Abstract
High-income countries are experiencing an obesity epidemic that follows a socioeconomic gradient, affecting groups of lower socioeconomic status disproportionately. Recent clinical findings have suggested new perspectives for the prevention and treatment of obesity, using personalized dietary approaches. Precision nutrition (PN), also called personalized nutrition, has been developed to deliver more preventive and practical dietary advice than ‘one-size-fits-all’ guidelines. With interventions becoming increasingly plausible at a large scale thanks to artificial intelligence and smartphone applications, some have begun to view PN as a novel way to deliver the right dietary intervention to the right population. We argue that large-scale PN, if taken alone, might be of limited interest from a public health perspective. Building on Geoffrey Rose’s theory regarding the differences in individual and population causes of disease, we show that large-scale PN can only address some individual causes of obesity (causes of cases). This individual-centred approach is likely to have a small impact on the distribution of obesity at a population level because it ignores the population causes of obesity (causes of incidence). The latter are embedded in the populations’ social, cultural, economic and political contexts that make environments obesogenic. Additionally, the most socially privileged groups in the population are the most likely to respond to large-scale PN interventions. This could have the undesirable effect of widening social inequalities in obesity. We caution public health actors that interventions based only on large-scale PN are unlikely, despite current expectations, to improve dietary intake or reduce obesity at a population level.
Keywords: Precision nutrition, personalized nutrition, obesity, population interventions, social inequalities in health, obesogenic environments
Key Messages
Some public health actors have begun to view large-scale precision nutrition as a novel opportunity to provide the right dietary intervention to the right population at the right time.
Large-scale precision nutrition is an individual-centred approach focusing on behavioural modification in large numbers, and not a true population approach as defined by Geoffrey Rose.
Large-scale precision nutrition is likely to have a limited impact on obesity at a population level as it neglects population causes of obesity that are rooted in obesogenic environments.
Early adoption and achievement of improved dietary habits based on precision nutrition are more likely among more socially privileged members of the population, which would exacerbate socioeconomic inequalities in diet and obesity.
If taken alone, interventions based on large-scale precision nutrition are unlikely to improve dietary intake or reduce obesity at a population level.
Introduction
Most high-income countries are experiencing an obesity epidemic, since 1975.1 For example, in the USA, more than one in three adults and one in six children were estimated to be obese in 2015.2 Obesity has been linked to numerous non-communicable diseases such as diabetes, cardiovascular disease, osteoarthritis and certain forms of cancer.3–5 According to the 2016 Global Burden of Disease,6 an unbalanced diet, obesity, and high fasting plasma glucose were among the top six leading risk factors for disability-adjusted life-years in high-income countries. In these countries, the incidence and prevalence of obesity follows a socioeconomic gradient, whereby individuals with lower education, occupation and income are disproportionally affected.7–9 In Spain, Italy and France for instance, the least educated women are over four times as likely to be obese as the most educated ones.10
Diet is a major modifiable determinant of obesity. Multiple public health interventions to improve population dietary intake have been implemented to date. Some individual-centred interventions have aimed at providing information about healthy eating. They used, for example, mass campaigns to disseminate dietary guidelines (e.g. ‘5 a day’) and food guides (e.g. MyPlate in the USA).11–13 More recent interventions have focused on shaping the food environment through structural measures. Classical examples are compulsory nutritional standards for school meals14,15 or taxes on sugar-sweetened beverages.16–18 So far outcomes have been disappointing. People largely fail to follow the dietary guidelines.19–22 As for obesity, the prevalence has not declined,2,23 and social inequalities in diet24 and obesity10,25,26 have persisted or even increased.
Recent research findings,27–35 particularly by Zeevi et al.36 have suggested new perspectives for the prevention and treatment of obesity-related diseases, using personalized dietary approaches. Precision nutrition (PN), also called personalized nutrition, is based on the postulate that the optimal diet is not the same for everyone. In brief, PN aims at delivering tailored nutritional recommendations based on combined information from individuals’ gut microbiota, genetic, physiological, and behavioural backgrounds.37–42
Following these promising results in clinical research,27–36 some large public research funders, such as the EU Horizon 2020 programme,43 have encouraged researchers to test solutions providing tailored nutritional advice to large numbers of people, including healthy individuals. An international trial, Food4Me, was recently launched with 1600 volunteers to test the opportunities and challenges of PN in the general population.44 Within this context, some39,42,45,46 have begun to consider PN as an emerging tool for public health to reduce obesity and obesity-related diseases, notably because precision approaches have a marked preventive component.
In parallel, advances in ‘omics’ technologies and wearable devices facilitate less costly collection and analysis of massive data. This makes scaleable delivery of tailored nutritional advice increasingly plausible.38,39,42 Thanks to these technical developments and the clinical context explained above, PN could be viewed as a novel opportunity to provide the right dietary intervention to the right population at the right time, and on a large scale.47–49 In this paper, we explore the promises and potential limitations of interventions based on large-scale PN. We question their relevance in balancing individuals’ diet and addressing obesity at a population level. We build our argument on Geoffrey Rose’s theory50,51 regarding the differences in individual and population causes of disease. We finally argue that large-scale PN could possibly have the unintended effect of exacerbating social inequalities in obesity.
What is large-scale precision nutrition?
Modelled after PN in clinical settings,36 large-scale PN relies on the collection and analysis of several types of data from eating behaviour, physical activity, deep phenotyping, nutrigenomics, microbiomics/metagenomics and metabolomics37–42 (Table 1). These data serve to define the appropriate diet for each individual, or more realistically, each population sub-stratum.49,52 Different amounts of data can be collected and analysed depending on the infrastructure availability and financial resources. For example, in the Food4Me trial, the intervention involved the delivery of personalized nutrition advice based on data from: (i) current diet; or (ii) diet plus phenotypic traits such as waist circumference, serum glucose, total cholesterol, carotenes and omega 3 index; (iii) diet and phenotype plus genotype (i.e. specific variants on five diet-responsive genes).44
Table 1.
