Abstract
Introduction:
Since the initial discoveries of human embryonic and induced pluripotent stem cells, many strategies have been developed to utilize the potential of these cells for translational research and disease modeling. The success of these aims and the development of future applications in this area will depend on the ability to generate high-quality and large numbers of differentiated cell types that genetically, epigenetically, and functionally mimic the cells found in the body.
Areas covered:
In this review, we highlight the current strategies used to maintain stem cell pluripotency (a measure of stem cell quality), as well as provide an overview of the various differentiation strategies being used to generate cells from all three germ lineages. We also discuss the particular considerations that must be addressed when utilizing these cells for translational therapy, and provide an example of a cell type currently used in clinical trials.
Expert opinion:
The major challenge in regenerative medicine and disease modeling will be in generating functional cells of sufficient quality that are physiologically and epigenetically similar to the diverse cells that they are modeled after. By meeting these criteria, these differentiated products can be successfully used in disease modeling, drug/toxicology screens, and cellular replacement therapy.
Keywords: differentiation, hESC, hiPSC, self-renewal, stem cells
1. Introduction
Human pluripotent stem cells (hPSCs) can proliferate without limit and differentiate to all somatic cell types. There are two main types of hPSCs: embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs). hESCs are derived from the inner cell mass of an embryo [1], whereas hiPSCs are reprogrammed from somatic cells using defined factors [2,3]. Despite these differences, hiPSCs and hESCs share similar molecular regulation machineries, and the strategies for self-renewal and differentiation of these cells are very similar. In this review, we refer to both of these categories of cells together as hPSCs unless otherwise specified.
Recent successes in transgene-free iPSC reprogramming have brought attention to the potential of using patient-specific pluripotent cells for clinical applications [4]. Protocols for the directed differentiation of hPSCs to numerous cell types are now available, including methods for the generation of neurons, cardiomyocytes, adipocytes, endothelial cells (ECs), and hematopoietic cells. However, the particular methods that generate differentiated cell types suitable for translational research are still being developed. In this review, we will discuss general cell culture platforms for hPSCs, as well as several strategies for cell lineage differentiation.
2. General cell culture platforms of hPSCs
The pluripotency maintenance and differentiation potential of hPSCs maintained in the laboratory are dependent upon the applied culture condition [1,5,6]. hPSCs grow as adherent cells on solid surfaces with coating and are maintained in media supplemented with specific growth factors to either sustain cell pluripotency or induce cellular differentiation. Therefore, suitable conditions for maintenance or differentiation can be achieved by manipulating two major components: the extracellular matrix (ECM) and growth factors. As hPSCs are increasingly publicized for their potential in clinical applications, the combination of conditions is determined by the demands of downstream applications.
Historically, hPSCs have been successfully cultured and maintained on mouse feeder cells (usually fibroblasts) in serum or serum replacement-containing medium [1,7]. These feeder cells secrete specific factors, which in combination with the nonspecified factors in serum are empirically effective in promoting hPSC growth and survival. Additionally, these feeder cells generate an ECM, onto which hPSCs can attach and expand. However, feeder cell-based platforms are not suitable for clinical application as the inconsistency of undefined components hinders production and repeatability. In response, the field has embraced the development and use of feeder-free culture conditions using xeno-free media [8]. Specific ECM products that meet the more stringent requirements for clinical applications have also been produced. Concurrent advances are being developed to address the necessity of high-quality, large-scale production of hPSCs for clinical therapy. These include large-surface bioreactor or vessels and suspension cultures for cell maintenance and differentiation.
3. Pluripotency strategies
3.1. Cell culture media
hPSC culture requires the activation of three major families of growth factors to support cell pluripotency and expansion: fibroblast growth factor (FGF), TGF-β/nodal/activin and IGF/insulin [9]. Whereas the FGF and TGF-β/nodal/activin pathways maintain the expression of pluripotency genes such as NANOG and OCT4 [10,11], the FGF and IGF/insulin pathways activate cell survival signaling [12–14]. hPSCs are traditionally cultured on mouse feeder cells in knockout serum replacement (KOSR) medium that is supplemented with FGF. As stated previously, the feeder cells and KOSR provide essential stimuli that work with the FGF to maintain cell pluripotency. However, this type of culture is not ideal for translational applications because feeder cells and KOSR have issues with consistency, animal sourcing and safety. In the last 15 years, many laboratories have made major efforts to develop more consistent and defined hPSC culture conditions; an international consortium was formed in 2010 to evaluate popular formulas for consensus use [11,14–17]. In an attempt to keep culture conditions animal-free, all hPSC-related growth factors are now available as recombinant proteins. Similarly, albumin (which is also used in some hPSC cultures) is also now available as recombinant albumin [18] or it can be totally removed from cell culture. To further improve the consistency of the medium, strategies have been developed to improve the stability of growth factors such as FGF through point mutation, use of heparin or heparan sulfate supplement [10,19–21] or slow-release mechanisms [21].
3.2. Extracellular matrix
As adherent cells, hPSCs require attachment to a specific ECM in order to activate the integrin receptors essential for cell survival. This ECM can be supplied by feeder cells in culture or by commercial products. Matrigel, which contains collagen and laminin from mouse sources, is the most commonly used ECM in feeder-free culture. Other recombinant proteins known to successfully support hPSCs include vitronectin, fibronectin and laminin. Xeno-free synthetic peptide matrices have also been shown to be successful [10,22–27]. Moving forward, the challenge is to optimize ECMs for defined stem cell culture.
3.3. Handling methods
Cell-to-cell contact is essential for hPSC survival; hPSCs generally do not survive dissociation by trypsin/EDTA and instead are dissociated with the enzymes collagenase and dispase, which generate clumps of cells that preserve cell-to-cell contact. However, it is difficult to regulate the size of these clumps, which results in cell culture variability [5,7]. These animal-sourced enzymes, additionally, are not ideal for translational research. Animal-free recombinant enzymes TrypLE and Accutase have been generated to more gently dissociate cells [28], and the addition of ROCK inhibitor has been shown to suppress cell death, thereby allowing for dissociation methods with more consistent cell survival in regular expansion and differentiation [29–31]. At the same time, enzyme-free dissociation methods were developed to dissociate and harvest hPSCs with simple chemicals, such as EDTA and citric acid [30,32].
