Abstract
Resistant hypertension is defined as uncontrolled blood pressure despite the use of 3 or more antihypertensive agents of different classes, including a diuretic, usually thiazide-like, a long-acting calcium channel blocker, and a blocker of the renin- angiotensin system, either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker, at maximal or maximally tolerated doses. Antihypertensive medication non-adherence and the white coat effect, defined as elevated blood pressure when measured in clinic but controlled when measured outside of clinic, must be excluded to make the diagnosis. Resistant hypertension is a high risk phenotype, leading to increased all-cause mortality and cardiovascular disease (CVD) outcomes. Healthy lifestyle habits are associated with reduced CV risk in patients with resistant hypertension. Aldosterone excess is common in patients with resistant hypertension, and addition of spironolactone or amiloride to the standard 3-drug antihypertensive regimen is effective at getting the blood pressure to goal in most of these patients. Refractory hypertension is defined as uncontrolled blood pressure despite use of 5 or more antihypertensive agents of different classes, including a long acting thiazide-like diuretic and a mineralocorticoid receptor antagonist, at maximal or maximally tolerated doses. Fluid retention, mediated largely by aldosterone excess, is the predominant mechanism underlying resistant hypertension, while patients with refractory hypertension typically exhibit increased sympathetic nervous system activity.
Subject Terms: Hypertension
Keywords: resistant hypertension, refractory hypertension, hyperaldosteronism, chlorthalidone, spironolactone
DEFINITIONS
Resistant hypertension (RHTN) is defined as high blood pressure (BP) in a hypertensive patient that remains above goal despite use of three or more antihypertensive agents of different classes, typically including a long-acting calcium channel blocker (CCB), a blocker of the renin-angiotensin system (RAS), either an angiotensin- converting enzyme inhibitor (ACEi) or an angiotensin receptor blocker (ARB]), and a diuretic, given at maximal or maximally tolerated doses.1-3 The definition also includes BP that is controlled on ≥4 antihypertensive medications, controlled RHTN. The diagnosis of RHTN requires exclusion of common causes of “pseudoresistance”, which include improper BP measurement technique, which usually results in falsely elevated readings; white coat RHTN, defined as uncontrolled office BP but controlled out-of-office BP in a patient on ≥3 antihypertensive agents; undertreatment, including clinical inertia, which is the failure to establish appropriate BP targets and escalate treatment to achieve treatment goals; and medication non-adherence. The term “apparent treatment resistant hypertension” is used to indicate patients diagnosed as having RHTN based on the number of prescribed medications and the office BP but in whom pseudoresistance cannot be excluded ,i.e., when medication dose, adherence or out-of-office BP values are not documented.1 These definitions are summarized in Box 1.
The term RHTN has been used to identify patients with difficult-to-treat hypertension who might benefit from special diagnostic and/or therapeutic procedures or referral to a hypertension specialist. Observational studies and clinical trials of antihypertensive treatment have shown that patients with RHTN are at increased risk of CVD compared with patients with more easily controlled hypertension,4 as well as higher risk of incident CV events, even after effective BP control is achieved.5-9 This definition has also been useful for identifying patients with RHTN in a standardized fashion for research purposes, particularly in standardizing enrollment criteria worldwide for clinical trials of evolving treatment strategies for RHTN, including novel device-based approaches.10-12
PSEUDORESISTANCE
Most uncontrolled hypertension is not truly resistant to medical treatment, but results from factors that lead to or maintain elevated BP readings independent of prescribed pharmacologic treatment, termed pseudoresistance. The most common causes of pseudo-RHTN are inaccurate BP measurement, resulting in falsely elevated readings, the white coat effect, where in-office BP is persistently elevated but out-of-office BP is at goal, undertreatment, including clinical inertia, and medication nonadherence (Figure 1). Identification of factors that contribute to pseudoresistance is important in preventing costly and potentially risky diagnostic evaluations of patients who are not truly resistant to treatment, and avoiding inappropriate intensification of treatment, which can be costly and potentially increases the risk of adverse events.
Figure 1.
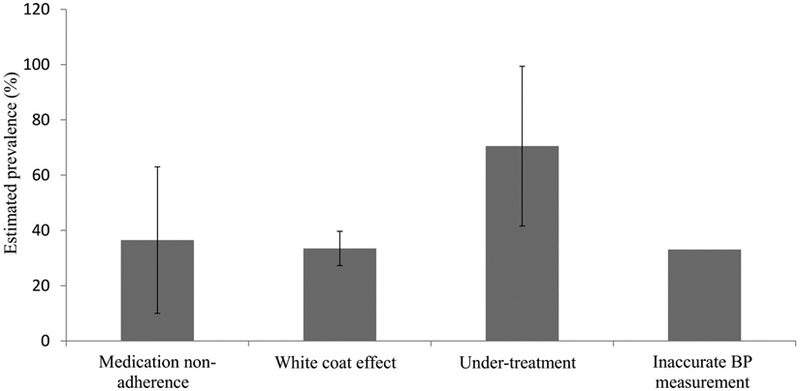
Estimated prevalence of common causes of pseudo-treatment resistance.
Reference: Bhatt H, Siddiqui M, Judd E, Oparil S and Calhoun D. Prevalence of pseudoresistant hypertension due to inaccurate blood pressure measurement. J Am Soc Hypertens. 2016;10:493-9.
Use of poor BP measurement technique is common in routine clinical settings, often resulting in inaccurate BP values. Some of the most common errors are not letting the patient rest in a quiet area, measuring the BP while the patient is standing or supine, engaging the patient in conversation during the BP measurement process, use of a BP cuff that is too small, placing the cuff over clothing, and introduction of operator biases through use of nonautomated devices. These errors often result in falsely elevated BP readings, and have been shown to be particularly common in patients with presumed uncontrolled RHTN.
In a prospective evaluation carried out in our hypertension referral clinic, Bhatt et al. compared BP measurements made routinely during the triage process with BP measurements made in the same patients by trained clinicians.13 The latter measurements used an automated oscillometric device BpTRU (VSM Med Tech Ltd. Coquitlam, Canada). A total of 130 consecutive patients referred for suspected uncontrolled RHTN were included in the analysis. After having their triage BP measured, patients were seated in a quiet room, a correctly sized cuff was placed directly over the brachial artery of their non-dominant arm, and then were left unattended for 3-5 minutes. Six serial, automated, unattended measurements were obtained, and the last five were averaged for the final reading. BP values obtained during the triage process were consistently higher compared with the unattended BP values obtained by the trained clinicians. The systolic and diastolic BP measurements were up to 33/21 mm Hg higher in the triage setting versus the standardized assessment, with a median difference of 23/13 mm Hg. Overall, 33% of the patients would have been misdiagnosed as having uncontrolled RHTN based solely on the triage assessments. These findings suggest that approximately one-third of patients referred to a hypertension specialty clinic may be falsely misidentified as having uncontrolled RHTN based on routine clinic BP measurements, highlighting the need for standardized, automated-based BP measurements to confirm true RHTN.
