Abstract
Objective
Obstructive sleep apnea syndrome (OSAS) is characterized by nocturnal intermittent hypoxemia and can increase the risk of Parkinson's disease. This study aimed to investigate the association between plasma α‐synuclein levels and hypoxia in the patients with OSAS.
Methods
We recruited 42 OSAS patients and 46 controls with simple snoring matched for age and gender. OSAS was diagnosed on the basis of the clinical symptoms as well as the nighttime polysomnography. Plasma total α‐synuclein and phosphorylated α‐synuclein levels were measured by ELISA kits.
Results
The OSAS patients had significant higher levels of plasma total α‐synuclein and phosphorylated α‐synuclein levels. Both of the above indexes were positively correlated with the apnea–hypopnea index and the oxygen desaturation index, while they were negatively correlated with the mean and lowest oxyhemoglobin saturations.
Interpretation
This study suggests that chronic intermittent hypoxia can increase the α‐synuclein levels, which may contribute to the pathogenesis of Parkinson's disease.
Introduction
Parkinson's disease (PD) is a common disease in the middle‐aged and elderly population, mainly affecting the motor system, as well as sleep problems and neuropsychiatric symptoms.1 The presence of Lewy bodies, which were accumulated by the α‐synuclein protein, is the major pathological process in PD.2 Thus α‐synuclein is considered to play a crucial role in the etiology and pathogenesis of PD. Many risk factors link to the etiology of PD.3 Obstructive sleep apnea syndrome (OSAS), a prevalent disease characterized by recurrent episodes of upper airway obstruction, can lead to intermittent hypoxemia during sleep.4 Recent researches found that OSAS was a risk factor for PD onset, and hypoxia may have contributed to it.5, 6 However, the detail mechanism remains to be further investigated. Previous studies both in vitro and in vivo reveal that hypoxia is able to induce overexpression of ɑ‐synuclein and its oligomer formation.7, 8, 9 So our study is aimed to investigate the association between α‐synuclein levels and hypoxia in OSAS patients.
Materials and Methods
Study participants
From September to December of 2014, we recruited 42 subjects diagnosed with OSAS from the Daping hospital. Among them, eight patients suffered with mild OSAS, 16 with moderate, and 18 with severe. Forty‐six subjects with simple snoring were recruited as the controls matched for age and gender. The exclusion criteria included: (1) a family history of PD; (2) other central nervous system disorders; (3) severe hepatic, renal, pulmonary, cardiac diseases, or neoplastic disorders; (4) long‐term smoker or drinker. This study was approved by the Institutional Review Board of Daping Hospital (Chongqing, China).
The diagnosis of OSAS
OSAS was diagnosed on the basis of the clinical symptoms and the polysomnography (PSG) recordings as described in a previous study.10 The apnea–hypopnea index (AHI) and oxygen desaturation index (ODI) could be measured based on the PSG recordings. Hypopnea was defined according to the American Academy of Sleep Medicine scoring manual11, 12: (1) The peak signal excursions decreased by more than 30% from pre‐event baseline using nasal pressure; (2) The duration of the decrease ≥ 30% in signal excursion is longer than 10 seconds; (3) There exists a ≥ 4% oxygen desaturation from baseline. Demographic data, including age, gender, weight, height, and educational levels, were gathered on admission. The medical histories of all subjects were also collected as described in our previous study.13
Blood sampling
To avoid the potential circadian rhythm effects, fasting blood was collected between 06:00 and 07:00. After drawn, the blood samples were immediately centrifuged and then reserved at −80°C. Before the acquisition of the blood samples, informed consent from each participant was obtained.
Measurements of plasma α‐synuclein levels
Plasma total α‐synuclein levels and phosphorylated α‐synuclein levels were measured by human total alpha‐synuclein ELISA Kit (Invitrogen) and phosphorylated alpha‐synuclein ELISA Kit (LSBio). All measurements were carried out in accordance with the manufacturers’ instructions. The standards and samples had received reduplicated measurements and analyses.