3.4. Large-scale culture
Cellular therapy typically utilizes billions of cells of interest----two or three orders of magnitude larger than the number used in regular laboratory research settings. To meet this need, specialized expansion platforms, such as bioreactors and suspension culture, are required [6,33] Bioreactors and multilayer vessels utilize the classical culture methods of hPSCs on adherent surfaces and enlarge usable culture surface area in limited space. An alternative platform is suspension culture, which is already commonly used for cell-based protein production of other cell types. As hPSCs usually grow as adherent cells, this platform utilizes ROCK inhibitor and IL-6 receptor treatment in order to limit cell death cause by suspension culture. Both platforms allow for large-scale expansion of hPSCs with greatly expanded capacity.
When culturing hPSCs in these conditions, it is important to consider the inherent potential for variability and mutation that accompanies large-scale culture. Theoretically, hPSCs can expand without limit while maintaining their pluripotency. It has been reported that normal karyotypes of hPSCs can be maintained up to 240 passages with cells in culture for up to 3 years, but abnormal karyotypes do arise in routine expansion [34]. In order to avoid potential abnormal karyotypes in hPSCs and maintain experimental consistency, we recommend the use of hPSCs within specific passages. hESCs are usually available at passage 30 – 40 upon reception; it is recommended to use them for an extra 30 passages until passage 50 – 70. At the same time, hiPSCs can often be obtained around passage 20 and can be used until passage 50.
4. Differentiation strategies
4.1. General platforms
There are three major platforms for stem cell differentiation: i) co-culture with other cell types; ii) formation of embryoid bodies (EBs) in suspension, which are subsequently directed to differentiate; and iii) monolayer differentiation [1,35,36]. The co-culture platform utilizes particular feeder cell types (chosen based on the differentiation protocol), which secrete essential factors to drive differentiation to specific lineages, thus initiating and determining cell fate. Because of the lack of control over feeder cells, it is difficult to regulate and optimize the differentiation process. The EB platform promotes differentiation of hPSCs in three-dimensional suspension culture by forming floating clumps of pure hPSCs. These EBs mimic natural embryogenesis, such that cells from all three germ lineages can emerge within the EB (though specific culture conditions can bias the cells to a particular lineage of interest). Under the monolayer system, researchers supplement differentiation media with specific factors, such as morphogens and growth factors, to drive adherent hPSCs to specific lineages. This simplified system makes it easier for researchers to understand regulatory mechanisms and to optimize protocols.
4.2. Strategies to improve purity
A pure population of differentiated cells from hPSCs is typically required for downstream applications, and as alternative cell types can contaminate these samples, multiple strategies are used to increase purity. Conventionally, cell sorting, by magnetic columns or flow cytometry, is used to enrich for cell types of interest. Gene targeting in combination with drug selection can also be used to engineer a cell-type-specific promoter to drive a selection marker. This can then be used to create a purified population. As both of these options are labor intensive, the ideal strategy is to optimize differentiation protocols such that cells can be generated with high purity.
4.3. Differentiation considerations
As discussed in the first section, pluripotency is maintained through the FGF, TGF-β/nodal/activin and IGF/insulin pathways. It has been recently proposed that pluripotency is achieved by balancing the signaling between the neural lineage and the mesoendodermal lineage [37]. The in vitro differentiation of pluripotent stem cells follows the same molecular regulation of the embryo in epiblast, gastrulation and further lineage determination stages. It has been long evident that disturbing the pluripotency growth factor pathways can directly induce the differentiation of cells to specific lineages. In general, activation of the bone morphogenic protein (BMP)4/ WNT pathway induces mesoendoderm, whereas inhibition of the BMP4/TGF-β pathway drives neural differentiation. In this review, we highlight several examples of hPSC differentiation to ectodermal, mesodermal and endodermal fates, and the key signaling pathways involved in differentiation to these cell types is summarized in Table 1. Although these differentiated cell types may be validated based on morphological and genetic criteria, it is important to note that not all cell types have been validated using functional criteria. Proper function of differentiated hPSCs is an important consideration moving forward and will be examined in further detail later on.
Table 1.
Summary of important signaling pathways related to hPSC differentiation to all three germ lineages.
| Cell type | Key signaling pathways | Ref. | |
|---|---|---|---|
| Ectoderm | Neurons | Notch, SHH, RA, WNT, FGF, TGF-β | [39–43] |
| Dopaminergic neurons | FGF8, SHH | [46,47] | |
| Motor neurons | RA, SHH | [43] | |
| Astrocytes | Heregulin, BMP, CNTF | [48] | |
| Oligodendrocytes | FGF2, RA, PDGF, IGF, NT3, SHH | [49] | |
| Keratinocytes | BMP4 | [50] | |
| Melanocytes | WNT3a | [51] | |
| Mesoderm | Cardiac cells | Activin, BMP4, SMAD, WNT | [59–62] |
| Mesenchymal stem cells | [65–67] | ||
| Endothelial cells | VEGF | [68–70] | |
| Hematopoietic stem/progenitor cells | BMP4, Activin, VEGF, FGF2 | [71–76] | |
| Endoderm | β cells | Activin, FGF2, RA, FGF, HGF, Notch | [80–83] |
| Hepatocytes | Activin, FGF2/4, BMP2/4, HGF, EGF | [86,87] |
BMP: Bone morphogenic protein; CNTF: Ciliary neurotrophic factor; FGF: Fibroblast growth factor; NT3: Neurotrophin-3; PDGF: Platelet-derived growth factor; RA: Retinoic acid; SHH: Sonic hedgehog.
4.4. Ectoderm specification
The ectoderm is the most distal of the three primary germ layers in the developing embryo, and its position in the early developing embryo is managed by selective affinity for the mesoderm layer and weak affinity for the endoderm layer. Ectoderm can be classified into two parts: surface ectoderm and neuroectoderm. Together, they give rise to neural cells, skin cells and pigment cells. Induction of ectoderm fate in hPSC populations can be considered a ‘default’ pathway due to the ease with which hPSCs can develop into neuroectodermal cells in basic cell culture conditions, including low serum [38].
