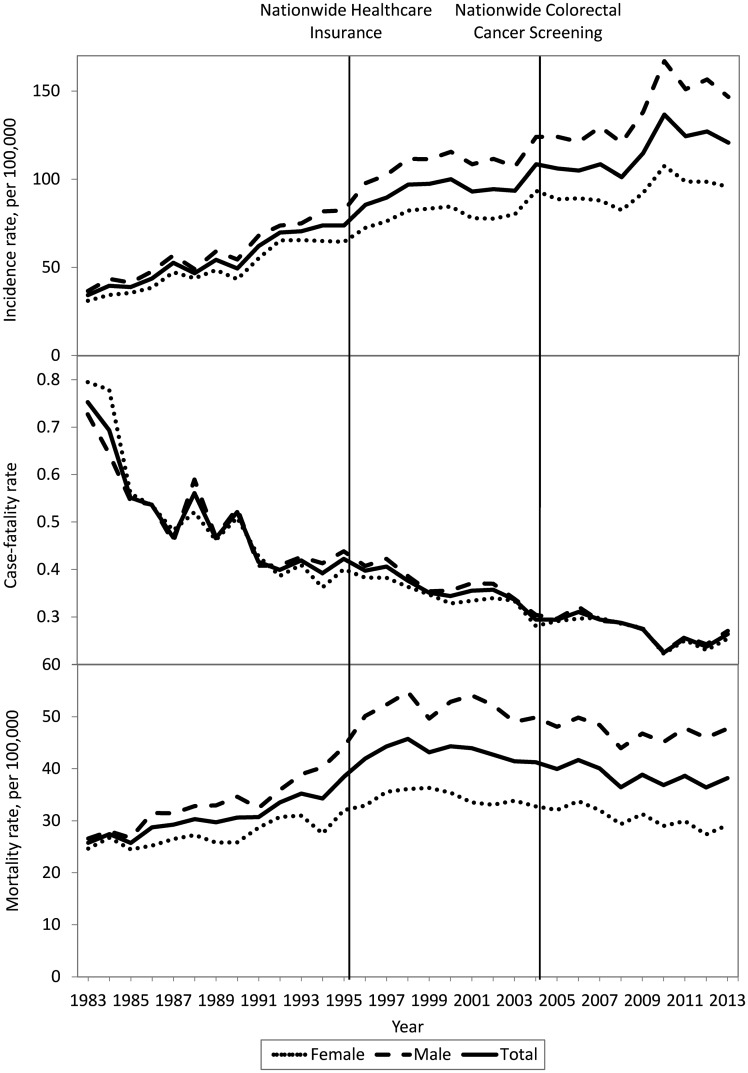
Results were then stratified according to age. In the middle-aged group (Figure 2), the trends for incidence and mortality rates were different from those of the two other age groups. The incidence rate reached a peak in 2010, the time of full implementation of NCCS, and gradually declined thereafter. Mortality reached a plateau during Period 2 and also was followed by a decreasing trend during Period 3. In contrast, results were similar in the other age groups to those of the overall group; the trends for incidence and mortality were increasing continuously (Supplementary Figures 2A and 2B, available as Supplementary Data at IJE online).
Figure 2.
Chronological trends regarding CRC incidence (upper), survival (case-fatality, middle) and mortality rates (lower) in Taiwan during 1983–2013 for the population aged 50–69 years, stratified by sex.
Sex differences
Similarly to differences shown in the age-and-period cross-tables, sex differences started at middle age and increased gradually over time. Similarly, improvements in survival rates were observed between the different age groups and between sexes.
Multivariable regression analysis
The results of multivariable regression analysis are shown in Table 3. The age-by-period interactions were significant but the sex-by-period interactions were not, so the results were stratified by age and were adjusted for sex.
Table 3.
