Abstract
Background
The purpose of this study was to examine if the smoking-related higher breast cancer risk was similar for the five race/ethnicity groups in the Multiethnic Cohort (MEC) study and by oestrogen (ER) and progesterone (PR) receptor status.
Methods
From 1993 to 2013, we followed 67 313 women who were enrolled in the MEC study at 45–75 years of age. We identified breast cancer cases and tumour receptor status via linkage to the Hawaii and California Surveillance, Epidemiology and End Results Program cancer registries through December 2013. We used Cox proportional hazards regression to estimate multivariable-adjusted hazard ratios with 95% confidence intervals (CI).
Results
During a mean follow-up of 16.7 years, we identified 4230 incident, invasive breast cancer cases. Compared with parous never smokers, parous ever smokers who had smoked more than 5 years before their first live childbirth had a higher risk of breast cancer overall of 31% (95% CI: 1.14–1.51). This higher risk was 51% (95% CI: 1.05–2.16) for African Americans, 66% (95% CI: 1.10–2.50) for Native Hawaiians, 42% (95% CI: 1.13–1.78) for Whites, 37% (95% CI: 1.17–1.61) for ER-positive (ER+) tumours and 33% (95% CI: 1.11–1.59) for PR+ tumours. No difference was suggested by racial/ethnic groups (Pheterogeneity = 0.15) or tumour receptor status (Pheterogeneity = 0.60 by ER status and 0.95 by PR status).
Conclusions
We find that the higher breast cancer risk related to smoking is similar across racial/ethnic groups and by ER and PR status, indicating that breast cancer should be considered as a smoking-related cancer.
Keywords: Breast cancer, cohort studies, ethnic differences, ER positive tumours, hormone receptor tumours, Multiethnic Cohort study, non-alcohol drinkers, PR positive tumours, smoking, smoking duration before first childbirth
Key Messages
Smoking is not an established risk factor for breast cancer.
Our main findings suggest that the smoking-related breast cancer risk is similar across racial/ethnic groups and by oestrogen and progesterone receptor status, indicating that breast cancer is a smoking-related cancer.
The results of the present study, together with those from other recent cohort studies, support the notion that women who start smoking as teenagers and continue until they get pregnant years later, have a higher risk of breast cancer.
Public health agencies reviewing the smoking and breast cancer data should reconsider the available evidence and update their conclusions.
Breast cancer prevention messages should address smoking by adolescent girls.
Introduction
Smoking is not an established risk factor for breast cancer, but increasing evidence supports an association especially for women who initiated smoking before first childbirth.1–10 In contrast to the developed world, tobacco consumption is increasing in the developing world and more women are initiating smoking in their teens than in previous generations.11,12 We previously reported that risk of breast cancer in the Multiethnic Cohort (MEC) study13 was directly associated with various measures of active smoking. The magnitude of the association among women who did not drink alcohol was similar to that in the overall study population, indicating that confounding by alcohol did not explain the smoking–breast cancer association.
Differences in risk by race/ethnicity have not been addressed in detail with regard to the smoking and breast cancer association. Most recent cohort studies reporting on this subject included only African Americans,14 Japanese15 or only16–21 or mostly Whites.22–27 Moreover, the 2014 US Surgeon General’s report raised the possibility of differences in the risk associated with smoking by hormone receptor status.4 This topic has been examined in several recent cohort studies,14,18–24,26 but the results have remained inconsistent.
The purpose of this study was to examine if the smoking-related higher breast cancer risk was similar for the five race/ethnicity groups in the MEC and by oestrogen (ER) and progesterone (PR) receptor status.
Methods
Study population
The MEC study consists of more than 215 000 men and women who were aged 45–75 years and living in California and Hawaii at time of cohort entry. It comprises mainly five racial/ethnic populations: African Americans, Japanese Americans, Latinos, Native Hawaiians and Whites. The cohort has been previously described in detail.28,29 Briefly, between 1993 and 1996, participants enrolled in the study by completing a 26-page mailed questionnaire asking detailed information about demographic factors, dietary habits, other lifestyle factors, prior medical conditions and family history of common cancers. We identified potential participants through driver’s license files from the state Department of Motor Vehicles, voter registration lists and Health Care Financing Administration (Medicare) data files. The Institutional Review Boards of the University of Hawaii and the University of Southern California approved the study.
Altogether, 96 137 postmenopausal women returned the questionnaire. Women who did not belong to one of the five targeted racial/ethnic groups (n = 5506), who had a prior breast cancer based on questionnaire reports or information from tumour registry linkages (n = 5455) or who had missing information on alcohol intake (n = 3588) and smoking status (n = 1698) were excluded. As a result, 79 890 participants remained for this analysis.
Data collection
At baseline, participants reported whether they had ever smoked at least 20 packs of cigarettes in their lifetime, the number of years they smoked cigarettes, the average number of cigarettes smoked per day during the period when they smoked, and the number of years since they quit smoking. We computed age at smoking initiation as age at questionnaire completion minus years smoking for current smokers, or as age at questionnaire completion minus the sum of years smoking and years since quitting for former smokers. We also calculated pack-years as number of cigarettes smoked per day, divided by 20 and multiplied by the duration of smoking in years. For parous smokers, we calculated ‘years of smoking before first childbirth’ as age at their first child’s birth minus age at smoking initiation.
The baseline questionnaire asked about years of education, height and current weight for calculating body mass index (BMI, kg/m2), age at and type of menopause, ever use of postmenopausal hormone therapy and alcohol consumption during the past year. We calculated mean alcohol intake in g/day based on the alcohol content of different beverages and usual portion sizes.
We identified invasive incident cancer cases by linkage to the Surveillance, Epidemiology, and End Results Program cancer registries covering Hawaii and California. We classified breast cancer cases according to the organ site code (C50) in the International Classification of Diseases, Tenth Revision and according to oestrogen and progesterone tumour receptor status categories [ER-positive (ER+), ER-negative (ER–), PR+, PR–] based on information from the registries. We identified deaths by linkage to death-certificate files in Hawaii and California and to the National Death Index. Case ascertainment and vital status were complete through December 31, 2013. We calculated person-years from the start of follow-up to the date of invasive breast cancer diagnosis, death or the end of follow-up (December 31, 2013), whichever occurred first.
