Abstract
Background
Nutritional perturbations during pregnancy may impact fetal and long-term childhood growth, although there are limited data on overall diet quality. We investigated whether diet quality, measured by the Healthy Eating Index-2010 (HEI-2010), during pregnancy was related to birthweight z-score (BWZ) and the clinically relevant birth outcomes of large- and small-for-gestational age (LGA and SGA).
Methods
In a prospective cohort of 2269 multi-racial/ethnic women from the Pregnancy Environment and Lifestyle Study (2014–2017), dietary intake was assessed by a food frequency questionnaire during early pregnancy. Offspring BWZ and LGA or SGA were derived based on gestational age-, sex-, and racial/ethnic-specific birthweight distributions. Multivariable linear and Poisson regression with robust standard errors were used.
Results
About 80% of women did not achieve good diet quality (HEI-2010 < 80). After adjusting for covariates, infants born to women in the lowest vs highest quartile of HEI-2010 (37.5–64.4 vs 78.7–94.2) had a 0.12 standard-deviation [95% confidence interval (CI) 0.01–0.23, P-for-trend = 0.023] greater BWZ and 1.76-fold (1.08–2.87, P-for-trend = 0.037) increased risk of LGA. No association was observed between HEI-2010 and SGA. Per-5-point substitution of the reversely coded empty calories component score with the whole grains component score in the HEI-2010 was related to a 25% (95% CI 0.66–0.86) lower risk of LGA.
Conclusions
Poor diet quality in pregnancy was associated with higher birthweight and increased risk of LGA independent of maternal obesity and other covariates. Substitution of empty calories with whole grains may mitigate the risk of excess fetal growth. Our findings may inform potential prevention strategies and dietary guidelines for pregnant women.
Keywords: Birth weight, diet quality, fetal growth extremes, healthy eating index, pregnancy
Key Messages
In this prospective multi-racial/ethnic cohort of 2269 women with singleton pregnancies, about 80% of women did not achieve good diet quality as measured by the Healthy Eating Index-2010 during early pregnancy, which is based on adherence to the Dietary Guidelines for Americans.
Poorer maternal diet quality in early pregnancy was associated with a greater offspring birthweight z-score and increased risk of the baby being large-for-gestational age at birth, independent of maternal obesity.
Per-5-point substitution of the empty calories component score with the whole grains component score in maternal Healthy Eating Index-2010 was related to a 25% lower risk of having a large-for-gestational age infant.
Our findings highlight the importance of promoting overall dietary quality adherence to the national dietary guidelines during pregnancy to mitigate the risk of excess fetal growth.
Introduction
Emerging evidence suggests that nutritional perturbations during pregnancy may impact fetal and long-term offspring growth and disease risk in later life.1–8 However, it remains unclear whether nutritional perturbation during pregnancy has an impact on fetal growth extremes as exemplified in large- or small-for-gestational age (LGA or SGA) infants, which in turn are important predictors of future childhood and adult health, including cardiometabolic outcomes.9–11 Previous research on fetal growth extremes and diet during pregnancy that focused on isolated foods or nutrients has reported inconsistent findings.5,12–14 In contrast, dietary patterns may better account for the likely synergism and interaction between foods and nutrients. However, empirically data-driven approaches to characterizing dietary patterns may still contribute to the inconsistency in findings,15,16 given the limited comparability across studies. In this regard, data on maternal a priori-based diet quality index during pregnancy in relation to fetal growth outcomes are warranted, but still scant.
Unlike data-driven dietary patterns, assessing overall diet quality with the Healthy Eating Index (HEI), a measure based on adherence to the United States Department of Agriculture (USDA) Dietary Guidelines for Americans, can be comparable across studies. Yet, studies assessing maternal diet quality during pregnancy to date have been inconclusive, with both inverse associations between maternal HEI and infant birthweight and adiposity17,18 and null findings.19,20 Notably, previous data were from cohorts one to two decades ago,19,20 that were predominantly Caucasian,18–20 had relatively small sizes18 or did not examine the clinically relevant outcome of LGA or SGA.17,18 Further, maternal obesity and gestational diabetes (GDM) are two major risk factors for fetal growth extremes.21,22 However, no study to date has examined whether maternal metabolic phenotype modifies the association between overall dietary quality during pregnancy and fetal growth extremes. In addition, no prior study has conducted a dietary substitution analysis to better quantify the effect of substituting HEI moderation components (components to decrease) with adequacy components (components to increase), which may inform the potential impact of healthier alternatives and prevention strategies.
In the present study, we aimed to fill these gaps in knowledge using 2269 women with a singleton pregnancy in the Pregnancy Environment and Lifestyle Study (PETALS), a contemporary multi-racial/ethnic prospective cohort study. We aimed to investigate whether diet quality measured by the Healthy Eating Index-2010 (HEI-2010) during early pregnancy was prospectively associated with birthweight z-score (BWZ) and risk of fetal growth extremes (LGA or SGA). We further examined effect modification by maternal obesity and GDM status and explored the impact of substituting the HEI moderation components with adequacy components.
Methods
Study population and design
The study participants were from PETALS, a longitudinal multi-racial/ethnic cohort of pregnant women. The study design and scope have been described in detail elsewhere.23 The source population was identified from the Kaiser Permanente Northern California (KPNC) membership, an integrated health care delivery system serving 4.2 million members, including about 30% of the underlining population, and is racially/ethnically and socio-economically diverse and representative of the population in the served geographic area.24,25 Briefly, after weekly search of the electronic health records (EHR), pregnant women aged 18–45 years of all races/ethnicities, carrying singleton infants and without recognized pre-existing diseases (i.e. diabetes, cancer, hepatitis C or liver cirrhosis) were recruited before gestational week 11 at five participating medical centres. Survey data were collected at study clinic visit 1 (gestational weeks 10–13) and clinic visit 2 (gestational weeks 16–19). The study was approved by the human subjects committee of the Kaiser Foundation Research Institute. Informed consent was obtained from all participants.