Potential sources of data for tailored nutritional advice in large-scale precision nutrition interventions
| Data | Aims of data collection | Methods to produce data | Infrastructures and tools to collect, analyse and store data |
|---|---|---|---|
| Eating behaviour | To evaluate:
|
Dietary assessment on several days using:
|
|
| Physical activity | To measure physical activity levelTo estimate energy expenditure | Accelerometry techniques using:
|
|
| Deep phenotyping | To assess:
|
Anthropometric measurements (e.g. weight, waist circumference, bone densitometry) Clinical chemistry from various bio-samples (e.g. plasma, urine, saliva) to assess visceral fat distribution, insulin resistance, low-density lipoprotein cholesterol, nutrient deficiencies, etc. | |
| Nutrigenomics | To look for genetic variants associated with diet-related diseases and/or responsive to dietary changes | DNA extraction and genotyping of selected loci from whole-blood samples | |
| Microbiomics/ metagenomics | To understand the interplay between diet and gut microbiota | Faeces collection to sequence the microorganisms present in the gut for microbial profiling and detection of dysbiosis | |
| Metabolomics | To understand how the body metabolizes/uses nutrients | Complex chemical analyses from biosamples (e.g. serum, plasma, urine) using:
|
Once the desired level of precision/information is defined, data can be collected on a large scale using personal smartphones and other relatively inexpensive and reliable wearable devices, such as an electronic food diary and wristband for accelerometry.38,39 In parallel, new tools (Table 1), such as dried blood spot testing42 already routinely used for the Guthrie test in newborns,53 and simple stool kits,36,54 enable biosample collection from home or a local pharmacy. The Food4Me intervention was entirely internet-delivered, for instance. Participants themselves collected both biosamples, using the saliva swabs for genotyping and dried blood spots for phenotyping. They followed online demonstrations, and sent their biological material by conventional mail.44 The advances of laboratory analytical techniques (e.g. DNA sequencing, mass spectrometry),39,42 bioinformatics, and artificial intelligence (e.g. machine-learning algorithms, deep learning)36,38,55,56 render the analysis and interpretation of large datasets less and less expensive and time-consuming.
Lastly, smartphone applications allow large-scale dissemination of personalized advice directly to individuals. For instance, the applications delivered by the companies DayTwo57 and Viome58 can provide a personal score for foods or recipes regarding their potential positive or negative impact on blood glucose level. The enterprise habit59 even offers detailed menu plans to comply with personalized recommended intake in terms of protein, carbohydrate and fat.
Large-scale precision nutrition: promises and challenges
The central promise of large-scale PN is personalized interventions based on more: (i) preventive (predictive and accurate); (ii) practical (understandable and implementable); and (iii) dynamic nutritional advice than ‘one-size-fits-all’ guidelines.39 First, PN advocates presume that nutritional advice is likely to be more predictive because the personal risk of developing specific diseases (e.g. based on polygenic risk scores) and biomedical context can be considered.40,49,60 Advice could also be more accurate due to more precise dietary intake and nutritional status assessment61–65 and better anticipation of interpersonal variability in food metabolic response.36,66,67 Second, personalized nutritional advice may be easily understood, as messages could be delivered in a simpler way using modern communication techniques.68,69 Advice may also be more implementable as adapted to actual food consumption, personal food preferences and lifestyle.68–70 Third, nutritional advice would evolve following the personal dietary and biomedical evolutions of each individual as automatically processed and refined over time through new data.39 In sum, large-scale PN promises better individual risk identification through comprehensive screening and behavioural modification in line with these identified risks.
At present, large-scale PN faces two main challenges, however. On the one hand, its application on a large scale raises organizational, legal and ethical questions, notably regarding biobank management, data protection and informed consent.42,52,71 However, these technical challenges are currently being addressed by some countries that have launched large-scale precision medicine projects, such as the Precision Medicine Initiative in one million US residents,72 and the human biomonitoring project (HBM4EU) in 28 European countries.73 On the other hand, the effectiveness associated with both identifying the individual risk and delivering personal messages for prevention and treatment of obesity-related disease is disputed.4,38,40,74–77 The 2018 Lancet review by Wang and Hu38 concluded that evidence is currently lacking to support the additional benefits of PN over ‘one-size-fits-all’ nutrition intervention in the prevention and treatment of type 2 diabetes. Evidence regarding effectiveness and cost-effectiveness of large-scale PN in the general population is even scarcer. To date, the Food4Me trial has determined that participants receiving personalized advice had a healthier diet compared with controls receiving standard guidelines after the 6-month intervention (completion rate: 79%).78 However, no significant changes in weight or waist circumference were observed, even when phenotypic or genotypic data were considered to personalized diet. The question of effectiveness on population health will probably remain open for some years.
Obese individuals and obese populations
In public health, two main traditional strategies have existed for preventive interventions: high-risk and population approaches.50,51 The traditional population approach seeks an improvement of overall population health by shifting the distribution of exposure risk in a favourable direction in the entire population (Figure 1A). With the assumption that ‘a large number of people at a small risk may give rise to more cases of disease than the small number who are at a high risk’, the population approach contrasts with the high-risk approach.50 The high-risk approach proposes targeted interventions addressed only to individuals screened for their higher probability of developing the disease.50
Figure 1.
