Abstract
Objective:
To assess the effects of protocolized sedation (algorithm or daily interruption) compared with usual care without protocolized sedation on clinical outcomes in mechanically ventilated adult intensive care unit (ICU) patients via a systematic review and meta-analysis of randomized controlled trials (RCTs).
Methods:
We searched Ovid MEDLINE, EMBASE, Cochrane CENTRAL, Web of Science, and ClinicalTrials.gov from their inception to February 28, 2013. A random-effects model was used to synthesize risk ratios (RRs) and weighted mean differences (WMDs).
Results:
Of 4782 records screened, 6 RCTs including 1243 patients met the inclusion criteria. Protocolized sedation was associated with significant reductions in overall mortality (RR, 0.85; 95% CI, 0.74 to 0.97; P=.02; number needed to treat, 20; P=.11), ICU length of stay (WMD, −1.73 days; 95% CI, −3.32 to −0.14 days; P=.03), hospital length of stay (WMD, −3.55 days; 95% CI, −5.98 to −1.12 days; P=.004), and tracheostomy (RR, 0.69; 95% CI, 0.50 to 0.96; P=.03; number needed to treat, 16.6; P=.04; 5 RCTs) compared with usual care. Protocolized sedation produced no significant differences in duration of mechanical ventilation (WMD, −1.04 days; 95% CI, −2.54 to 0.47 days; P=.18), reintubation (RR, 0.78; 95% CI, 0.52 to 1.15; P=.21; 3 RCTs), and self-extubation (RR, 1.49; 95% CI, 0.46 to 4.82; P=.51; 4 RCTs) compared with usual care. Included studies did not report delirium incidence.
Conclusion:
In mechanically ventilated adults in closed, nonspecialty ICUs, protocolized sedation seems to decrease overall mortality (15%), ICU and hospital lengths of stay (1.73 and 3.55 days, respectively), and tracheostomy (31%) compared with usual care without protocolized sedation.
More than 790,000 patients require mechanical ventilation each year in the United States.1 Sedation is commonly used in the intensive care unit (ICU) to facilitate the use of mechanical ventilation and mitigate the symptoms of pain and agitation.2 However, there is considerable variation in what constitutes optimal sedation; consequently, variation in sedation practice may lead to undersedation or, more likely, oversedation.3 Inappropriate sedation, for example, oversedation, is associated with adverse clinical outcomes, including a longer duration of mechanical ventilation, prolonged ICU length of stay (LOS), episodes of delirium, and increased mortality.4,5
Protocolized sedation (algorithm or daily interruption) intends to reduce variation in clinical care by reducing subjectivity in clinical decision making, thus replacing ICU staff discretion with evidence-based protocols that standardize sedation management.6 In 1999 and 2000, 2 landmark randomized controlled trials (RCTs) by Brook et al7 and Kress et al,8 respectively, suggested that protocolized (standardized) sedation significantly improved several clinical outcomes, including duration of mechanical ventilation, compared with usual care without protocolized (standardized) sedation. Between 2008 to 2011, 4 more RCTs9–12 were published; chief among them was the study by Girard et al,11 which reported more ventilator-free days; however, most studies had varying, and sometimes conflicting, results for common clinical outcomes reported across studies (eg, ICU LOS). To our knowledge, a quantitative summary of RCTs examining standardized vs nonstandardized discretionary sedation management does not exist.
We conducted a systematic review and meta-analysis of the current body of literature to summarize RCTs that evaluated protocolized sedation vs usual care without protocolized sedation in mechanically ventilated adult ICU patients and that reported 1 or more of the prespecified clinical outcomes.
METHODS
This systematic review and meta-analysis was guided by the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement.13
Study Eligibility Criteria
We included RCTs that studied the effect of protocolized sedation vs usual care without protocolized sedation in mechanically ventilated adult (≥18 years old) ICU patients and reported 1 or more of the following clinical outcomes: mortality, duration of mechanical ventilation, ICU or hospital LOS, and incidence of tracheostomy, reintubation, self-extubation, and delirium. We defined protocolized sedation as either of the following 2 standardized sedation management strategies used to treat ICU patients: a sedation algorithm or a daily sedation interruption. We defined usual care as nonprotocolized, discretion-based sedation management (eg, clinician-directed sedation).
Search Methods
We searched the following databases from inception through February 28, 2013: Ovid MEDLINE, EMBASE, Cochrane CENTRAL, Web of Science, and ClinicalTrials.gov. We used Cochrane’s Highly Sensitive Search Strategy for RCTs in MEDLINE.14 No search had language or other limitations. One reviewer (M.A.M.) excluded duplicate articles and clearly ineligible studies based on title and abstract. Two reviewers (M.A.M. and A.G.V.) independently screened the remaining articles in full text to determine review eligibility based on the inclusion criteria. In all cases, a third reviewer (A.K.), or group consensus, resolved disagreements. Last, the reference section of included studies was searched. The Supplemental Appendix (available online at http://www.mayoclinicproceedings.org) provides full details of the Ovid MEDLINE search strategy.