Between April 2014 and October 2017, 2525 eligible women were enrolled, of whom 37 had pregnancy losses and seven moved or changed health care provider, resulting in a sample of 2481 women (98.3%) who gave birth. We sequentially excluded women whose child had missing data on birthweight from the EHR (n = 94), missing data from Food Frequency Questionnaire (FFQ) (n = 87), implausible daily energy intake (<400 or >6000 kcal/day, n = 23) and FFQ completed after the diagnosis of GDM (n = 8), rendering a pool of 2269 women as the analytical sample.
Exposure assessment
Dietary intakes during pregnancy were assessed by the Block FFQ,26 administered at study clinic visit 1 (gestational weeks 10–13), that collected information on habitual dietary intake during the previous 3 months. Women reported their usual intake and portion sizes of foods and beverages, including items modified to accommodate the diverse dietary habits of the multi-racial/ethnic cohort and used in previous studies.27,28 The Block FFQ has demonstrated adequate reliability and validity in comparison with multiple dietary records,29 serving as a useful instrument for analyses of the energy, food and nutrient levels among diverse populations, including pregnant women.28,30,31 Nutrient intakes were adjusted for total energy intake using the residual method.32
To assess the overall diet quality, we calculated the HEI-2010 score, which is a valid and reliable measure for assessing adherence to the 2010 Dietary Guidelines for Americans.33 Notably, we used HEI-2010, not HEI-2015, because the 2010 guidelines covered most of the study period from 2014 to 2017, whereas the 2015 guidelines were not released until 2016. The HEI-2010 consists of 12 components (total fruit, whole fruit, total vegetables, greens and beans, whole grains, dairy, total protein foods, seafood and plant proteins, fatty acids, refined grains, sodium and empty calories from solid fats, alcohol and added sugars), with a maximum possible score of 100.34 To make the index more appropriate for the pregnant population, given that alcohol is not recommended during pregnancy, we excluded alcohol intake from the empty calories component.
Outcome measures
Infant birthweight was collected from the EHR, which has served as the gold standard measure in various settings, including research studies.35,36 Gestational age at birth was extracted from the EHR based on first-trimester ultrasound data. As demonstrated previously, we have validated gestational age at birth from the EHR against fetal ultrasonographic examinations among both preterm and term infants.37 BWZ was derived according to sex-, gestational age- and racial/ethnic-specific distribution of birthweight in the underlying population.38 Accordingly, LGA and SGA were defined as a birthweight >90th or <10th percentile of the aforementioned birthweight distribution, respectively.
Covariates
A comprehensive list of covariates, including demographic, lifestyle and medical factors, was obtained from structured questionnaires administered at gestational weeks 10–13: maternal age (<25, 25–29, 30–34, 35–40, ≥40 years); race/ethnicity (non-Hispanic White, Hispanic, non-Hispanic Black, Asian/Pacific Islander, other); education (≤high school, some college, ≥college); household income (<$50 000, $50 000–99 999, $100 000–149 999, ≥$150 000); nulliparity; prenatal supplement use, smoking and alcohol intake during pregnancy (yes/no); total daily energy intake during pregnancy (kcal/day) from the FFQ;26 and physical activity (metabolic equivalent of task/week) from the Pregnancy Physical Activity Questionnaire.39 Additional covariates were extracted from the EHR: prepregnancy body mass index (BMI, <18.5, 18.5–24.9, 25.0–29.9, ≥30.0 kg/m2); gestational weight gain (GWG, kg); pre-existing hypertension (yes/no); and gestational age at delivery (week). Presence of GDM was identified from the KPNC GDM Registry40,41 and ascertained according to the Carpenter and Coustan criteria with two or more values meeting or exceeding the following thresholds: fasting glucose, 5.3 mmol/L; 1-h, 10.0 mmol/L; 2-h, 8.6 mmol/L; and 3-h, 7.8 mmol/L.42 Covariates meeting the inclusion criteria of ≥10% change in the main effect estimates were retained in the multivariable model.
Statistical analysis
Distribution of maternal characteristics and offspring outcomes across quartiles of the HEI-2010 score were assessed by ANOVA for continuous variables and χ2 test for categorical variables. Multivariable linear regression models estimated the beta coefficients (β) and 95% confidence intervals (CIs) for offspring BWZ in association with maternal HEI-2010 in quartiles [with the highest quartile (HEI-2010 ≥ 78.7) indicating the highest diet quality as the reference], after adjustment for aforementioned covariates. Similar models were constructed for BWZ in relation to good vs poorer (poor or improvement needed) diet (i.e. HEI-2010 score ≥ vs <80) based on the USDA recommendations.43
Similarly, multivariable Poisson regression with robust standard errors calculated relative risks (RR) and 95% CIs for offspring risk of LGA and SGA in relation to HEI-2010 in quartiles and good vs poorer diet, respectively. To assess effect modification by maternal metabolic phenotypes, we performed a priori stratified analyses by maternal prepregnancy overweight/obesity and GDM status, respectively. We also examined effect modification by race/ethnicity. P-for-interaction was obtained by the likelihood ratio test. In a sensitivity analysis, we additionally adjusted for GWG in the multivariable models to test against potential residual confounding, despite the potential for over-adjustment given the plausibility that GWG is in the causal pathway between maternal diet in early pregnancy and fetal growth. To further test the robustness of the findings, we conducted sensitivity analyses restricted to women with term deliveries (≥37 gestational weeks) and without hypertensive complications during pregnancy, respectively.