Statistical analysis
Statistical comparisons of demographic characteristics and plasma α‐synuclein levels between OSAS patients and controls were tested by two‐tailed independent‐sample t‐tests, Mann–Whitney U test, or chi‐squared test as appropriate. The correlations of plasma α‐synuclein levels and AHI, ODI, MSaO2 as well as LSaO2 values were analyzed by partial correlation analyses. Data were shown as the mean ± standard deviation (SD). Statistically significant was defined by two‐sided P‐value less than 0.05. The data were analyzed with GraphPad Prism 6 (GraphPad Software, USA).
Results
Characteristics of the subjects
This study included 42 OSAS patients and 46 controls with simple snoring matched for age and gender (Table 1). No significant difference in body mass index (BMI) (P = 0.126), total sleep time (P = 0.696), and sleep efficiency (P = 0.864) was seen between the OSAS patients and controls. Compared with the controls, the OSAS group showed remarkably higher AHI (P < 0.001), ODI (P < 0.001), and arousal index (ArI) (P < 0.001). Also the OSAS group had dramatically lower mean SaO2 (P < 0.001) and lowest SaO2 (P < 0.001) than controls. And the significant differences between these two groups were also found in the proportions of stages 1 and 2 of sleep (P = 0.005) and rapid eye movement (REM) sleep (P < 0.001), but not in the stages 3 sleep (P = 0.197).
Table 1.
Characteristics of the subjects
| Controls (N = 46) | OSAS (N = 42) | P value | |
|---|---|---|---|
| Age, years | 43.46 (9.70) | 45.02 (9.80) | 0.453 |
| Gender, male, n (%) | 33 (71.74) | 31 (73.81) | 0.862 |
| BMI, kg/m2 | 26.10 (2.09) | 26.96 (2.57) | 0.126 |
| Total sleep time, min | 403.41 (52.15) | 399.33 (41.58) | 0.696 |
| Sleep efficiency, % | 84.84 (7.20) | 84.51 (10.13) | 0.864 |
| AHI, events/h | 2.22 (1.35) | 33.96 (21.56) | <0.001 |
| ArI, events/h | 8.70 (4.25) | 33.03 (17.02) | <0.001 |
| ODI, events/h | 2.14 (1.21) | 28.07 (15.17) | <0.001 |
| Mean SaO2, % | 95.65 (1.18) | 90.64 (2.69) | <0.001 |
| Lowest SaO2, % | 91.30 (1.66) | 80.24 (6.38) | <0.001 |
| Sleep stages 1 and 2, % | 56.26 (10.24) | 62.41 (15.76) | 0.005 |
| Sleep stages 3, % | 25.73 (10.32) | 25.50 (17.99) | 0.197 |
| REM, % | 17.99 (5.67) | 12.09 (6.83) | <0.001 |
Statistical comparisons were tested using two‐tailed independent‐sample t‐tests, Mann–Whitney U test, or chi‐squared test as appropriate. BMI, body mass index; AHI, apnea–hypopnea index; ArI, arousal index; ODI, oxygen desaturation index; SaO2, oxyhemoglobin saturation; REM, rapid eye movement.
Plasma α‐synuclein levels in the participants
As the Figure 1 showed, OSAS patients had significant higher levels of plasma total α‐synuclein (37.68 ± 18.35 ng/mL vs. 21.08 ± 16.21 ng/mL, P < 0.001) and phosphorylated α‐synuclein (26.87 ± 15.42 ng/mL vs. 14.61 ± 12.98 ng/mL, P < 0.001) levels than the controls.
Figure 1.
