Abstract
Innate lymphoid cells (ILCs) constitute a heterogeneous population of cytokine-secreting cells that colonize different tissues and are heavily reliant on cytokines and other secreted factors for their development, maintenance and effector functions. Most ILCs are tissue resident and differentiate in non-lymphoid peripheral tissues. As tissue-resident sentinels, ILCs must rapidly identify pathogens or malignancy in an effort to return the tissue to homeostasis. Here we review the mechanisms that ILCs employ to sense cytokines and other potent immunoregulatory factors that promote their development in different tissues as well as the ability to distinguish pathogenic versus healthy tissue microenvironments and highlight the importance of these pathways for human disease.
Introduction
ILCs are recombination activating gene (RAG)-independent lymphocytes that originate from a common lymphoid progenitor and are present throughout the body with higher frequencies at mucosal surfaces (Figure 1). ILCs have emerged as critical regulators of mucosal barrier integrity, particularly in the intestine, lungs and skin where they reside and respond rapidly to environmental stimuli and impact subsequent adaptive immune responses [1–3] Moreover, ILCs have been shown to contribute to viral immunosurveillance of the liver [4] and homeostasis of adipose tissue [5–7] Thus, their functions range from host defense to metabolic homeostasis.
Figure 1.
Development and functions of the ILC subsets. ILC1s, ILC2s, and ILC3s derive from the ILCP, whereas NK cells derive from an upstream EILP [105,106]. In contrast, ILCreg originate from the CHILP but not ILCPs and depend on the transcriptional factors Id3 and SOX4. ILCreg secrete IL-10 that inhibits ILC1 and ILC3 activation to alleviate intestinal inflammation and produce autocrine TGF-β during inflammation to promote self-expansion. Tissue-resident T-bet+ ILC1s may include: (1) ILC1s derived directly from ILCP; (2) converted ILC2s exposed to IL-1β and IL-12 (or IL-13 + TSLP and IL-12) that have downregulated GATA3 and upregulated T-bet; (3) converted ILC3s (ex-ILC3) exposed to IL-2 or IL-15, and IL-23 that have downregulated RORγt and upregulated T-bet; (4) NK cells that downregulate EOMES when exposed to a TGF-β-rich environment. Inflammatory ILC2 (ilLC2) produce IL-17 and are derived from ILC2 that have upregulated RORγt upon exposure to IL-25. ILC210 produce IL-10, IL-5 and IL-13 but not IL-9 and are derived from ILC2s that have upregulated RELMα after exposure to either IL-33 or papain in vivo that may be enhanced by IL-2 and RA. Abbreviations: AhR, aryl hydrocarbon receptor; CLP, common lymphoid progenitor; common helper-like innate lymphoid progenitor, CHILP; early innate lymphoid precursor EILP; GATA3, GATA-binding protein 3; Hobit, Homologue of Blimp-1 in T cells; ID2, inhibitor of DNA binding 2; ID3, inhibitor of DNA binding 3; ILCP, ILC precursor; NKP, NK cell progenitor; NFIL3, nuclear factor interleukin 3; RELMα, resistin-like molecule α RORα, retinoic acid receptor-related orphan receptor α; PLZF, promyelocytic leukemia zinc finger; SOX4, sex-determining region Y-box 4; T-bet, T-box expressed in T cells; TCF1, T cell factor-1.
Cytokines – potent mediators of ILC function