Further, assuming the observed association between maternal HEI-2010 score and offspring risk of LGA reflected a true effect, we used the substitution regression models44,45 to examine whether any of the HEI-2010 adequacy components could serve as potential healthier alternatives for the reversely coded moderation components (i.e. greater the intakes, greater the component score, after reverse coding). Poisson regression estimated adjusted RRs and 95% CIs for the substitution association using the computed difference in β coefficients and variances, and the covariance between the two components of interest per 5-point-score (e.g. lowering 5% of energy from empty calories and increasing whole grains by 0.75 ounces), while adjusting for aforementioned covariates and the HEI-2010 score by subtracting the two components of interest.44 Post hoc multiple-comparison adjustment for P values was performed using the Benjamini–Hochberg false discovery rate-controlling method.46 All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Among 2269 women in the PETALS cohort, the mean HEI-2010 score was 71.3 (SD 10.0, range 37.5–94.2), with 21.1% of women meeting the USDA recommendations for good diet quality (HEI-2010 score >80). Across the increasing HEI-2010 quartiles, the proportions of women aged ≥35 years, of non-Hispanic White origin, with a college degree or above, with household income ≥$100 000, being nulliparous and taking prenatal supplement increased, while the proportions of women with a prepregnancy BMI ≥25 kg/m2 and who reported smoking during pregnancy decreased (Table 1).
Table 1.
Characteristics of 2269 women in the Pregnancy Environment and Lifestyle Study who delivered between 2014 and 2017 by quartile of the Healthy Eating Index-2010 score during pregnancy
| Quartile 1 (n = 567)a | Quartile 2 (n = 567) | Quartile 3 (n = 568) | Quartile 4 (n = 567) | P-valueb | |
|---|---|---|---|---|---|
| Age, years, n (%) | <0.001 | ||||
| 18-24 | 138 (24.3)c | 110 (19.4) | 66 (11.6) | 51 (9.0) | |
| 25-29 | 164 (28.9) | 157 (27.7) | 132 (23.2) | 148 (26.1) | |
| 30-34 | 164 (28.9) | 191 (33.7) | 241 (42.4) | 217 (38.3) | |
| ≥35 | 101 (17.8) | 109 (19.2) | 129 (22.7) | 151 (26.6) | |
| Race/ethnicity, n (%) | <0.001 | ||||
| Non-Hispanic White | 98 (17.3) | 108 (19.0) | 138 (24.3) | 161 (28.4) | |
| Hispanic | 245 (43.2) | 254 (44.8) | 218 (38.4) | 220 (38.8) | |
| African American | 77 (13.6) | 63 (11.1) | 45 (7.9) | 28 (4.9) | |
| Asian/Pacific Islander | 124 (21.9) | 124 (21.9) | 149 (26.2) | 138 (24.3) | |
| Other | 23 (4.1) | 18 (3.2) | 18 (3.2) | 20 (3.5) | |
| Education, n (%) | <0.001 | ||||
| High school or less | 120 (21.2) | 88 (15.5) | 57 (10.0) | 48 (8.5) | |
| Some college | 244 (43.0) | 245 (43.2) | 210 (37.0) | 171 (30.2) | |
| College graduate or above | 203 (35.8) | 234 (41.3) | 301 (53.0) | 348 (61.4) | |
| Household income, $, n (%) | <0.001 | ||||
| <50 000 | 241 (42.5) | 192 (33.9) | 169 (29.8) | 140 (24.7) | |
| 50 000-99 999 | 174 (30.7) | 204 (36.0) | 184 (32.4) | 163 (28.7) | |
| 100 000-149 999 | 81 (14.3) | 84 (14.8) | 114 (20.1) | 129 (22.8) | |
| ≥150 000 | 60 (10.6) | 77 (13.6) | 96 (16.9) | 127 (22.4) | |
| Missing | 11 (1.9) | 10 (1.8) | 5 (0.9) | 8 (1.4) | |
| Nulliparity, n (%) | 243 (42.9) | 237 (41.8) | 249 (43.8) | 272 (48.0) | 0.18 |
| Prepregnancy BMI, kg/m2, n (%) | 0.003 | ||||
| <18.5 | 22 (3.9) | 13 (2.3) | 11 (1.9) | 16 (2.8) | |
| 18.5-24.9 | 199 (35.1) | 198 (34.9) | 256 (45.1) | 261 (46.0) | |
| 25.0-29.9 | 171 (30.2) | 176 (31.0) | 152 (26.8) | 156 (27.5) | |
| ≥30.0 | 175 (30.9) | 180 (31.7) | 149 (26.2) | 134 (23.6) | |
| Gestational weight gain, kg | 13.2 ± 6.7 | 13.2 ± 6.2 | 12.9 ± 6.2 | 13.1 ± 6.0 | 0.69 |
| Smoking in pregnancy, n (%) | 7 (1.2) | 2 (0.4) | 1 (0.2) | 0 (0.0) | 0.008 |
| Alcohol in pregnancy, n (%) | 94 (16.6) | 85 (15.0) | 79 (13.9) | 82 (14.5) | 0.64 |
| Prenatal supplement use, n (%) | 526 (92.8) | 543 (95.8) | 552 (97.2) | 557 (98.2) | <0.001 |
| Physical activity, METs/week | 150.2 ± 115.0 | 161.7 ± 126.0 | 148.6 ± 103.9 | 148.0 ± 104.1 | 0.43 |
| Pre-existing hypertension, n (%) | 17 (3.0) | 33 (5.8) | 29 (5.1) | 17 (3.0) | 0.03 |
| Hypertensive disorders in pregnancy, n (%) | 59 (10.4) | 72 (12.7) | 65 (11.4) | 48 (8.5) | 0.13 |
| Gestational diabetes, n (%) | 58 (10.2) | 54 (9.5) | 68 (12.0) | 66 (11.6) | 0.55 |
| Gestational age at delivery, week | 38.8 ± 1.7 | 38.8 ± 1.7 | 38.8 ± 1.8 | 39.0 ± 1.7 | 0.04 |
| Preterm birth, n (%) | 43 (7.6) | 35 (6.2) | 37 (6.5) | 26 (4.6) | 0.21 |
| Large-for-gestational age, n (%) | 51 (9.0) | 53 (9.3) | 52 (9.2) | 31 (5.5) | 0.064 |
| Small-for-gestational age, n (%) | 55 (9.7) | 49 (8.6) | 63 (11.1) | 65 (11.5) | 0.46 |
MET, metabolic equivalent of task.