Data Extraction
Two reviewers (M.A.M. and A.G.V.) independently conducted unblinded data extraction for each included study using standardized pro forma. Discrepancies were resolved via group discussion. Information extracted from each study included publication date, hospital location, sample size, ICU type and setup, sedatives used, sedation scale and weaning protocol use, follow-up, patient characteristics, inclusion and exclusion criteria, and clinical outcomes listed under study eligibility criteria.
Assessment of Bias Risk
We assessed methodological quality using Cochrane’s domain-based risk of bias tool (Supplemental Figure; available online at http://www.mayoclinicproceedings.org).15 Two reviewers (M.A.M. and A.K.) independently extracted data from each included study for the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias risk. A third reviewer (A.G.V.) resolved assessment disagreements.
Data Synthesis and Analysis
We used Cochrane Review Manager version 5.2 software to calculate summary risk ratios (RRs) and the number needed to treat (NNT) for dichotomous outcomes and weighted mean differences (WMDs) for continuous outcomes, along with their respective 95% CIs. A random effects model was used to account for variations in protocol design among the included studies.
Overall mortality was analyzed as follows: we pooled the longest followed mortality measure reported in each study, to avoid double counting. For example, Bucknall et al12 reported both ICU and hospital mortality, but only hospital mortality was included in the overall mortality summary estimate.
Missing Data
We emailed all 6 study corresponding authors for missing or supplemental data. Girard et al11 provided mean duration of mechanical ventilation data, and 5 authors7–11 provided supplemental data. However, Bucknall et al12 did not reply to requests for supplemental data. When means and standard deviations were not published, we estimated those values from other information contained in the published report as follows: if the study provided a median, we used that value as an appropriate estimate of central tendency and estimated the standard deviation from the interquartile range8,9,11,12 or, if the interquartile range was not provided, the range,10,12 using established formulas.14,16
In the study by Girard et al,11 the upper quartile for hospital LOS was not quantifiable in the control group because 75% or less of patients had been discharged by the end of the 28-day hospital follow-up. We imputed a worst-case estimate for the upper quartile by extrapolating the control arm of the outcome’s published Kaplan-Meier curve.11 The aforementioned methods were applied to the interquartile range.
Identifying and Addressing Heterogeneity
We identified the presence of between-study heterogeneity in studies contributing to each outcome’s summary estimate via a standard χ2 test, where P<.10 indicated significant heterogeneity. Owing to the low power of this test, we also quantified between-study inconsistency via the I2 statistic, which ranges from 0% to 100% and assesses what percentage of the variation in the effect estimates is due to heterogeneity. An I2≥50% indicated substantial heterogeneity.14 We addressed heterogeneity by examining, in full text, individual studies contributing to a summary estimate and excluded the single most outlying study in a sensitivity analysis. All other statistical tests were considered significant at 2-tailed P≤.05.
Assessment of Reporting Bias
We aimed to assess for publication bias by plotting each included RCT’s effect size by its sample size for duration of mechanical ventilation. The resulting funnel plot was to be visually inspected for symmetry and evidence of publication bias.17 However, the meta-analysis included fewer than 10 studies; therefore, funnel plots to assess for publication bias were not produced because they would be inadequately powered to detect real asymmetry—and evidence of publication bias.14
Subgroup Analyses
We did not perform any subgroup analyses.
Sensitivity Analyses
We performed a sensitivity analysis to explore the impact that a single excluded study had on I2, if I2≥50% in studies contributing to an outcome with a heterogeneous summary estimate.
RESULTS
Identification and Selection of Studies
As outlined in Figure 1, the initial search produced 4782 studies; of these, 894 duplicates were removed. We further excluded 3720 studies on the basis of title and abstract and retrieved 168 studies for full-text review. Ultimately, 6 RCTs with 1243 randomized patients reporting 461 nonduplicated deaths met the inclusion criteria.7–12
FIGURE 1.