4.4.1. General considerations for the generation of neural cells
Many groups have succeeded in developing protocols for the differentiation of neurons from hPSCs. In vitro, BMP/SMAD inhibition (including chordin, noggin and follistatin) promotes the neural fate [39]. Careful management and manipulation of key signaling pathways induces generation of neuronal subtypes, including motor neurons or dopaminergic neurons, as well as develop other neural cell types, including astrocytes and oligodendrocytes. These cell types may hold relevance for future studies focusing on particular diseases. Other neural cells are developed from the neural crest fate, including sensory neurons and Schwann cells.
4.4.2. Generation of neural stem cells
The inherent heterogeneity associated with direct differentiation of neurons from hPSCs has led to the utilization of neural stem cells (NSCs) as an intermediate multipotent cell type, which can differentiate to mature neurons and glia. Traditionally, NSCs are generated through a rosette-based method, by which EBs are primed to form adherent neural rosettes; this is done using neural induction media containing FGF2 to promote self-renewal [40,41]. Purified rosettes are then dissociated to establish NSCs, which can be identified by the expression of specific markers such as SOX1/2, NESTIN and PAX6.
4.4.3. Generation of neurons
The key signaling pathways involved in neuronal differentiation involve Notch, sonic hedgehog (SHH), retinoic acid (RA), Wnt, FGF and TGF-β. Several groups have shown that neurons can be created through a variety of methods, including co-culture with stromal cells [42], directed differentiation from hPSCs [43] and differentiation from a stable NSC stage [40]. Generally, neurons can be formed by the removal of FGF from the cell culture medium. Other factors, such as activin [44], the Notch inhibitor DAPT [45] and cAMP, have also been shown to induce the differentiation of neuronal precursors. In order to expedite the differentiation process, neuronal differentiation medium can be supplemented with growth factors, including brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. These factors mimic the support rendered from surrounding astrocytes in vivo (Figure 1). As it is difficult to sort these cells, researchers rely on alternate methods to generate pure populations of neurons. These include optimizing protocols or adding small molecules to inhibit the growth or proliferation of other cell types.
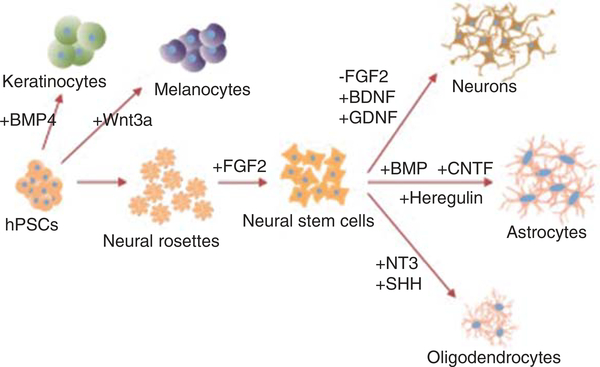
Figure 1. hPSCs can be differentiated to ectodermal lineages.
To generate neural cells, hPSC first pass through a neural rosette stage of NSCs, which are supported by the addition of FGF2. Reduction of FGF2 and addition of BDNF and GDNF to support neuronal survival can generate neurons. Astrocytes can be generated through the addition of BMP and CNTF or through the addition of heregulin. Oligodendrocytes require the addition of neurotrophin-3 and SHH. hPSCs can also generate non-neural ectodermal cells such as keratinocytes with the addition of BMP4, and melanocytes through the addition of Wnt3a.
BMP: Bone morphogenic protein; BDNF: Brain-derived neurotrophic factor; CNTF: Ciliary neurotrophic factor; GDNF: Glial cell line-derived neurotrophic factor; hPSCs: Human pluripotent stem cells; SHH: Sonic hedgehog.
4.4.4. Generation of neural subtypes of cells
Culture conditions can be adapted to generate other neural cells, including subtypes of neuronal cells and glial cells. hPSCs are differentiated to dopaminergic neurons through the addition of signaling factors FGF8 and SHH [46,47]. Motor neurons are developed through activation of the RA and SHH pathways [43]. Glial cells can also be created through the addition of particular growth factors to the cell culture medium. Astrocytes are generated through the addition of heregulin, or a combination of BMP and ciliary neurotrophic factor (CNTF) to culture medium [48]. Oligodendrocytes progenitor cells can be derived from hPSCs as well through a carefully timed introduction of growth factors including FGF2, RA, platelet-derived growth factor, IGF, neurotrophin-3 and SHH [49].
4.4.5. Generation of non-neural cells from ectoderm
Surface ectoderm cells can also be derived from hPSCs. Activation of BMP4 results in development of skin, including keratinocytes [50]. Pigment cells, such as melanocytes, are generated from hiPSCs by supplementing culture medium with WNT3a [51].
4.5. Mesoderm and endoderm specification
The development of the mesoderm and endoderm lineages is very closely related. The specific lineage that these cells commit to is also dependent upon modulating the essential hPSC regulatory pathways [52–54] (FGF, TGF-β/nodal/activin and IGF/insulin). In mouse embryogenesis, embryonic stem cells give rise to primitive streak cells with Brachyury (T) expression, which then further develop into endoderm lineage cells with Foxa2-positive expression and mesoderm lineage with Flk expression. Early mesoderm/endoderm differentiation in hPSCs is also initiated through modulation of BMP/activin pathway to induce T. It was recently found that FGF and insulin/IGF pathways play important roles in fine-tuning differentiation and that inhibition of IGF pathways promotes differentiation to T [52]. Insulin/IGF pathway activation subsequently activates AKT and sustains pluripotency, whereas the inhibition of IGF pathways allows for easier transition to mesoendoderm lineages with subsequent activation of WNT signaling [55,56]. FGF plays a different role during BMP4/activin-induced differentiation, and it controls the cell fate between extra embryonic lineage and mesoderm lineages. Upon BMP4 activation, cells are promoted toward a mesoderm (T+) fate with FGF2 and toward primitive endoderm (CDX2) in its absence [56]. In later stages, FGF collaborates with BMP4 to prevent endoderm differentiation while promoting mesodermal lineages.
4.5.1. General considerations for mesoderm induction
Several methods for derivation of mesoderm lineage cells from hPSCs have been recorded. Original EB differentiation methods in media supplemented with fetal bovine serum (FBS) and different growth factors or chemical combinations in culture are known to generate mesodermal cells after weeks of induction. Recently, more efficient differentiation strategies have been developed through the utilization of a monolayer method. It has been established that BMP4 activation plays a central role to drive mesodermal differentiation, along with active FGF and TGF-β/nodal pathways (Figure 2). In addition, it has also been recently shown that direct WNT activation is sufficient to generate cardiac and hematopoietic lineages [57].