Ranges for quartiles 1–4 were 37.5–64.4, 64.5–71.7, 71.8–78.6 and 78.7–94.2, respectively.
P-values were obtained by ANOVA for continuous variables and by Chi-square test for categorical variables.
Values are mean ± SD for continuous variables or otherwise specified as n (%) for categorical variables.
Maternal HEI-2010 score was positively associated with percent of energy from carbohydrates and protein and intakes of polyunsaturated fat, dietary fibre, vitamins C and E, calcium, iron and magnesium, and inversely associated with total fat (percent of energy), total and added sugars, glycaemic index and glycaemic load (Supplementary Table 1, available as Supplementary data at IJE online). All individual component scores monotonously increased across the increasing quartiles of the total HEI-2010.
Overall, lower maternal HEI-2010 score was associated with greater BWZ (Table 2). Infants born to women in the lowest quartile vs highest quartile (37.5–64.4 vs 78.7–94.2) of the HEI-2010 score were 0.12 SD (95% CI 0.01, 0.23) heavier at birth (P-for-trend across quartiles = 0.023), equivalent to a greater birthweight of 62.1 g. Similarly, poor diet or diet needs improvement compared to good diet quality (HEI-2010 ≤ 80 vs >80) was associated with a 0.11 SD (95% CI 0.02, 0.19) or 57.0 g increase in birthweight.
Table 2.
Crude and adjusted beta coefficients for offspring birthweight z-score in association with maternal Healthy Eating Index-2010 score during pregnancy in the Pregnancy Environment and Lifestyle Study, 2014–2017
| Maternal diet quality during pregnancy | Beta coefficients (95% CIs) for birthweight z-score |
|
|---|---|---|
| Crude | Multivariable modela | |
| HEI-2010 score in quartiles | ||
| Quartile 1 (37.5-64.4) | 0.17 (0.06, 0.28) | 0.12 (0.01, 0.23) |
| Quartile 2 (64.5-71.7) | 0.15 (0.04, 0.26) | 0.11 (-0.002, 0.23) |
| Quartile 3 (71.8-78.6) | 0.11 (0.001, 0.22) | 0.07 (-0.04, 0.18) |
| Quartile 4 (78.7-94.2) | 0 (Reference) | 0 (Reference) |
| P-for-trend | 0.006 | 0.023 |
| HEI-2010 score ratings | ||
| Poor diet or diet needs improvement (≤80) | 0.14 (0.04, 0.23) | 0.11 (0.02, 0.19) |
| Good diet (>80) | 0 (Reference) | 0 (Reference) |
HEI, health eating index.
Adjusted for maternal age, race/ethnicity, parity, prepregnancy body mass index, pre-existing hypertension, total daily energy intake during pregnancy, physical activity during pregnancy, prenatal supplement use and gestational age at delivery.
For fetal growth extremes, women in each of the lowest three quartiles vs the highest quartile of the HEI-2010 had a greater risk of LGA births, with risk estimates ranging from 1.67 to 1.76 in the multivariable model (Table 3). Specifically, women in the lowest vs highest quartile of the HEI-2010 and women with poorer vs good diet quality had a 1.76-fold (95% CI: 1.08, 2.87; P-for-trend = 0.04) and 1.81-fold (1.15, 2.84) greater risk of LGA births, respectively. No associations were observed for HEI-2010 and SGA.
Table 3.
Crude and adjusted relative risks of offspring risk of large- or small-for-gestational age in association with maternal Healthy Eating Index-2010 score during pregnancy in the Pregnancy Environment and Lifestyle Study, 2014–2017
| Maternal diet quality during pregnancy | Relative risks (95% CIs) |
|
|---|---|---|
| Crude | Multivariable modela | |
| Large-for-gestational age | ||
| HEI-2010 score in quartiles | ||
| Q1 (37.5-64.4) | 1.83 (1.14, 2.94) | 1.76 (1.08, 2.87) |
| Q2 (64.5-71.7) | 1.82 (1.14, 2.90) | 1.71 (1.06, 2.75) |
| Q3 (71.8-78.6) | 1.80 (1.14, 2.85) | 1.67 (1.03, 2.69) |
| Q4 (78.7-94.2) | 1 (reference) | 1 (reference) |
| P-for-trend | 0.025 | 0.037 |
| HEI-2010 score ratings | ||
| Poor diet or diet needs improvement (≤80) | 1.88 (1.21, 2.92) | 1.81 (1.15, 2.84) |
| Good diet (>80) | 1 (reference) | 1 (reference) |
| Small-for-gestational age | ||
| HEI-2010 score in quartiles | ||
| Q1 (37.5-64.4) | 0.87 (0.59, 1.28) | 0.92 (0.61, 1.38) |
| Q2 (64.5-71.7) | 0.77 (0.52, 1.14) | 0.83 (0.55, 1.25) |
| Q3 (71.8-78.6) | 1.01 (0.70, 1.47) | 1.09 (0.75, 1.60) |
| Q4 (78.7-94.2) | 1 (reference) | 1 (reference) |
| P-for-trend | 0.290 | 0.454 |
| HEI-2010 score ratings | ||
| Poor diet or diet needs improvement (≤80) | 0.89 (0.64, 1.24) | 0.96 (0.69, 1.36) |
| Good diet (>80) | 1 (reference) | 1 (reference) |
HEI, health eating index. Q, quartile.