The relative risk for colorectal cancer incidence, survival and mortality rates by age and period, adjusted for sex
| Age | Period | Incidence rate |
Survival rate |
Mortality rate |
|||
|---|---|---|---|---|---|---|---|
| aRR (95% CI) | aRR (95% CI) | aRR (95% CI) | |||||
| 30–49 | 1984–1993 | 1 | – | 1 | – | 1 | – |
| 1994–2003 | 1.56 (1.51–1.62) | 1 | 0.79 (0.75–0.82) | 1 | 1.24 (1.18–1.32) | 1 | |
| 2004–2013 | 2.27 (2.20–2.35) | 1.46 (1.42–1.49) | 0.62 (0.60–0.65) | 0.79 (0.76–0.82) | 1.43 (1.36–1.51) | 1.15 (1.10–1.21) | |
| 50–69 | 1984–1993 | 1 | – | 1 | – | 1 | – |
| 1994–2003 | 1.71 (1.68–1.74) | 1 | 0.76 (0.74–0.78) | 1 | 1.41 (1.37–1.44) | 1 | |
| 2004–2013 | 2.21 (2.17–2.25) | 1.29 (1.28-1.31) | 0.55 (0.54–0.57) | 0.72 (0.71–0.74) | 1.30 (1.27–1.33) | 0.93 (0.91–0.95) | |
| 70–84 | 1984–1993 | 1 | – | 1 | – | 1 | – |
| 1994–2003 | 2.01 (1.95–2.06) | 1 | 0.71 (0.69–0.73) | 1 | 1.51 (1.45–1.57) | 1 | |
| 2004–2013 | 2.81 (2.74–2.88) | 1.40 (1.38–1.42) | 0.56 (0.55–0.58) | 0.79 (0.77–0.81) | 1.64 (1.57–1.70) | 1.08 (1.06–1.11) | |
aRR, adjusted relative risk; CI, confidence interval.
Effects of healthcare policies on CRC incidence
Using Period 1 as the baseline comparator, the incremental percentages (95% CIs) of CRC incidence during Period 2 were 56% (51–62%), 71% (68–74%) and 101% (95–106%), respectively, for the young-, middle- and older-age subgroups, and results for all subgroups indicated an increasing trend in incidence. Using Period 2 as the reference group, the incremental percentages during Period 3 were 46% (42–49%), 29% (28–31%) and 40% (38–42%) for the young-, middle- and older-age subgroups, respectively. Although the increasing trend was seen in the middle-aged subgroup, the increase was attenuated compared with that in the other age subgroups that were not eligible for the NCCS programme.
Effects of healthcare policies on CRC survival
Using the baseline comparator of Period 1, improvements in the survival rate during Period 2 were 21% (18–25%), 24% (22–26%) and 29% (27–31%), respectively, for the young-, middle- and old-age subgroups. Using Period 2 as the baseline, the improvements during Period 3 were 21% (18–24%), 28% (26–29%) and 21% (19–23%), respectively, for the young-, middle- and older-age subgroups; the middle-aged subgroup had greater improvement in CRC survival.
Effects of healthcare policies on CRC mortality
As compared with Period 1, the incremental percentages for the young-, middle- and older-age subgroups were 24% (18–32%), 41% (37–44%) and 51% (45–57%), respectively, during Period 2. Using Period 2 as the baseline, the incremental percentages during Period 3 were 15% (10–21%) and 8% (6–11%) for the young- and older-age subgroups, respectively. In contrast with the increased mortality rate in the other age subgroups that were not eligible for NCCS, the mortality rate of the middle-aged subgroup decreased by 7% (5–9%), with reductions of 5% (3–8%) and 9% (6–13%), respectively, for men and women.