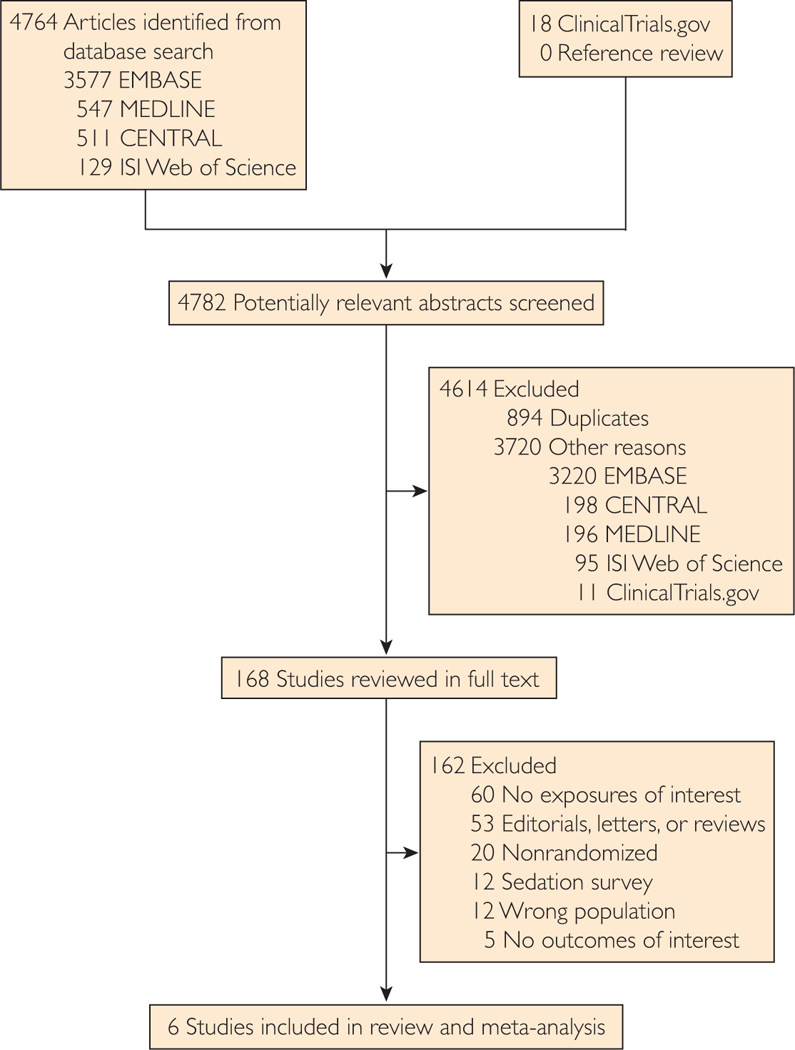
Study selection flowchart.
Characteristics of the Included Studies
Characteristics of the included studies are summarized in the Table. Three studies were conducted in the United States,7,8,11 2 in Australia,9,12 and 1 in Greece.10 The first 2 studies evaluating sedation protocols were published in 1999 and 2000; the remaining 4 were published between 2008 and 2011. Only 2 of the 6 studies were multicentered.9,11 Three studies had medical ICUs,7,8,11 2 had medical ICUs combined with 1 or more specialties,9,10 and 1 had a general ICU.12 Girard et al11 used both open and closed ICUs, and the remaining studies had closed ICUs. Follow-up was variable and ranged from hospital discharge to 1 year. Follow-up was unclear in the study by Bucknall et al,12 so we estimated duration based on the longest followed mortality measure.
TABLE.
Characteristics of Included Randomized Controlled Trials Comparing Protocoled Sedation vs Usual Care Without Protocoled Sedation on Clinical Outcomes in Mechanically Ventilated Adult ICU Patientsa
| Reference, year, location |
Sample size |
ICU type (setup) |
Age (y). mean |
Male sex (%) |
APACHE II score, Mean |
Most cases | Drugs used | Sedation scale (Weaning protocol) |
Intervention | Control | Follow-up | Reported outcomesb |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | I | C | ||||||||||
| Brook et al,7
1999 Single center United States |
321 | Medical (closed) |
57.8 | 58.1 | 51.2 | 47.2 | 23.1 | 23.2 | Pneumonia | Diazepam, fentanyl, lorazepam, midazolam, morphine, haloperidol |
Ramsay (Yes) |
Nurse- implemented continuous sedation algorithm (n=162) |
Physician-directed continuous sedation (n= 159) |
Hospital dischargec |
1, 3–7 |
| Kress et al,8
2000 Single center United States |
128 | Medical (closed)c |
57d | 6ld | 50 | 43.3 | 20d | 22d | COPD or ventilatory failure |
Midazolam, morphine, propofol |
Ramsay (Yesc) |
Daily interruption of sedation until patients were awake (n=68) |
ICU physician— directed sedative interruption (n=60) |
Hospital discharge |
1, 3–8 |
| Bucknall et al,12 2008 Single center Australia |
312 | General (closed) |
58.2 | 56.1 | 64 | 58 | 18.6 | 19.5 | Medical | Diazepam, haloperidol, fentanyl, midazolam, morphine, propofol |
SAS (No) |
Nurse- implemented sedation algorithm (n=159) |
Sedation medication type and dose limits for infusion and boluses prescribed by ICU medical staff (n=153) |
Hospital dischargee |
0, 1, 3–6, 8 |
| Girard et al,11
2008 Multicenter United States |
335 | Medical (mixed)c |
60d | 64d | 54 | 51 | 26d | 26.5d | Sepsis/ARDS | Benzodiazepines,f haloperidol, opiates,f propofol |
RASS (Yes) |
Nurse- implemented daily interruption of sedation plus a daily spontaneous breathing trial (n=167) |
Sedation per usual care plus a daily spontaneous breathing trial (n=168) |
1 y | 2, 3c, 4, 5g, 6–8 |
| Anifantaki et al,10 2009 Single center Greece |
97 | Medical; surgical (closed)c |
52.1 | 56.1 | 71.4 | 72.9 | 17.4 | 18.9 | Trauma | Fentanyl, midazolam, morphine, propofol, remifentanil |
Ramsay (NR) |
Nurse- implemented daily interruption of sedation (n=49) |
ICU medical staff— directed sedation (n=48) |
Hospital discharge |
1, 3–5 |
| Weisbrodt et al,9 2011 Multicenter Australia |
50 | Medical surgical trauma (closed)c |
65.1 | 69.1 | 54 | 71 | 23.4 | 21.4 | Respiratory | Fetanyl, midazolam, propofol |
RASS (No) | Daily interruption of fentanyl or midazolam infusions for up to 6 h (n=26) |
Usual management of sedation in Australia (n=24) |
6 moc | 0, 2–6 |
APACHE II = Acute Physiology and Chronic Health Evaluation II; ARDS = acute respiratory disease syndrome; C = control; COPD = chronic obstructive pulmonary disease; 1 = intervention; ICU = intensive care unit; NR = not reported; RASS = Richmond Agitation Sedation Scale; SAS = Riker Sedation-Agitation Scale.