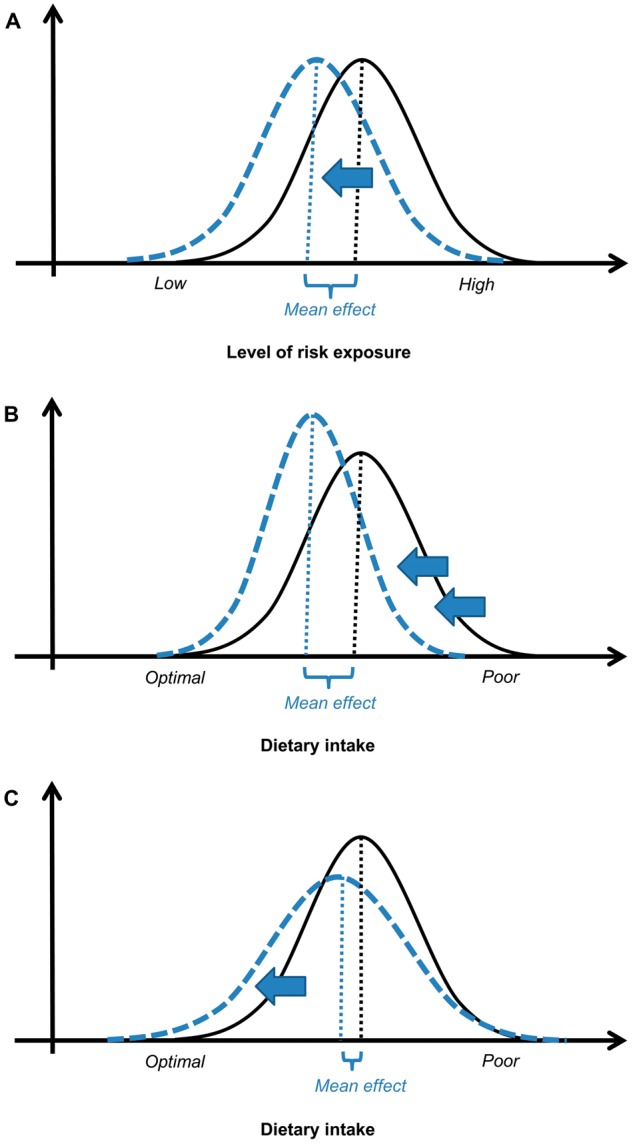
Impact of public health interventions on health. A. Intended effect of Rose’s population strategy on risk of exposure (i.e. large mean effect and unchanged standard deviation after the intervention). B. Desirable impact of public health interventions on dietary intake (i.e. large mean effect and decreased standard deviation after the intervention). C. Probable impact of large-scale precision nutrition on dietary intake (i.e. small mean effect and increased standard deviation after intervention). Solid line: distribution of risk/dietary intake before the intervention. Dashed line: distribution of risk/dietary intake after the intervention.
Large-scale PN targets the whole population in the spirit of a traditional population approach. Both preventive strategies can be used for primary and secondary prevention. However, large-scale PN interventions substantially differ from the traditional population interventions, in the way of achieving the distribution shift. The former targets individual risk with precision behavioural measures in large numbers, whereas the latter targets overall population risk with structural/environmental measures, as shown below.
In the 1985 seminal article ‘Sick individuals and sick populations’,50 still considered relevant for modern public health,79 Geoffrey Rose suggested a distinction be made between two kinds of disease determinants. First, the causes of cases explain why individuals become sick (i.e. individual risk). Second, the causes of incidence explain why certain populations become sick, whereas others do not (i.e. population risk). Rose,50,51 and later Schwartz and Diez-Roux,80 demonstrated that the causes of cases and of incidence are not necessarily the same, even if they are often related. Using empirical examples for hypertension and hypercholesterolaemia, Rose showed that causes of cases originate generally from the individual variation in genetic, social and behavioural factors, or a mixture of them (i.e. what we call today gene-environment interactions).51 As for the causes of incidence, they originate instead from the population variation in collective and societal characteristics.51
Returning to the issue of obesity, Rose would argue that the causes of some individuals becoming obese differ from the causes of some populations becoming obese. Table 2 provides examples of the distinction between causes of obesity in individuals and those in populations, knowing that inadequate diet and lack of physical activity are common causes at both individual and population levels. Based on the determinants listed in Table 2, we observe that the causes of incidence are largely related to the living conditions encouraging excessive food intake and discouraging physical activity. Others have grouped these determinants under the umbrella term of obesogenic environments.81–88 As for large-scale PN, it accounts only for some of the causes of obesity in individuals: e.g. genetic predisposition, gut microbial dysbiosis and lack of food and nutrition literacy regarding the meaning of healthy eating. By definition, as PN is an individualized approach, it does not address any causes of incidence. That is why we define large-scale PN as an individual-centred approach in large numbers, rather than a true population approach.
Table 2.
Non-exhaustive list of determinants of obesity in individuals vs those of obese populations in most high-income countries
| Causes of cases: individual risk Why do some individuals in a population become obese? | Causes of incidence: population risk Why do some populations become obese whereas others do not? | |
|---|---|---|
| Common causes | Quantitative and qualitative imbalance in diet Lack of physical activity | |
| Distinctive causes |
|
|
Individual-centred interventions targeting behaviour change in large numbers can bring benefits to some individuals or sub-strata in the population. For example, it could allow early detection of rare forms of monogenic obesity, such as leptin deficiency due to LEP gene mutations.89,90 However, such interventions are less valuable for overall population health, especially in the case of common diseases with reduced penetrance, such as obesity.51,91 We will now discuss these limitations.
Limitations of individual-centred strategies for population health
Individual-centred strategies often offer temporary and palliative, rather than radical, success at a population level because they do not alter the conditions that affect the overall distribution.50,79 In other words, helping individuals to reduce their individual level of risk exposure does not address the root of the problem determining population risk exposure.51 For obesity, the root of the problem of inadequate dietary intake in most high-income populations is mainly the obesogenic food environment, as mentioned previously.