Statistical analysis
We calculated age-adjusted breast cancer incidence rates per 100 000 person-years, truncated to ages 45–85 years, weighted by the age distribution of the 2000 US standard population.30 We used Cox proportional hazards regression to model time to breast cancer, with age as the underlying time scale. Hazard ratios (HRs) with 95% confidence intervals (CIs) were computed for the associations with different measures of smoking exposure [smoking status at cohort entry (never, former, current, ever); and among ever smokers, age at smoking initiation (<20, 20–24, ≥25 years), smoking duration (≤20, 21–30, ≥31 years), number of cigarettes smoked per day (≤10, 11–20, ≥21) and number of pack-years (≤10, 11–20, ≥21)], with never smokers as the reference group. We included as covariates race/ethnicity (African American, Native Hawaiian, Japanese, Latina and White, adjusted as a strata variable), age at cohort entry (continuous), family history of breast cancer (no, yes,), education (≤12; >12 years), BMI (<25; 25–<30; ≥30 kg/m2), age at menarche (≤12; 13–14; ≥15 years), age at first childbirth (no children; ≤20; 21–30; ≥31 years), number of children for parous women (1; 2–3; ≥4), age at and type of menopause (natural: age <45, 45–<50, 50–<55, ≥55 years; oophorectomy: age <45, 45–<50, ≥50 years; hysterectomy: age <45, 45–<50, ≥50 years), postmenopausal hormone therapy (no current oestrogen use; past oestrogen use with or without progestin; current oestrogen use without progestin; current oestrogen use with past/current progestin) and alcohol consumption (continuous as ethanol g/day). The proportional hazards assumption was tested using Schoenfeld residuals and was found to hold.31,32
We conducted tests for linear trends by including an ordinal exposure variable with equally spaced scores in models and never smokers as the first category. We assessed heterogeneity in the association of breast cancer risk with smoking variables by race/ethnicity by testing the vector of parameters for the pairwise product terms between smoking and race against zero using a Wald test.31 For parous women, we estimated breast cancer risk by smoking initiation in relation to first childbirth (after or <1 year before first childbirth, 1–5 years before, >5 years before), compared with parous never smokers overall and stratified by the five racial/ethnic groups, adjusting for the applicable covariates described above. We repeated these multivariable analyses with three categories of smoking exposure (never, initiation at time of /after first birth, initiation before first birth). We then performed competing risk analysis using cause-specific models for time to receptor status breast cancer outcomes, with censoring at diagnosis for any breast cancer cases with a receptor status other than that being considered.32–34 The receptor status outcomes considered were ER+ and ER–, PR+ and PR–, and a combination of positive and negative hormone receptor statuses as the outcomes. Cases with missing information on both ER and PR status (n = 466) were excluded from these analyses. In order to compare the parameters by tumour receptor status, an augmented data approach as described in Lunn and McNeil35 was implemented that computes simultaneous models for breast cancer of each receptor status type. Heterogeneity by tumour receptor status categories is assessed by a Wald test comparing the interaction between tumour receptor event type and smoking exposure, using robust variance estimates.
The primary analysis used a complete case approach which excluded women with missing data on any of the covariates (n = 12 577), leaving 67 313 women for the multivariable analyses. The analyses were also rerun using multiple imputation models and five iterations, assuming the missing data were missing completely at random, conditional on age and ethnicity.36 The results of the complete case (excluding altogether 28 824 women) and multiple imputation models (excluding altogether 16 247 women) were very similar. We present the complete case analysis in the main tables and figure and in Supplementary Tables 1 and 2, available as Supplementary data at IJE online. The imputation results are available in Supplementary Tables 3–5, available as Supplementary data at IJE online.
We performed the analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
During a mean follow-up of 16.7 years, we identified 4230 incident, invasive breast cancer cases with at least one tumour hormone receptor type ascertained. Table 1 shows that the age-adjusted incidence rates for breast cancer ranged from 403 among Native Hawaiians to 217 per 100 000 person-years (truncated to ages 45–85) among Latinas. African Americans, Native Hawaiians and Whites were more likely to be ever smokers than Japanese Americans and Latinas (Table 1).