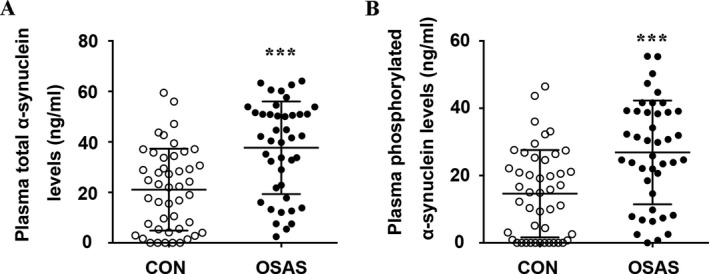
Comparison of the plasma total α‐synuclein (A) and phosphorylated α‐synuclein levels (B) between the controls and patients with obstructive sleep apnea syndrome (OSAS). *** P < 0.001.
Correlations of plasma α‐synuclein levels with AHI
We used the partial correlation analyses to analyze the relationships between plasma α‐synuclein levels and AHI by adjusting for age, gender, and BMI. In all participants, both of total α‐synuclein and phosphorylated α‐synuclein levels in plasma were positively correlated with AHI (Table 2). In the OSAS patients, the plasma levels of total α‐synuclein (γ = 0.550, P < 0.001) and plasma phosphorylated α‐synuclein (γ = 0.613, P < 0.001) levels were also positively correlated with AHI (Fig. 2).
Table 2.
The partial correlation analyses of the plasma α‐synuclein levels with AHI, ODI, mean SaO2, and lowest SaO2 in all subjects
| Total α‐synuclein | Phosphorylated α‐synuclein | |||
|---|---|---|---|---|
| γ | P | γ | P | |
| AHI, events/h | 0.609 | <0.001 | 0.610 | <0.001 |
| ODI, events/h | 0.511 | <0.001 | 0.486 | <0.001 |
| mean SaO2 | −0.502 | <0.001 | −0.514 | <0.001 |
| lowest SaO2 | −0.520 | <0.001 | −0.496 | <0.001 |
Partial correlation analysis, adjusted for age, gender, body mass index, and comorbidities. AHI, apnea‐hypopnea index; ArI, arousal index; ODI, oxygen desaturation index; SaO2, oxyhemoglobin saturation.
Figure 2.
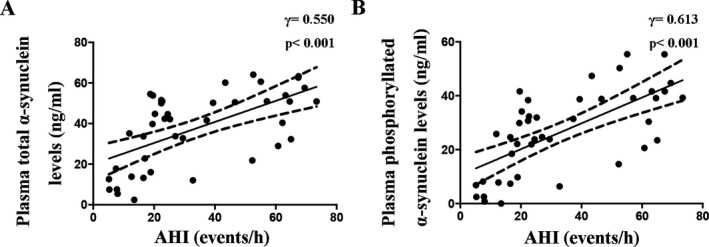
Partial correlations of the plasma total α‐synuclein levels (A) and phosphorylated α‐synuclein levels (B) with AHI in the patients with obstructive sleep apnea syndrome adjusted for age, gender, BMI, and comorbidities.
Correlations of plasma α‐synuclein levels with ODI, mean SaO2, and lowest SaO2
Then, we analyzed the associations between plasma α‐synuclein levels and the extent of hypoxia. In all participants, the plasma total α‐synuclein and phosphorylated α‐synuclein levels were positively correlated with ODI, while negatively correlated with mean SaO2 and lowest SaO2 (Table 2). In OSAS patients, the plasma total α‐synuclein (γ = 0.373, P = 0.019) and phosphorylated α‐synuclein (γ = 0.364, P = 0.023) levels were both positively correlated with ODI (Fig. 3A and B). In addition, the plasma total α‐synuclein (γ = −0.318, P = 0.048) and plasma phosphorylated α‐synuclein (γ = −0.346, P = 0.031) levels were both negatively correlated with mean SaO2 (Fig. 3C and D). We also found negative correlations of the lowest SaO2 with plasma total α‐synuclein (γ = −0.407, P = 0.010) and phosphorylated α‐synuclein (γ = −0.368, P = 0.021) levels (Fig. 3E and F). The above results suggested that the plasma α‐synuclein levels were associated with the degree of hypoxia.
Figure 3.