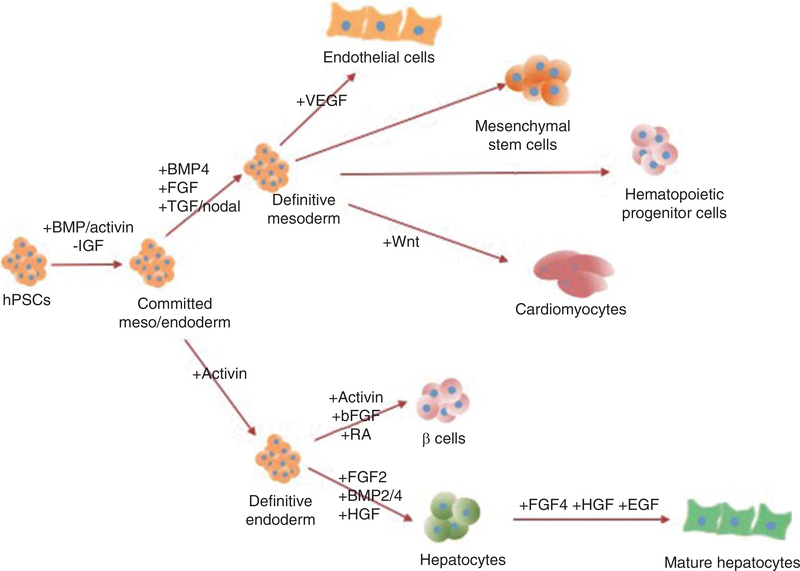
Figure 2. hPSCs can be differentiated to mesodermal and endodermal lineages.
The induction of the mesoderm and endoderm is first established through the activation of the BMP/activin pathway and inhibition of IGF pathway. The addition of BMP4, FGF and TGF-β/nodal creates definitive mesoderm, which can be differentiated to endothelial cells, MSCs, HPCs and cardiomyocytes. Definitive endoderm is created through the addition of activin A. β cells can be generated through addition of additional activin, FGF2 and RA. Hepatocytes can be generated by the addition of FGF2, BMP2/4 and HGF, and can be further matured by addition of FGF4, HGF and EGF.
BMP: Bone morphogenic protein; EGF: Epidermal growth factor; FGF: Fibroblast growth factor; HGF: Hepatocyte growth factor; MSCs: Mesenchymal stem cells; RA: retinoic acid; SHH: Sonic hedgehog.
4.5.2. Generation of cardiac cells
Both EB suspension culture, co-culture and monolayer platforms are currently used in production of cardiac cells [58–60]. During generation of cardiac cells, the dynamic crucial stage-specific markers are shown as following: T → MESP1/KDR/FLK → NKX2–5/ISL1 → TNNT2. Activation through activin/BMP4/SMAD to activate WNT signaling or direct WNT activation and subsequent WNT inhibition allows for effective generation of cardiomyocytes using EB [59,61] and monolayer [62] methods. hPSC-derived cardiomyocytes have been successfully obtained for in vitro disease models of Timothy syndrome, long QT syndrome and LEOPARD syndrome [63]. hPSC-derived cardiomyocytes in these disease models displayed manifested irregular electrical activity and contraction. Electrophysiological recording and calcium imaging studies can be performed on these cells, thus establishing them as a useful tool for investigation of molecular and cellular mechanisms of cardiac pathophysiology in these diseases and for drug screening. The current challenge for cardiac differentiation is in developing more mature cells and subtypes of cardiac cells.
4.5.3. Generation of mesenchymal stem cells (hPSC-MSCs)
Mesenchymal stem cell (MSC)-mediated therapy is a fast-growing field that has demonstrated itself to be safe and effective in cell-based therapies for various degenerative diseases and tissue injuries. Although MSCs are recognized as easy to access from bone marrow and other adult tissue, the differentiation potential of such harvested MSCs is gradually reduced when these cells are expanded in culture, thereby limiting their therapeutic efficacy. Derivation of MSC from hPSCs would be advantageous for clinical application as one can theoretically obtain unlimited cell numbers without senescence [64]. Several investigators have successfully generated MSCs from hPSCs, and those cells have been shown to possess the same in vitro and in vivo phenotype and functions as MSCs derived from adult sources [65–67]. These include differentiating into osteoblasts, adipocytes and chondrocytes, as well as promoting vascular and muscle regeneration in the hind limb ischemia mouse model. Future studies should focus on the efficiency of hPSC-MSCs based on different clinically relevant protocols, as well as in vivo safety and efficacy studies.
Despite the great potential of MSCs, it is important to functionally validate these cells beyond common markers and assays. MSCs are multipotent stem cell types that share some common markers but contain diverse potential. MSCs are often referred to as conceptual progenitors with mesodermal origins and display great diversity in their lineage potential and organ sources. In vitro, the potential of MSCs could be greatly affected by available differentiation conditions. Therefore, more vigorous functional characterization assays should be developed before the MSCs can be used in clinical practices.
4.5.4. Generation of endothelial cells (hPSC-ECs)
ECs are located at the luminal surface of all blood and lymphatic vessels as a monolayer. They control the interaction of the vessel wall with circulating blood components and regulate vascular responses to hemodynamic forces. Several methods for derivation of ECs from hPSCs have been recorded. Original EB differentiation methods supplemented with FBS and a high dose of VEGF. However, previously developed methods have been difficult to replicate among independent hPSC lines due to protocol complexity, batch variation in induction reagents and various other unexplained factors. With improved and reproducible methods, hPSC-derived ECs display compatible molecular markers and similar gene expression profiles when compared across several lines [68]. hPSC-ECs possess a repertoire of functional phenotypic plasticity and are amenable to cell-based assays probing endothelial contributions to inflammatory and cardiovascular diseases [69]. Additionally, hPSC-ECs offer a unique model to study developmental specification of vascular heterogeneity in tissue-specific stem and progenitor cells at steady states and during organ regeneration. The discovery of phenotypically similar ECs with unique overlapping signatures between adult mice and hPSC-derived EC cultures demonstrates the developmentally conserved pathways common to tissue-specific ECs [68]. Transplanted hPSC-ECs have led to increased function and vascularization in multiple animal disease models, including in hind limb perfusion and myocardial infarction, in addition to stably carrying blood up to 150 days after transplantation with no safety issues reported [70].