0 = ICU mortality, 1 = hospital mortality 2 = 6- to 12-month mortality, 3 = duration of mechanical ventilation, 4 = ICU length of stay, 5 = hospital length of stay, 6 = incidence of tracheostomy, 7 = incidence of reintubation, 8 = incidence of self-extubation, 9 = incidence of delirium.
Reported via email.
Values are medians not means.
Estimated based on the longest followed mortality measure.
Benzodiazepines as lorazepam and opiates as fentanyl equivalents.
Median hospital length of stay is reported; however, interquartile range is missing in the control group.
The total number of patients per study ranged from 50 to 335; US trials generally enrolled more patients. The mean age ranges of the intervention and control groups of the included studies were 52.1 to 65.1 years and 56.1 to 69.1 years, respectively. The percentages of males in the intervention and control groups were 50.0% to 71.4% and 43.3% to 72.9%, respectively. The mean APACHE II (Acute Physiology and Chronic Health Evaluation II) score ranges of the intervention and control groups were 17.4 to 26.0 and 18.9 to 26.5, respectively. Except for the study by Anifantaki et al,10 in which trauma was more prominent, most patients were admitted to the ICU for medical reasons.
Sedatives varied, but the most commonly used drugs were fentanyl, midazolam, and propofol. All the studies used sedation assessment scales, the most common being the Ramsay sedation scale, which was used in 3 studies.7,8,10 Three studies used weaning protocols.7,8,11 Sedation strategies varied in design (algorithm vs daily interruption) and implementation (nurse vs non-nurse) but were consistent in their intention to standardize sedation management. Four studies used daily interruption,8–11 and the remaining 2 used an algorithm.7,12 Similarly, 4 studies were nurse implemented,7,10–12 and 2 were not.8,9 Studies varied in reporting clinical outcomes (Table); the duration of mechanical ventilation and ICU and hospital LOSs were universally reported, whereas the incidence of delirium was not reported at all.
Risk of Bias Assessment
The risk of bias for included studies is summarized in the Supplemental Figure. The overall quality of evidence was moderate. Five of the 6 studies provided sufficient information to assess 5 or more bias categories. Two studies described participant randomization but did not discuss the technique.7,12 Except for Anifantaki et al,10 all the studies adequately described allocation concealment, and only the study by Weisbrodt et al9 was double blinded. Two studies8,10 provided insufficient attrition information to assess for incomplete outcome data. Only 2 studies11,12 published their protocols, allowing for selective outcome reporting assessment; however, most reported the expected clinical outcomes. The basis for “other bias” varied, but the most common reason for high risk included aberrations in ICU bed and nurse supply.
Study Outcomes
Overall Mortality.
After pooling the longest followed mortality from each included study, protocolized sedation was associated with a significant reduction in overall mortality compared with usual care without protocolized sedation (RR, 0.85; 95% CI, 0.74 to 0.97; P=.02; I2=0%; NNT, 20; P=.11) (Figure 2).
FIGURE 2.
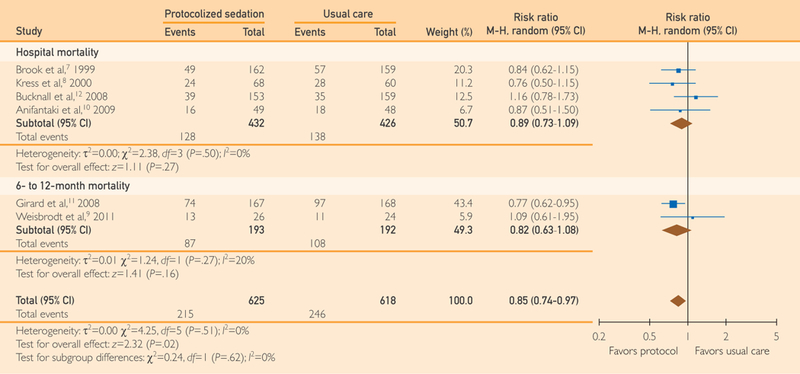
Forest plot of the effect of protocolized sedation compared with usual care without protocolized sedation on overall mortality. Diamond summary estimate pooling the longest followed mortality from each study (ie, pooled subtotals) is presented last. Square size is proportional to study weight in this random effects meta-analysis. M-H indicates Mantel-Haenszel.
Duration of Mechanical Ventilation.
Among the included studies, protocolized sedation produced no significant difference in mean days of mechanical ventilation compared with usual care without protocolized sedation (WMD, –1.04 days; 95% CI, –2.54 to 0.47 days; P=.18; I2=74%) (Figure 3).