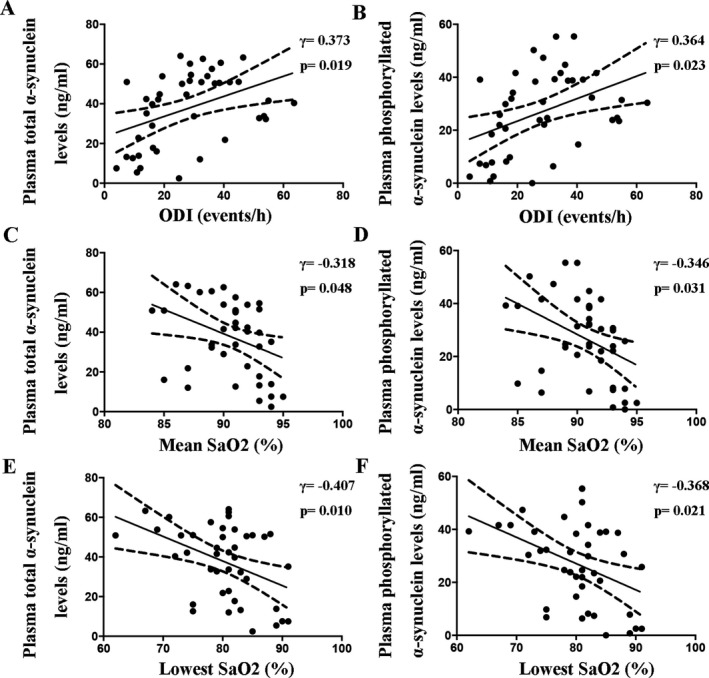
Partial correlations of the plasma α‐synuclein levels with the ODI (A and B), mean SaO2 (C and D), and lowest SaO2 (E and F) in the patients with obstructive sleep apnea syndrome adjusted for age, gender, BMI, and comorbidities.
Discussion
In the present study, we for the first time found that the OSAS patients had significantly higher plasma total α‐synuclein and phosphorylated α‐synuclein levels. Besides, both of them were positively correlated with disease severity and the degree of hypoxia. These results suggest that hypoxia may be involved in the pathogenesis of PD.
Recent epidemiological studies found that there was a significantly elevated risk of developing PD in the patients with OSAS,5, 6 especially in female patients with OSAS.14 On the other hand, PD patients exhibited a high prevalence of OSAS (20%–60%), which may be related to specific phenotype and rapid progression of PD.15, 16, 17 Thus, the cause and effect of α‐synuclein levels changes in OSAS require further investigation. Recent researches suggested that OSA and REM sleep behavior disorder can manifest with similar symptoms and coincide in a certain number of cases.18, 19, 20 So in OSAS, except for nocturnal intermittent hypoxia, REM sleep behavior disorder may also be associated with the pathophysiology of the disease and involved in the development of α‐synucleinopathy.21, 22 These further indicated that OSAS may aggravate the pathological process in early stage of PD.
Alpha‐synuclein, abundant in the brain, is composed of 140 amino acids and encoded by the SNCA gene.23 The aggregation of α‐synuclein forms Lewy bodies, which is the typical pathological feature of PD.2 Although the function of α‐synuclein is still not well understood, studies have shown that α‐synuclein plays a essential role in the pathogenesis of PD.24, 25 It is related to apoptosis suppression, glucose regulation, and the modulation of calmodulin activity.26 The phosphorylation of α‐synuclein may accelerate this process by ubiquitinated and the disruption of internalized vesicle membranes.27, 28 In addition, plasma levels of total α‐synuclein and phosphorylated α‐synuclein are increased in PD and have been thought to be potential biomarkers of disease progression.29, 30, 31 Whether peripheral α‐synuclein has pathogenic capacity remains unclear. Previous study found that peripheral injection of α‐synuclein was able to induce brain α‐synucleinopathy and a motor phenotype in mice.32 So the elevated α‐synuclein levels in OSAS patients in the present study indicate that OSAS may facilitate the initiation of PD pathogenesis.