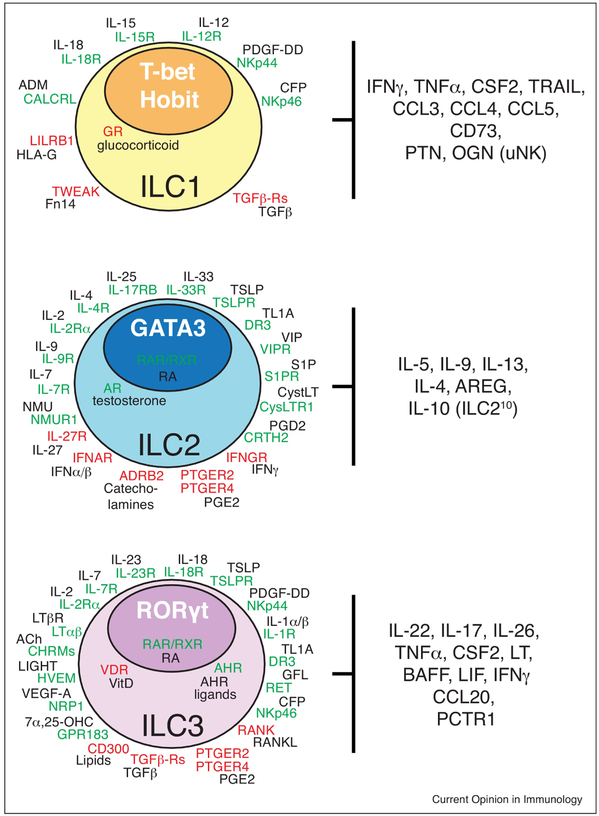
The different ILC players are portrayed in three categories, ILC1, ILC2 and ILC3, based on the cytokines they produce and transcription factors (TFs) that guide their differentiation [8] (Figure 1). Cytokines are the most extensively studied stimuli for ILCs. Seminal studies have shown that each ILC group can be activated via specific cytokine receptors that trigger the secretion of signature cytokine modules (Figure 2). For example, T-bet+ ILC1 produce IFN-γ in response to IL-12, IL-15 and IL-18, facilitating the control of intracellular pathogens by classical macrophage activation [1–3], ILC2, which express high levels of GATA3, respond to IL-25, IL-33 and Thymic Stromal Lymphopoietin (TSLP) by secreting interleukin-5 (IL-5), IL-9, and IL-13 that promote alternative macrophage activation, eosinophilia, and goblet cell hyperplasia to limit helminth infections [9–12], Rorγt+ ILC3 react to IL-23 and IL-1β stimulation by producing IL-17 and IL-22 that trigger epithelial defense mechanisms and granulocytic responses to combat extracellular bacterial and fungal infections [1–3]. Collectively, these functional ILC modules mirror the functional polarizations of CD4+ T helper (Th)1, Th2, and Th17 cells. Natural Killer (NK) cells are also innate lymphocytes that produce IFN-γ but are distinct from ILC1 because they specialize in the cytolysis of malignant or pathogen-infected cells and are therefore considered innate counterparts of cytotoxic CD8+ T cells. The lymphoid tissue-inducer (LTi) cells that spark lymphoid tissue organogenesis during development are Rorγt+ and produce IL-17 and IL-22; therefore, LTi are frequently categorized as ILC3, but may represent a different lineage that emerges from the common lymphoid progenitor prior to ILCs [13]
Figure 2.
Cell-surface and intracellular receptors expressed by the different subsets of ILCs. Activating receptors (green) and inhibitory receptors (red) adjacent to their cognate ligands (black) are indicated for each ILC type. Effector molecules and TFs (white bold) that guide the differentiation of each ILC subset are indicated. This scheme is comprehensive for both human and mouse data (Ach, acetylcholine; ADRB2, adrenoceptor Beta 2; AREG, amphiregulin; AR, androgen receptor; AHR, aryl hydrocarbon receptor; BAFF, B-cell activating factor; CFP, complement factor properdin; CHRM, cholinergic receptor muscarinic; CRTH2, chemoattractant receptor-homologous molecule expressed on Th2 cells (prostaglandin D2 receptor 2); CysLTR1, cysteinyl leukotriene receptor 1; DR3, death receptor 3; GFL, glial cell-derived neurotrophic factor family of ligands; GPR183, G protein-coupled receptor 183; GR, glucocorticoid receptor; NMUR1, LT, lymphotoxin; neuromedin U receptor 1; OGN, osteoglycin; ; PCTR1, protectin conjugates in tissue regeneration 1; PDGF-DD, platelet-derived growth factor-DDPTN, Pleiotrophin; PTGER, prostaglandin E receptor; RANKL, RAR/RXR, retinoic acid receptor; Receptor activator of NF-κB ligand; RET, Ret proto-oncogene; S1PR, shingosine-1-phosphate receptor; TRAIL, TNF-related apoptosis inducing ligand; TSLPR, thymic stromal lymphopoietin receptor; VIPR, vasoactive intestinal peptide receptor; 7α,25-OHC, 7α,25-hydroxycholesterol).