White coat RHTN is another common cause of pseudo-treatment resistance. De la Sierra et al. determined the prevalence of white coat RHTN among >8200 patients with apparent RHTN included in the Spanish Ambulatory Blood Pressure Monitoring Registry.14 Overall, 62.5% were classified as having true RHTN, based on having sustained uncontrolled hypertension both in clinic and during 24-hour ambulatory BP monitoring (ABPM), while the remaining 37.5% were identified as having white coat RHTN. These findings clearly indicate that a prominent white coat effect is common in patients with suspected uncontrolled RHTN, and accurate out-of-office assessment of BP, ideally with ABPM, is essential to confirm true RHTN. A study by Muxfeldt et al. further highlighted the need for continued out-of-office BP monitoring of patients with white coat RHTN.15 Among 198 patients with white coat RHTN whose ABPM was repeated 18 months later, approximately 50% had persistence of their white coat effect, but 50% had been reclassified as having true sustained RHTN. These studies indicate that out-of-office BP assessments are necessary to exclude a white coat effect in order to confirm true RHTN and avoid unnecessary treatment in those with pseudo-RHTN. These findings also emphasize that continued surveillance of out-of-office BP levels is necessary, as many patients with white coat RHTN may develop true RHTN and will require intensification of antihypertensive treatment.
Undertreatment of hypertension, including lack of appropriate treatment intensification in uncontrolled patients, termed clinical inertia, is common and becomes more prevalent as the number of prescribed medications increases.16 Egan et al identified over 44,000 patients who were enrolled in community-based practices in the Southeastern United States as having apparent uncontrolled RHTN using electronic medical records.17 Of these, only 15% had been prescribed an optimal antihypertensive regimen, defined as a diuretic and two or more additional antihypertensive agents at 50% or higher of the recommended maximum dose for treating hypertension. Among patients receiving an optimal treatment regimen, including a diuretic, the mean number of prescribed medications was 4.2 in those whose BP remained uncontrolled versus 4.9 agents in those with controlled BP. These findings provide evidence that clinical inertia contributes importantly to lack of BP control in usual clinical practice.
Another common cause of pseudoresistance is poor medication adherence. Jung et al. assessed adherence with prescribed antihypertensive regimens by measuring antihypertensive drug or drug metabolite levels in urine samples from patients referred to a hypertension specialty clinic for uncontrolled RHTN.18 After excluding patients whose BP was controlled with optimization of therapy or who had white coat RHTN or secondary causes of hypertension, the 76 patients thought to have true RHTN were tested. Based on the presence of detectable urinary drug or drug metabolite levels, only 36 (47%) of these patients were adherent with all prescribed agents, whereas 40 (53%) were considered non-adherent. Twelve of the 40 non-adherent patients were taking none of their prescribed antihypertensive medications based on the complete absence of detectable urinary drug or metabolites, and the majority of those considered partially adherent were taking less than half of the prescribed medications. Other studies based on measurement of serum or urinary drug levels confirmed poor medication adherence levels in patients with apparent RHTN.19, 20
It is well established that as the number of the prescribed medications increases and as the dosing schedule becomes increasingly complex, adherence with prescribed regimens declines.16 Accordingly, treatment of RHTN is often complicated by worsening adherence as therapy is intensified. A major challenge in the management of these patients is accurate assessment of medication adherence. Clinician impression is often incorrect and self-reported adherence is often overstated. Increased use of electronic medical records allows for better monitoring of prescription refill rates, but having a medication refilled, especially if done automatically through a mail order service, does not ensure adequate adherence. Observed pill ingestion to document lack of adequate BP response is not likely viable for most clinics, given space and staffing constraints. Commercial laboratories are increasingly offering standardized testing of blood and/or urine for the presence of prescribed medications and their metabolites, but issues of patient consent and lack of insurance coverage for the testing need to be resolved to allow for routine clinical use.
Prognosis
RHTN is a high-risk phenotype of hypertension. Uncontrolled BP is the single most important modifiable risk factor for CV morbidity and mortality worldwide.21 Patients with RHTN typically have long histories of severe BP elevation, predisposing them to higher CV risk than treated hypertensive patients with controlled BP. A retrospective cohort study carried out by Daugherty et al. in two integrated health plans examined the CVD outcomes of patients with incident hypertension who went on to develop RHTN.5 They found that patients who developed RHTN are more likely to experience clinical outcomes of death, myocardial infarction, heart failure, stroke or CKD compared to treated hypertensive patients with controlled BP (18% versus 13.5%, p<0.001, hazard ratio 1.47, 95% confidence interval [CI] 1.33-1.62) during a mean 3.8 years follow up. In another retrospective analysis of over 400,000 persons, patients with RHTN had increased risk of developing end stage renal disease, ischemic heart disease, heart failure, stroke, or death compared to treated hypertensive patients with controlled BP (multivariable adjusted hazard ratios of 1.32 [95% CI 1.27-1.37], 1.24 [1.20-1.28], 1.46 [1.40-1.52], 1.14 [1.10-1.19], and 1.06 [1.03-1.08], respectively).7
Patients with RHTN also have a higher prevalence of comorbid conditions, including diabetes (48% vs 30% in hypertensive patients with controlled BP), CKD (45% vs 24%), ischemic heart disease (41% vs 22%), and cerebrovascular disease (16% vs 9%), which greatly increase the risk of clinical events.7 In the Chronic Renal Insufficiency Cohort (CRIC), a multicenter, prospective, observational study of risk factors for progression of CKD, patients with CKD, but not on dialysis, and RHTN had a higher risk of a composite outcome of myocardial infarction, stroke, peripheral arterial disease, congestive heart failure, and all-cause mortality than hypertensive patients with CKD and controlled BP, even when the BP was at goal.22 In hypertensive patients with coronary artery disease (CAD), the presence of RHTN is associated with a higher risk of all-cause mortality, nonfatal myocardial infarction and nonfatal stroke compared to treated hypertensive patients with controlled BP.8 In another study of patients with CAD, the risk of death and morbidity was increased by as much as 64-73% in patients with RHTN compared to treated hypertensive patients with controlled BP.23 Women with RHTN and signs and symptoms of myocardial ischemia had worse outcomes, including an increased risk of death, than treated hypertensive women with controlled BP.9 Further, the mortality risk persisted for at least 10 years from the determination of treatment resistant status, regardless of the presence of obstructive CAD.9
It is uncertain whether the increased CV risk seen in RHTN is related solely to the persistent BP elevation per se, or whether specific pathophysiologic factors are involved. The high CVD burden in RHTN results from an interplay of multiple processes, e.g., increased RAS and sympathetic nervous system activity, hyperaldosteronism and increased arterial stiffness, that have been associated with increased CV risk. In a post hoc analysis of the INternational Verapamil SR-Trandolapril STudy (INVEST), a prospective, randomized, open-label, blinded endpoint trial that compared clinical outcomes in 22,576 participants with CAD and HTN who were randomly assigned to a CCB-based or β-blocker-based antihypertensive treatment strategy, there was no difference in outcomes between patients with uncontrolled RHTN versus those with controlled RHTN, despite a difference in mean BP of approximately 27/10 mm Hg between the two groups.8 Further, it has been shown that patients who are prescribed a greater number of antihypertensive medications have increased CV risk independent of BP level.24 The Reduction of Atherothrombosis for Continued Health (REACH) Registry followed persons ≥45 years old with ≥3 risk factors for atherosclerosis and with established CAD, CVD, or peripheral arterial disease prospectively for 4 years.24 Participants in REACH with RHTN on 4 antihypertensive agents had a 15% higher risk of the primary endpoint, a composite of CV death, myocardial infarction, or stroke at 4 years, when compared with treated hypertensive patients with controlled BP (20.1 vs. 13.9%; HR: 1.15; 95% CI: 1.06–1.24; P = 0.0004). Participants on ≥5 agents were at even higher risk compared to those with controlled BP; 20% higher hazard of the primary endpoint (21.3 vs. 14.7%; HR: 1.20; 95% CI: 1.01–1.43; P = 0.036). In the Reasons for Geographic And Racial Differences in Stroke (REGARDS) Study, differences in BP control among patients with RHTN were not associated with differences in stroke or mortality.25 Further, BP lowering may confer less improvement in the CV risk profile of patients with RHTN than in hypertensive patients without RHTN.26 Collectively, these studies provide evidence that the presence of RHTN, more than the level of the BP alone, is an important predictor of CV risk in patients with hypertension.