Adjusted for maternal age, race/ethnicity, parity, prepregnancy body mass index, pre-existing hypertension, total daily energy intake during pregnancy, physical activity during pregnancy, prenatal supplement use and gestational age at delivery.
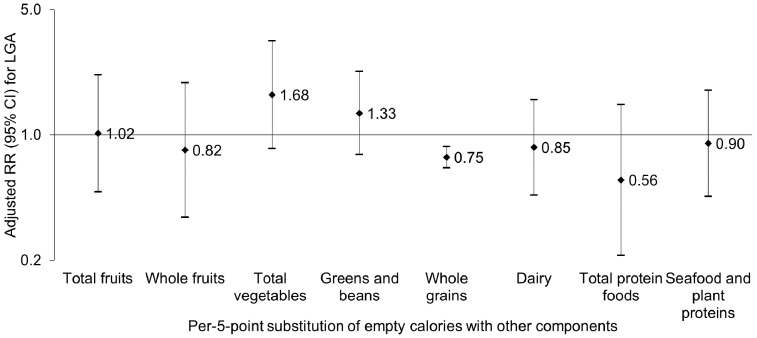
Substitution of per-5-point score of the reversely coded empty calories component with the whole grains component was associated with a 25% (RR 0.75, 95% CI 0.66, 0.86) decreased risk of LGA, which remained significant after false discovery rate adjustment for multiple comparisons (Figure 1), whereas no other individual HEI-2010 component exhibited comparable substitutional effect for empty calories or any other moderation components.
Figure 1.
Risk of offspring large-for-gestational age associated with substitution of per-5-point maternal empty calories component reverse score with other food component scores in the Healthy Eating Index-2010 score during pregnancy. Relative risks (RR) and 95% confidence intervals (CI) were shown in a logarithmic scale and adjusted for maternal age, race/ethnicity, parity, prepregnancy body mass index, pre-existing hypertension, total daily energy intake during pregnancy, physical activity during pregnancy, prenatal supplement use, gestational age at delivery, and the Healthy Eating Index-2010 score without counting the empty calories component and other individual food component of interest.
In stratified analyses, the inverse association between the HEI-2010 and risk of LGA was significant among women without GDM (P-for-interaction<0.001, Supplementary Figure 1, available as Supplementary data at IJE online); no clear trend was observed for women with GDM, which may be attributed to the smaller sample size or treatment effect. No effect modification was observed by prepregnancy overweight/obesity or race/ethnicity (P-for-interaction= 0.37 and 0.25, respectively). Further, sensitivity analyses showed similar and robust results after additionally adjusting for GWG. Results remained similar in sensitivity analyses restricted to women with term births or without hypertensive complications during pregnancy, respectively (Supplementary Table 2, available as Supplementary data at IJE online).
Discussion
In this contemporary multi-racial/ethnic prospective cohort study, a majority (79%) of pregnant women did not adhere to the Dietary Guidelines for Americans. Lower maternal dietary quality during early pregnancy was associated with greater infant BWZ and risk of LGA, independent of maternal obesity and other major covariates. Notably, the association was particularly pronounced among women without GDM, reflecting the possible effect of nutritional and medication treatment after the diagnosis of GDM, and highlighting the importance of promoting overall dietary quality even among low-risk women to mitigate the risk of excess fetal growth. Moreover, per-5-point substitution of the reversely coded empty calories component score with the whole grains component score was associated with a 25% decreased risk of LGA births.
Several indexes have been developed to assess the global effect of diet quality on health outcomes in the general population.47 Consistent with our findings, two recent studies in the USA reported inverse associations between maternal HEI-2010 during pregnancy and infant birthweight and adiposity, although with no comparable data on BWZ or clinically relevant outcomes (LGA or SGA).17,18 On the other hand, among 787 mother–child dyads in Spain, the Alternate Healthy Eating Index in the first trimester was associated with lower risk of fetal growth restriction, suggesting that higher diet quality in pregnancy favours fetal growth.48 In addition, two others reported null findings.19,20 The discordant findings may be partially attributable to variations in the dietary assessment tool, diet quality indexes, dietary behaviours and participant characteristics in the underlying population and potential residual confounding. Notably, most previous data were from cohorts that were predominantly Caucasian,18–20,48,49 whereas our cohort was predominantly racial-ethnic minorities (78%). In contrast to our contemporary cohort of women who gave birth between 2014 and 2017, some previous data were from cohorts one to two decades ago.19,20,48,49 Moreover, some previous cohorts had 70% of women with a normal prepregnancy BMI,19,48 whereas 57% of our cohort were overweight/obese before pregnancy, consistent with the obesity epidemic among reproductive-aged women in the USA.50 The current study extends the literature by examining the overall diet quality in early pregnancy in relation to birth size for gestational age among a large contemporary, multi-racial/ethnic prospective cohort, with consideration of a comprehensive list of covariates.