Cellular sources of cytokines that trigger and maintain ILC
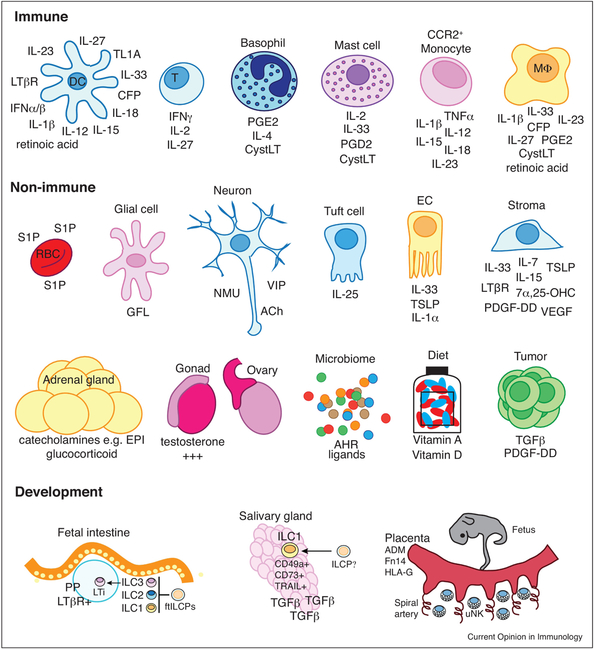
Many studies are now beginning to address the cellular sources and tissue microenvironmental conditions that may imprint ILC identity and trigger their responses during development and in either steady state or pathogenic tissue conditions [14–16], Interactions of epithelial and mesenchymal tissues with ILCs are therefore important during fetal and adult life (Figure 3). In addition to providing crucial structural support and barrier protection, epithelial and stromal cells are prominent tissue-resident sensors and potent drivers of ILC function. Thus, ILCs readily form positive feedback loops by sensing cytokines released by specialized epithelial cells and fibroblasts. For example, tuft cells are chemosensory epithelial cells that line the intestine and respiratory tract and provide an innate source of IL-25 to drive type 2 immune responses. Tuft cells can sense succinate fermented by intestinal parasites to drive an IL-25-ILC2-IL-13-dependent immune circuit that controls parasite infections and initiates intestinal remodeling [17,18], Conversely, tuft cells are targets for norovirus infection in the intestine and IL-25 induced upon co-infection with parasites or helminths can promote type 2 cytokines that induce tuft cell proliferation and perpetuate norovirus infection [19]
Figure 3.
Immune and non-immune cell sources of immunoregulatory molecules that serve as stimuli for activating and inhibitory ILC receptors in fetal and adult tissue development. Abbreviations: DC, dendritic cell; T, T cell; MΦ, macrophage; RBC, red blood cell; EC, epithelial cells; EPI, epinephrine.
IL-33 is a member of the IL-1 family that is released by damaged epithelial and endothelial cells that induces strong ILC2 activation and resistance to helminth infections or promote allergen-induced lung and skin inflammation [1], Interestingly, IL-33 derived from islet mesenchymal cells activated the expression of IL-13 and GM-CSF by ILC2, which induced retinoic acid (RA) production by macrophages and dendritic cells (DCs) that signaled β cells to increase insulin secretion. IL-33 injections rescued islet function in obese mice with a defective IL-33-ILC2 axis, suggesting that immunometabolic crosstalk between IL-33, ILC2s, and myeloid cells regulates insulin production in pancreatic islets [20].
IL-1α is another inflammatory cytokine produced by virus-infected intestinal epithelial cells that stimulates IL-22 production from ILC3 and enhances the clearance of rotavirus [21] Fibroblastic reticular cells (FRC) are stromal cells in lymphoid tissues that express IL-15 and are essential for ILC1 maintenance in Peyer’s patches and mesenteric lymph nodes. FRCs can sense the intestinal microflora through Toll-like receptors (TLRs), which suppress the secretion of IL-15. Thus, FRC-specific deficiency of the TLR signaling adaptor Myd88 elicited increased IL-15 production and ILC1 hyperactivation that accelerated clearance of an enteropathogenic virus yet also precipitated severe intestinal inflammatory disease [22]
In contrast to inflammatory cytokines, TGF-β has immunosuppressive functions yet is also important for tissue imprinting immune cell function during development [23]. Salivary gland (SG) ILCs, in addition to liver and intestinal intraepithelial ILC1, express markers denoting tissue residency and TGF-β imprinting, such as CD49a, TRAIL and CD73 [24] (Figure 2). TGF-β promotes the differentiation of SG ILC1 by suppressing the TF Eomes required for the differentiation of conventional NK cells (Figure 1). Moreover, TGF-β imprinting of SG ILC1 was found to be concurrent with SG development [24] (Figure 3).
The lymphotoxin (LT)-pathway is a critical mechanism by which fetal LTi regulate lymphoid organogenesis, such as lymph nodes and Peyer’s patches (PP), during development and polymorphisms in the genes encoding LTα are linked to several phenotypes that contribute to metabolic syndrome [25], Fetal ILC precursors (ftILCPs) with the potential for differentiation into either ILC1s, ILC2s or ILC3s reside in the intestine during the development of PP [26]. The ftILCPs aggregate at PP anlagen in a LTα-dependent manner forming a localized source for ILC populations (Figure 3). Thus, the LT-pathway may link the host immune response, microbiota, and metabolic syndrome [25].
Using II7-lineage trace mice, a subset of ICAM+VCAM+ murine fetal lymphoid tissue organizer (LTo) cells that express LTβR and RANKL were shown to give rise to a population of adult marginal reticular cells (MRCs) that form a dedicated stromal niche for resident ILC3 in secondary lymphoid tissues throughout life [27]. A population of PDGFRα+gp38+ mesenchymal cells provides an optimal microenvironment for the terminal differentiation of fetal liver-derived ILC2s in peripheral tissues. However, the specific factors produced by these mesenchymal cells that can promote terminal ILC2 differentiation and maturation were not identified [28].