Table 1.
Distribution of selected characteristics given as %a and mean (SD)b for postmenopausal women at baseline in 1993–96, by race/ethnicity and breast cancer status in the Multiethnic Cohort study, followed to 2013 (n = 67 313)
| African American (n = 12 776) |
Native Hawaiian (n = 4286) |
Japanese American (n = 19 043) |
Latina (n = 14 371) |
White (n = 16 837) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Non-cases | Cases | Non-cases | Cases | Non-cases | Cases | Non-cases | Cases | Non-cases | |
| No. of participants | 754 | 12 022 | 375 | 3911 | 1306 | 17 737 | 667 | 13 704 | 1128 | 15 709 |
| Age at cohort entry, yearsb | 63.0 (7.4) | 62.8 (7.8) | 58.9 (7.4) | 59.1 (8.0) | 62.6 (7.1) | 63.2 (7.6) | 61.1 (6.7) | 60.7 (6.8) | 61.7 (7.6) | 61.3 (8.0) |
| Person-years of follow-up | 7790 | 207 873 | 3883 | 69 225 | 13 064 | 331 685 | 6848 | 254 293 | 11 007 | 284 427 |
| Follow-up yearsb | 9.3 (5.7) | 16.3 (5.8) | 9.3 (5.5) | 16.7 (5.1) | 9.0 (5.5) | 17.7 (4.1) | 9.3 (5.6) | 17.6 (4.4) | 8.8 (5.4) | 17.1 (4.8) |
| Incidence/100 000c | 247.5 | 402.6 | 325.8 | 216.8 | 288.0 | |||||
| Age at diagnosis, yearsb | 72.9 (8.5) | 68.7 (8.3) | 72.1 (8.6) | 70.8 (8.3) | 71.0 (8.5) | |||||
| Family history of breast cancera | 17.2 | 10.7 | 18.1 | 13.1 | 15.1 | 10.2 | 15.4 | 8.4 | 18.0 | 11.8 |
| Current smokera | 18.0 | 20.0 | 20.5 | 22.5 | 6.6 | 8.4 | 11.5 | 10.4 | 15.3 | 16.6 |
| Ever smokera | 56.2 | 56.3 | 55.7 | 55.8 | 30.9 | 30.5 | 36.4 | 36.2 | 57.4 | 55.7 |
| Age at smoking initiation, yearsb,d | 31.6 (11.2) | 31.5 (11.1) | 26.6 (11.1) | 27.3 (10.3) | 30.3 (9.8) | 30.7 (10.1) | 31.9 (10.6) | 33.1 (11.4) | 27.8 (9.9) | 27.8 (9.6) |
| Smoking duration, yearsb,d | 22.3 (12.6) | 23.4 (12.3) | 23.9 (11.8) | 23.9 (12.1) | 19.7 (12.3) | 21.0 (12.5) | 20.1 (12.3) | 18.7 (12.9) | 23.5 (12.6) | 23.5 (12.8) |
| No. of cigarettes smoked/dayb,d | 10.9 (6.3) | 11.4 (6.6) | 14.8 (7.7) | 14.0 (7.6) | 12.2 (6.5) | 12.3 (6.9) | 10.0 (6.5) | 9.2 (6.2) | 15.3 (8.3) | 15.8 (8.4) |
| Pack-years of smokingb,d | 14.0 (12.5) | 14.8 (12.8) | 19.5 (15.1) | 18.4 (14.9) | 13.9 (12.7) | 14.7 (13.3) | 11.5 (11.5) | 10.1 (11.5) | 20.4 (17.1) | 20.9 (17.3) |
| Smokers who started to smoke before the first childbirtha,d,e | 21.9 | 19.2 | 35.1 | 30.1 | 35.3 | 30.1 | 13.9 | 17.2 | 36.9 | 33.4 |
| Years of smoking before first childbirthb,d,e | 5.7 (3.9) | 5.1 (4.2) | 5.7 (5.0) | 5.1 (4.1) | 5.8 (3.8) | 5.4 (4.2) | 5.4 (5.2) | 5.5 (4.5) | 6.2 (4.3) | 5.5 (4.3) |
| Body mass index (kg/m2)b | 29.0 (5.4) | 28.8 (5.7) | 29.3 (6.2) | 28.2 (6.1) | 24.3 (3.9) | 23.4 (3.7) | 28.1 (5.2) | 27.8 (5.1) | 25.7 (4.9) | 25.7 (5.2) |
| ≥13 years of educationa | 65.8 | 59.0 | 41.9 | 41.1 | 59.6 | 52.9 | 32.8 | 27.7 | 69.1 | 68.2 |
| Age at menarche, yearsb | 13.1 (1.6) | 13.2 (1.7) | 12.8 (1.7) | 12.9 (1.7) | 13.1 (1.6) | 13.3 (1.7) | 13.3 (1.7) | 13.2 (1.7) | 13.1 (1.7) | 13.1 (1.6) |
| Parous womena | 85.8 | 86.6 | 90.7 | 93.1 | 84.9 | 87.1 | 89.2 | 91.7 | 83.1 | 84.2 |
| Number of childrenb,e | 3.2 (1.8) | 3.4 (1.9) | 4.0 (1.8) | 4.0 (1.8) | 2.7 (1.1) | 2.8 (1.2) | 3.9 (1.8) | 4.1 (1.9) | 2.8 (1.4) | 3.0 (1.5) |
| Age at first childbirth, yearsb,e | 21.8 (4.6) | 21.4 (4.6) | 21.6 (4.0) | 21.6 (3.8) | 25.7 (4.2) | 25.2 (4.1) | 22.6 (4.7) | 22.1 (4.5) | 24.0 (4.5) | 23.4 (4.4) |
| Ever postmenopausal hormone therapy usea | 49.7 | 46.0 | 54.9 | 51.7 | 67.0 | 59.2 | 52.8 | 47.7 | 72.3 | 66.5 |
| Age at menopause, yearsb,f | 48.7 (5.4) | 48.3 (5.4) | 48.6 (5.3) | 48.1 (5.4) | 50.2 (4.5) | 49.7 (4.7) | 48.5 (5.4) | 48.0 (5.3) | 49.4 (4.8) | 48.7 (5.0) |
| Menopause typea | ||||||||||
| Natural | 56.1 | 55.5 | 72.0 | 65.9 | 72.7 | 71.3 | 69.3 | 68.7 | 69.6 | 65.7 |
| Oophorectomy | 21.9 | 23.0 | 17.9 | 22.7 | 17.5 | 18.4 | 14.5 | 15.2 | 16.8 | 20.8 |
| Hysterectomy | 22.0 | 21.5 | 10.1 | 11.4 | 9.9 | 10.2 | 16.2 | 16.1 | 13.6 | 13.5 |
| Non-drinkersa | 63.3 | 63.4 | 65.1 | 64.6 | 78.1 | 79.2 | 60.9 | 64.7 | 35.2 | 40.9 |
| Alcohol consumption, g/dayg | ||||||||||
| Mean (SD) | 14.7 (28.1) | 12.1 (27.7) | 11.5 (20.7) | 12.4 (27.1) | 6.6 (12.3) | 6.1 (11.7) | 7.2 (15.2) | 7.1 (17.3) | 17.6 (24.3) | 15.4 (24.2) |
| Median (min, max) | 4.1 (0.4, 208) | 3.3 (0.0, 392) | 3.9 (0.2, 154) | 3.7 (0.0, 403) | 1.6 (0.4, 106) | 1.7 (0.2, 248) | 2.4 (0.4, 186) | 2.2 (0.0, 328) | 9.8 (0.4, 263) | 6.6 (0.2, 428) |
aValues are percents.
bValues are means (standard deviations).
cRates, truncated to ages 45–85, were adjusted to the 2000 US standard population.
dAmong ever smokers.
eAmong parous women.
fNatural menopause.
gAmong drinkers.