High-energy and ultra-processed foods rich in sodium, added sugars and saturated fats are widely available in shops and restaurants, and hence in households.92,93 This is particularly applicable for people of lower socioeconomic status (SES), who tend to experience a more prominent obesogenic food environment in their neighbourhoods.85,94,95 For example, lack of access to shops/supermarkets to buy fresh healthy products and over-exposure to fast-food restaurants have been documented in the US poorer neighbourhoods.85,94,95 Moreover, high-energy and ultra-processed foods tend to cost less than healthier alternatives.96–99 High-energy and ultra-processed foods are also heavily advertised,100,101 promoting their over-consumption especially in children.102,103 In addition, food is sold in large portion sizes encouraging overeating.87 Of note, social and cultural norms (e.g. reduction of time and/or skills to shop, prepare and eat food, and frequent snacking) tend to favour imbalanced diets and excessive food intake.104,105 These social, cultural, economic and political barriers hinder healthy eating on a daily basis. If these barriers persist at a population level, the weight loss success of some individuals, thanks to large-scale PN, might be attenuated by the future weight gain of their neighbours or children who are exposed to the same unchanged obesogenic environments. This puts them continually at risk of obesity.106
Similarly, if the root causes of disease in the population are not addressed, individual-centred strategies tend to be behaviourally and culturally difficult to maintain over time.50,79 Namely, implementing behaviour change at an individual level becomes challenging when ‘social norms’ (i.e. peers and environment) are not altered. Deviation from norms necessitates constant effort to sustain alternative behaviours.107–110 This might enlighten us as to why individual-centred programmes, aimed at changing eating behaviour and/or maintaining weight loss in a priori motivated people, have regularly yielded disappointing results in the long term.111–113
PN advocates could argue that knowing the higher personal risk of obesity might further motivate people to change their diet. A systematic review of seven randomized and quasi-randomized controlled trials114 and a more recent trial115 however did not support this hypothesis. They found that communicating DNA-based risk estimates for common complex diseases did not enhance eating behaviour compared with non-DNA-based risk estimates or no risk estimates at all. It seems, indeed, that targeting individual eating behaviour with rational advice on food choices without simultaneously tackling the social, cultural, economic and political conditions in which behaviours occur is unlikely to generate large, long-term dietary changes at a population level.
Together with efficacy, public health interventions aim at maximizing equity or at least mitigating inequity.91,116 In other words, desirable population interventions should have a large mean effect size together with a decreased standard deviation (Figure 1B). Applied to large-scale PN, desirable interventions should reduce the gap between those with the best and worse dietary intake. This means that they should have the most impact on groups with poorer dietary intake, often those of lower SES.24,117,118 However, several reviews have shown that individual-centred public health interventions targeting behavioural changes to improve nutrition119,120 or health121,122 provide less benefit to lower SES groups. For example, Sumar and McLaren123 demonstrated that public information campaigns about the importance of folic acid intake among childbearing-aged women (i.e. an intervention requiring individual decisions to change behaviour) were more likely to increase socioeconomic inequalities in folate status than staple food fortification with folic acid (i.e. an intervention at a policy level requiring no individual decision making).
Inequalities resulting from individual-centred interventions targeting the entire population can be understood through the ‘capability approach’, developed by Amartya Sen.124,125 He stated that people with the same amount of resources at hand are not equal in capacity, that is in what they are able to actually achieve with these resources. Specifically to health, Link and Phelan’s fundamental cause theory126,127 states that individuals of higher SES have a wider range of ‘flexible resources’ with regard to knowledge, wealth, power and social networks than individuals of lower SES. Thanks to these resources, they can better understand information, afford and become motivated to engage in a larger range of activities focusing on their health improvement. In essence, control over the determinants of diet and the motivation to act on it is unequally distributed within a population.
These theories, plus the role of obesogenic neighbourhoods, may partly explain why individuals of higher SES have already taken the most advantage of previous public health individual-centred interventions and thus have lower obesity prevalence than less privileged individuals.10,25,26 From this observation, and building on the fundamental cause theory,126,127 we believe that smartphone applications delivering tailored nutritional advice, albeit free, may be more or less attractive and differentially used according to SES. Early adoption and achievement of improved dietary habits is hence more likely among more socially privileged members of the population. This could exacerbate socioeconomic inequalities in diet and in obesity. This is not only an equity concern, but also one of efficacy. Indeed, if mostly privileged groups in the population improve their eating habits, this would have a limited impact on overall population health, since they already demonstrate a lower risk of obesity and obesity-related disease.
Conclusion
Some public health actors have become enthused by the central promise of PN to better identify the individual risks and suggest targeted dietary modification. They expect PN applied at a large scale to improve populations’ diet and health. We showed, however, that individual-centred interventions directed to behaviour change, such as large-scale PN, are likely to have a limited and unequal impact on diet and obesity incidence at a population level (Figure 1C), particularly if the obesogenic environments are not addressed in the first place.
We nevertheless believe that knowledge and technologies from large-scale PN (Table 1) may provide improved solutions to two recurring concerns in nutritional epidemiology: (i) the accurate assessment of food and nutrients intakes, together with physical activity, in relation to energy intake and expenditure; and (ii) the long-term monitoring of nutritional status at individual and population levels. This may improve or validate our understanding of the impact of dietary intakes and changes on the personal risk of diseases and related biological pathways.36,38,41,65,128 Similarly, methods used in large-scale PN could complement traditional subjective and/or memory-based dietary assessment methods, such as food frequency questionnaires and 24-h dietary recalls.38,39,41,61,63,64 Overall, this may help confirm or refine dietary guidelines for specific population sub-strata. Despite the potential for causal inference and population surveillance, we conclude that PN on a large scale would be of limited interest for public health interventions in the prevention of common polygenic diseases, such as obesity. The impact of large-scale PN on populations’ health is likely to be minor and unevenly distributed in the populations in the absence of complementary social and structural/environmental measures.
Funding
The costs related to AC’s fellowship with KLF at the Ecole de Santé Publique, Université de Montréal, was supported by the Foundation of Lausanne University. The funder had no role in the writing and publication of this paper.
Acknowledgements
We warmly thank Thierry Gagné and Josée Lapalme at the University of Montreal for their precious advice during the manuscript writing. Dr Silvia Stringhini, Saman Khalatbari Soltani and Fabiën Belle at the Lausanne Institute of Social and Preventive Medicine are also thanked for their valuable inputs.
Author Contributions
AC conceptualized the manuscript under the supervision of KLF. MB provided inputs to the manuscript. All authors read and approved the final manuscript.
Conflict of interest: None declared.
References
- 1.World Health Organization. Obesity and Overweight. Key Facts http://www.who.int/mediacentre/factsheets/fs311/en/ (13 June 2018, date last accessed).
- 2. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL.. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA 2018;319:1723–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pi-Sunyer X. The medical risks of obesity. Postgrad Med 2009;121:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol 2018;6:223–36. [DOI] [PubMed] [Google Scholar]
- 5. Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH.. The disease burden associated with overweight and obesity. JAMA 1999;282:1523–29. [DOI] [PubMed] [Google Scholar]
- 6.Global Burden of Disease Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1345–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drewnowski A. Obesity, diets, and social inequalities. Nutr Rev 2009;67(Suppl 1):S36–39. [DOI] [PubMed] [Google Scholar]
- 8. Robertson A, Lobstein T, Knai C.. Obesity and ocio-economic Groups in Europe: Evidence Review and Implications for Action. Report SANCO/2005/C4-NUTRITION-03. Brussels: European Commission, 2007.