FIGURE 3.
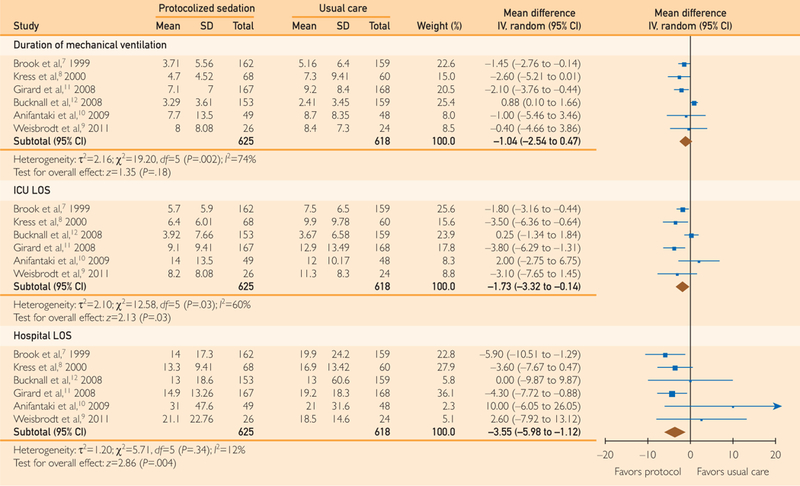
Forest plot of the effect of protocolized sedation compared with usual care without protocolized sedation on days of mechanical ventilation and intensive care unit (ICU) and hospital lengths of stay (LOSs). Diamond summary estimate is presented last for each outcome. Square size is proportional to study weight in this random effects meta-analysis. IV indicates inverse variance.
ICU and Hospital LOSs.
Among the included studies, protocolized sedation was associated with a significant reduction in mean ICU LOS (WMD, –1.73 days; 95% CI, –3.32 to –0.14 days; P=.03; I2=60%) and mean hospital LOS (WMD, –3.55 days; 95% CI, –5.98 to –1.12 days; P=.004; I2=12%) compared with usual care without protocolized sedation (Figure 3).
Tracheostomy.
Among the 5 reporting studies7–9,11,12 protocolized sedation was associated with a significant reduction in tracheostomy compared with usual care without protocolized sedation (RR, 0.69; 95% Cl, 0.50 to 0.96; P=.03; I2=30%; NNT, 16.6; P=.04; 5 RCTs) (Figure 4).
FIGURE 4.
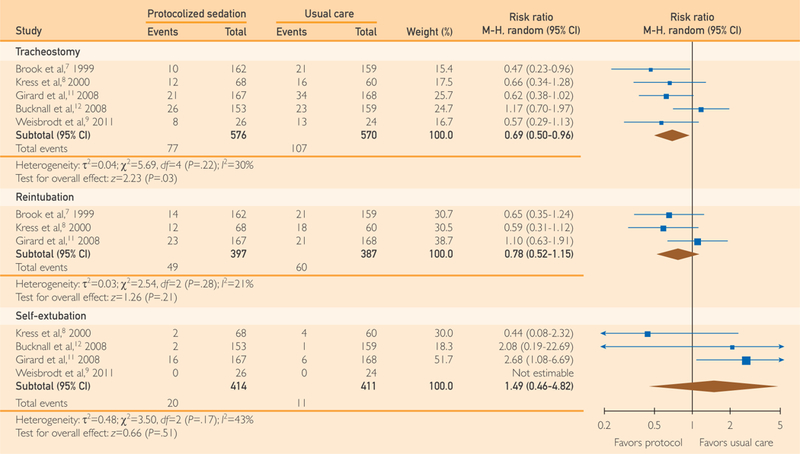
Forest plot of the effect of protocolized sedation compared with usual care without protocolized sedation on tracheostomy, reintubation, and self-extubation. Diamond summary estimate is presented last for each outcome. Square size is proportional to study weight in this random effects meta-analysis. M-H indicates Mantel-Haenszel.
Reintubation.
Among the 3 reporting studies,7,8,11 protocolized sedation produced no significant difference in reintubation compared with usual care without protocolized sedation (RR, 0.78; 95% Cl, 0.52 to 1.15; P=.21; I2=21%;3 RCTs) (Figure 4).
Self-extubation.
Among the 4 reporting studies,8,11,12 protocolized sedation produced no significant difference in self-extubation compared with usual care without protocolized sedation (RR, 1.49; 95% Cl, 0.46 to 4.82; P=.51; I2=43%; 4 RCTs) (Figure 4).
Delirium.
The included studies did not report the incidence of delirium; however, Girard et al11 reported no significant difference in days of delirium between groups in their trial.
Sensitivity Analysis
The summary estimates for duration of mechanical ventilation and ICU LOS showed I2≥50%. We suspected that Bucknall et al12 were causing substantial heterogeneity because of their lack of respiratory therapists and (contrary to Weisbrodt et al’s9 similar lacking) their large sample size. A sensitivity analysis excluding the study by Bucknall et al12 from both outcome summary estimates resolved the heterogeneity; the mean duration of mechanical ventilation was significantly reduced (WMD, –1.72 days; 95% Cl, –2.64 to –0.81 days; P=.0002; I2=0%; 5 RCTs), and mean ICU LOS was further reduced (WMD, –2.37 days; 95% Cl, –3.84 to –0.90 days; P=.0002; I2=32%; 5 RCTs) (figures not shown).