Table 2 shows that compared with never smokers, ever smokers had a 9% higher breast cancer risk (95% CI: 1.02–1.16). The results did not suggest risk differences across the five racial/ethnic groups for ever versus never smokers (Pheterogeneity = 0.65). We observed direct associations with breast cancer risk overall, for smoking duration (Ptrend < 0.001), number of cigarettes smoked daily (Ptrend = 0.004) and number of pack-years (Ptrend < 0.001) and an inverse association for age at smoking initiation (Ptrend < 0.001). When we restricted the analyses to parous women, ever smokers who had smoked more than 5 years before their first live childbirth had a higher risk of breast cancer overall of 31% (95% CI: 1.14–1.51) compared with never smokers. This higher risk was 51% (95% CI: 1.05–2.16) for African Americans, 66% (95% CI: 1.10–2.50) for Native Hawaiians and 42% (95% CI: 1.13–1.78) for Whites. Similar results were found for all five racial/ethnic groups (Pheterogeneity = 0.15) (Table 2).
Table 2.
Multivariable adjusted hazard ratios (HR) and 95% confidence intervals (CI) for breast cancer by race/ethnicity according to different measures of smoking exposure, the Multiethnic Cohort study, 1993–2013a
| Smoking exposure | All women (n = 67 313) |
African American (n = 12 776) |
Native Hawaiian (n = 4286) |
Japanese American (n = 19 043) |
Latina (n = 14 371) |
White (n = 16 837) |
P heterogeneity b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | HR (95% CI) | Cases | HR (95% CI) | Cases | HR (95% CI) | Cases | HR (95% CI) | Cases | HR (95% CI) | Cases | HR (95% CI) | ||
| Common reference group | |||||||||||||
| Never smokers | 2303 | 1.00 (ref) | 330 | 1.00 (ref) | 166 | 1.00 (ref) | 903 | 1.00 (ref) | 424 | 1.00 (ref) | 480 | 1.00 (ref) | |
| Smoking status | |||||||||||||
| Former | 1378 | 1.08 (1.01–1.16) | 288 | 1.12 (0.96–1.32) | 132 | 1.08 (0.86–1.37) | 317 | 1.05 (0.92–1.20) | 166 | 0.98 (0.82–1.17) | 475 | 1.13 (0.99–1.28) | |
| Current | 549 | 1.11 (1.00–1.22) | 136 | 1.09 (0.89–1.34) | 77 | 1.05 (0.79–1.39) | 86 | 0.89 (0.71–1.12) | 77 | 1.28 (1.00–1.64) | 173 | 1.20 (1.00–1.43) | 0.45 |
| Ever | 1927 | 1.09 (1.02–1.16) | 424 | 1.11 (0.96–1.29) | 209 | 1.07 (0.87–1.32) | 403 | 1.01 (0.90–1.14) | 243 | 1.06 (0.90–1.24) | 648 | 1.14 (1.01–1.29) | 0.65 |
| Ever smokers | |||||||||||||
| Smoking duration, years | |||||||||||||
| ≤20 | 855 | 1.04 (0.96–1.13) | 182 | 1.11 (0.93–1.34) | 78 | 1.05 (0.80–1.39) | 208 | 1.04 (0.89–1.21) | 119 | 0.91 (0.74–1.12) | 268 | 1.06 (0.91–1.24) | |
| 21–30 | 460 | 1.20 (1.08–1.33) | 105 | 1.22 (0.97–1.52) | 52 | 0.96 (0.70–1.32) | 93 | 1.04 (0.83–1.29) | 64 | 1.68 (1.29–2.20) | 146 | 1.25 (1.03–1.50) | |
| ≥31 | 581 | 1.12 (1.02–1.24) | 127 | 1.07 (0.87–1.32) | 75 | 1.21 (0.91–1.61) | 95 | 0.94 (0.76–1.16) | 53 | 1.09 (0.82–1.45) | 231 | 1.23 (1.05–1.44) | |
| Ptrend | <0.001 | 0.26 | 0.30 | 0.82 | 0.07 | 0.004 | 0.26 | ||||||
| Number of cigarettes | |||||||||||||
| ≤10/day | 961 | 1.07 (0.99–1.15) | 259 | 1.15 (0.98–1.36) | 81 | 0.96 (0.73–1.26) | 211 | 0.99 (0.85–1.16) | 165 | 0.98 (0.82–1.18) | 245 | 1.15 (0.99–1.35) | |
| 11–20/day | 626 | 1.13 (1.03–1.24) | 123 | 1.08 (0.87–1.33) | 79 | 1.14 (0.87–1.50) | 145 | 1.08 (0.91–1.30) | 52 | 1.33 (1.00–1.78) | 227 | 1.13 (0.97–1.33) | |
| ≥21/day | 316 | 1.12 (0.99–1.26) | 33 | 0.98 (0.68–1.40) | 48 | 1.27 (0.91–1.76) | 43 | 0.90 (0.66–1.23) | 19 | 1.29 (0.81–2.05) | 173 | 1.17 (0.98–1.40) | |
| Ptrend | 0.004 | 0.53 | 0.13 | 0.89 | 0.11 | 0.045 | 0.63 | ||||||
| Pack-years | |||||||||||||
| ≤10 | 748 | 1.05 (0.96–1.14) | 178 | 1.16 (0.97–1.40) | 56 | 0.91 (0.67–1.24) | 169 | 0.98 (0.83–1.16) | 127 | 0.93 (0.76–1.13) | 218 | 1.14 (0.97–1.34) | |
| 11–20 | 602 | 1.11 (1.01–1.21) | 135 | 1.02 (0.83–1.25) | 78 | 1.14 (0.86–1.50) | 141 | 1.05 (0.87–1.25) | 68 | 1.31 (1.01–1.69) | 180 | 1.12 (0.95–1.34) | |
| ≥21 | 531 | 1.19 (1.08–1.32) | 95 | 1.21 (0.96–1.53) | 70 | 1.20 (0.90–1.60) | 84 | 1.01 (0.80–1.27) | 38 | 1.50 (1.08–2.10) | 244 | 1.21 (1.03–1.42) | |
| Ptrend | <0.001 | 0.19 | 0.16 | 0.77 | 0.01 | 0.02 | 0.45 | ||||||
| Age at smoking initiation, years | |||||||||||||