Distribution of CRC stages at diagnosis
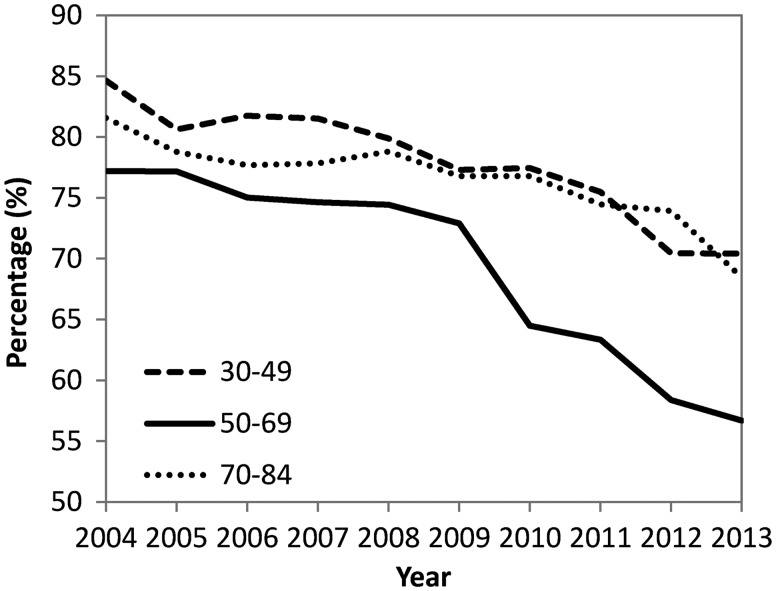
The cancer staging at diagnosis during Period 3 is shown in Figure 3. The proportions of advanced-stage to overall CRCs decreased with time in all three age subgroups (Supplementary Table 3, available as Supplementary Data at IJE online). However, the decrease in the middle-aged subgroup (declined from 77 to 56%, difference: 21%, 95% CI: 19–22%) was greater than those in both the younger-age subgroup (declined from 84 to 70%, difference: 14%, 11–18%) and older-age subgroup (declined from 81 to 68%, difference: 13%, 11–15%), indicating that NCCS had remarkable effects on the stage shift towards earlier diagnosis. If stages 0–II were defined as the early stage, the results were similar.
Figure 3.
Proportions of advanced-stage (stage II–IV) to overall CRCs during 2004–13 in Taiwan.
Attributable proportion of CRC mortality
The overall attributable proportion of mortality between Periods 2 and 3 was –6.9% (95% CI: –9.2 to –4.4%) in the middle-aged subgroup whereas those in the younger- and older-age subgroups were +11.8% (7.1–16.3%) and +5.4% (2.8–8.0%), respectively (Supplementary Table 4, available as Supplementary Data at IJE online). When the mortality change was separated according to the impact of incidence and survival, the results showed an increase of +23.0% (95% CI: 21.7–24.2%) in CRC-related deaths in the middle-aged subgroup after NCCS implementation was related to the emergence of new cases; however, that was outweighed by a reduction in case fatality (–28.3%, 95% CI: –30.4 to –26.1%), resulting in a net decrease in the mortality rate. Both figures were lower than those in the younger-age (+31.0 and –21.7%) and older-age subgroups (+26.4 and –22.1%), in which the survival improvement was apparently outweighed by the emergence of new cases, leading to a continuous increase in the mortality rate.
Cohort analysis
As shown in Supplementary Figure 3, available as Supplementary Data at IJE online, the incidence rates of CRC increased in the same age range in successive birth cohorts. The incidence in the younger-age subgroup increased by 4.10–4.51% per year of birth cohort (Supplementary Table 5, available as Supplementary Data at IJE online). When the cohorts entered middle age and old age, the increases in CRC incidence were 4.55–4.82 and 5.40–8.79%, respectively. Consistently with findings from age-and-period cross-tables, sex differences in outcomes began to increase starting in middle age.
Discussion
In addition to the contribution of universal healthcare that is conducive to improving CRC survival, results of the present study demonstrate a significant reduction in CRC mortality occurring only 10 years after the implementation of a FIT-based screening programme in the eligible subpopulation. This reduction offers a striking contrast with the trend towards increasing mortality among ineligible subjects. This finding offers solid evidence to support the nationwide use of this screening strategy and can likely be generalized to most populations in developed and developing regions, in which similar dynamic changes in the CRC burden and healthcare use are taking place.