DISCUSSION
This systematic review and meta-analysis included 6 RCTs involving 1243 patients and 461 deaths. Although we summarized multiple clinical outcomes, only 3 outcomes were reported by all 6 studies. Pooled analysis suggests that among mechanically ventilated adults treated in closed, nonspecialty ICUs, standardized sedation management via protocolized sedation (algorithm or daily interruption) is associated with a significant decrease in overall mortality, ICU and hospital LOSs, and tracheostomy compared with usual care without protocolized sedation. Self-extubation, reintubation, and duration of mechanical ventilation were not significantly different; however, the latter had a heterogeneous summary estimate; a sensitivity analysis excluding the study by Bucknall et al12 resolved heterogeneity, and duration of mechanical ventilation was significantly reduced. The incidence of delirium was not reported in the included studies.
To our knowledge, this study is the first to summarize RCTs comparing protocolized sedation (algorithm or daily interruption) with usual care without protocolized sedation (nonstandardized, discretionary sedation). However, these results are comparable with the 2011 meta-analysis by Augustes et al18 of 5 RCTs comparing daily interruption with no daily interruption. The summary estimates for most reported clinical outcomes were similar to ours in effect size, statistical significance, and heterogeneity. The similarities are likely due to the fact that of the 5 RCTs included in that meta-analysis, the 3 largest8,10,11 were also included in the present study. In sharp contrast, their ICU and hospital LOS summary estimates were remarkably heterogeneous and small in effect size compared with our results. These stark differences can partly be ascribed to the remaining 2 RCTs,19,20 which were excluded from the present study because the control groups also had protocolized sedation.18
A subsequent Cochrane review by Burry et al21 included 9 RCTs and investigated the same question. Our reviews shared 4 studies8–11; the remaining 5 studies19,20,22–24 were excluded because the control groups also had protocolized sedation via algorithm. Although the Cochrane review included 3 more studies than the present review, the total number of patients included was almost the same. Again, most reported clinical outcomes were similar except ICU and hospital LOSs—particularly with respect to effect for each outcome. Square size is proportional to study weight in this random effects meta-analysis. IV indicates inverse variance. size and statistical significance. For example, whereas the present results indicated a significant 1.72-day reduction in ICU LOS, the Cochrane review found a nonsignificant 0.5-day reduction.21 This repeated discrepancy—regarding ICU and hospital LOSs—between the present findings and those of the 2 previously published reviews18,21 may partly be explained by the fact that both previous reviews also included RCTs in which the control group received protocolized sedation via algorithm instead of discretion. Evidence that choice of control group affects results comes from a subgroup analysis in the Cochrane review in which the duration of mechanical ventilation was significantly attenuated in the summary estimate of RCTs in which the control group was a sedation algorithm (as opposed to discretionary sedation).21 Interestingly, sedation dose is reduced by a larger margin when discretionary sedation is compared with protocolized sedation11 as opposed to comparing differing methods of the latter strategy.22
A recent review suggested that clinical outcomes in the ICU improve as sedation is minimized.2 The review incorporated evidence from 2 large RCTs8,11 included in this study; however, the strongest line of evidence supporting this hypothesis comes from a 2010 study by Strom et al25 in which mechanically ventilated adults randomized to a protocol of no sedation had significantly more ventilator-free days and fewer ICU and hospital days compared with a protocol of sedation with daily interruption. Given the apparent inverse relationship between sedation and clinical benefit, we suspected that sedation reduction was part of the mechanism of protocolized sedation, which, in the present meta-analysis, was associated with several improved clinical outcomes. A post hoc analysis of this study showed that 3 of the 6 included RCTs had significantly less sedation (lower total benzodiazepine dose8,11 and fewer days of continuous infusion7) in the protocolized sedation group. Seemingly, standardization reduces sedative use compared with discretionary sedation. However, this does not seem to be the case when comparing 2 standardized sedation strategies; a large 2012 multicenter RCT by Mehta et al22 found that daily interruption plus a sedation protocol (vs a sedation protocol alone) resulted in significantly more total sedation and no clinical benefit. The true mechanism is likely multifaceted, and we caution against the assumption that decreased sedation is a corollary of standardized sedation management.