4.5.5. Generation of hematopoietic stem/progenitor cells (hPSC-HSCs)
Hematopoietic stem/progenitor cell (HSCs) derivation from hPSCs is initiated by activation of BMP4/activin to generate mesoderm precursors [71,72]. These mesoderm precursors are directed to hematopoietic linage cells in media supplemented with various hematopoietic growth factors. Multiple blood cell types, including myelomonocytic cells, megakaryocytes, T/B lymphocytes and hPSC-HSCs, can be experimentally derived from hPSCs by using either EB formation and monolayer culture in the presence of hematopoietic cytokines or coculture with stromal cells [73–75]. However, the generation of self-renewing multipotent HSCs from hPSCscontinuesto challenge researchers as this process is extremely inefficient and to date has been unsuccessful at differentiating hPSCs into iPSC-HSCs in vitro. Several studies have reported derivation of CD34+ HSCs from hPSCs, showing low engraftment potential after transplantation to immunocompromised mice. Giovanni Amabile et al. have reported a novel in vivo system in which human iPS cells differentiate within teratomas into NOD. Cg-Prkdcscid Il2rgtm1Wjl/SzJ immunocompromised (NSG) mice to derive functional myeloid and lymphoid cells [76]. Generation of hPSC-HSCs from teratomas could be useful for human antibody generation and drug screening applications. However, these cells bear a concern of safety issues in clinical transplantation.
4.5.6. Endoderm lineage induction
The enrichment of endoderm progenitors or definitive endoderm (DE) cells is crucial for the development of efficient strategies to generate functional endoderm lineage cells such as pancreatic cells and hepatocytes. Activation of activin is the driving force for induction of endoderm progenitors or DE cells in vitro. However, activin A-induced endoderm differentiation from hPSCs depends on concentration, culture conditions, time of application, as well as interactions of activin A with various signaling pathways (WNT3a, noggin and FGF2/4) and differentiation factors [77,78].
4.5.7. Insulin producing cells (β cells)
Several studies have reported the generation of DE or foregut endoderm, pancreatic progenitors or insulin producing cells (β cells) from hPSCs in vitro [79,80]. These cells were further matured into functional β cells, which showed insulin secretion in response to various reagents and glucose stimulation in vivo. It has been demonstrated that several specific signaling molecules including activin, FGF2 and RA, along with an appropriate extracellular environment, were important for the differentiation of pancreatic progenitor cells. The induction of activin A-induced DE cells into pancreatic endocrine progenitor cells is facilitated by various growth and differentiation factors (such as the pancreas-specific inducers cyclopamine and RA) and by proliferation inducers (including sodium butyrate, betacellulin, FGFs and hepatocyte growth factor (HGF)). Such differentiation begins with activin-driven DE specification, followed by RA activation and SHH inhibition for pancreatic specification, with high expression of PDX1, and FGF10 and Notch inhibition driving the maturation of pancreatic progenitor [81,82]. However, all those factors do not efficiently allow for terminal differentiation of hPSCs cells into functional β cells in vitro. There are additionally aspects of β cell development that are not yet well understood, thus hampering generation of PSC-derived β cells. In particular, the signaling pathways that instruct endocrine progenitor cells to differentiate into mature and functional β cells are poorly understood. Other significant obstacles include the need for safe and cost-effective differentiation methods for large-scale generation of transplantation quality β cells, methods to prevent immune rejection of grafted tissues and amelioration of the risks of tumorigenesis [83].
4.5.8. Hepatocytes
Multiple protocols for hPSC differentiation into cells of hepatic lineage have been studied [84–86]. Activin is the main driving factor for hepatocyte derivation. Other growth factors such as FGF2, BMP2/4, HGF, dexamethasone and oncostatin M have been used to trigger hepatocyte specification, and FGF4, HGF and EGF are known to promote hepatic maturation [87]. The vascular endothelial system (ECs and endothelial cell-released growth factors) and mesenchymal stem cells (MSCs) in cell-matrix cultivation conditions are required to generate sufficient amounts of functionally engrafted hepatocytes [86].
5. Translation towards clinical use
The first clinical trial using hPSCs was to be performed by the Geron Corporation and involved the use of hESCs to treat spinal cord injury. However, the company announced in late 2011 that it would discontinue its Phase 1 trial. Now, it seems the focus of the first human trials will be the eye, specifically in patients with inherited disorders associated with the loss of retinal pigment epithelial cells [88]. These trials are supported by companies such as Advanced Cell Technology and academic centers including the RIKEN Center for Developmental Biology and will be conducted in the UK, the US and Japan.
As hPSCs advance towards clinical use, regulatory agencies will be required to implement additional safeguards and regulations regarding the use of these cell types. Although guidelines for investigational cellular therapy products are available, these guidelines are related to the assessment of human clinical grade somatic cells. Major challenges of hPSC-related cellular therapy include the tumorigenicity of hPSCs, genetic stability and epigenetic drift. The determination of tumor producing cell dosages is required for each hPSC-derived differentiated cell type. In order to compare these levels to adequate positive and negative controls, specific assays tailored towards downstream clinical different applications are necessary [89]. Unfortunately, these challenges can only be partially addressed in an in vitro cellular state. Adequately addressing larger safety considerations such as dosage efficacy and the effects of the immune system will require large analogue animal models. However, this also presents a hurdle, as the knowledge of pluripotent stem cell systems in larger animal species is lacking.
6. Expert opinion
hPSCs have enormous potential to identify and validate new disease mechanisms, to develop accurate and relevant drug and toxicology screens, and to provide new treatment strategies through cell therapy approaches. The success, however, of hPSCs in these areas will be contingent upon the characteristics and quality of the hPSC product. Quality control is therefore of major importance and a major challenge of the field moving forward.
Stable and widely available control hPSC-derived cell lines are necessary to provide a relative measurement of basic characteristics of newly generated cell products. Completely defined, publicly available protocols are required to ensure reproducibility of cellular characteristics from cell products generated by different entities. However, despite the importance of conformity, the new area of individualized medicine must deal with the inherited genetic variance in each individual cellular product. The challenge will be to distinguish between inherited and experimentally generated variances in the cellular products.