In the brain, α‐synuclein is highly expressed in the neurons. Additionally, α‐synuclein is also expressed in various peripheral tissues such as the skin, liver, kidney, adrenal gland, and so on.33, 34 A previous study quantified α‐synuclein levels in the different fractions of blood and found that more than 99% of α‐synuclein was from the red blood cells with less than 1% of the total α‐synuclein from the platelets and peripheral blood mononuclear cells.35 The oligomeric α‐synuclein in red blood cells can be a potential diagnostic biomarker for PD.36, 37 It has been shown that short‐term or mild hypoxic stress is able to cause ɑ‐synuclein overexpression and reduce the cell viability in HEK293T cells.7, 9 Studies in vivo also revealed that the α‐synuclein level was significantly increased in rat cortex under hypoxic conditions.8 However, more studies are needed to prove the effect of hypoxia on α‐synuclein expression in animal model and humans. At this point, our findings are consistent with those findings that hypoxia is related to increased production of ɑ‐synuclein. The systemic inflammation in OSAS can change the function and properties of red blood cells, which may also contribute to the increased expression of α‐synuclein.38 Additionally, oxidative stress can promote the uptake of extracellular α‐synuclein in oligodendrocytes, accelerating the accumulation and oligomerization of it.39
Continuous positive airway pressure (CPAP) is currently thought to be the standard treatment for OSAS. Recent researches have shown that CPAP can alleviate sleep problems and retard cognitive decline in PD patients.40, 41 However, whether this benefit is associated with the decrease in α‐synuclein levels by CPAP remains unknown. Further prospective studies are needed to explore whether CPAP can reduce plasma α‐synuclein levels in OSAS patients and delay the course of PD.
However, our study has some limitations. First, as this study used a cross‐sectional design, we were unable to determine the cause and effect of hypoxia and α‐synuclein. In addition, the confounders such as sleep disruption may also affect the data. Moreover, more prospective studies with larger sample size are required to explore the clinical significance of elevated plasma α‐synuclein in OSAS patients for predicting the risk of developing into PD.
In summary, the present study found that increased α‐synuclein levels in the plasma are correlated with the degree of hypoxia in OSAS, indicating that chronic hypoxia caused by OSAS may be involved in the pathogenesis of PD.
Conflicts of Interests
The authors declare no financial or other conflicts of interests.
Acknowledgments
This study was supported by the National Key R & D Program of China (2018YFA0109600) and the National Natural Science Foundation of China (Grant no. 81701043).
Funding Information
This study was supported by the National Key R & D Program of China (2018YFA0109600) and the National Natural Science Foundation of China (Grant no. 81701043).
Funding Statement
This work was funded by National Key R & D Program of China grant 2018YFA0109600; National Natural Science Foundation of China grant 81701043.
Contributor Information
Yan‐Jiang Wang, Email: yanjiang_wang@tmmu.edu.cn.
Xian‐Le Bu, Email: buxianle@sina.cn.