| ≥25 | 1150 | 1.05 (0.98–1.13) | 278 | 1.10 (0.93–1.29) | 95 | 0.98 (0.76–1.26) | 262 | 1.00 (0.87–1.15) | 163 | 1.01 (0.84–1.22) | 352 | 1.09 (0.95–1.25) | |
| 20–24 | 398 | 1.19 (1.07–1.33) | 75 | 1.18 (0.91–1.52) | 49 | 1.15 (0.83–1.60) | 82 | 1.09 (0.87–1.38) | 47 | 1.50 (1.11–2.04) | 145 | 1.17 (0.97–1.41) | |
| <20 | 340 | 1.20 (1.06–1.35) | 59 | 1.21 (0.91–1.61) | 61 | 1.26 (0.93–1.72) | 50 | 0.95 (0.71–1.27) | 24 | 0.98 (0.65–1.49) | 146 | 1.33 (1.10–1.61) | |
| Ptrend | <0.001 | 0.09 | 0.13 | 0.90 | 0.22 | 0.003 | 0.55 | ||||||
| Smoking initiation in relation to first childbirth for parous women | |||||||||||||
| Neverc | 2006 | 1.00 (ref) | 303 | 1.00 (ref) | 151 | 1.00 (ref) | 775 | 1.00 (ref) | 385 | 1.00 (ref) | 392 | 1.00 (ref) | |
| During/after | 1141 | 1.04 (0.96–1.12) | 267 | 0.99 (0.84–1.17) | 128 | 1.03 (0.81–1.31) | 217 | 0.94 (0.81–1.10) | 178 | 1.08 (0.90–1.30) | 351 | 1.12 (0.97–1.30) | |
| ≤5 years before | 203 | 1.03 (0.89–1.19) | 30 | 0.97 (0.66–1.42) | 26 | 0.99 (0.65–1.51) | 50 | 0.98 (0.73–1.31) | 13 | 0.80 (0.46–1.39) | 84 | 1.16 (0.91–1.47) | |
| >5 years before | 242 | 1.31 (1.14–1.51) | 37 | 1.51 (1.05–2.16) | 31 | 1.66 (1.10–2.50) | 59 | 1.22 (0.93–1.60) | 10 | 0.63 (0.33–1.19) | 105 | 1.42 (1.13–1.78) | |
| Ptrend | 0.002 | 0.18 | 0.08 | 0.52 | 0.45 | 0.002 | 0.15 | ||||||
aAdjusted for age at cohort entry, race/ethnicity where applicable, body mass index, family history of breast cancer, age at first birth, number of children, age at menarche, age at and type of menopause, hormone replacement therapy, alcohol intake and education.
b P for heterogeneity across race/ethnicity.
cParous never smokers as reference group.
As shown in Supplementary Table 1, available as Supplementary data at IJE online, the distribution of tumours by receptor status was similar for ever smokers compared with all cases and by racial/ethnic group. Native Hawaiians were more likely to be diagnosed with ER+ and PR+ tumours, and less likely to be diagnosed with ER– and PR– tumours. The opposite was true for African Americans (Supplementary Table 1, available as Supplementary data at IJE online).
Table 3 shows that compared with never smokers, ever smokers had an 8 or 9% higher breast cancer risk for all four tumour subtypes, with corresponding CIs all including the null value. We observed positive trends for higher breast cancer risk with duration of smoking ER+ (Ptrend = 0.01) and PR + (Ptrend = 0.02) tumours, for number of cigarettes per day for PR + (Ptrend = 0.04) tumours, and for pack-years for ER+ (Ptrend = 0.013) and PR+ (Ptrend = 0.01) tumours. Similarly, we found an inverse association for age at smoking initiation and both ER+ (Ptrend < 0.001) and PR+ (Ptrend < 0.001) tumours.
Table 3.
Multivariable adjusted hazard ratios (HR) and 95% confidence intervals (CI) for ER+, ER‒, PR+, PR‒ breast cancer according to different measures of smoking exposures, the Multiethnic Cohort study, 1993–2013a
| Smoking exposure | ER-positive (n = 3095) |
ER-negative (n = 659) |
P heterogeneity b | PR-positive (n = 2502) |
PR-negative (n = 1063) |
P heterogeneity b | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | HR (95% CI) | Cases | HR (95% CI) | Cases | HR (95% CI) | Cases | HR (95% CI) | |||
| Common reference group | ||||||||||
| Never smokers | 1695 | 1.00 (ref) | 358 | 1.00 (ref) | 1366 | 1.00 (ref) | 581 | 1.00 (ref) | ||
| Smoking status | ||||||||||
| Former | 1020 | 1.09 (1.00–1.18) | 206 | 1.06 (0.89–1.27) | 838 | 1.11 (1.02–1.21) | 329 | 1.04 (0.91–1.20) | ||
| Current | 380 | 1.06 (0.94–1.19) | 95 | 1.13 (0.89–1.43) | 0.65 | 298 | 1.03 (0.90–1.17) | 153 | 1.19 (0.99–1.43) | 0.42 |
| Ever | 1400 | 1.08 (1.00–1.16) | 301 | 1.08 (0.92–1.27) | 0.97 | 1136 | 1.09 (1.00–1.18) | 482 | 1.08 (0.96–1.23) | 0.86 |
| Ever smokers | ||||||||||
| Smoking duration, years | ||||||||||
| ≤20 | 627 | 1.04 (0.95–1.14) | 130 | 1.01 (0.82–1.24) | 509 | 1.05 (0.94–1.16) | 212 | 1.03 (0.87–1.21) | ||
| 21–30 | 337 | 1.19 (1.06–1.35) | 77 | 1.27 (0.99–1.64) | 283 | 1.24 (1.09–1.41) | 113 | 1.18 (0.96–1.45) | ||