It is therefore most important to avoid artificially generated abnormal karyotypes and functional single nucleotide polymorphisms (SNPs). Although karyotyping assays by G-banding and SKY allow the identification of large-scale genomic changes [90–93], SNPs cannot be detected by these approaches. An increasing number of researchers are favoring exome sequencing and whole-genome sequencing to identify point mutations accumulated in somatic cells from reprogramming or the expansion process [94–96]. However, these technologies are expensive and their analysis and interpretation complex. Next-generation sequencing demonstrates a surprisingly high rate of individual genetic variants, most of them silent and not disease causing. It becomes increasingly obvious that the generation of genetic variability in cells is common and may be even seen as part of their biological characteristics. hPSCs are not an exception, and their short population doubling time combined with high generation numbers make these cells even more prone to acquire genetic variability. The challenge will be to better understand the impact of genetic variability and to identify functional/disease causing from silent SNPs for a safe cell product. hPSC technology provides a unique opportunity to study individual disease mechanisms and provide personalized therapy. However, the generation of hPSCs from parental lines is a complex and long process. It is therefore essential to establish identity between each patient and his or her corresponding hPSC products.
Even with correct genetic composition, the potential of pluripotent stem cells is also affected by the presence of epigenetic modifications. Therefore, it is essential to evaluate the quality of pluripotent stem cells after derivation and expansion. Surface markers and nuclear markers are usually used as quick tools to identify abnormal cells [1–3]. Epigenetic analyses were introduced for a more thorough examination that could reflect global gene regulation more accurately [3,13,97]. Such approaches have effectively helped people understand important phenomena such as clonal differences and tissue memory [98]. However, these assays still need validation in EBs, teratoma and monolayer platforms. Multiplex assays have been developed to analyze the potential of these cells [98,99].
Challenges remain in obtaining functional cells at suitable stages of differentiation that can be used for cellular therapy. Though researchers are currently able to generate cells that match the target cell line by criteria such as an expected gene expression profile, such signatures cannot guarantee that the developed cells are functionally identical to cells obtained from our bodies. Functional assays are thus required for cells such as cardiomyocytes, neurons, blood cells and hepatocytes in order to verify cell identity. Despite the use of existing assays to measure cell function, the maturation of differentiated cells remains a major challenge and a necessity for using these cells in future drug screening and cell-based therapy [100]. Functional assays should be developed to verify all differentiated cells, including subtypes of cells, before they can be safely used in clinical applications.
We believe that hPSC technology will revolutionize medicine and public health with great impact on our communities. In order to achieve this goal in a reasonable timeframe, we advocate that private and government-funding agencies work together in order to provide the required resources that will support and promote translational use of hPSCs.
Article highlights.
hPSC quality for small and large culture systems is dependent upon appropriate culture methods.
Knowledge of key signaling pathways in development is necessary to optimize differentiation strategies of hPSC to specific lineages.
Functional assays on hPSC-derived differentiated cells will be an important prerequisite for future hPSC-based therapies.
Footnotes
Declaration of interest
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
Bibliography
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282(5391):1145–7 [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131(5):861–72 [DOI] [PubMed] [Google Scholar]
- 3.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318(5858):1917–20 [DOI] [PubMed] [Google Scholar]
- 4.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 2008;132(4):661–80. Available from: http://www.cell.com/cell/abstract/S0092-8674(08)00216-X [DOI] [PubMed] [Google Scholar]
- 5.Thomson JA, Kalishman J, Golos TG, et al. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA 1995;92(17):7844–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen KG, Mallon BS, McKay RD, Robey PG. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell 2014;14(1):13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reubinoff BE, Pera MF, Fong CY, et al. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 2000;18(4):399–404 [DOI] [PubMed] [Google Scholar]
- 8.Akopian V, Andrews PW, Beil S, et al. Comparison of defined culture systems for feeder cell free propagation of human embryonic stem cells. In vitro Cell Dev Biol Anim 2010;46(3–4):247–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen G, Gulbranson DR, Hou Z, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods 2011;8(5):424–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, Gulbranson DR, Yu P, et al. Thermal stability of fibroblast growth factor protein is a determinant factor in regulating self-renewal, differentiation, and reprogramming in human pluripotent stem cells. Stem Cells 2012;30(4):623–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci 2005;118(Pt 19):4495–509 [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Lin G, Martins-Taylor K, et al. Inhibition of caspase-mediated anoikis is critical for basic fibroblast growth factorsustained culture of human pluripotent stem cells. J Biol Chem 2009;284(49):34054–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eiselleova L, Matulka K, Kriz V, et al. A complex role for FGF-2 in self-renewal, survival, and adhesion of human embryonic stem cells. Stem Cells 2009;27(8):1847–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Schulz TC, Sherrer ES, et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood 2007;1;110(12):4111–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Powell S, Brunette E, et al. Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol Bioeng 2005;91(6):688–98 [DOI] [PubMed] [Google Scholar]
- 16.Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol 2006;24(2):185–7 [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Hou R, Booth CJ, et al. Defined culture conditions of human embryonic stem cells. Proc Natl Acad Sci USA 2006;103(15):5688–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ng ES, Davis R, Stanley EG, Elefanty AG. A protocol describing the use of a recombinant protein-based, animal product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat Protoc 2008;3(5):768–76 [DOI] [PubMed] [Google Scholar]