The prognosis of patients with RHTN may be improved with lifestyle modification. Healthy lifestyle factors, including having a normal waist circumference, engaging in physical activity ≥4 times/week, nonsmoking, moderate alcohol consumption, high Dietary Approaches to Stop Hypertension diet score, and low sodium-to-potassium intake ratio; all of which are recommended for all patients with hypertension, are associated with a lower risk for CV events and mortality among individuals with RHTN.27 In particular, nonsmokers and those who engage in physical activity ≥4 times/week had the lowest associated risk for CV events during a mean follow up of 4.5 years.
Pharmacologic Treatment
Initial Three-Drug Regimens
Pharmacologic therapy of RHTN is based on use of effective combinations of three or more antihypertensive medications. While particular combinations have to be individualized based on patients’ comorbidities, prior medication intolerances and financial considerations, the initial three-drug regimen should be standardized as much as possible to include a RAS blocker, specifically an ACEi or ARB, a long-acting CCB, most commonly amlodipine, and a long-acting thiazide-like diuretic, preferably chlorthalidone or indapamide. The standard three-drug regimen of ACEi/ARB, amlodipine, and chlorthalidone combines classes of agents with complementary mechanisms of action that have been shown to be effective both individually and in combination in lowering BP and in preventing CVD and death. All of the recommended agents are available as generics and in long-acting formulations and are generally well tolerated. These particular agents have the advantage of being available in various dual or triple pill combinations, allowing for simplified regimens, reduction in pill burden, and sometimes lesser out-of-pocket , including co-payment, costs. The standardized triple-drug combination of a RAS blocker, amlodipine, and a thiazide-like diuretic was used as baseline therapy in multiple studies of RHTN, including the Prevention And Treatment of Hypertension With Algorithm based Therapy (PATHWAY-2) study, which demonstrated the superiority of spironolactone when added as a fourth drug.28 The benefit of adding spironolactone to combinations of pills other than an ACEi or ARB, amlodipine, and chlorthalidone or indapamide, has not been systematically determined. In addition, this particular triple combination continues to be widely used as the standard baseline therapy for ongoing clinical trials assessing the efficacy and tolerability of potential new pharmacologic therapies for treatment of RHTN, as well as the multiple studies evaluating new device-based therapies for RHTN. Widespread use of the triple combination in prior and ongoing studies assessing novel treatment strategies for RHTN provides important clinical validation of the three-drug regimen in terms of clinical benefit, including ease of use.
ACEi and ARBs play important roles in the treatment of patients with RHTN because of their efficacy and tolerability, as well as the prevention and management of common comorbidities, such as diabetes, heart failure, and CKD. Both ACEis and ARBs reduce the rate of incident diabetes by approximately 20-30% compared to other classes of antihypertensive drugs.29, 30 Further, ACEis and ARBs are indicated for the management of patients with CKD and heart failure, which are common comorbidities in patients with RHTN.31-33
A long-acting thiazide-like diuretic, specifically chlorthalidone, if available, is recommended over HCTZ given its superior efficacy and clear benefit demonstrated in multiple outcome studies of hypertension.4, 34-36 While the efficacy of chlorthalidone has not been prospectively compared to HCTZ in patients with well characterized RHTN, some reports demonstrate that substituting chlorthalidone for HCTZ in patients uncontrolled on multiple-drug combinations provides further BP reduction and improves overall control rates.37 The superiority of chlorthalidone over HCTZ to reduce BP, particularly nighttime BP, is likely related to its longer half-life. However, chlorthalidone use is more frequently associated with adverse metabolic effects, particularly hypokalemia and hyponatremia, compared to HCTZ. These adverse effects can be severe enough to require withdrawal of chlorthalidone, and it is important to monitor electrolytes regularly when prescribing chlorthalidone.
Fixed dose combinations of ACEis or ARBs, amlodipine, and HCTZ are widely available, allowing for simplification of dosing, reduction in pill number, and possibly less cost, advantages which are well known to improve medication adherence. Combinations with chlorthalidone are less common but should be used preferentially if available and affordable for the patient.
Use of Spironolactone as the Fourth Agent for Treating Resistant Hypertension
A large body of literature demonstrates that aldosterone excess is a common cause of RHTN.38-42 Multiple studies indicate that true, classical primary aldosteronism is present in approximately 20% of patients with confirmed RHTN. More importantly, lesser degrees of aldosterone excess that do not fulfill strict criteria for classical primary aldosteronism may contribute to resistance to commonly used antihypertensive medications.43 This aldosterone excess is related to being overweight or obese, common comorbidities in patients with RHTN. 44 There is a positive relationship between weight gain and aldosterone levels in both women and men, but this effect is more pronounced in men (Figure 2).44 This finding suggests that accumulation of excess abdominal adiposity, more characteristic of men, may either directly release aldosterone or indirectly stimulate the release of aldosterone secretagogues. Such a possibility is consistent with cell culture studies suggesting that adipocytes, particularly abdominal adipocytes, are hormonally active and release agents that stimulate the secretion of aldosterone from isolated zona glomerulosa cells.45, 46
Figure 2.
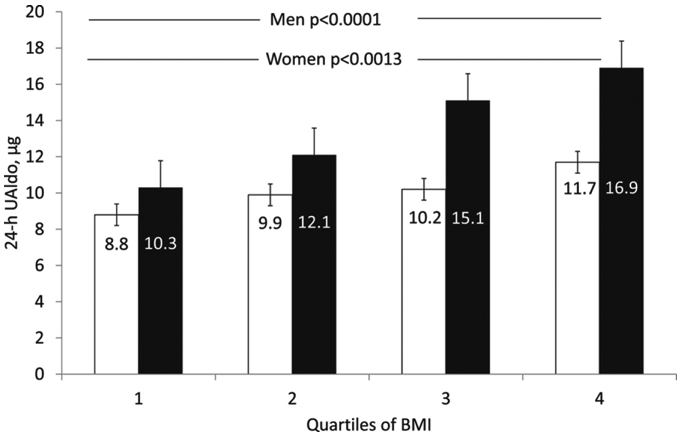
Mean 24-hour urinary aldosterone (UAldo) levels to quartiles of body mass index (BMI) in men (black columns) and women (white columns) with resistant hypertension.