Although the mechanism underlying the inverse association between maternal diet quality during pregnancy, as measured by the HEI-2010, and neonatal LGA risk remains to be elucidated, higher HEI-2010 has been strongly linked to greater dietary variety,51 greater blood nutrient concentrations (carotenoids and vitamins C and E)51,52 and lower blood cholesterol, glucose, insulin resistance and inflammatory biomarkers,53–56 that in turn have been linked to a more favourable cardiometabolic profile among varied populations, including pregnant women.57–59 Notably, assuming a causal relationship between maternal diet quality as assessed by HEI-2010 and risk of LGA, our findings suggest that 5-point-score substitution of the reversely coded empty calories component with the whole grains component (i.e. lowering 5% of energy from empty calories and increasing whole grains by 0.75 ounces) was associated with 25% decreased risk of LGA. This is consistent with data from a randomized clinical trial that showed a diet rich in whole grains and low in simple sugars, fats and oils appeared to be effective in reducing offspring birthweight.60 Similarly, a randomized crossover trial showed that a high-complex carbohydrate, low-fat diet significantly lowered postprandial free fatty acids among pregnant women, suggesting potential implications for preventing excess fetal growth.61 On the other hand, the substitution of the empty calories component with other adequacy components of the HEI was not associated with a lower risk of having an LGA infant. Although the mechanisms underlying the varied substitutional associations remain to be elucidated, it is plausible that varied recall accuracy and intake distributions of different food groups may partially contribute to the differential associations.
A major strength of this study is the prospective data collection, allowing for examination of the temporal relationship between maternal diet quality in early pregnancy and infant size at birth. The measure of diet quality is based a priori on national recommendations and generalizable and comparable across different studies, as opposed to data-driven methods such as population-specific dietary patterns or indexes.47 Our results are robust against a series of sensitivity analyses restricted to term births and pregnancies free of pregnancy hypertensive complications, respectively. Moreover, our findings of the substitution analysis could be of particular relevance to women seeking healthier alternatives for empty calories during pregnancy, informing potential prevention strategies and dietary guidelines for pregnant women.
Several potential limitations of the study need to be noted. Maternal dietary intakes were self-reported using the FFQ in early pregnancy, with potential recall bias and exposure misclassification. However, the FFQ was validated against three 4-day diet records and demonstrated applicable to analyses on food group and nutrient level.29,30 We did not have data on maternal diet in mid-to-late pregnancy; however, diet quality in early pregnancy may be a harbinger of that in later pregnancy62 and previous studies suggest that dietary patterns change little across pregnancy.62,63 Further, despite the potential over-adjustment bias, we conducted analyses additionally adjusting for GWG, an indicator of intrauterine nutrition environment across gestation; reassuringly, results remained similar and robust. Of note, the substitution analysis results should be interpreted with caution, given the underlying assumption of causality between maternal diet quality in pregnancy and offspring risk of LGA. Nonetheless, given that an effective population-based dietary intervention study would be costly and pragmatically challenging, such data are currently lacking; therefore, carefully conducted observational studies could serve as a reasonable approach to assessing the putative association. Finally, we did not have measures of neonatal adiposity, but provided information on important clinically relevant outcomes such as LGA and SGA.
In conclusion, poorer maternal diet quality as measured by the HEI-2010 in early pregnancy was related to increased offspring BWZ and risk of LGA infants, independent of covariates including maternal obesity. Substitution of empty calories with whole grains may mitigate the risk of excess fetal growth. Our findings may inform potential prevention strategies and dietary guidelines for pregnant women. Future prospective studies with longer follow-up are warranted to determine whether maternal diet quality may impact longer-term childhood growth beyond infant size at birth.
Funding
This work was supported by a research grant to Dr Ferrara from the National Institute of Environmental Health Sciences [grant number 5R01ES019196], a career development training award to Dr Zhu from the National Institutes of Health (NIH) Building Interdisciplinary Research Careers in Women's Health Program [grant number 5K12HD052163], and a research grant to Dr Hedderson from the Health Resources and Services Administration [grant number R40MC21515]. Drs. Ferrara and Hedderson are also supported by a contract award [grant number UG3OD023289] from the NIH Environmental Influences on Child Health Outcomes (ECHO) Program.
Supplementary Material
Acknowledgements
The authors thank the PETALS staff and participants for their valuable contributions.
Authors’ Contributions
Y.Z. and M.M.H. conceptualized the study and drafted the manuscript. Y.Z. analyzed data. S.S. and A.F. contributed to acquisition, interpretation of data analyses and critical revision of the manuscript. F.X. and J.F. contributed to data management and analysis and critical revision of the manuscript. Y.Z. and M.M.H. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest: None declared.