References
- 1. Sveinbjornsdottir S. The clinical symptoms of Parkinson's disease. J Neurochem 2016;139(Suppl 1):318–324. [DOI] [PubMed] [Google Scholar]
- 2. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 1988;51:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lauretti E, Di Meco A, Merali S, Praticò D. Circadian rhythm dysfunction: a novel environmental risk factor for Parkinson's disease. Mol Psychiatry 2017;22:280–286. [DOI] [PubMed] [Google Scholar]
- 4. Andreou G, Vlachos F, Makanikas K. Effects of chronic obstructive pulmonary disease and obstructive sleep apnea on cognitive functions: evidence for a common nature. Sleep Disord 2014;2014:768210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeh NC, Tien KJ, Yang CM, et al. Increased risk of parkinson's disease in patients with obstructive sleep apnea: a population‐based, propensity score‐matched, longitudinal follow‐up study. Medicine (Baltimore) 2016;95:e2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen JC, Tsai TY, Li CY, Hwang JH. Obstructive sleep apnea and risk of Parkinson's disease: a population‐based cohort study. J Sleep Res 2015;24:432–437. [DOI] [PubMed] [Google Scholar]
- 7. Chen T, Li J, Chao D, et al. delta‐Opioid receptor activation reduces alpha‐synuclein overexpression and oligomer formation induced by MPP(+) and/or hypoxia. Exp Neurol 2014;255:127–136. [DOI] [PubMed] [Google Scholar]
- 8. Hu X, Rea HC, Wiktorowicz JE, Perez‐Polo JR. Proteomic analysis of hypoxia/ischemia‐induced alteration of cortical development and dopamine neurotransmission in neonatal rat. J Proteome Res 2006;5:2396–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen T, Wang Q, Chao D, et al. delta‐opioid receptor activation attenuates the oligomer formation induced by hypoxia and/or alpha‐synuclein overexpression/mutation through dual signaling pathways. Mol Neurobiol 2018:1–13. 10.1007/s12035-018-1316-1 [DOI] [PubMed] [Google Scholar]
- 10. Qureshi A, Ballard RD. Obstructive sleep apnea. J Allergy Clin Immunol 2003;112:643–651.; quiz 652. [DOI] [PubMed] [Google Scholar]
- 11. Thornton AT, Singh P, Ruehland WR, Rochford PD. AASM criteria for scoring respiratory events: interaction between apnea sensor and hypopnea definition. Sleep 2012;35:425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American academy of sleep medicine. J Clin Sleep Med 2012;8:597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bu XL, Liu YH, Wang QH, et al. Serum amyloid‐beta levels are increased in patients with obstructive sleep apnea syndrome. Sci Rep 2015;5:13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sheu JJ, Lee HC, Lin HC, et al. A 5‐year follow‐up study on the relationship between obstructive sleep apnea and parkinson disease. J Clin Sleep Med 2015;11:1403–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zoccolella S, Savarese M, Lamberti P, et al. Sleep disorders and the natural history of Parkinson's disease: the contribution of epidemiological studies. Sleep Med Rev 2011;15:41–50. [DOI] [PubMed] [Google Scholar]
- 16. Norlinah MI, Afidah KN, Noradina AT, et al. Sleep disturbances in Malaysian patients with Parkinson's disease using polysomnography and PDSS. Parkinsonism Relat Disord 2009;15:670–674. [DOI] [PubMed] [Google Scholar]
- 17. Harmell AL, Neikrug AB, Palmer BW, et al. Obstructive sleep apnea and cognition in Parkinson's disease. Sleep Med 2016;21:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang J, Zhang J, Lam SP, et al. Amelioration of obstructive sleep apnea in REM sleep behavior disorder: implications for the neuromuscular control of OSA. Sleep 2011;34:909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCarter SJ, Louis EK, Sandness DJ, et al. Diagnostic REM sleep muscle activity thresholds in patients with idiopathic REM sleep behavior disorder with and without obstructive sleep apnea. Sleep Med 2017;33:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schaefer C, Kunz D, Bes F. Melatonin effects in REM sleep behavior disorder associated with obstructive sleep apnea Syndrome: a case series. Curr Alzheimer Res 2017;14:1084–1089. [DOI] [PubMed] [Google Scholar]