- 9. Siddiqi A, Brown R, Nguyen QC, Loopstra R, Kawachi I.. Cross-national comparison of socioeconomic inequalities in obesity in the United States and Canada. Int J Equity Health 2015;14:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Devaux M, Sassi F.. Social inequalities in obesity and overweight in 11 OECD countries. Eur J Public Health 2013;23:464–69. [DOI] [PubMed] [Google Scholar]
- 11.Food and Agriculture Organization. Food-based Dietary Guidelines http://www.fao.org/nutrition/nutrition-education/food-dietary-guidelines/en/ (13 June 2018, date last accessed).
- 12.United States Department of Agriculture. 2015–2020 Dietary Guidelines for Americans https://www.choosemyplate.gov/dietary-guidelines (13 June 2018, date last accessed).
- 13.Public Health England. The Eatwell Guide https://www.gov.uk/government/publications/the-eatwell-guide (13 June 2018, date last accessed).
- 14.United States Department of Agriculture. Nutrition standards in the national school lunch and school breakfast programs. Federal Register 2012;77:4088–167. [PubMed]
- 15.United Kingdom Department of Education. The Requirements for School Food Regulations, 2014, No. 1603.
- 16. Backholer K, Blake M, Vandevijvere S.. Sugar-sweetened beverage taxation: an update on the year that was 2017. Public Health Nutr 2017;20:3219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Silver LD, Ng SW, Ryan-Ibarra S, Taillie LS, Induni M, Miles DR.. Changes in prices, sales, consumer spending, and beverage consumption one year after a tax on sugar-sweetened beverages in Berkeley, California, US: a before-and-after study. PLoS Med 2017;14:e1002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colchero MA, Molina M, Guerrero-Lopez CM.. After Mexico implemented a tax, purchases of sugar-sweetened beverages decreased and water increased: difference by place of residence, household composition, and income level. J Nutr 2017;147:1552–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rehm CD, Penalvo JL, Afshin A, Mozaffarian D.. Dietary intake among US adults, 1999-2012. JAMA 2016;315:2542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bates B, Lennox A, Prentice A. et al. National Diet and Nutrition Survey: Results from Years 1, 2, 3 and 4 (Combined) of the Rolling Programme (2008/2009–2011/2012). London: Public Health England & Food Standard Agency, 2014. [Google Scholar]
- 21. Diethelm K, Jankovic N, Moreno LA. et al. Food intake of European adolescents in the light of different food-based dietary guidelines: results of the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr 2012;15:386–98. [DOI] [PubMed] [Google Scholar]
- 22. Chatelan A, Beer-Borst S, Randriamiharisoa A. et al. Major differences in diet across three linguistic regions of Switzerland: results from the First National Nutrition Survey menuCH. Nutrients 2017;9. doi: 10.3390/nu9111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Non-communicable Disease Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390:2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang DD, Li Y, Chiuve SE, Hu FB, Willett WC.. Improvements in US diet helped reduce disease burden and lower premature deaths, 1999-2012; overall diet remains poor. Health Aff (Millwood) 2015;34:1916–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stamatakis E, Primatesta P, Chinn S, Rona R, Falascheti E.. Overweight and obesity trends from 1974 to 2003 in English children: what is the role of socioeconomic factors? Arch Dis Child 2005;90:999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh GK, Siahpush M, Hiatt RA, Timsina LR.. Dramatic increases in obesity and overweight prevalence and body mass index among ethnic-immigrant and social class groups in the United States, 1976-2008. J Community Health 2011;36:94–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qi Q, Chu AY, Kang JH. et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med 2012;367:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Price ND, Magis AT, Earls JC. et al. A wellness study of 108 individuals using personal, dense, dynamic data clouds. Nat Biotechnol 2017;35:747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang T, Huang T, Zheng Y. et al. Genetic variation of fasting glucose and changes in glycemia in response to 2-year weight-loss diet intervention: the POUNDS LOST trial. Int J Obes 2016;40:1164–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L.. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 2011;124:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang T, Ley SH, Zheng Y. et al. Genetic susceptibility to diabetes and long-term improvement of insulin resistance and beta cell function during weight loss: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Am J Clin Nutr 2016;104:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Corella D, Arregui M, Coltell O. et al. Association of the LCT-13910C>T polymorphism with obesity and its modulation by dairy products in a Mediterranean population. Obesity (Silver Spring) 2011;19:1707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goni L, Cuervo M, Milagro FI, Martinez JA.. A genetic risk tool for obesity predisposition assessment and personalized nutrition implementation based on macronutrient intake. Genes Nutr 2015;10:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu GD, Compher C, Chen EZ. et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016;65:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ortega-Azorin C, Sorli JV, Estruch R. et al. Amino acid change in the carbohydrate response element binding protein is associated with lower triglycerides and myocardial infarction incidence depending on level of adherence to the Mediterranean diet in the PREDIMED trial. Circ Cardiovasc Genet 2014;7:49–58. [DOI] [PubMed] [Google Scholar]
- 36. Zeevi D, Korem T, Zmora N. et al. Personalized nutrition by prediction of glycemic responses. Cell 2015;163:1079–94. [DOI] [PubMed] [Google Scholar]
- 37. Ferguson LR, De Caterina R, Gorman U. et al. Guide and position of the International Society of Nutrigenetics/Nutrigenomics on Personalised Nutrition: Part 1—Fields of Precision Nutrition. J Nutrigenet Nutrigenomics 2016;9:12–27. [DOI] [PubMed] [Google Scholar]
- 38. Wang DD, Hu FB.. Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol 2018;6:416-26. [DOI] [PubMed] [Google Scholar]
- 39. de Toro-Martin J, Arsenault BJ, Despres JP, Vohl MC.. Precision nutrition: a review of personalized nutritional approaches for the prevention and management of metabolic syndrome. Nutrients 2017;9. doi: 10.3390/nu9080913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heianza Y, Qi L.. Gene-diet interaction and precision nutrition in obesity. Int J Mol Sci 2017;18. doi: 10.3390/ijms18040787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ferguson JF, Allayee H, Gerszten RE. et al. Nutrigenomics, the microbiome, and gene-environment interactions: new directions in cardiovascular disease research, prevention, and treatment: a scientific statement from the American Heart Association. Circ Cardiovasc Genet 2016;9:291–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kohlmeier M, De Caterina R, Ferguson LR. et al. Guide and position of the International Society of Nutrigenetics/Nutrigenomics on Personalized Nutrition: Part 2—ethics, challenges and endeavors of precision nutrition. J Nutrigenet Nutrigenomics 2016;9:28–46. [DOI] [PubMed] [Google Scholar]
- 43.European Commission. EU Programmes 2014–2020. Horizon2020. TOPIC: Personalized Nutrition http://ec.europa.eu/research/participants/portal/desktop/en/opportunities/h2020/topics/dt-sfs-14–2018.html (13 June 2018, date last accessed).