The prominent effects of FIT screening seen in the present study are related to its high accuracy in detecting CRC.13 FIT-based screening programmes have additional merits, including a convenient design that helps to enhance patients’ acceptance;10 results that are specific to lower gastrointestinal lesions;30 quantitative outcomes that can be used either for risk stratification of screening candidates31 or for setting priorities among colonoscopy candidates;22 a cut-off point that can be adjusted according to the limited number of colonoscopists available;32 and a favourable cost–benefit ratio.33,34 Nonetheless, a FIT-based screening programme, in comparison with the approach of one-off colonoscopy screening, entails additional programmatic considerations.11,35–39 Research evidence to support the effectiveness about results remains limited.12,16
In the FIT screening programme in Taiwan, which has a current annual volume of about 1 million participants, the FIT positivity rate is about 8% and, in nearly 50 000 confirmatory colonoscopies performed per year, the number of screening-detected CRC cases is >2000 per year, of which about half were at stage 0–I.25 This results in two major benefits that contribute to decreasing the mortality rate. First, early detection leads to a substantial shift in CRC stages at diagnosis, which is associated with improvements in patients’ survival. Second, a peak in CRC incidence was seen after full implementation of NCCS, which was then followed by a reducing trend, which was likely due to the detection and removal of colon polyps.40
Results of the present cohort analysis indicate a strong driving force on CRC incidence over decades; that seems to continuously purge the number of new CRC cases without active intervention. The finding that men have the more rapid increase of CRC after 50 years of age indicates extensive exposure to risk factors before cancer onset. This interpretation is supported by two important findings. First, in a nationwide survey in Taiwan, the prevalence of cigarette smoking reached a peak of about 40% at 41–45 years among men whereas, in contrast, the corresponding rate was only 5.5% for women.41 Additional compelling evidence is available from a colonoscopy-based study in which the prevalence of advanced polyps (2.1%) in men aged 40–49 years was similar to that of women aged 50–59 years.42
Strengths of the present study include the large sample size, long follow-up time, execution of different interventions on a nationwide scale and the availability of a registry of cancer incidence and mortality so the unique independent effects of NCCS and NHI could be distinguished and statistically estimated in association with age and time periods. However, the present study has certain limitations. First, some of the programme indicators regarding NCCS still have room for improvement, such as the suboptimal referral rate after a positive FIT22 and the colonoscopy quality issue associated with the occurrence of post-colonoscopy CRC,43 both of which may decrease the programme’s effectiveness. Nevertheless, the magnitude of benefit will likely become larger when the coverage rate is increased. Second, regarding primary prevention, ongoing interventions are already in place. However, because development of CRC that follows the adenoma–carcinoma sequence may require decades to occur, the observation time in the present study may be insufficient to evaluate the benefits of primary prevention that have great potential impact on CRC incidence.
Taking into consideration the common global phenomenon of increased CRC incidence but with improved survival over time as demonstrated by the effectiveness of universal healthcare in Taiwan, results of the present study suggest, in particular, that active screening with FIT can efficiently reduce the number of deaths from CRC on a nationwide scale. Results of the present study add important implications for informing healthcare policy towards efficient resource allocation in the campaign to reduce the enormous burden of CRC.
Funding
This work was supported by the Health Promotion Administration, Ministry of Health and Welfare, Taiwan (A1011119, A1021227, A1031135, A1041122, A1051013, and A1061224), which had no role in the study’s design, conduct and reporting. This work was also supported by the Innovation and Policy Center for Population Health and Sustainable Environment (Population Health Research Center, PHRC), College of Public Health, National Taiwan University from the Ministry of Science and Technology (MOST 107–3017-F-002–003) and Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (NTU-107L9003).
Supplementary Material
Acknowledgements
Y.-C.L., C.-Y.H. and H.-H.C. have full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis. Conception and design of the study: all authors. Generation, collection, assembly, analysis and/or interpretation of data: all authors. Drafting or revision of the manuscript: Y.-C.L., C.-Y.H. and H.-H.C. Critical revision of the manuscript for important intellectual content: all authors. Administrative, technical or material support: all authors. Approval of the final version of the manuscript: all authors. Study supervision: H.-H.C. Y.-C.L. and H.-H.C. had the final responsibility for the decision to submit for publication. H.-H.C. acted as guarantor for the paper. The references have been checked for accuracy and completeness by Y.-C.L. The lead author and manuscript’s guarantor (HHC) affirm that this manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. This study was approved by the Health Promotion Administration, Ministry of Health and Welfare, prior to data retrieval and analysis (1069907397). Patient records/information were anonymized and de-identified prior to analysis, and signed informed consent was not needed. The Research Ethics Committee of National Taiwan University Hospital approved the protocol for this project and granted a waiver of informed consent (201511034W) pursuant to the regulation of the Institutional Review Board.
Conflict of interest: The authors report no conflicts of interest.