References
- 1. Parlee SD, MacDougald OA.. Maternal nutrition and risk of obesity in offspring: the Trojan horse of developmental plasticity. Biochim Biophys Acta 2014;1842:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Symonds ME, Stephenson T, Gardner DS, Budge H.. Long-term effects of nutritional programming of the embryo and fetus: mechanisms and critical windows. Reprod Fertil Dev 2007;19:53–63. [DOI] [PubMed] [Google Scholar]
- 3. Stephenson J, Heslehurst N, Hall J. et al. Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018;391:1830–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dabelea D, Harrod CS.. Role of developmental overnutrition in pediatric obesity and type 2 diabetes. Nutr Rev 2013;71(Suppl 1):S62–67. [DOI] [PubMed] [Google Scholar]
- 5. Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW.. Associations of seafood and elongated n-3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort. Am J Epidemiol 2004;160:774–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu Y, Olsen SF, Mendola P. et al. Maternal dietary intakes of refined grains during pregnancy and growth through the first 7 y of life among children born to women with gestational diabetes. Am J Clin Nutr 2017;106:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu Y, Olsen SF, Mendola P. et al. Maternal consumption of artificially sweetened beverages during pregnancy, and offspring growth through 7 years of age: a prospective cohort study. Int J Epidemiol 2017;46:1499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu Y, Olsen SF, Mendola P. et al. Growth and obesity through the first 7 y of life in association with levels of maternal glycemia during pregnancy: a prospective cohort study. Am J Clin Nutr 2016;103:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stettler N. Nature and strength of epidemiological evidence for origins of childhood and adulthood obesity in the first year of life. Int J Obes (Lond) 2007;31:1035.. [DOI] [PubMed] [Google Scholar]
- 10. Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA.. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003;111:e221–26. [DOI] [PubMed] [Google Scholar]
- 11. Rogers I. The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. Int J Obes Relat Metab Disord 2003;27:755.. [DOI] [PubMed] [Google Scholar]
- 12. Olsen SF, Grandjean P, Weihe P, Videro T.. Frequency of seafood intake in pregnancy as a determinant of birth weight: evidence for a dose dependent relationship. J Epidemiol Community Health 1993;47:436–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mitchell EA, Robinson E, Clark PM. et al. Maternal nutritional risk factors for small for gestational age babies in a developed country: a case-control study. Arch Dis Child Fetal Neonatal Ed 2004;89:F431–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Eijsden M, Hornstra G, van der Wal MF, Vrijkotte TG, Bonsel GJ.. Maternal n-3, n-6, and trans fatty acid profile early in pregnancy and term birth weight: a prospective cohort study. Am J Clin Nutr 2008;87:887–95. [DOI] [PubMed] [Google Scholar]
- 15. Knudsen VK, Orozova-Bekkevold IM, Mikkelsen TB, Wolff S, Olsen SF.. Major dietary patterns in pregnancy and fetal growth. Eur J Clin Nutr 2008;62:463–70. [DOI] [PubMed] [Google Scholar]
- 16. van den Broek M, Leermakers ET, Jaddoe VW. et al. Maternal dietary patterns during pregnancy and body composition of the child at age 6 y: the Generation R Study. Am J Clin Nutr 2015;102:873–80. [DOI] [PubMed] [Google Scholar]
- 17. Shapiro AL, Kaar JL, Crume TL. et al. Maternal diet quality in pregnancy and neonatal adiposity: the Healthy Start Study. Int J Obes (Lond) 2016;40:1056–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grandy M, Snowden JM, Boone-Heinonen J, Purnell JQ, Thornburg KL, Marshall NE.. Poorer maternal diet quality and increased birth weight. J Maternal-Fetal Neonatal Med 2017;1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW.. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc 2009;109:1004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poon AK, Yeung E, Boghossian N, Albert PS, Zhang C.. Maternal dietary patterns during third trimester in association with birthweight characteristics and early infant growth. Scientifica 2013;2013:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ryan EA. Diagnosing gestational diabetes. Diabetologia 2011;54:480–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hernandez TL, Mande A, Barbour LA.. Nutrition therapy within and beyond gestational diabetes. Diabetes Research and Clinical Practice 2018;145:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu Y, Hedderson MM, Feng J, Mevi AA, Ferrara A.. The pregnancy environment and lifestyle study (PETALS): a population-based longitudinal multi-racial birth cohort. BMC Pregnancy and Childbirth 2017;17:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gordon N, Lin T.. The kaiser permanente Northern California adult member health survey. The Permanente Journal 2016;20:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH.. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA: J Am Med Assoc 2006;296:2105–11. [DOI] [PubMed] [Google Scholar]
- 26. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L.. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124:453–69. [DOI] [PubMed] [Google Scholar]
- 27. Ferrara A, Hedderson MM, Albright CL. et al. A pragmatic cluster randomized clinical trial of diabetes prevention strategies for women with gestational diabetes: design and rationale of the Gestational Diabetes' Effects on Moms (GEM) study. BMC Pregnancy and Childbirth 2014;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrara A, Hedderson MM, Brown SD. et al. The comparative effectiveness of diabetes prevention strategies to reduce postpartum weight retention in women with gestational diabetes mellitus: the Gestational Diabetes’ Effects on Moms (GEM) cluster randomized controlled trial. Dia Care 2016;39:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Block G, Woods M, Potosky A, Clifford C.. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 1990;43:1327–35. [DOI] [PubMed] [Google Scholar]