- 19.Furue MK, Na J, Jackson JP, et al. Heparin promotes the growth of human embryonic stem cells in a defined serumfree medium. Proc Natl Acad Sci USA 2008;105(36):13409–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levenstein ME, Berggren WT, Lee JE, et al. Secreted proteoglycans directly mediate human embryonic stem cell-basic fibroblast growth factor 2 interactions critical for proliferation. Stem Cells 2008;26(12):3099–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lotz S, Goderie S, Tokas N, et al. Sustained levels of FGF2 maintain undifferentiated stem cell cultures with biweekly feeding. PLoS One 2013;8(2):e56289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manton KJ, Richards S, Van Lonkhuyzen D, et al. A chimeric vitronectin: IGF-I protein supports feeder-cell-free and serum-free culture of human embryonic stem cells. Stem Cells Dev 2010;19(9):1297–305 [DOI] [PubMed] [Google Scholar]
- 23.Braam SR, Zeinstra L, Litjens S, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells 2008;26(9):2257–65 [DOI] [PubMed] [Google Scholar]
- 24.Melkoumian Z, Weber JL, Weber DM, et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol 2010;28(6):606–10 [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki T, Futaki S, Suemori H, et al. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat Commun 2012;3:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prowse AB, Doran MR, Cooper-White JJ, et al. Long term culture of human embryonic stem cells on recombinant vitronectin in ascorbate free media. Biomaterials 2010;31(32):8281–8 [DOI] [PubMed] [Google Scholar]
- 27.Rodin S, Domogatskaya A, Strom S, et al. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol 2010;28(6):611–15 [DOI] [PubMed] [Google Scholar]
- 28.Ellerstrom C, Strehl R, Noaksson K, et al. Facilitated expansion of human embryonic stem cells by single-cell enzymatic dissociation. Stem Cells 2007;25(7):1690–6 [DOI] [PubMed] [Google Scholar]
- 29.Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 2007;25(6):681–6 [DOI] [PubMed] [Google Scholar]
- 30.Chen G, Hou Z, Gulbranson DR, Thomson JA. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell 2010;7(2):240–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohgushi M, Matsumura M, Eiraku M, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell 2010;7(2):225–39 [DOI] [PubMed] [Google Scholar]
- 32.Beers J, Gulbranson DR, George N, et al. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat Protoc 2012;7(11):2029–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Chou BK, Dowey S, et al. Scalable expansion of human induced pluripotent stem cells in the defined xeno-free E8 medium under adherent and suspension culture conditions. Stem Cell Res 2013;11(3):1103–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taapken SM, Nisler BS, Newton MA, et al. Karotypic abnormalities in human induced pluripotent stem cells and embryonic stem cells. Nat Biotechnol 2011;29(4):313–14 [DOI] [PubMed] [Google Scholar]
- 35.Kaufman DS, Hanson ET, Lewis RL, et al. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA 2001;98(19):10716–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells 2001;19(3):193–204 [DOI] [PubMed] [Google Scholar]
- 37.Shu J, Deng H. Lineage specifiers: new players in the induction of pluripotency. Genomics Proteomics Bioinformatics 2013;11(5):259–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozair MZ, Kintner C, Brivanlou AH. Neural induction and early patterning in vertebrates. Wiley Interdiscip Rev Dev Biol 2013;2(4):479–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bond AM, Bhalala OG, Kessler JA. The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Dev neurobiol 2012;72(7):1068–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koch P, Opitz T, Steinbeck JA, et al. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc Natl Acad Sci USA 2009;106(9):3225–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joannides AJ, Fiore-Heriche C, Battersby AA, et al. A scaleable and defined system for generating neural stem cells from human embryonic stem cells. Stem Cells 2007;25(3):731–7 [DOI] [PubMed] [Google Scholar]
- 42.Kawasaki H, Mizuseki K, Nishikawa S, et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 2000;28(1):31–40 [DOI] [PubMed] [Google Scholar]
- 43.Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell 2002;110(3):385–97 [DOI] [PubMed] [Google Scholar]
- 44.Reubinoff BE, Itsykson P, Turetsky T, et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol 2001;19(12):1134–40 [DOI] [PubMed] [Google Scholar]
- 45.Borghese L, Dolezalova D, Opitz T, et al. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells 2010;28(5):955–64 [DOI] [PubMed] [Google Scholar]
- 46.Zeng X, Cai J, Chen J, et al. Dopaminergic differentiation of human embryonic stem cells. Stem Cells 2004;22(6):925–40 [DOI] [PubMed] [Google Scholar]
- 47.Yan Y, Yang D, Zarnowska ED, et al. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells 2005;23(6):781–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaltouki A, Peng J, Liu Q, et al. Efficient generation of astrocytes from human pluripotent stem cells in defined conditions. Stem Cells 2013;May;31(5):941–52 [DOI] [PubMed] [Google Scholar]
- 49.Hu BY, Du ZW, Zhang SC. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat Protoc 2009;4(11):1614–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Itoh M, Kiuru M, Cairo MS, Christiano AM. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc Natl Acad Sci USA 2011;108(21):8797–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohta S, Imaizumi Y, Okada Y, et al. Generation of human melanocytes from induced pluripotent stem cells. PLoS One 2011;6(1):e16182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gadue P, Huber TL, Paddison PJ, Keller GM. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci USA 2006;103(45):16806–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tada S, Era T, Furusawa C, et al. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development 2005;132(19):4363–74 [DOI] [PubMed] [Google Scholar]
- 54.Singh AM, Reynolds D, Cliff T, et al. Signaling network crosstalk in human pluripotent cells: a Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell 2012;10(3):312–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bechard M, Trost R, Singh AM, Dalton S. Frat is a phosphatidylinositol 3-kinase/Akt-regulated determinant of glycogen synthase kinase 3beta subcellular localization in pluripotent cells. Mol Cell Biol 2012;32(2):288–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bernardo AS, Faial T, Gardner L, et al. BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell 2011;9(2):144–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sakurai H, Sakaguchi Y, Shoji E, et al. In vitro modeling of paraxial mesodermal progenitors derived from induced pluripotent stem cells. PLoS One 2012;10:e47078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 2012;10(1):16–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burridge PW, Thompson S, Millrod MA, et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One 2011;6(4):e18293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mummery CL, Zhang J, Ng ES, et al. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ res 2012;111(3):344–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kattman SJ, Witty AD, Gagliardi M, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 2011;8(2):228–40 [DOI] [PubMed] [Google Scholar]