| ≥31 | 415 | 1.10 (0.98–1.23) | 88 | 1.09 (0.86–1.39) | 328 | 1.08 (0.95–1.22) | 147 | 1.14 (0.94–1.37) | ||
| Ptrend | 0.01 | 0.20 | 0.78 | 0.02 | 0.09 | 0.84 | ||||
| Number of cigarettes | ||||||||||
| ≤10/day | 712 | 1.10 (1.00–1.20) | 144 | 0.99 (0.82–1.21) | 568 | 1.10 (0.99–1.21) | 238 | 1.04 (0.89–1.21) | ||
| 11–20/day | 437 | 1.07 (0.96–1.19) | 105 | 1.23 (0.98–1.54) | 353 | 1.07 (0.95–1.20) | 169 | 1.22 (1.02–1.45) | ||
| ≥21/day | 237 | 1.10 (0.95–1.26) | 46 | 1.11 (0.81–1.53) | 204 | 1.15 (0.99–1.34) | 66 | 0.96 (0.74–1.25) | ||
| Ptrend | 0.07 | 0.16 | 0.68 | 0.04 | 0.28 | 0.69 | ||||
| Pack–years | ||||||||||
| ≤10 | 553 | 1.06 (0.96–1.17) | 109 | 0.96 (0.78–1.20) | 439 | 1.05 (0.94–1.17) | 188 | 1.04 (0.88–1.23) | ||
| 11–20 | 447 | 1.12 (1.00–1.24) | 98 | 1.13 (0.90–1.42) | 371 | 1.15 (1.02–1.29) | 149 | 1.08 (0.90–1.30) | ||
| ≥21 | 373 | 1.12 (1.00–1.26) | 82 | 1.23 (0.96–1.58) | 304 | 1.13 (0.99–1.28) | 129 | 1.18 (0.97–1.44) | ||
| Ptrend | 0.01 | 0.09 | 0.62 | 0.01 | 0.10 | 0.95 | ||||
| Age at smoking initiation, years | ||||||||||
| ≥25 | 806 | 1.02 (0.93–1.11) | 191 | 1.12 (0.93–1.34) | 647 | 1.02 (0.93–1.12) | 298 | 1.08 (0.94–1.25) | ||
| 20–24 | 307 | 1.23 (1.09–1.40) | 52 | 0.98 (0.73–1.32) | 258 | 1.27 (1.11–1.46) | 81 | 0.97 (0.76–1.23) | ||
| <20 | 261 | 1.23 (1.07–1.41) | 51 | 1.14 (0.83–1.54) | 211 | 1.21 (1.04–1.41) | 90 | 1.27 (1.01–1.61) | ||
| Ptrend | <0.001 | 0.42 | 0.29 | <0.001 | 0.11 | 0.49 | ||||
| Smoking initiation in relation to first childbirth for parous women | ||||||||||
| Neverc | 1459 | 1.00 (ref) | 324 | 1.00 (ref) | 1172 | 1.00 (ref) | 517 | 1.00 (ref) | ||
| During/after | 810 | 1.03 (0.94–1.12) | 182 | 0.99 (0.82–1.20) | 658 | 1.04 (0.94–1.15) | 283 | 0.99 (0.85–1.15) | ||
| ≤5 years before | 155 | 1.05 (0.89–1.24) | 32 | 1.01 (0.70–1.47) | 133 | 1.10 (0.91–1.32) | 45 | 0.90 (0.66–1.23) | ||
| >5 years before | 191 | 1.37 (1.17–1.61) | 39 | 1.44 (1.02–2.04) | 149 | 1.33 (1.11–1.59) | 73 | 1.60 (1.23–2.08) | ||
| Ptrend | 0.001 | 0.15 | 0.60 | 0.004 | 0.03 | 0.95 | ||||
aAfter excluding 466 (11.0 %) cases with missing data on both ER and PR status. Adjusted for age at cohort entry, race/ethnicity, body mass index, family history of breast cancer, age at first birth, number of children, age at menarche, age at and type of menopause, hormone replacement therapy, alcohol intake and education.
b P for heterogeneity between receptor status in a competing risk model.
cParous never smokers as reference group.
When we restricted the analyses to parous women, women who initiated smoking >5 years before their first childbirth had a higher risk for all four hormone receptor categories: for ER+ tumours 37% (95% CI: 1.17, 1.61), for ER– 44% (95% CI: 1.02–2.04, Pheterogeneity = 0.60), for PR+ 33 % (95% CI: 1.11–1.59) and for PR– 60% (95% CI: 1.23–2.08, Pheterogeneity = 0.95) (Table 3).
Supplementary Table 2, available as Supplementary data at IJE online, shows that when we stratified according to race/ethnicity and hormone receptor status, Whites who had smoked >5 years before their first birth, had a higher risk of similar magnitude for ER+ 51% (95% CI: 1.18–1.94) and PR+ 52% (95% CI: 1.15–2.01) (Supplementary Table 2, available as Supplementary data at IJE online). The results did not suggest differences in the smoking and breast cancer risk associations across the five race/ethnic subgroups (Pheterogeneity = 0.27 for ER+, 0.32 for PR+, 0.33 for ER+/PR+ and 0.09 for ER+/PR– tumours) for smoking initiation before first childbirth among parous ever smokers.
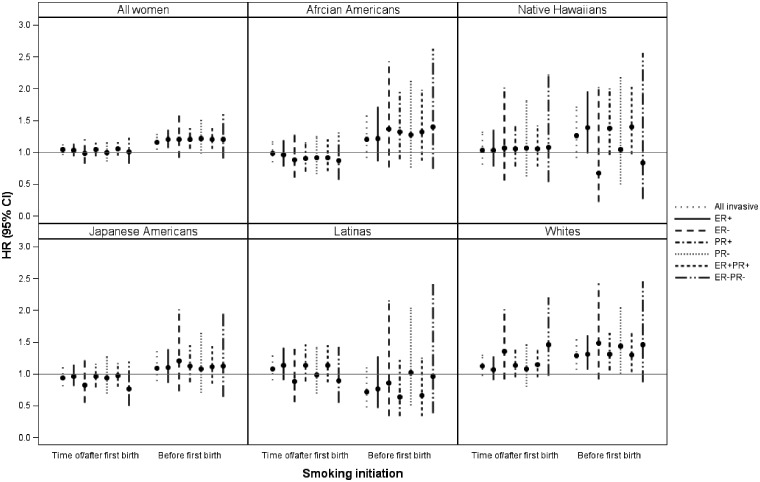
Figure 1 displays the association for ever compared with never parous smokers by two categories of smoking initiation (after or at the time of, and before first childbirth), for all invasive cases and according to six (ER+, ER–, PR+, PR–, ER+/PR+, ER–/PR–) hormone receptor tumour categories overall and stratified by race/ethnic groups. The figure shows that for those who started before first live birth the association with breast cancer risk shows similar patterns for both positive and negative hormone receptor tumours overall and when stratified by race/ethnicity (Figure 1).