Despite widespread consensus supporting the use of standardized sedation management, adoption has been suboptimal.26,27 The 2013 pain, agitation/sedation, and delirium (PAD) guidelines explicitly recommend light sedation via “daily sedative interruption or a light target level.”5 These guidelines were published in January 2013 and cited a 2009 survey by Patel et al28 that showed that 71% of health care professionals used a sedation protocol. To understand current practice patterns, we conducted a small survey in September 2014, approximately 19 months after the guidelines were published. We called 123 hospitals—a 3% random sample of 4089 acute care hospitals the United States in 2010—and asked whether protocolized sedation was used in the ICU. The response rate was 72% after excluding 40 hospitals without an ICU. Of 60 responding hospitals, 71% used protocolized sedation via daily interruption (63%), algorithm (47%), or both (12%). The present findings—the same as those of Patel et al28—are not encouraging for 2 reasons: first, the present results are self-reported, and actual vs self-reported use of protocolized sedation has been shown to be a conservative 20% lower27; and second, it seems that the 2013 PAD guidelines have not compelled protocolized sedation adoption, at least not in the first 19 months after publication. A survey of perceived barriers found unplanned extubation to be a major concern preventing daily interruption adoption.29 This concern may be warranted as we found that protocolized sedation increased self-extubation risk by 49%; although nonsignificant, this result is underpowered. However, this review does suggest that the benefits of protocolized sedation outweigh the harms, and we hope that this work will provide the remaining 29% of health care professionals more confidence in adopting the previously mentioned recommendations from the 2013 PAD guidelines. In addition, we hope that this impartial meta-analysis might convince skeptical clinicians to standardize sedation management, particularly clinicians who are skeptical of the objectivity of clinical practice guidelines that may be formulated by researchers with financial or intellectual conflicts of interest.30
The overall quality of evidence was moderate; in other words, there is a need for further high-quality research. Five of the 6 included studies were not blinded; patients in either group may have received more or less diligent care, especially if providers cared for patients in both study arms. Consequently, particular attention should be given to minimizing performance and detection bias. The study by Weisbrodt et al9 was the most recently conducted, and despite claims of the impossibility of a blinded study from investigators of several included RCTs, the authors demonstrated the feasibility of a double-blinded RCT. Their method provides a framework for future investigators to curtail these critical biases.
This study has several limitations. First, the results are not applicable to specialty ICUs or to patients with severe illness. Most included patients were treated for medical conditions. Thus, these results are best generalized to adults admitted to medical ICUs with an APACHE II range of approximately 18 to 26. Second, these results are not applicable to children; however, recent randomized evidence supported protocolized sedation use in the pediatric population.31,32 Third, we did not search abstracts from conference proceedings, which may have introduced bias into this review. Last, although we found a significant 15% reduction in overall mortality, the results should be interpreted cautiously because the analytical method does not account for variable follow-up, which is better analyzed using hazard ratios. In addition, statistical significance in overall mortality was driven by the study by Girard et al,11 which had a weight of 43.4% (Figure 2). We do not find this particularly concerning because their study was the largest and highest-quality RCT included in this review. Hospital mortality was the most consistently reported mortality measure (reported by 4 of 6 RCTs), and although there was no difference between groups, it provides a more conservative, albeit underpowered, estimate of the direction of mortality benefit (Figure 2).
CONCLUSION
Protocolized sedation (algorithm or daily interruption) is associated with a 15% reduction in overall mortality, a 1.73-day reduction in ICU LOS, a 3.55-day reduction in hospital LOS, and a 31% reduction in tracheostomy compared with usual care without protocolized sedation. In other words, a standardized approach to sedation management seems better than a nonstandardized, discretionary approach. This conclusion is most generalizable to older mechanically ventilated adults who are not severely ill and are being treated for medical conditions in a closed, nonspecialty ICU. Given the unsatisfactory implementation rate found in these survey results and the apparent benefits of protocolized sedation suggested by this review, we recommend that ICUs strongly consider implementing either daily sedation interruption or a sedation algorithm. Further research investigating protocolized sedation should quantify delirium incidence, as it was not reported in the included RCTs.
Supplementary Material
ACKNOWLEDGMENTS
We thank Thomas L. Mead for helping conduct the literature search, Robin J. Larson for critically reviewing earlier drafts of the manuscript, and Daniel M. Pearlman for providing technical advice.
Mr Minhas and Dr Velasquez contributed equally and are both first authors.
Grant Support: Dr Celi receives financial support through the National Institutes of Health grant R01 EB017205–01A1.
Abbreviations and Acronyms:
- APACHE II
Acute Physiology and Chronic Health Evaluation II
- C
control
- COPD
chronic obstructive pulmonary disease
- I
intervention
- ICU
intensive care unit
- LOS
length of stay
- NNT
number needed to treat
- NR
not reported
- PAD
pain, agitation/sedation, and delirium
- RASS
Richmond Agitation Sedation Scale
- RCT
randomized controlled trial
- RR
risk ratio (relative risk)
- SAS
Riker Sedation-Agitation Scale
- WMD
weighted mean difference
Footnotes
SUPPLEMENTAL ONLINE MATERIAL
Supplemental material can be found online at http://www.mayoclinicproceedings.org.
Data Previously Presented: An outdated abstract of this paper titled “Do Sedation Protocols Decrease Mortality in the ICU: A Systematic Review & Meta-analysis” was presented at the 43rd Critical Care Congress in San Francisco, CA.