- 62.Lian X, Hsiao C, Wilson G, et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci USA 2012;109(27):E1848–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bellin M, Marchetto MC, Gage FH, Mummery CL. Induced pluripotent stem cells: the new patient? Nature reviews. Mol cell biol 2012;13(11):713–26 [DOI] [PubMed] [Google Scholar]
- 64.Hemmati M, Abbaspour A, Alizadeh AM, et al. Rat xenograft chondrosarcoma development by human tissue fragment. Exp Oncol 2011;33(1):52–4 [PubMed] [Google Scholar]
- 65.Niibe K, Kawamura Y, Araki D, et al. Purified mesenchymal stem cells are an efficient source for iPS cell induction. PLoS One 2011;3:e17610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gruenloh W, Kambal A, Sondergaard C, et al. Characterization and in vivo testing of mesenchymal stem cells derived from human embryonic stem cells. Tissue Eng Part A 2011;17(11–12):1517–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lian Q, Zhang Y, Zhang J, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 2010;121(9):1113–23 [DOI] [PubMed] [Google Scholar]
- 68.Nolan DJ, Ginsberg M, Israely E, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 2013;26(2):204–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White MP, Rufaihah AJ, Liu L, et al. Limited gene expression variation in human embryonic stem cell and induced pluripotent stem cell-derived endothelial cells. Stem Cells 2013;31(1):92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoo J, Kim HS, Hwang DY. Stem cells as promising therapeutic options for neurological disorders. J Cell Biochem 2013;114(4):743–53 [DOI] [PubMed] [Google Scholar]
- 71.Park SW, Jun Koh Y, Jeon J, et al. Efficient differentiation of human pluripotent stem cells into functional CD34+ progenitor cells by combined modulation of the MEK/ERK and BMP4 signaling pathways. Blood 2010;116(25):5762–72 [DOI] [PubMed] [Google Scholar]
- 72.Pick M, Azzola L, Mossman A, et al. Differentiation of human embryonic stem cells in serum-free medium reveals distinct roles for bone morphogenetic protein 4, vascular endothelial growth factor, stem cell factor, and fibroblast growth factor 2 in hematopoiesis. Stem Cells 2007;25(9):2206–14 [DOI] [PubMed] [Google Scholar]
- 73.Chicha L, Feki A, Boni A, et al. Human pluripotent stem cells differentiated in fully defined medium generate hematopoietic CD34− and CD34+ progenitors with distinct characteristics. PLoS One 2011;6(2):e14733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi KD, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells 2009;27(3):559–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Y, Liu L, Zhang L, et al. Efficient commitment to functional CD34+ progenitor cells from human bone marrow mesenchymal stem-cell-derived induced pluripotent stem cells. PLoS One 2012;7(4):e34321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Amabile G, Welner RS, Nombela-Arrieta C, et al. In vivo generation of transplantable human hematopoietic cells from induced pluripotent stem cells. Blood 2013;121(8):1255–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grapin-Botton A, Constam D. Evolution of the mechanisms and molecular control of endoderm formation. Mech Dev 2007;124(4):253–78 [DOI] [PubMed] [Google Scholar]
- 78.Toivonen S, Lundin K, Balboa D, et al. Activin A and Wnt-dependent specification of human definitive endoderm cells. Exp Cell Res 2013;319(17):2535–44 [DOI] [PubMed] [Google Scholar]
- 79.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26(4):443–52 [DOI] [PubMed] [Google Scholar]
- 80.Jiang J, Au M, Lu K, et al. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells 2007;25(8):1940–53 [DOI] [PubMed] [Google Scholar]
- 81.Offield MF, Jetton TL, Labosky PA, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 1996;122(3):983–95 [DOI] [PubMed] [Google Scholar]
- 82.Shiraki N, Yoshida T, Araki K, et al. Guided differentiation of embryonic stem cells into Pdx1-expressing regional-specific definitive endoderm. Stem Cells 2008;26(4):874–85 [DOI] [PubMed] [Google Scholar]
- 83.Sulzbacher S, Schroeder IS, Truong TT, Wobus AM. Activin A-induced differentiation of embryonic stem cells into endoderm and pancreatic progenitors-the influence of differentiation factors and culture conditions. Stem Cell Rev 2009;5(2):159–73 [DOI] [PubMed] [Google Scholar]
- 84.Si-Tayeb K, Noto FK, Nagaoka M, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 2010;51(1):297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Espejel S, Roll GR, McLaughlin KJ, et al. Induced pluripotent stem cell-derived hepatocytes have the functional and proliferative capabilities needed for liver regeneration in mice. J Clin Invest 2010;120(9):3120–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 2013;499(7459):481–4 [DOI] [PubMed] [Google Scholar]
- 87.Chiang CH, Huo TI, Sun CC, et al. Induced pluripotent stem cells and hepatic differentiation. J Chin Med Assoc 2013;76(11):599–605 [DOI] [PubMed] [Google Scholar]
- 88.Schwartz SD, Hubschman JP, Heilwell G, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 2012;379(9817):713–20 [DOI] [PubMed] [Google Scholar]
- 89.Kanemura H, Go MJ, Shikamura M, et al. Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration. PLoS One 2014;9(1):e85336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Draper JS, Smith K, Gokhale P, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol 2004;22(1):53–4 [DOI] [PubMed] [Google Scholar]
- 91.Martins-Taylor K, Nisler BS, Taapken SM, et al. Recurrent copy number variations in human induced pluripotent stem cells. Nat biotechnol 2011;29(6):488–91 [DOI] [PubMed] [Google Scholar]
- 92.Taapken SM, Nisler BS, Newton MA, et al. Karotypic abnormalities in human induced pluripotent stem cells and embryonic stem cells. Nat biotechnol 2011;29(4):313–14 [DOI] [PubMed] [Google Scholar]
- 93.Draper JS, Smith K, Gokhale P, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat biotechnol 2004;22(1):53–4 [DOI] [PubMed] [Google Scholar]
- 94.Howden SE, Gore A, Li Z, et al. Genetic correction and analysis of induced pluripotent stem cells from a patient with gyrate atrophy. Proc Natl Acad Sci USA 2011;108(16):6537–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng L, Hansen NF, Zhao L, et al. Low incidence of DNA sequence variation in human induced pluripotent stem cells generated by nonintegrating plasmid expression. Cell Stem Cell 2012;10(3):337–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gore A, Li Z, Fung HL, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature 2011;471(7336):63–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature 2010;467(7313):285–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bock C, Kiskinis E, Verstappen G, et al. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 2011;144(3):439–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boulting GL, Kiskinis E, Croft GF, et al. A functionally characterized test set of human induced pluripotent stem cells. Nat biotechnol 2011;29(3):279–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem cells dev 2013;22(14):1991–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]