Reference: Dudenbostel T, Ghazi L., Liu M., Li P., Oparil S., Calhoun DA. Body mass index predicts 24-hr urinary aldosterone levels in patients with resistant hypertension. Hypertension. 2016.
Given that excess aldosterone is a common cause of antihypertensive treatment resistance, it is not surprising that mineralocorticoid receptor antagonists (MRAs), which block the action of aldosterone on mineralocorticoid receptors, are especially effective for treatment of RHTN.47-51 Three MRAs: spironolactone, eplerenone, and canrenone (not available in the United States) are available for the treatment of hypertension, and spironolactone is the most studied in RHTN. Spironolactone is the most effective fourth medication for treating RHTN in patients already on treatment with triple regimens that include an ACEi or ARB, amlodipine, and a thiazide-like diuretic.28 Early studies that assessed the add-on benefit of spironolactone as the fourth drug for treating RHTN demonstrated substantial antihypertensive benefit, with mean BP reductions often exceeding 20/10 mm Hg.49, 50 These studies typically had important limitations, including open-label assessments of medication use, lack of an active comparator, failure to confirm true RHTN, and inclusion of small numbers of patients. Nonetheless, they were critical in showing consistent benefit of spironolactone for treatment of hypertension uncontrolled on multiple-drug regimens.
The landmark PATHWAY-2 study demonstrated a major benefit of spironolactone in treating patients with RHTN uncontrolled on a standardized three-drug regimen of an ACEi or ARB, amlodipine, and the thiazide-like diuretic indapamide.28 PATHWAY-2 was a double-blind, four-way cross-over study that compared 3 months of treatment with spironolactone 25 to 50 mg daily to the beta-blocker bisoprolol 5-10 mg, the alpha-blocker doxazosin 5-10 mg, or placebo as add-on therapy for RHTN. True RHTN was confirmed after home BP remained uncontrolled following observed ingestion of maximum tolerated doses of the standardized triple-drug regimen. Adherence during the study was monitored by pill counts and by measurement of serum ACE activity. PATHWAY-2 showed that spironolactone was superior to placebo and the two active comparators for reducing BP in patients with uncontrolled RHTN. On average, spironolactone reduced home systolic BP by 8.70 mm Hg more than placebo, 4.48 mm Hg more than bisoprolol, and 4.03 mm Hg more than doxazosin (p<0.0001). The percentage of patients whose BP was controlled was 58.0% for spironolactone compared to 23.9% for placebo, 43.3% for bisoprolol, and 41.5% for doxazosin (p<0.001 for all groups).
Spironolactone was well tolerated in those PATHWAY-2 study participants who had normal renal function (estimated glomerular filtration rate [eGFR] >45 ml/min and mean eGFR 91.1 ml/min). Overall, there was no difference in the occurrence of adverse events between treatments, including adverse events that might be expected to limit spironolactone use, such as gynecomastia or hyperkalemia. Six of the 285 patients (2%) receiving spironolactone developed a serum potassium level >6.0 mmol/L on a single occasion. No gynecomastia was reported during the study, but study participants had only 3 months of exposure to spironolactone. Similarly, in our experience, the risk of spironolactone-induced hyperkalemia is low in patients with normal renal function, particularly if they are already receiving chlorthalidone, which promotes potassium excretion. However, risk of hyperkalemia increases with declining renal function.
PATHWAY-2 provided two additional findings that are clinically important. First, it clearly demonstrated an additional BP-lowering effect of titrating spironolactone dosage up to 50 mg. Prior studies assessing the benefit of spironolactone for treating RHTN were generally limited to the 25 mg dose. In PATHWAY-2, spironolactone was titrated to 50 mg and at week 12 produced greater BP reductions than the other treatments after titration to higher doses (−3.86 mm Hg [CI −5.28 to −2.45] versus −0.88 mm Hg [−2.32 to 0.56] for doxazosin, −1.49 mm Hg [−2.94 to −0.04] for bisoprolol, and −0.68 mm Hg [−2·10 to 0·75] for placebo, p<0.0001). The other important finding was the enhanced benefit of spironolactone in patients with suppressed renin levels. While spironolactone reduced BP in patients at all renin levels, there was a strong inverse relation between baseline renin levels and the magnitude of BP reduction, with patients with the lowest renin levels exceeding on average 20 mmHg reduction in home systolic BP. This level of BP reduction is extraordinary, especially in patients already treated with three other classes of agents, and in being predicted by a routine biochemical assessment.
Pathogenesis of Resistant Hypertension: Role of Aldosterone-Induced Fluid Retention
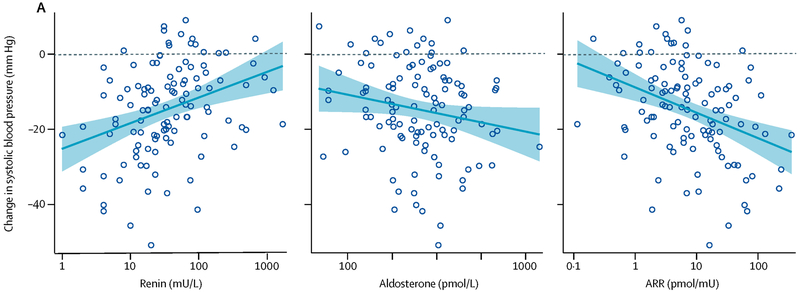
PATHWAY-2 included several substudies designed to prospectively explore the pathogenesis of RHTN.52 The first substudy showed that the baseline aldosterone-renin ratio (ARR) and baseline renin level strongly predicted the BP response to spironolactone, while the baseline aldosterone level only weakly predicted the BP response (Figure 3). In contrast, the BP responses to the beta blocker and the alpha blocker were unrelated to the baseline ARR. The second substudy demonstrated that the antihypertensive effect of spironolactone was associated with a significant reduction in thoracic fluid content, an index of fluid retention. In contrast, beta-blockade had no significant effect on thoracic fluid content, while alpha-blockade increased it. The three add-on agents were associated with similar small reductions in vascular resistance.
Figure 3.
Relation between baseline plasma renin, aldosterone, and the serum aldosterone and renin concentration (ARR) ratio and the home systolic blood pressure response to spironolactone in the PATHWAY-2 study.
Reference: Williams B, MacDonald TM, Morant SV, Webb DJ, Sever P, McInnes GT, Ford I, Cruickshank JK, Caulfield MJ, Padmanabhan S, Mackenzie IS, Salsbury J, Brown MJ. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6(6):464-475. doi: 10.1016/S2213-8587(18)30071-8.