- 44. Celis-Morales C, Livingstone KM, Marsaux CF. et al. Design and baseline characteristics of the Food4Me study: a web-based randomised controlled trial of personalised nutrition in seven European countries. Genes Nutr 2015;10:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Newell A, Zlot A, Silvey K, Arail K.. Addressing the obesity epidemic: a genomics perspective. Prev Chronic Dis 2007;4:A31.. [PMC free article] [PubMed] [Google Scholar]
- 46. Claus SP. Development of personalized functional foods needs metabolic profiling. Curr Opin Clin Nutr Metab Care 2014;17:567–73. [DOI] [PubMed] [Google Scholar]
- 47. Khoury MJ, Iademarco MF, Riley WT.. Precision public health for the era of precision medicine. Am J Prev Med 2016;50:398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vaithinathan AG, Asokan V.. Public health and precision medicine share a goal. J Evid Based Med 2017;10:76–80. [DOI] [PubMed] [Google Scholar]
- 49. Belsky DW, Moffitt TE, Caspi A.. Genetics in population health science: strategies and opportunities. Am J Public Health 2013;103(Suppl 1):S73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rose G. Sick individuals and sick populations. Int J Epidemiol 1985;14:32–38. [DOI] [PubMed] [Google Scholar]
- 51. Rose G. The Strategy of Preventive Medicine. Oxford, UK: Oxford University Press, 1992. [Google Scholar]
- 52. Burton H, Sagoo GS, Pharoah P, Zimmern R-L.. Time to revisit Geoffrey Rose: strategies for prevention in the genomic era? Ital J Public Health 2012;9. doi: 10.2427/8665. [Google Scholar]
- 53. Guthrie R, Susi A.. A simple phenylalanine method for detecting phenylketonuria in large populations of newborn infants. Pediatrics 1963;32:338–43. [PubMed] [Google Scholar]
- 54.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012;486:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rimbach G, Minihane AM.. Nutrigenetics and personalised nutrition: how far have we progressed and are we likely to get there? Proc Nutr Soc 2009;68:162–72. [DOI] [PubMed] [Google Scholar]
- 56. Beam AL, Kohane IS.. Big data and machine learning in health care. JAMA 2018;319:1317–18. [DOI] [PubMed] [Google Scholar]
- 57.DayTwo. DayTwo: Personalized Nutrition Based On Your Gut Microbiome https://www.daytwo.com (13 June 2018, date last accessed).
- 58.Viome. Viome: Gut Microbiome and Wellness https://www.viome.com (13 June 2018, date last accessed).
- 59.Habit. Habit: Personalized Nutrition Designed for Better Health & Weight Loss https://habit.com (13 June 2018, date last accessed).
- 60. Weber GM, Mandl KD, Kohane IS.. Finding the missing link for big biomedical data. JAMA 2014;311:2479–80. [DOI] [PubMed] [Google Scholar]
- 61. Garcia-Perez I, Posma JM, Gibson R. et al. Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endocrinol 2017;5:184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vernocchi P, Vannini L, Gottardi D. et al. Integration of datasets from different analytical techniques to assess the impact of nutrition on human metabolome. Front Cell Infect Microbiol 2012;2:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lloyd AJ, Beckmann M, Haldar S, Seal C, Brandt K, Draper J.. Data-driven strategy for the discovery of potential urinary biomarkers of habitual dietary exposure. Am J Clin Nutr 2013;97:377–89. [DOI] [PubMed] [Google Scholar]
- 64. Martin CK, Correa JB, Han H. et al. Validity of the Remote Food Photography Method (RFPM) for estimating energy and nutrient intake in near real-time. Obesity (Silver Spring) 2012;20:891–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vazquez-Fresno R, Llorach R, Urpi-Sarda M. et al. Metabolomic pattern analysis after mediterranean diet intervention in a nondiabetic population: a 1- and 3-year follow-up in the PREDIMED study. J Proteome Res 2015;14:531–40. [DOI] [PubMed] [Google Scholar]
- 66. Connaugton RM, McMorrow AM, Healy ML, McGillicuddy FC, Lithander FE, Roche HM (eds). An anti-inflammatory nutritional intervention selectively improves insulin sensitivity in overweight and obese adolescents wherein baseline metabotype predicts response Proceedings of the Irish Section Meeting, Changing Dietary Behaviour: Physiology through to Practice, 18-20 June 2014 University of Ulster, UK, 2014. [Google Scholar]
- 67. Sevilla-Villanueva B, Gibert K, Sanchez-Marre M, Fito M, Covas MI.. Evaluation of adherence to nutritional intervention through trajectory analysis. IEEE J Biomed Health Inform 2017;21:628–34. [DOI] [PubMed] [Google Scholar]
- 68. Nielsen DE, El-Sohemy A.. A randomized trial of genetic information for personalized nutrition. Genes Nutr 2012;7:559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Brug J, Campbell M, van Assema P.. The application and impact of computer-generated personalized nutrition education: a review of the literature. Patient Educ Couns 1999;36:145–56. [DOI] [PubMed] [Google Scholar]
- 70. Afshin A, Babalola D, McLean M. et al. Information technology and lifestyle: a systematic evaluation of internet and mobile interventions for improving diet, physical activity, obesity, tobacco, and alcohol use. J Am Heart Assoc 2016;5. doi: 10.1161/JAHA.115.003058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stewart-Knox B, Kuznesof S, Robinson J. et al. Factors influencing European consumer uptake of personalised nutrition. Results of a qualitative analysis. Appetite 2013;66:67–74. [DOI] [PubMed] [Google Scholar]
- 72. Collins FS, Varmus H.. A new initiative on precision medicine. N Engl J Med 2015;372:793–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.German Environment Agency. Human Biomonitoring for Europe. HBM4EU https://www.hbm4eu.eu (13 June 2018, date last accessed).