- 30. Harley K, Eskenazi B, Block G.. The association of time in the US and diet during pregnancy in low-income women of Mexican descent. Paediatr Perinat Epidemiol 2005;19:125–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brown SD, Ehrlich SF, Kubo A. et al. Lifestyle behaviors and ethnic identity among diverse women at high risk for type 2 diabetes. Soc Sci Med 2016;160:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Willett W, Stampfer MJ.. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 33. Guenther PM, Kirkpatrick SI, Reedy J. et al. The Healthy Eating Index-2010 is a valid and reliable measure of diet quality according to the 2010 dietary guidelines for Americans. J Nutr 2014;144:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guenther PM, Casavale KO, Kirkpatrick SI. et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet 2013;113. doi:10.1016/j.jand.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Northam S, Knapp TR.. The reliability and validity of birth certificates. J Obstetr Gynecol Neonatal Nurs 2006;35:3–12. [DOI] [PubMed] [Google Scholar]
- 36. DiGiuseppe DL, Aron DC, Ranbom L, Harper DL, Rosenthal GE.. Reliability of birth certificate data: a multi-hospital comparison to medical records information. Matern Child Hlth J 2002;6:169–79. [DOI] [PubMed] [Google Scholar]
- 37. Hedderson MM, Ferrara A, Sacks DA.. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: association with increased risk of spontaneous preterm birth. Obstet Gynecol 2003;102:850–56. [DOI] [PubMed] [Google Scholar]
- 38. Ehrlich SF, Crites YM, Hedderson MM, Darbinian JA, Ferrara A.. The risk of large for gestational age across increasing categories of pregnancy glycemia. Am J Obstet Gynecol 2011;204:240.e1–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS.. Development and validation of a pregnancy physical activity questionnaire. Med Sci Sports Exerc 2004;36:1750–60. [DOI] [PubMed] [Google Scholar]
- 40. Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007;30(Suppl 2):S141–46. [DOI] [PubMed] [Google Scholar]
- 41. Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM.. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstet Gynecol 2004;103:526–33. [DOI] [PubMed] [Google Scholar]
- 42. Carpenter MW, Coustan DR.. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982;144:768–73. [DOI] [PubMed] [Google Scholar]
- 43.United States Department of Agriculture Center for Nutrition Policy and Promotion. The Healthy Eating Index. Washington, D.C: United States Department of Agriculture, 1995. [Google Scholar]
- 44. Pan A, Sun Q, Bernstein AM. et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med 2012;172:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Willett WC, Howe GR, Kushi LH.. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220S–8S; discussion 9S–31S. [DOI] [PubMed] [Google Scholar]
- 46. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 47. Liese AD, Krebs-Smith SM, Subar AF. et al. The dietary patterns methods project: synthesis of findings across cohorts and relevance to dietary guidance. J Nutr 2015;145:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rodriguez-Bernal CL, Rebagliato M, Iniguez C. et al. Diet quality in early pregnancy and its effects on fetal growth outcomes: the Infancia y Medio Ambiente (Childhood and Environment) Mother and Child Cohort Study in Spain. Am J Clin Nutr 2010;91:1659–66. [DOI] [PubMed] [Google Scholar]
- 49. Ferland S, O'Brien HT.. Maternal dietary intake and pregnancy outcome. J Reprod Med 2003;48:86–94. [PubMed] [Google Scholar]
- 50. Branum A, Kirmeyer SE, Gregory ECW.. Prepregnancy Body Mass Index by Maternal Characteristics and State: Data from the Birth Certificate, 2014. Hyattsville, MD: National Center for Health Statistics; 2016. [PubMed] [Google Scholar]
- 51. Hann CS, Rock CL, King I, Drewnowski A.. Validation of the Healthy Eating Index with use of plasma biomarkers in a clinical sample of women. Am J Clin Nutr 2001;74:479–86. [DOI] [PubMed] [Google Scholar]
- 52. Weinstein SJ, Vogt TM, Gerrior SA.. Healthy Eating Index scores are associated with blood nutrient concentrations in the third National Health and Nutrition Examination Survey. J Am Diet Assoc 2004;104:576–84. [DOI] [PubMed] [Google Scholar]
- 53. Kant AK, Graubard BI.. A comparison of three dietary pattern indexes for predicting biomarkers of diet and disease. J Am Coll Nutr 2005;24:294–303. [DOI] [PubMed] [Google Scholar]
- 54. Gesteiro E, Rodriguez Bernal B, Bastida S, Sanchez-Muniz FJ.. Maternal diets with low Healthy Eating Index or Mediterranean diet adherence scores are associated with high cord-blood insulin levels and insulin resistance markers at birth. Eur J Clin Nutr 2012;66:1008–15. [DOI] [PubMed] [Google Scholar]
- 55. Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS.. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis 2009;203:311–19. [DOI] [PubMed] [Google Scholar]
- 56. Ford ES, Mokdad AH, Liu S.. Healthy Eating Index and C-reactive protein concentration: findings from the National Health and Nutrition Examination Survey III, 1988–1994. Eur J Clin Nutr 2005;59:278–83. [DOI] [PubMed] [Google Scholar]
- 57. Scholl TO, Sowers M, Chen X, Lenders C.. Maternal glucose concentration influences fetal growth, gestation, and pregnancy complications. Am J Epidemiol 2001;154:514–20. [DOI] [PubMed] [Google Scholar]
- 58. Romero R, Gotsch F, Pineles B, Kusanovic JP.. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev 2007;65: S194–202. [DOI] [PubMed] [Google Scholar]
- 59. Sattar N, Greer IA.. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening?. BMJ: British Medical Journal 2002;325:157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Asemi Z, Samimi M, Tabassi Z, Esmaillzadeh A.. The effect of DASH diet on pregnancy outcomes in gestational diabetes: a randomized controlled clinical trial. Eur J Clin Nutr 2014;68:490–95. [DOI] [PubMed] [Google Scholar]
- 61. Hernandez TL, Van Pelt RE, Anderson MA. et al. A higher-complex carbohydrate diet in gestational diabetes mellitus achieves glucose targets and lowers postprandial lipids: a randomized crossover study. Dia Care 2014;37:1254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Kleinman KP, Oken E, Gillman MW.. Changes in dietary intake from the first to the second trimester of pregnancy. Paediatr Perinat Epidemiol 2006;20:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM.. Women's dietary patterns change little from before to during pregnancy. J Nutr 2009;139:1956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.