- 21. Barone DA, Henchcliffe C. Rapid eye movement sleep behavior disorder and the link to alpha‐synucleinopathies. Clin Neurophysiol 2018;129:1551–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shprecher DR, Adler CH, Zhang N, et al. Predicting alpha‐synuclein pathology by REM sleep behavior disorder diagnosis. Parkinsonism Relat Disord 2018;55:92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burre J, Sharma M, Sudhof TC. Definition of a molecular pathway mediating alpha‐synuclein neurotoxicity. J Neurosci 2015;35:5221–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Erskine D, Patterson L, Alexandris A, et al. Regional levels of physiological alpha‐synuclein are directly associated with Lewy body pathology. Acta Neuropathol 2018;135:153–154. [DOI] [PubMed] [Google Scholar]
- 25. Shaltouki A, Hsieh CH, Kim MJ, Wang X. Alpha‐synuclein delays mitophagy and targeting Miro rescues neuron loss in Parkinson's models. Acta Neuropathol 2018;136:607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nouri F. Alpha‐synuclein structure, functions, and interactions. J Res Med Sci 2016;21:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hasegawa M, Fujiwara H, Nonaka T, et al. Phosphorylated alpha‐synuclein is ubiquitinated in alpha‐synucleinopathy lesions. J Biol Chem 2002;277:49071–49076. [DOI] [PubMed] [Google Scholar]
- 28. Samuel F, Flavin WP, Iqbal S, et al. Effects of Serine 129 phosphorylation on alpha‐synuclein aggregation, membrane association, and internalization. J Biol Chem 2016;291:4374–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin CH, Yang SY, Horng HE, et al. Plasma alpha‐synuclein predicts cognitive decline in Parkinson's disease. J Neurol Neurosurg Psychiatry 2017;88:818–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Foulds PG, Mitchell JD, Parker A, et al. Phosphorylated alpha‐synuclein can be detected in blood plasma and is potentially a useful biomarker for Parkinson's disease. FASEB J 2011;25:4127–4137. [DOI] [PubMed] [Google Scholar]
- 31. Foulds PG, Diggle P, Mitchell JD, et al. A longitudinal study on alpha‐synuclein in blood plasma as a biomarker for Parkinson's disease. Sci Rep 2013;3:2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sacino AN, Brooks M, Thomas MA, et al. Intramuscular injection of alpha‐synuclein induces CNS alpha‐synuclein pathology and a rapid‐onset motor phenotype in transgenic mice. Proc Natl Acad Sci U S A 2014;111:10732–10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ltic S, Perovic M, Mladenovic A, et al. Alpha‐synuclein is expressed in different tissues during human fetal development. J Mol Neurosci 2004;22:199–204. [DOI] [PubMed] [Google Scholar]
- 34. Malek N, Swallow D, Grosset KA. Alpha‐synuclein in peripheral tissues and body fluids as a biomarker for Parkinson's disease ‐ a systematic review. Acta Neurol Scand 2014;130:59–72. [DOI] [PubMed] [Google Scholar]
- 35. Barbour R, Kling K, Anderson JP, et al. Red blood cells are the major source of alpha‐synuclein in blood. Neurodegener Dis 2008;5:55–59. [DOI] [PubMed] [Google Scholar]
- 36. Daniele S, Frosini D, Pietrobono D, et al. Alpha‐Synuclein Heterocomplexes with beta‐Amyloid are increased in red blood cells of Parkinson's disease patients and correlate with disease severity. Front Mol Neurosci 2018;11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang X, Yu S, Li F, Feng T. Detection of alpha‐synuclein oligomers in red blood cells as a potential biomarker of Parkinson's disease. Neurosci Lett 2015;599:115–119. [DOI] [PubMed] [Google Scholar]
- 38. Sokucu SN, Karasulu L, Dalar L, et al. Can red blood cell distribution width predict severity of obstructive sleep apnea syndrome? J Clin Sleep Med 2012;8:521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pukaß K, Richter‐Landsberg C. Oxidative stress promotes uptake, accumulation, and oligomerization of extracellular α‐synuclein in oligodendrocytes. J Mol Neurosci 2014;52:339–352. [DOI] [PubMed] [Google Scholar]
- 40. Terzaghi M, Spelta L, Minafra B, et al. Treating sleep apnea in Parkinson's disease with C‐PAP: feasibility concerns and effects on cognition and alertness. Sleep Med 2017;33:114–118. [DOI] [PubMed] [Google Scholar]
- 41. Kaminska M, Mery VP, Lafontaine AL, et al. Change in cognition and other non‐motor symptoms with obstructive sleep apnea treatment in Parkinson disease. J Clin Sleep Med 2018;14:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]