Figure 1.
Multivariable adjusted hazard ratios (HR) and 95% confidence intervals (CI) for breast cancer among parous women according to timing of smoking initiation in relation to first childbirth (never, at time of/after and before) by tumour receptor status and race/ethnicity, the Multiethnic Cohort study, 1993–2013a,b,c. aAdjusted for race/ethnicity where applicable, age at cohort entry, body mass index, family history of breast cancer, age at first childbirth, number of children, age at menarche, age at and type of menopause, postmenopausal hormone therapy, alcohol intake, and education. bParous women (n = 58 119) and 5 racial/ethnic groups for all invasive breast cancer cases (n = 3 592), with at least one tumour receptor status. cParous never smokers as reference group.
Discussion
In this prospective study with three additional years of incident breast cancer cases, we confirm our previous13 findings showing that various measures of smoking exposure, i.e. age at smoking initiation, smoking duration, number of cigarettes/day, pack-years and smoking before first childbirth, are associated with an elevated breast cancer risk for all five racial/ethnic groups. Among parous women, the magnitude of the higher breast cancer risk for those who initiated smoking before first birth was very consistent across racial/ethnic groups, except for Latinas for whom no association was observed. Furthermore, we show that these associations seem to be of similar magnitude by ER and PR status.
Past cohort studies that included only Whites all found a positive association with either active,17,20,21 or active and passive16,18,19 smoking and breast cancer risk. A past study in African Americans14 found a higher breast cancer risk for both active and passive smoking, while a study in Japanese15 reported a higher risk for passive, but not for active smoking. Also, the Sister cohort study conducted in the USA and Puerto Rico reported a higher risk for passive, but not active smoking.27
In the MEC, four out of five tumours were hormone receptor positive, and the associations with smoking for this type of tumour were more consistent than for those with hormone receptor negative tumours, possibly because of the smaller number of cases for the latter. In European Prospective Investigation into Cancer and Nutrition Cohort,18 we found the strongest association with smoking for ER+/PR– breast tumours, as was reported in the USA24 and in Denmark.20 In all of these three cohorts, the vast majority of breast cancer cases were also either ER or PR positive tumours.18,20,24 In the present study, we used the same categories of smoking exposure as in our recent report from the Norwegian Woman and Cancer study.19 In that study, we found associations between smoking before first birth, and a higher breast cancer risk for both ER and PR positive and negative tumours. Also, a Dutch study reported similar associations for the smoking and breast cancer associations for the different hormone receptor subtypes.21
The two US studies,14,23 as well as the previously mentioned pooled analysis,26 reported a smoking-related higher breast cancer risk with ER+, but not with ER–, tumours. In all three studies,14,23,26 >80% of the tumours were ER+, like in the present study. The pooled analysis, including data from 14 cohort studies, had over 36 000 invasive breast cancer cases, of which 5000 were ER–. Such a sample size would have been sufficient to detect a modest higher risk with smoking in ER-tumours. We may have lacked power to detect a difference in association by ER status.
Our study has several major strengths. It focuses on the smoking-related risk of breast cancer in a multi-ethnic population, in which close to 90% of tumours were classified according to ER and PR status. In addition, all women were postmenopausal, the majority was non-drinkers of alcohol and we were able to adjust for most established breast cancer risk factors.
The main limitation of this study is that despite more than 4000 incident postmenopausal breast cancer cases, the numbers of cases were relatively small for important subset analyses. The low proportion of ever smokers among Latinas and Japanese Americans, the late age of smoking initiation for African Americans, Japanese Americans and Latinas, and the low proportion of women who started to smoke before their first childbirth, particularly among African Americans and Latinas, reduced the power to examine these associations in more detail. Nevertheless, our study also displays strong positive associations for several of these subgroup analyses.
In a report from the Norwegian Women and Cancer Study, with a similar follow-up time as in the present study, we found that one in three deaths among middle-aged Norwegian women was smoking related.37 Smokers in the present study may have died from different smoking-related causes, before they were diagnosed with breast cancer. The reduction in life expectancy associated with smoking may conceal or obscure the association between the different measures of smoking exposure and breast cancer risk.
The association between active smoking and breast cancer risk became stronger when women exposed to passive smoking were excluded from the reference group in six cohort studies.14–16,18,19,27 Thus, our risk estimates may have been attenuated since women exposed to passive smoking could not be excluded from our reference group due to the lack of information on this potential risk factor. Our main findings suggest that the higher breast cancer risk related to smoking is similar across racial/ethnic groups and for oestrogen and progesterone receptor status, indicating that breast cancer is a smoking-related cancer. The previously cited expert reports1–4 have described the biological mechanisms by which smoking may be a cause of breast cancer. All four conclude that these mechanisms provide plausibility to the causal nature of a smoking–breast cancer association.1–4 The results of the present study, together with those from other recent cohort studies, support the notion that women who start smoking as teenagers and continue until they get pregnant years later, are at a higher risk of breast cancer. Public health agencies reviewing the smoking and breast cancer data should reconsider the available evidence and update their conclusions. Breast cancer prevention messages should address smoking by adolescent and young women.
Funding
This work was supported in part by US Public Health Service, National Cancer Institute grant U01 CA164973. The tumour registries were supported by National Cancer Institute contracts N01 PC 35137 and N01 PC 35139.
Supplementary Material
Acknowledgements
This work was mainly carried out while Professor Gram was a Visiting Scholar in the Population Sciences in the Pacific Epidemiology Program, University of Hawaii Cancer Center, Honolulu, Hawaii.
Conflict of interest: None declared.