Given that the ARR is strong index of volume status, with suppressed renin and a corresponding high ARR indicating excess volume retention, the PATHWAY-2 substudy findings indicate that RHTN is attributable in large part to excess fluid retention mediated by aldosterone excess. The benefit of spironolactone in patients with RHTN is thus related to reversal of aldosterone-induced fluid retention. These results are consistent with prior findings of increased intravascular fluid retention evidenced by high natriuretic peptide levels and increased intracardiac volumes measured by magnetic resonance imaging in patients with RHTN.53 In addition, a study of salt sensitivity in patients with RHTN has demonstrated that the excess fluid retention characteristic of these patients can be overcome by dietary sodium restriction.54 The prior study results, combined with the PATHWAY-2 findings, strongly implicate inappropriate fluid retention secondary to high dietary sodium intake and aldosterone excess as an important mediator of antihypertensive treatment resistance that is best overcome by the natriuretic and diuretic effects of spironolactone in patients with RHTN.
The third substudy of PATHWAY-2 assessed the antihypertensive effect of amiloride as an alternative to spironolactone.52 This was an open-label assessment performed after completion of the double-blind, randomized main study protocol, in which participants who were willing to continue in the study were crossed-over from spironolactone to amiloride for 6-12 weeks. In these 146 subjects, amiloride10-20 mg daily reduced home systolic BP by 20.4 mm Hg, an effect comparable to the 18.3 mm Hg reduction induced by spironolactone, suggesting that amiloride can be as effective as spironolactone as a fourth medication in treating RHTN. The amiloride substudy lacked the scientific rigor of the main protocol because of its open-label design and the lower number of participants. Nevertheless, it does provide compelling support for preferential use of amiloride as an alternative to spironolactone, if the latter is not tolerated, in patients with RHTN.
Overall, PATHWAY-2 and its substudies provide clinically important guidance on how to treat RHTN. The study clearly establishes spironolactone as the most appropriate fourth medication for treating RHTN uncontrolled on a standardized triple combination of an ACE inhibitor or ARB, amlodipine, and a long-acting thiazide-like diuretic (chlorthalidone or indapamide). Additional findings demonstrate an effective dose-range for spironolactone up to 50 mg daily, and support use of amiloride as an effective alternative if spironolactone is not tolerated. From a mechanistic standpoint, the findings are important in providing compelling evidence that treatment resistance to the most commonly used classes of antihypertensive agents is broadly attributable to inappropriate volume retention secondary to aldosterone excess, even at low levels. This effect is clearly reflected in the large BP reduction induced by mineralocorticoid receptor (MR) blockade and predicted by suppressed renin levels. This mechanistic insight highlights the need to overcome underlying fluid retention in patients with RHTN through a treatment strategy based on effective dietary sodium restriction and combined use of thiazide-like diuretics and MRAs. Eplerenone, which is more selective for the MR, has also been shown in small studies to be effective in treating patients with RHTN,49 and may be considered when adverse effects such as gynecomastia or vaginal bleeding are noted with spironolactone therapy.
Non-Pharmacologic Therapies
A large body of literature demonstrates consistent benefit of dietary sodium restriction on BP reduction in patients with hypertension.55 This benefit may be greater in patients with RHTN, given the broad role that aldosterone-induced sodium and fluid retention play in contributing to development of RHTN. Such large benefit was suggested in a small study that compared extreme dietary sodium restriction (50 mEq/day) for 7 days to high dietary sodium intake (250 mEq/day) in cross-over evaluation of 12 patients with confirmed RHTN.54 The low compared to the high sodium intake induced a substantial reduction in BP, reducing 24-hr ABP by 20.1/9.8 mm Hg. While the study was limited by the small number of subjects, the findings suggest that some patients with RHTN may be especially salt-sensitive and may have large BP benefit with intensive sodium restriction, consistent with the well-recognized role that aldosterone plays in the pathogenesis of RHTN.
Lifestyle changes known to provide antihypertensive effects in the general hypertensive population, such as weight loss and regular exercise, have not been adequately evaluated in patients with RHTN. Such interventions clearly provide overall CV and metabolic benefits, and likely will provide BP reductions in RHTN patients similar to or greater than those seen in the general hypertensive population, and so should be recommended.
Obstructive sleep apnea (OSA) is especially common in patients with RHTN, with observational studies suggesting prevalence rates as high as 90%, especially in men.56, 57 Studies link this high prevalence of OSA to the aldosterone excess that is common in patients with RHTN.58-60 Antihypertensive treatment resistance and OSA are hypothesized to share a common mechanism, in that aldosterone promotes intravascular fluid retention, thus increasing BP and accumulation of fluid in the parapharyngeal region, promoting increases in upper airway resistance and thereby worsening OSA severity.60 Such an mechanistic effect is supported by studies demonstrating that spironolactone reduces the severity of OSA by about 50% in patients with RHTN.60-62
Use of continuous positive airway pressure (CPAP) to treat OSA tends to induce relatively modest decreases in BP in patients with RHTN, as well as in the general population of patients with hypertension. In a rigorous comparison of CPAP use to no treatment in 194 subjects with RTHN, CPAP reduced 24-hr diastolic BP by 3.2 mmHg and had no significant effect on 24-hr systolic BP overall.63 However, CPAP use was <4 hours/night in 28% of the participants. Among those who used CPAP >4 hours/night, CPAP significantly reduced 24-hr systolic and diastolic BP by 4.4 and 4.1 mm Hg, respectively. The benefit was especially prominent at night, with 7.7 and 4.1 mm Hg reductions in systolic and diastolic BP, respectively. Importantly, there was a significant positive correlation between CPAP use and BP reduction: patients fully adherent with CPAP (>8 hours/night) manifested a reduction in 24-hr systolic BP > 10 mm Hg. Overall, studies indicate that OSA is common in patients with RHTN, and so these patients should be screened intensively for OSA and, when present, should be treated with CPAP. The BP benefit of CPAP in patients with RHTN and OSA rivals that of adding another BP medication in fully adherent patients.