References
- 1. Bosman FT. Colorectal cancer In: Stewart BW, Wild CP (eds). World Cancer Report 2014. Lyon: International Agency for Research on Cancer, 2014, pp. 392–402. [Google Scholar]
- 2. Edwards BK, Ward E, Kohler BA. et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schoen RE, Pinsky PF, Weissfeld JL. et al. ; PLCO Project Team. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med 2012;366:2345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atkin W, Wooldrage K, Parkin DM. et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet 2017;389:1299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nishihara R, Wu K, Lochhead P. et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med 2013;369:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L.. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009;150:01–08. [DOI] [PubMed] [Google Scholar]
- 7. Kahi CJ, Imperiale TF, Juliar BE, Rex DK.. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol 2009;7:770–75. [DOI] [PubMed] [Google Scholar]
- 8. Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M.. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med 2011;154:22–30. [DOI] [PubMed] [Google Scholar]
- 9. Shaukat A, Mongin SJ, Geisser MS. et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013;369:1106–14. [DOI] [PubMed] [Google Scholar]
- 10. Quintero E, Castells A, Bujanda L. et al. ; COLONPREV Study Investigators. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012;366:697–706. [DOI] [PubMed] [Google Scholar]
- 11. Chiang TH, Chuang SL, Chen SL. et al. Difference in performance of fecal immunochemical tests with the same hemoglobin cutoff concentration in a nationwide colorectal cancer screening program. Gastroenterology 2014;147:1317–26. [DOI] [PubMed] [Google Scholar]
- 12. Zorzi M, Fedeli U, Schievano E. et al. Impact on colorectal cancer mortality of screening programmes based on the faecal immunochemical test. Gut 2015;64:784–90. [DOI] [PubMed] [Google Scholar]
- 13. Lee JK, Liles EG, Bent S, Levin TR, Corley DA.. Accuracy of fecal immunochemical tests for colorectal cancer. Ann Intern Med 2014;160:171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellis L, Woods LM, Estève J, Eloranta S, Coleman MP, Rachet B.. Cancer incidence, survival and mortality: explaining the concepts. Int J Cancer 2014;135:1774–82. [DOI] [PubMed] [Google Scholar]
- 15. Tsoi KKF, Hirai HW, Chan FCH, Griffiths S, Sung JJY.. Predicted increases in incidence of colorectal cancer in developed and developing regions, in association with ageing populations. Clin Gastroenterol Hepatol 2017;15:892–900.e4. [DOI] [PubMed] [Google Scholar]
- 16. Chiu HM, Chen SL, Yen AM. et al. Effectiveness of fecal immunochemical testing in reducing colorectal cancer mortality from one million Taiwanese screening program. Cancer 2015;121:3221–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wen CP, Tsai SP, Chung WS.. A 10-year experience with universal health insurance in Taiwan: measuring changes in health and health disparity. Ann Intern Med 2008;148:258–67. [DOI] [PubMed] [Google Scholar]
- 18. Chiang CJ, Chen YC, Chen CJ. et al. Taiwan cancer registry task force: cancer trends in Taiwan. Jpn J Clin Oncol 2010;40:897–904. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Registry. On-line Interactive Data Acquisition System, Health Promotion Administration, Ministry of Health and Welfare, Taiwan. https://cris.hpa.gov.tw/ (20 February 2017, date last accessed).
- 20.World Health Organizaton. International Statistical Classification of Diseases and Related Health Problems. http://www.who.int/classifications/icd/en/ (20 February 2017, date last accessed).