- 74. Gardner CD, Trepanowski JF, Del Gobbo LC. et al. Effect of low-fat vs low-carbohydrate diet on 12-month weight loss in overweight adults and the association with genotype pattern or insulin secretion: the DIETFITS randomized clinical trial. JAMA 2018;319:667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Livingstone KM, Celis-Morales C, Papandonatos GD. et al. FTO genotype and weight loss: systematic review and meta-analysis of 9563 individual participant data from eight randomised controlled trials. BMJ 2016;354:i4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Look AHEAD Research Group. Prospective association of GLUL rs10911021 with cardiovascular morbidity and mortality among individuals with type 2 diabetes: the Look AHEAD study. Diabetes 2016;65:297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Tyrrell J, Wood AR, Ames RM. et al. Gene-obesogenic environment interactions in the UK Biobank study. Int J Epidemiol 2017;46:559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Celis-Morales C, Livingstone KM, Marsaux CF. et al. Effect of personalized nutrition on health-related behaviour change: evidence from the Food4Me European randomized controlled trial. Int J Epidemiol 2017;46:578–88. [DOI] [PubMed] [Google Scholar]
- 79. Doyle YG, Furey A, Flowers J.. Sick individuals and sick populations: 20 years later. J Epidemiol Community Health 2006;60:396–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schwartz S, Diez-Roux AV.. Commentary: causes of incidence and causes of cases—a Durkheimian perspective on Rose. Int J Epidemiol 2001;30:435–39. [DOI] [PubMed] [Google Scholar]
- 81. Hill JO, Peters JC.. Environmental contributions to the obesity epidemic. Science 1998;280:1371–74. [DOI] [PubMed] [Google Scholar]
- 82. Egger G, Swinburn B.. An “ecological” approach to the obesity pandemic. BMJ 1997;315:477–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Swinburn B, Egger G, Raza F.. Dissecting obesogenic environments: the development and application of a framework for identifying and prioritizing environmental interventions for obesity. Prev Med 1999;29(6 Pt 1):563–70. [DOI] [PubMed] [Google Scholar]
- 84. Drewnowski A. Obesity and the food environment: dietary energy density and diet costs. Am J Prev Med 2004;27(Suppl 3):154–62. [DOI] [PubMed] [Google Scholar]
- 85. Papas MA, Alberg AJ, Ewing R, Helzlsouer KJ, Gary TL, Klassen AC.. The built environment and obesity. Epidemiol Rev 2007;29:129–43. [DOI] [PubMed] [Google Scholar]
- 86. Booth KM, Pinkston MM, Poston WS.. Obesity and the built environment. J Am Diet Assoc 2005;105(5 Suppl 1):S110–17. [DOI] [PubMed] [Google Scholar]
- 87. Osei-Assibey G, Dick S, Macdiarmid J. et al. The influence of the food environment on overweight and obesity in young children: a systematic review. BMJ Open 2012;2. doi: 10.1136/bmjopen-2012-001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Glanz K, Sallis JF, Saelens BE, Frank LD.. Healthy nutrition environments: concepts and measures. Am J Health Promot 2005;19:330–33, ii. [DOI] [PubMed] [Google Scholar]
- 89. Fruhbeck G, Kiortsis DN, Catalan V.. Precision medicine: diagnosis and management of obesity. Lancet Diabetes Endocrinol 2018;6:164–66. [DOI] [PubMed] [Google Scholar]
- 90. ShabanaHasnain S. The p. N103K mutation of leptin (LEP) gene and severe early onset obesity in Pakistan. Biol Res 2016;49:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Keyes KM, Galea S.. Population Health Science. New York, NY: Oxford University Press, 2016. [Google Scholar]
- 92.Pan American Health Organization (PAHO). Ultra-processed Food and Drink Products in Latin America: Trends, Impact on Obesity, Policy Implications. Chapter 3. Ultra-processed Product Sales. Washington, DC: PAHO, 2015. [Google Scholar]
- 93. Monteiro CA, Moubarac JC, Levy RB, Canella DS, Louzada M, Cannon G.. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr 2018;21:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lovasi GS, Hutson MA, Guerra M, Neckerman KM.. Built environments and obesity in disadvantaged populations. Epidemiol Rev 2009;31:7–20. [DOI] [PubMed] [Google Scholar]
- 95. Cummins S, Macintyre S.. Food environments and obesity—neighbourhood or nation? Int J Epidemiol 2006;35:100–04. [DOI] [PubMed] [Google Scholar]
- 96. Rao M, Afshin A, Singh G, Mozaffarian D.. Do healthier foods and diet patterns cost more than less healthy options? A systematic review and meta-analysis. BMJ Open 2013;3. doi: 10.1136/bmjopen-2013-004277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jones NR, Conklin AI, Suhrcke M, Monsivais P.. The growing price gap between more and less healthy foods: analysis of a novel longitudinal UK dataset. PLoS One 2014;9:e109343.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Drewnowski A, Darmon N.. The economics of obesity: dietary energy density and energy cost. Am J Clin Nutr 2005;82(Suppl 1):265S–73S. [DOI] [PubMed] [Google Scholar]
- 99. Andrieu E, Darmon N, Drewnowski A.. Low-cost diets: more energy, fewer nutrients. Eur J Clin Nutr 2006;60:434–36. [DOI] [PubMed] [Google Scholar]
- 100. Batada A, Seitz MD, Wootan MG, Story M.. Nine out of 10 food advertisements shown during Saturday morning children’s television programming are for foods high in fat, sodium, or added sugars, or low in nutrients. J Am Diet Assoc 2008;108:673–78. [DOI] [PubMed] [Google Scholar]
- 101. Sixsmith R, Furnham A.. A content analysis of British food advertisements aimed at children and adults. Health Promot Int 2010;25:24–32. [DOI] [PubMed] [Google Scholar]