Refractory Hypertension
Refractory hypertension (RfHTN) is a proposed phenotype of antihypertensive treatment failure in which BP remains uncontrolled on maximal or near-maximal therapy.64-66 The current definition of RfHTN is based on failure to control BP with use of 5 or more antihypertensive agents of different classes, including a long-acting thiazide-like diuretic such as chlorthalidone and spironolactone. Observational studies of patients with uncontrolled RHTN who were referred to our hypertension clinic and who were managed by two hypertension specialists, with continued titration and optimization of antihypertensive therapy, suggest that RfHTN is rare, affecting <5% of patients.64,-66 Patients with RfHTN, identified after routine clinical follow-up of ≥3 visits for ≥ 6 months, had uncontrolled BP in spite of being adherent to a regimen of >5 classes of antihypertensive agents, including chlorthalidone 25 mg daily and an MRA (spironolactone 25 mg daily or eplerenone 50 mg twice daily) without evidence of underlying secondary causes of hypertension.64 Risk factors for RfHTN, including African American race, obesity, diabetes, and CKD overlap with those for RHTN. However, patients with RfHTN tend to be younger and more often female than those with RHTN. Not surprisingly, given their long history of poorly controlled, often severe hypertension, patients with RfHTN frequently manifest target organ damage, including left ventricular hypertrophy and heart failure with preserved left ventricular ejection fraction.65-67
The patients with RfHTN in our study underwent transthoracic impedance cardiography to measure thoracic fluid content and systemic vascular resistance, as well as assessment of pulse wave velocity, a measure of arterial stiffness. Indirect evidence suggests that antihypertensive treatment failure in RfHTN, as opposed to the much larger category of patients with RHTN, may not be secondary to persistent excess fluid retention, but instead is likely neurogenic in etiology, and attributable to heightened sympathetic outflow. This potentially important mechanistic distinction is based on persistently higher heart rates, greater vascular stiffness as indexed by pulse wave velocity, and greater 24-hour excretion of urinary norepinephrine typical of patients with RfTHN compared to patients with controlled RHTN.68 In contrast, indices of volume status, including renin activity, aldosterone levels, urinary sodium excretion, natriuretic peptide levels, and intracardiac volumes, are similar or lower in patients with RfHTN compared to patients with controlled RHTN, suggesting that persistent fluid retention does not contribute to their antihypertensive treatment failure.65, 68 These findings suggest that with use of effective doses of chlorthalidone and spironolactone, any underlying fluid retention is largely overcome, and the continued failure to control BP in patients with RfHTN is attributable to other causes, particularly excess sympathetic outflow.
Effective treatment for RfHTN, by definition, remains unavailable. By having RfHTN these patients have failed all or nearly all available classes of antihypertensive agents. Apparent normalization of volume status with use of chlorthalidone and spironolactone fails to control BP in patients with RfHTN, suggesting that further intensification of diuretic therapy is unlikely to be effective. Based on the evidence implicating heightened sympathetic tone as an important mediator of the treatment failure, sympatholytic agents should be beneficial, but use of effective doses of currently available agents that inhibit sympathetic output, such as clonidine, is often precluded by intolerable adverse effects. Accordingly, effective management of RfHTN may be dependent on development of more effective and better tolerated sympatholytic compounds, or perhaps use of evolving, but still unproven device-based strategies, such as renal denervation or carotid sinus activation.
Box 1:
Definitions of terms
| Resistant hypertension (RHTN) | BP that remains elevated above goal in spite of the concurrent use of ≥3 antihypertensive agents of different classes, commonly including a long-acting calcium channel blocker, a blocker of the renin-angiotensin system and a diuretic, administered at maximal or maximally tolerated doses, and requires exclusion of common causes of pseudoresistance. |
| Pseudoresistance | Factors that can cause a falsely elevated BP in a patient on ≥3 antihypertensive agents, such as improper BP measurement technique, white coat RHTN, undertreatment, clinical inertia, and medication non-adherence |
| Clinical inertia | The failure to establish appropriate targets and escalate treatment to achieve treatment goals |
| Controlled resistant hypertension | BP that is controlled on ≥4 antihypertensive medications at maximal or maximally tolerated doses. |
| Apparent treatment resistant hypertension | Term used when medication dose, adherence, or out-of-office BP is not documented or accounted for, and pseudoresistance cannot be excluded in a patient on ≥3 antihypertensive agents. |
| White coat resistant hypertension | Term used when office BP is uncontrolled but out-of-office BP monitoring shows controlled SBP and DBP values in a patient on ≥3 antihypertensive agents. |
| Refractory hypertension | BP that remains uncontrolled on maximal or near-maximal therapy, which is the use of 5 or more antihypertensive agents of different classes, including a long-acting thiazide-like diuretic (such as chlorthalidone) and spironolactone. |
Abbreviations: BP – blood pressure, SBP systolic blood pressure, DBP – diastolic blood pressure
Acknowledgments
Sources of funding
The National Institutes of Health (NIH R01 HL113004) and the American Heart Association Strategically Focused Research Network (AHA 5SFRN2390002) supported this research.
Nonstandard Abbreviations and Acronyms:
- ACE
angiotensin-converting enzyme
- ARB
angiotensin receptor blocker
- BP
blood pressure
- CAD
coronary artery disease
- CPAP
continuous positive airway pressure
- CRIC
Chronic Renal Insufficiency Cohort
- CVD
cardiovascular disease
- INVEST
International Verapamil SR-Trandolapril Study
- MR
mineralocorticoid receptor
- OSA
obstructive sleep apnea
- PATHWAY-2
Prevention and Treatment of Hypertension With Algorithm Based Therapy
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
- RfHTN
refractory hypertension
- RHTN
resistant hypertension
Footnotes
Conflicts of Interest/Disclosures
Dr. Calhoun has received research support from ReCor Medical and has served as a consultant Selenity Therapeutics and Idorsia Pharmaceuticals.
Dr. Oparil reports grant/personal fees/non-financial support from NIH/NIAMS, NIH/NHLBI, 98point6, Inc., Actelion/George Clinical, Bayer, Idorsia Pharmaceuticals Ltd., Novartis, Pfizer, ROX Medical.
Dr. Acelajado and Zachary Hughes do not have any potential conflict of interest.