References
- 1.California Environmental Protection Agency’s (CalEPA). Proposed Identification of Environmental Tobacco Smoke as a Toxic Air Contaminant. Part B: Health Effects. Sacramento, CA: California Environmental Agency, Office of Environmental Health Hazard Assessment. 2005. [Google Scholar]
- 2. Johnson KC, Miller AB, Collishaw NE, Palmer JR, Hammond SK, Salmon AG. Active smoking and secondhand smoke increase breast cancer risk: the report of the Canadian Expert Panel on Tobacco Smoke and Breast Cancer Risk (2009). Tob Control 2011;20:e2. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. International Agency for Research on Cancer (IARC) Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 100 E.: A Review of Human Carcinogens: Personal Habits and Indoor Combustions. Lyon, France: International Agency for Research on Cancer, 2012. [PMC free article] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, 2014. [Google Scholar]
- 5. Johnson KC, Glantz SA. Evidence secondhand smoke causes breast cancer in 2005 stronger than for lung cancer in 1986. Prev Med 2008;46:492–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iwasaki M, Tsugane S. Risk factors for breast cancer: epidemiological evidence from Japanese studies. Cancer Sci 2011;102:1607–14. [DOI] [PubMed] [Google Scholar]
- 7. Glantz SA, Johnson KC. The surgeon general report on smoking and health 50 years later: breast cancer and the cost of increasing caution. Cancer Epidemiol Biomarkers Prev 2014;23:37–46. [DOI] [PubMed] [Google Scholar]
- 8. Macacu A, Autier P, Boniol M, Boyle P. Active and passive smoking and risk of breast cancer: a meta-analysis. Breast Cancer Res Treat 2015;154:213–24. [DOI] [PubMed] [Google Scholar]
- 9. Kispert S, McHowat J. Recent insights into cigarette smoking as a lifestyle risk factor for breast cancer. Breast Cancer (Dove Med Press) 2017;9:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gray JM, Rasanayagam S, Engel C, Rizzo J. State of the evidence 2017: an update on the connection between breast cancer and the environment. Environ Health 2017;16:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giovino GA, Mirza SA, Samet JM et al. . Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet 2012;380:668–79. [DOI] [PubMed] [Google Scholar]
- 12. Eriksen MP, Mackay J, Schluger N, Islami F, Drope J. The Tobacco Atlas. 5th edn Atlanta, GA: American Cancer Society, 2015. [Google Scholar]
- 13. Gram IT, Park SY, Kolonel LN et al. . Smoking and risk of breast cancer in a racially/ethnically diverse population of mainly women who do not drink alcohol: the MEC study. Am J Epidemiol 2015;182:917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenberg L, Boggs DA, Bethea TN, Wise LA, Adams-Campbell LL, Palmer JR. A prospective study of smoking and breast cancer risk among African-American women. Cancer Causes Control 2013;24:2207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wada K, Kawachi T, Hori A et al. . Husband’s smoking status and breast cancer risk in Japan: from the Takayama study. Cancer Sci 2015;106:455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gram IT, Braaten T, Terry PD et al. . Breast cancer risk among women who start smoking as teenagers. Cancer Epidemiol Biomarkers Prev 2005;14:61–66. [PubMed] [Google Scholar]
- 17. Bjerkaas E, Parajuli R, Weiderpass E et al. . Smoking duration before first childbirth: an emerging risk factor for breast cancer? Results from 302 865 Norwegian women. Cancer Causes Control 2013;24:1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dossus L, Boutron-Ruault MC, Kaaks R et al. . Active and passive cigarette smoking and breast cancer risk: results from the EPIC cohort. Int J Cancer 2014;134:1871–88. [DOI] [PubMed] [Google Scholar]
- 19. Gram IT, Little MA, Lund E, Braaten T. The fraction of breast cancer attributable to smoking: the Norwegian women and cancer study 1991–2012. Br J Cancer 2016;115:616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Andersen ZJ, Jorgensen JT, Gron R, Brauner EV, Lynge E. Active smoking and risk of breast cancer in a Danish nurse cohort study. BMC Cancer 2017;17:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van den Brandt PA. A possible dual effect of cigarette smoking on the risk of postmenopausal breast cancer. Eur J Epidemiol 2017;32:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xue F, Willett WC, Rosner BA, Hankinson SE, Michels KB. Cigarette smoking and the incidence of breast cancer. Arch Intern Med 2011;171:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun MJ. Active smoking and breast cancer risk: original cohort data and meta-analysis. J Natl Cancer Inst 2013;105:515–25. [DOI] [PubMed] [Google Scholar]
- 24. Nyante SJ, Gierach GL, Dallal CM et al. . Cigarette smoking and postmenopausal breast cancer risk in a prospective cohort. Br J Cancer 2014;110:2339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Catsburg C, Miller AB, Rohan TE. Active cigarette smoking and risk of breast cancer. Int J Cancer 2015;136:2204–09. [DOI] [PubMed] [Google Scholar]
- 26. Gaudet MM, Carter BD, Brinton LA et al. . Pooled analysis of active cigarette smoking and invasive breast cancer risk in 14 cohort studies. Int J Epidemiol 2016;46:881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. White AJ, D’Aloisio AA, Nichols HB, DeRoo LA, Sandler DP. Breast cancer and exposure to tobacco smoke during potential windows of susceptibility. Cancer Causes Control 2017;28:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kolonel LN, Henderson BE, Hankin JH et al. . A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151:346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pike MC, Kolonel LN, Henderson BE et al. . Breast cancer in a multiethnic cohort in Hawaii and Los Angeles: risk factor-adjusted incidence in Japanese equals and in Hawaiians exceeds that in whites. Cancer Epidemiol Biomarkers Prev 2002;11:795–800. [PubMed] [Google Scholar]
- 30. Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2010 Stat Notes 2001;20:1–10. [PubMed] [Google Scholar]
- 31. Vittinghoff E, Glidden DV, Shiboski SC, McCullough CE. Regression Methods in Biostatistics: Linear, Logistic, Survival, and Repeated Measures Models. New York: Springer Science + Business Media, LLC., 2012. [Google Scholar]
- 32. Therneau TM, Grambsch P. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer-Verlag, Inc., 2001. [Google Scholar]
- 33. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009;170:244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics 1995;51:524–32. [PubMed] [Google Scholar]
- 36. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, Inc., 1987. [Google Scholar]
- 37. Gram IT, Sandin S, Braaten T, Lund E, Weiderpass E. The hazards of death by smoking in middle-aged women. Eur J Epidemiol 2013;28:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.