- 102. Dovey TM, Taylor L, Stow R, Boyland EJ, Halford JC.. Responsiveness to healthy television (TV) food advertisements/commercials is only evident in children under the age of seven with low food neophobia. Appetite 2011;56:440–46. [DOI] [PubMed] [Google Scholar]
- 103. Halford JC, Boyland EJ, Hughes G, Oliveira LP, Dovey TM.. Beyond-brand effect of television (TV) food advertisements/commercials on caloric intake and food choice of 5-7-year-old children. Appetite 2007;49:263–67. [DOI] [PubMed] [Google Scholar]
- 104. Smith LP, Ng SW, Popkin BM.. Trends in US home food preparation and consumption: analysis of national nutrition surveys and time use studies from 1965-1966 to 2007-2008. Nutr J 2013;12:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Higgs S, Thomas J.. Social influences on eating. Curr Opin Behav Sci 2016;9:1–6. [Google Scholar]
- 106. Syme SL. Strategies for health promotion. Prev Med 1986;15:492–507. [DOI] [PubMed] [Google Scholar]
- 107. Frohlich KL, Poland B, Sharek M, Contrasting entry points for intervention in health promotion practice: situating and working with context In: Rootman I, Pederson A, Frohlich KL, Dupéré S (eds). Health Promotion in Canada New Perspectives on Theory, Practice, Policy, and Research. 4th edn Toronto, ON: Canadian Scholars, 2017. [Google Scholar]
- 108. Higgs S. Social norms and their influence on eating behaviours. Appetite 2015;86:38–44. [DOI] [PubMed] [Google Scholar]
- 109. Delormier T, Frohlich KL, Potvin L.. Food and eating as social practice—understanding eating patterns as social phenomena and implications for public health. Sociol Health Illn 2009;31:215–28. [DOI] [PubMed] [Google Scholar]
- 110. Hargreaves K, Amos A, Highet G. et al. The social context of change in tobacco consumption following the introduction of ‘smokefree’ England legislation: a qualitative, longitudinal study. Soc Sci Med 2010;71:459–66. [DOI] [PubMed] [Google Scholar]
- 111. Dombrowski SU, Knittle K, Avenell A, Araujo-Soares V, Sniehotta FF.. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ 2014;348:g2646.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mead E, Brown T, Rees K. et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst Rev 2017;6:CD012651.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Douketis JD, Macie C, Thabane L, Williamson DF.. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes 2005;29:1153.. [DOI] [PubMed] [Google Scholar]
- 114. Hollands GJ, French DP, Griffin SJ. et al. The impact of communicating genetic risks of disease on risk-reducing health behaviour: systematic review with meta-analysis. BMJ 2016;352:i1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Godino JG, van Sluijs EM, Marteau TM, Sutton S, Sharp SJ, Griffin SJ.. Lifestyle advice combined with personalized estimates of genetic or phenotypic risk of type 2 diabetes, and objectively measured physical activity: a randomized controlled trial. PLoS Med 2016;13:e1002185.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Benach J, Malmusi D, Yasui Y, Martinez JM.. A new typology of policies to tackle health inequalities and scenarios of impact based on Rose’s population approach. J Epidemiol Community Health 2013;67:286–91. [DOI] [PubMed] [Google Scholar]
- 117. Darmon N, Drewnowski A.. Does social class predict diet quality? Am J Clin Nutr 2008;87:1107–17. [DOI] [PubMed] [Google Scholar]
- 118. Giskes K, Avendaňo M, Brug J, Kunst AE.. A systematic review of studies on socioeconomic inequalities in dietary intakes associated with weight gain and overweight/obesity conducted among European adults. Obes Rev 2009;11:413–29. [DOI] [PubMed] [Google Scholar]
- 119. Adams J, Mytton O, White M, Monsivais P.. Why are some population interventions for diet and obesity more equitable and effective than others? The role of individual agency. PLoS Med 2016;13:e1001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lorenc T, Petticrew M, Welch V, Tugwell P.. What types of interventions generate inequalities? Evidence from systematic reviews. J Epidemiol Community Health 2013;67:190–93. [DOI] [PubMed] [Google Scholar]
- 121. Beauchamp A, Backholer K, Magliano D, Peeters A.. The effect of obesity prevention interventions according to socioeconomic position: a systematic review. Obes Rev 2014;15:541–54. [DOI] [PubMed] [Google Scholar]
- 122. Frohlich KL, Potvin L.. Transcending the known in public health practice: the inequality paradox: the population approach and vulnerable populations. Am J Public Health 2008;98:216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Sumar N, McLaren L.. Impact on social inequalities of population strategies of prevention for folate intake in women of childbearing age. Am J Public Health 2011;101:1218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sen A. Inequality Re-examined. Cambridge, MA: Harvard University Press, 1992. [Google Scholar]
- 125. Frohlich KL. The social determinants of what? Int J Public Health 2010;55:235–36. [DOI] [PubMed] [Google Scholar]
- 126. Link BG, Phelan J.. Social conditions as fundamental causes of disease. J Health Soc Behav 1995;Spec No:80–94. [PubMed] [Google Scholar]
- 127. Phelan JC, Link BG, Tehranifar P.. Social conditions as fundamental causes of health inequalities: theory, evidence, and policy implications. J Health Soc Behav 2010;51(Suppl):S28–40. [DOI] [PubMed] [Google Scholar]
- 128. Esko T, Hirschhorn JN, Feldman HA. et al. Metabolomic profiles as reliable biomarkers of dietary composition. Am J Clin Nutr 2017;105:547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]