References
- 1.Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB; on behalf of the American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Peripheral Vascular Disease; Council on Quality Care and Outcomes Research; and Stroke Council. Resistant hypertension: detection, evaluation and management: a scientific statement from the American Heart Association. Hypertension. 2018;72:e53–e90. DOI: 10.1161/HYP.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acelajado MC and Calhoun DA. Aldosteronism and resistant hypertension. Int J Hypertens. 2011; 2011:837817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B and Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–19. [DOI] [PubMed] [Google Scholar]
- 4.Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). ALLHAT Collaborative Research Group. JAMA. 2000;283:1967–75. [PubMed] [Google Scholar]
- 5.Daugherty SL, Powers JD, Magid DJ, Tavel HM, Masoudi FA, Margolis KL, O’Connor PJ, Selby JV and Ho PM. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmqvist L, Bostrom KB, Kahan T, Schioler L, Hasselstrom J, Hjerpe P, Wettermark B and Manhem K. Cardiovascular outcome in treatment-resistant hypertension: results from the Swedish Primary Care Cardiovascular Database (SPCCD). J Hypertens. 2018; 36(2):402–409. [DOI] [PubMed] [Google Scholar]
- 7.Sim JJ, Bhandari SK, Shi J, Reynolds K, Calhoun DA, Kalantar-Zadeh K and Jacobsen SJ. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int. 2015;88:622–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith SM, Gong Y, Handberg E, Messerli FH, Bakris GL, Ahmed A, Bavry AA, Pepine CJ and Cooper-Dehoff RM. Predictors and outcomes of resistant hypertension among patients with coronary artery disease and hypertension. J Hypertens. 2014;32:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith SM, Huo T, Delia Johnson B, Bittner V, Kelsey SF, Vido Thompson D, Noel Bairey Merz C, Pepine CJ and Cooper-Dehoff RM. Cardiovascular and mortality risk of apparent resistant hypertension in women with suspected myocardial ischemia: a report from the NHLBI-sponsored WISE Study. J Am Heart Assoc. 2014;3:e000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, Saxena M, Feyz L, Rader F, Lurz P, Sayer J, Sapoval M, Levy T, Sanghvi K, Abraham J, Sharp ASP, Fisher NDL, Bloch MJ, Reeve-Stoffer H, Coleman L, Mullin C, Mauri L and Investigators R-H. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391:2335–2345. [DOI] [PubMed] [Google Scholar]
- 11.Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K and Investigators SH-OMT. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. [DOI] [PubMed] [Google Scholar]
- 12.Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017; 390:2160–2170. [DOI] [PubMed] [Google Scholar]
- 13.Bhatt H, Siddiqui M, Judd E, Oparil S and Calhoun D. Prevalence of pseudoresistant hypertension due to inaccurate blood pressure measurement. J Am Soc Hypertens. 2016;10:493–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Sierra A, Segura J, Banegas JR, Gorostidi M, de la Cruz JJ, Armario P, Oliveras A and Ruilope LM. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902. [DOI] [PubMed] [Google Scholar]
- 15.Muxfeldt ES, Fiszman R, de Souza F, Viegas B, Oliveira FC, Salles GF. Appropriate time interval to repeat ambulatory blood pressure monitoring in patients with white-coat resistant hypertension. Hypertension. 2012; 59(2):384–9. [DOI] [PubMed] [Google Scholar]
- 16.Mu L, Mukamal KJ. Treatment intensification for hypertension in US ambulatory medical care. J Am Heart Assoc. 2016;5:e004188. doi: 10.1161/JAHA.116.004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan BM, Zhao Y, Li J, Brzezinski WA, Todoran TM, Brook RD and Calhoun DA. Prevalence of optimal treatment regimens in patients with apparent treatment-resistant hypertension based on office blood pressure in a community-based practice network. Hypertension. 2013;62:691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung O, Gechter JL, Wunder C, Paulke A, Bartel C, Geiger H and Toennes SW. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens. 2013;31:766–74. [DOI] [PubMed] [Google Scholar]
- 19.Brinker S, Pandey A, Ayers C, Price A, Raheja P, Arbique D, Das SR, Halm EA, Kaplan NM and Vongpatanasin W. Therapeutic drug monitoring facilitates blood pressure control in resistant hypertension. J Am Coll Cardiol. 2014;63:834–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceral J, Habrdova V, Vorisek V, Bima M, Pelouch R and Solar M. Difficult-to-control arterial hypertension or uncooperative patients? The assessment of serum antihypertensive drug levels to differentiate non-responsiveness from non-adherence to recommended therapy. Hypertens Res. 2011;34:87–90. [DOI] [PubMed] [Google Scholar]
- 21.Forouzanfar MH et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016; 388: 1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas G, Xie D, Chen HY, Anderson AH, Appel LJ, Bodana S, Brecklin CS, Drawz P, Flack JM, Miller ER 3rd, Steigerwalt SP, Townsend RR, Weir MR, Wright JT Jr. and Rahman M. Prevalence and prognostic significance of Apparent Treatment Resistant Hypertension in Chronic Kidney Disease: Report from the Chronic Renal Insufficiency Cohort Study. Hypertension. 2016;67:387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bangalore S, Fayyad R, Laskey R, Demicco DA, Deedwania P, Kostis JB and Messerli FH. Prevalence, predictors, and outcomes in treatment-resistant hypertension in patients with coronary disease. Am J Med. 2014;127:71–81. [DOI] [PubMed] [Google Scholar]
- 24.Kumbhani DJ, Steg PG, Cannon CP, Eagle KA, Smith SC Jr, Crowley K, Goto S, Ohman EM, Bakris GL, Perlstein TS, Kinlay S, Bhatt DL; REACH Registry Investigators. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J. 2013; 34(16):1204–14. [DOI] [PubMed] [Google Scholar]
- 25.Irvin MR, Booth JN 3rd, Shimbo D, Lackland DT, Oparil S, Howard G, Safford MM, Muntner P and Calhoun DA. Apparent treatment-resistant hypertension and risk for stroke, coronary heart disease, and all-cause mortality. J Am Soc Hypertens. 2014;8:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egan BM, Kai B, Wagner CS, Henderson JH, Chandler AH and Sinopoli A. Blood pressure control provides less cardiovascular protection in adults with than without apparent treatment-resistant hypertension. J Clin Hypertens. (Greenwich). 2016;18:817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz KM, Booth JN III, Calhoun DA, Irvin MR, Howard G, Safford MM, Muntner P, Shimbo D. Healthy lifestyle factors and risk of cardiovascular events and mortality in treatment-resistant hypertension: The Reasons for Geographic and Racial Differences in Stroke Study. Hypertension. 2014;64:465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S, Brown MJ and British Hypertension Society’s PSG. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheen AJ. Renin-angiotensin system inhibition prevents type 2 diabetes mellitus. Part 1. A meta-analysis of randomised clinical trials. Diabetes Metab. 2004;30:487–96. [DOI] [PubMed] [Google Scholar]
- 30.Abuissa H, Jones PG, Marso SP and O’Keefe JH Jr. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J Am Coll Cardiol. 2005;46:821–6. [DOI] [PubMed] [Google Scholar]
- 31.Fu M, Zhou J, Sun A, Zhang S, Zhang C, Zou Y, Fu M and Ge J. Efficacy of ACE inhibitors in chronic heart failure with preserved ejection fraction--a meta-analysis of 7 prospective clinical studies. Int J Cardiol. 2012;155:33–8. [DOI] [PubMed] [Google Scholar]
- 32.Angiotensin Sleight P. II and trials of cardiovascular outcomes. Am J Cardiol. 2002;89:11A–16A; discussion 16A-17A. [DOI] [PubMed] [Google Scholar]
- 33.Sleight P The renin-angiotensin system: a review of trials with angiotensin-converting enzyme inhibitors and angiotensin receptor blocking agents. Eur Heart J Supplements. 2002;Suppl A:A53–A57. [Google Scholar]