- 21. Edge SB, Compton CC.. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–74. [DOI] [PubMed] [Google Scholar]
- 22. Lee YC, Li-Sheng Chen S, Ming-Fang Yen A. et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy noncompliers. J Natl Cancer Inst 2017;109:dwj269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benson VS, Atkin WS, Green J. et al. International Colorectal Cancer Screening Network: toward standardizing and reporting colorectal cancer screening indicators on an international level: the International Colorectal Cancer Screening Network. Int J Cancer 2012;130:2961–73. [DOI] [PubMed] [Google Scholar]
- 24. Bosman FT, Carneiro F, Hruban RH, Theise ND (eds). WHO Classification of Tumours of the Digestive System. Lyon: IARC, 2010. [Google Scholar]
- 25. Chou CK, Chen SL, Yen AM. et al. Outreach and inreach organized service screening programs for colorectal cancer. PLoS One 2016;11:e0155276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu TY, Majeed A, Kuo KN.. An overview of the healthcare system in Taiwan. London J Prim Care (Abingdon) 2010;3:115–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hsing AW, Ioannidis JP.. Nationwide population science: lessons from the Taiwan National Health Insurance Research Database. JAMA Intern Med 2015;175:1527–29. [DOI] [PubMed] [Google Scholar]
- 28. Bray I. Application of Markov Chain Monte Carlo methods to projecting cancer incidence and mortality. J R Stat Soc Ser C Appl Stat 2002;51:151–64. [Google Scholar]
- 29. Lunn DJ, Thomas A, Best N, Spiegelhalter D.. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325–37. [Google Scholar]
- 30. Chiang TH, Lee YC, Tu CH, Chiu HM, Wu MS.. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ 2011;183:1474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen LS, Yen AM, Chiu SY, Liao CS, Chen HH.. Baseline faecal occult blood concentration as a predictor of incident colorectal neoplasia: longitudinal follow-up of a Taiwanese population-based colorectal cancer screening cohort. Lancet Oncol 2011;12:551–58. [DOI] [PubMed] [Google Scholar]
- 32. Toes-Zoutendijk E, van Leerdam ME, Dekker E. et al. ; Dutch National Colorectal Cancer Screening Working Group. Real-time monitoring of results during first year of Dutch colorectal cancer screening program and optimization by altering fecal immunochemical test cut-off levels. Gastroenterology 2017;152:767–75.e2. [DOI] [PubMed] [Google Scholar]
- 33. Chen LS, Liao CS, Chang SH, Lai HC, Chen TH.. Cost-effectiveness analysis for determining optimal cut-off of immunochemical faecal occult blood test for population-based colorectal cancer screening (KCIS 16). J Med Screen 2007;14:191–99. [DOI] [PubMed] [Google Scholar]
- 34. Wilschut JA, Hol L, Dekker E. et al. Cost-effectiveness analysis of a quantitative immunochemical test for colorectal cancer screening. Gastroenterology 2011;141:1648–55.e1. [DOI] [PubMed] [Google Scholar]
- 35. Rabeneck L, Lansdorp-Vogelaar I.. Assessment of a cancer screening program. Best Pract Res Clin Gastroenterol 2015;29:979–85. [DOI] [PubMed] [Google Scholar]
- 36. Grazzini G, Ventura L, Zappa M. et al. Influence of seasonal variations in ambient temperatures on performance of immunochemical faecal occult blood test for colorectal cancer screening: observational study from the Florence district. Gut 2010;59:1511–15. [DOI] [PubMed] [Google Scholar]
- 37. Corley DA, Jensen CD, Quinn VP. et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA 2017;317:1631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jensen CD, Corley DA, Quinn VP. et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med 2016;164:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scholefield JH, Moss SM, Mangham CM. et al. Nottingham trial of faecal occult blood testing for colorectal cancer: a 20-year follow-up. Gut 2012;61:1036–40. [DOI] [PubMed] [Google Scholar]
- 40. Ventura L, Mantellini P, Grazzini G. et al. The impact of immunochemical faecal occult blood testing on colorectal cancer incidence. Dig Liver Dis 2014;46:82–86. [DOI] [PubMed] [Google Scholar]
- 41.Assessment for the Enforcement Performance of the Tobacco Hazards Prevention Act. 2016 Taiwan Tobacco Control Annual Report. Health Promotion Administration, Ministry of Health and Welfare, Taiwan, pp. 76–80.
- 42. Chang LC, Wu MS, Tu CH, Lee YC, Shun CT, Chiu HM.. Metabolic syndrome and smoking may justify earlier colorectal cancer screening in men. Gastrointest Endosc 2014;79:961–69. [DOI] [PubMed] [Google Scholar]
- 43. Chiu SY, Chuang SL, Chen SL. et al. Faecal haemoglobin concentration influences risk prediction of interval cancers resulting from inadequate colonoscopy quality: analysis of the Taiwanese Nationwide Colorectal Cancer Screening Program. Gut 2017;66:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.