REFERENCES
- 1.Wunsch H, Linde-Zwirble WT, Angus DC, et al. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38(10):1947–1953. [DOI] [PubMed] [Google Scholar]
- 2.Reade MC, Finfer S Sedation and delirium in the intensive care unit. N Engl J Med. 2014;370(5):444–454. [DOI] [PubMed] [Google Scholar]
- 3.Jackson DL, Proudfoot CW, Cann KF, Walsh TS. The incidence of sub-optimal sedation in the ICU: a systematic review. Crit Care. 2009;13(6):R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186(8):724–731. [DOI] [PubMed] [Google Scholar]
- 5.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41 (1):263–306. [DOI] [PubMed] [Google Scholar]
- 6.Morris AH. Treatment algorithms and protocolized care. Curr Opin Crit Care. 2003;9(3):236–240. [DOI] [PubMed] [Google Scholar]
- 7.Brook AD, Ahrens TS, Schaiff R, et al. Effect of a nursing-implemented sedation protocol on the duration of mechanical ventilation. Crit Care Med. 1999;27(12):2609–2615. [DOI] [PubMed] [Google Scholar]
- 8.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–1477. [DOI] [PubMed] [Google Scholar]
- 9.Weisbrodt L, McKinley S, Marshall AP, et al. Daily interruption ofsedation in patients receiving mechanical ventilation. Am J Crit Care. 2011;20(4):e90–e98. [DOI] [PubMed] [Google Scholar]
- 10.Anifantaki S, Prinianakis G, Vitsaksaki E, et al. Daily interruption of sedative infusions in an adult medical-surgical intensive care unit: randomized controlled trial. J Adv Nurs. 2009;65(5): 1054–1060. [DOI] [PubMed] [Google Scholar]
- 11.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008; 371(9607):126–134. [DOI] [PubMed] [Google Scholar]
- 12.Bucknall TK, Manias E, Presneill JJ. A randomized trial of protocol-directed sedation management for mechanical ventilation in an Australianintensivecareunit.CritCareMed.2008;36(5):1444–1450. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W264. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. London, UK: The Cochrane Collaboration; 2009. [Google Scholar]
- 15.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodo!. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. [DOI] [PubMed] [Google Scholar]
- 18.Augustes R, Ho KM. Meta-analysis of randomised controlled trials on daily sedation interruption for critically ill adult patients. Anaesth Intensive Care. 2011;39(3):401–409. [DOI] [PubMed] [Google Scholar]
- 19.Mehta S, Burry L, Martinez-Motta JC, et al. A randomized trial of daily awakening in critically ill patients managed with a sedation protocol: a pilot trial. Crit Care Med. 2008;36(7): 2092–2099. [DOI] [PubMed] [Google Scholar]
- 20.de Wit M, Gennings C, Jenvey WI, Epstein SK. Randomized trial comparing daily interruption of sedation and nursing-implemented sedation algorithm in medical intensive care unit patients. Crit Care. 2008;12(3):R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burry L, Rose L, McCullagh IJ, et al. Daily sedation interruption versus no daily sedation interruption for critically ill adult patients requiring invasive mechanical ventilation. Cochrane Data-base Syst Rev. 2014;7:CD009176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta S, Burry L, Cook D, et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA. 2012; 308(19):1985–1992. [DOI] [PubMed] [Google Scholar]
- 23.Yiliaz C, Kelebek Girgin N, Ozdemir N, Kutlay O. The effect of nursing-implemented sedation on the duration of mechanical ventilation in the ICU. Ulus Travma Acil Cerrahi Derg. 2010; 16(6):521–526. [PubMed] [Google Scholar]
- 24.Nassar Junior AP, Park M. Daily sedative interruption versus intermittent sedation in mechanically ventilated critically ill patients: a randomized trial. Ann Intensive Care. 2014;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375(9713):475–480. [DOI] [PubMed] [Google Scholar]
- 26.Miller MA, Bosk EA, Iwashyna TJ, Krein SL. Implementation challenges in the intensive care unit: the why, who, and how of daily interruption of sedation. J Crit Care. 2012;27(2):218. e211–218.e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta S, McCullagh I, Burry L. Current sedation practices: lessons learned from international surveys. Crit Care Clin. 2009; 25(3):471–488, vii-viii. [DOI] [PubMed] [Google Scholar]
- 28.Patel RP, Gambrell M, Speroff T, et al. Delirium and sedation in the intensive care unit: survey of behaviors and attitudes of 1384 healthcare professionals. Crit Care Med. 2009;37(3): 825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanios MA, de Wit M, Epstein SK, Devlin JW. Perceived barriers to the use of sedation protocols and daily sedation interruption: a multidisciplinary survey. J Crit Care. 2009;24(1):66–73. [DOI] [PubMed] [Google Scholar]
- 30.Steinbrook R Improving clinical practice guidelines.JAMA Intern Med. 2014;174(2):181. [DOI] [PubMed] [Google Scholar]
- 31.Gupta K, Gupta VK, Jayashree M, Singhi S. Randomized controlled trial of interrupted versus continuous sedative infusions in ventilated children. Pediatr Crit Care Med. 2012; 13(2):131–135. [DOI] [PubMed] [Google Scholar]
- 32.Verlaat CW, Heesen GP, Vet NJ, et al. Randomized controlled trial of daily interruption of sedatives in critically ill children. Paediatr Anaesth. 2014;24(2):151–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