- 34.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–64. [PubMed] [Google Scholar]
- 35.Ernst ME, Carter BL, Goerdt CJ, Steffensmeier JJ, Phillips BB, Zimmerman MB and Bergus GR. Comparative antihypertensive effects of hydrochlorothiazide and chlorthalidone on ambulatory and office blood pressure. Hypertension. 2006;47:352–8. [DOI] [PubMed] [Google Scholar]
- 36.Group SR, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK and Ambrosius WT. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khosla N, Chua DY, Elliott WJ and Bakris GL. Are chlorthalidone and hydrochlorothiazide equivalent blood-pressure-lowering medications? J Clin Hypertens. 2005;7:354–6. [DOI] [PubMed] [Google Scholar]
- 38.Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB and Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–6. [DOI] [PubMed] [Google Scholar]
- 39.Eide IK, Torjesen PA, Drolsum A, Babovic A and Lilledahl NP. Low-renin status in therapy-resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22:2217–26. [DOI] [PubMed] [Google Scholar]
- 40.Gallay BJ, Ahmad S, Xu L, Toivola B and Davidson RC. Screening for primary aldosteronism without discontinuing hypertensive medications: plasma aldosterone-renin ratio. Am J Kidney Dis. 2001;37:699–705. [DOI] [PubMed] [Google Scholar]
- 41.Strauch B, Zelinka T, Hampf M, Bernhardt R and Widimsky J Jr. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J Hum Hypertens. 2003;17:349–52. [DOI] [PubMed] [Google Scholar]
- 42.Chapman N, Dobson J, Wilson S, Dahlof B, Sever PS, Wedel H, Poulter NR and Anglo-Scandinavian Cardiac Outcomes Trial I. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839–45. [DOI] [PubMed] [Google Scholar]
- 43.Gaddam KK, Nishizaka MK, Pratt-Ubunama MN, Pimenta E, Aban I, Oparil S and Calhoun DA. Characterization of resistant hypertension: association between resistant hypertension, aldosterone, and persistent intravascular volume expansion. Arch Intern Med. 2008;168:1159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dudenbostel T, Ghazi L., Liu M, Li P, Oparil S, Calhoun DA. Body mass index predicts 24-hr urinary aldosterone levels in patients with resistant hypertension. Hypertension. 2016. 68(4):995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, Hauner H, McCann SM, Scherbaum WA and Bornstein SR. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci USA. 2003;100:14211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huby AC, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA and Belin de Chantemele EJ. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation. 2015;132:2134–45. [DOI] [PubMed] [Google Scholar]
- 47.Alvarez-Alvarez B, Abad-Cardiel M, Fernandez-Cruz A and Martell-Claros N. Management of resistant arterial hypertension: role of spironolactone versus double blockade of the renin-angiotensin-aldosterone system. J Hypertens. 2010;28:2329–35. [DOI] [PubMed] [Google Scholar]
- 48.Calhoun DA and White WB. Effectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertension. J Am Soc Hypertens. 2008;2:462–8. [DOI] [PubMed] [Google Scholar]
- 49.Nishizaka MK, Zaman MA and Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–30. [DOI] [PubMed] [Google Scholar]
- 50.Ouzan J, Perault C, Lincoff AM, Carre E and Mertes M. The role of spironolactone in the treatment of patients with refractory hypertension. Am J Hypertens. 2002;15:333–9. [DOI] [PubMed] [Google Scholar]
- 51.Vaclavik J, Sedlak R, Jarkovsky J, Kocianova E and Taborsky M. Effect of spironolactone in resistant arterial hypertension: a randomized, double-blind, placebo-controlled trial (ASPIRANT-EXT). Medicine (Baltimore). 2014;93:e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams B, MacDonald TM, Morant SV, Webb DJ, Sever P, McInnes GT, Ford I, Cruickshank JK, Caulfield MJ, Padmanabhan S, Mackenzie IS, Salsbury J, Brown MJ; British Hypertension Society programme of Prevention And Treatment of Hypertension With Algorithm based Therapy (PATHWAY) Study Group. Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6(6):464–475. doi: 10.1016/S2213-8587(18)30071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaddam K, Corros C, Pimenta E, Ahmed M, Denney T, Aban I, Inusah S, Gupta H, Lloyd SG, Oparil S, Husain A, Dell’Italia LJ and Calhoun DA. Rapid reversal of left ventricular hypertrophy and intracardiac volume overload in patients with resistant hypertension and hyperaldosteronism: a prospective clinical study. Hypertension. 2010;55:1137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell’Italia LJ and Calhoun DA. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009;54:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He FJ and MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2004:CD004937. [DOI] [PubMed] [Google Scholar]
- 56.Logan AG, Perlikowski SM, Mente A, Tisler A, Tkacova R, Niroumand M, Leung RS and Bradley TD. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19:2271–7. [DOI] [PubMed] [Google Scholar]
- 57.Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM and Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131:453–9. [DOI] [PubMed] [Google Scholar]
- 58.Gonzaga CC, Gaddam KK, Ahmed MI, Pimenta E, Thomas SJ, Harding SM, Oparil S, Cofield SS and Calhoun DA. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med. 2010;6:363–8. [PMC free article] [PubMed] [Google Scholar]
- 59.Ke X, Guo W, Peng H, Hu C, Zhang H, Peng C and Wang X. Association of aldosterone excess and apnea-hypopnea index in patients with resistant hypertension. Sci Rep. 2017;7:45241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaddam K, Pimenta E, Thomas SJ, Cofield SS, Oparil S, Harding SM and Calhoun DA. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens. 2010;24:532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krasinska B, Miazga A, Cofta S, Szczepaniak-Chichel L, Trafas T, Krasinski Z, Pawlaczyk-Gabriel K and Tykarski A. Effect of eplerenone on the severity of obstructive sleep apnea and arterial stiffness in patients with resistant arterial hypertension. Pol Arch Med Wewn. 2016;126:330–9. [DOI] [PubMed] [Google Scholar]
- 62.Yang L, Zhang H, Cai M, Zou Y, Jiang X, Song L, Liang E, Bian J, Wu H and Hui R. Effect of spironolactone on patients with resistant hypertension and obstructive sleep apnea. Clin Exp Hypertens. 2016;38:464–8. [DOI] [PubMed] [Google Scholar]
- 63.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310:2407–15. [DOI] [PubMed] [Google Scholar]
- 64.Acelajado MC, Pisoni R, Dudenbostel T, Dell’Italia LJ, Cartmill F, Zhang B, Cofield SS, Oparil S and Calhoun DA. Refractory hypertension: definition, prevalence, and patient characteristics. Journal of clinical hypertension. 2012;14:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dudenbostel T, Acelajado MC, Pisoni R, Li P, Oparil S and Calhoun DA. Refractory hypertension: Evidence of heightened sympathetic activity as a cause of antihypertensive treatment failure. Hypertension. 2015;66:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao G, Chen C, Lin Q, Chen Y, Zhen Z, Zou Y, Liu J, Wu M, Wang R, Liu M, Zhao C, Lu S, Ng MY, Tse HF, Yiu KH. Prevalence, clinical characteristics and echocardiography parameters of non-resistant, resistant and refractory hypertension in Chinese. Postgrad Med. 2017;129:187–192. [DOI] [PubMed] [Google Scholar]
- 67.Modolo R, de Faria AP, Sabbatini AR, Barbaro NR, Ritter AM and Moreno H. Refractory and resistant hypertension: characteristics and differences observed in a specialized clinic. J Am Soc Hypertens. 2015;9:397–402. [DOI] [PubMed] [Google Scholar]
- 68.Velasco A, Siddiqui M, Kreps E, Kolakalapudi P, Dudenbostel T, Arora G, Judd EK, Prabhu SD, Lloyd SG, Oparil S and Calhoun DA. Refractory hypertension Is not attributable to intravascular fluid retention as determined by intracardiac volumes. Hypertension. 2018;72:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]