Macrophages and DCs are prominent sensors of the tissue environment due to their extensive expression of pattern recognition receptors and can also instruct ILC acquisition of specialized tissue-specific functions. For example, ILC3 are located in close spatial proximity to intestinal CX3CR1+ mononuclear phagocytes, which produce more IL-23 and IL-1β than conventional CD103+ DC, and are more efficient in stimulating IL-22 production by ILC3 [29]. Similarly, CD11c+ DCs expressing IL-18 are found in close proximity to ILC3s in human tonsils. IL-18 cooperated with an ILC3 survival factor, IL-15, to induce proliferation of human ILC3s, and production of IL-22 [30]. Cross-talk between ILC3 and intestinal macrophages is critical for intestinal homeostasis. Microbiota-driven IL-1β production by intestinal macrophages stimulated ILC3-mediated release of GM-CSF, which acted on DCs and macrophages to maintain colonic Treg numbers [31]. Interestingly, TNFSF15 (also know as TL1A) is selectively expressed by human intestinal mononuclear phagocytes and is associated with ulcerative colitis and Crohn’s disease. TNFSF15 binds to TNFRSF25 (also know as DR3) to enhance IL-23 and IL-1β-induced production of IL-22 and GM-CSF by ILC3 [29]. TNFSF15 also promotes the expansion, survival, and functionality of ILC2s. Consequently, Tnfrsf25−/− mice fail to control gut helminthic infections or mount ILC2 responses in the lung after nasal challenge with papain [32]. In another example of interplay between TNF-TNFR superfamily members regulating ILCs, TNFRSF14 (HVEM) binding to TNFSF14 (LIGHT) promotes IFN-γ production from ILC3s and protection from Yersinia enterocolitica [33].
Recent studies using IL-22 reporter mice suggest that high IL-22 expression is a unique feature of LTi cells, which colocalize with a population of activated macrophages constitutively positive for IL-23p40 in the isolated lymphoid follicles of the intestine that appear at weaning and are maintained by the microbiota [34]. The interaction of LTαβ on LTi cells with LTβR expressed on DCs in cryptopatches promotes an amplification loop resulting in IL-23 secretion by DCs and enhanced IL-22 production by LTi during Citrobacter rodentium infection [35,36].
In addition to tissue-resident stromal and myeloid cell populations, ILCs also readily engage in positive-feedback loops with myeloid cells recruited from the circulation. TNF-α produced by inflammatory monocytes recruited to the lungs of mice infected with Klebsiella pneumoniae markedly increased the frequency of ILCs producing IL-17, which enhanced monocyte-mediated bacterial uptake and killing [37]. Basophils are an important source of IL-4 and are recruited from the circulation to provide protection against helminths but may also mediate detrimental tissue functions, such as allergen-induced inflammation [38]. ILC2s express the IL-4R and IL-4 released by basophils can stimulate ILC2s to promote allergen-induced airway inflammation [39] and atopic dermatitis-like disease [40]. Notably, the genes encoding IL-33, IL-33R (ST2), TSLP, IL-4, IL-5 and IL-13 have all been linked to atopic disease [41]. Collectively, these studies illustrate that ILCs express a variety of different cytokine receptors that sense cytokines released by specialized epithelial and stromal cells or myeloid cells (either resident or recruited) to engage in positive feedback loops with the aim of rapidly returning the tissue to homeostasis. Such interactions of stromal tissues and tissue-resident macrophage populations with ILCs may be important in both fetal and adult life. Moreover, polymorphisms in the genes encoding proteins in these circuits may predispose to inflammatory diseases.
Regulation of ILC functions by cytokines
Whilst many cytokines are potent drivers of ILC activation, some may also inhibit ILC functions to limit inflammatory responses or be usurped by pathogens or tumor cells to promote immunosuppression. For example, tumor-derived TGF-β can suppress NK cell functions and drive their differentiation into pro-tumorigenic ILC1 [42] (Figure 1). SMAD4 has been identified as playing an unexpected role in regulating non-canonical TGF-β signaling in conventional NK cells. SMAD4-deficient NK cells unexpectedly acquired an ILC1-like gene signature and were unable to control tumor metastasis or viral infection [43]. TGF-β also impairs the development NKp46+ ILC3 suggesting that TGF-β cross-inhibits different ILC subsets [44]. Autocrine TGF-β production has been proposed to maintain and expand a population of regulatory ILC (ILCreg) that secrete IL-10 during intestinal inflammation and can suppress the activity of ILC1s and ILC3s [45] (Figure 1). Type I IFNs (i.e. IFN-α and IFN-β), IFN-γ and IL-27 can inhibit ILC2 responses, which may be critical for limiting type 2 immunopathology following viral or bacterial infection [46–48]. Conversely, ILC2 activating cytokines e.g. epithelial cell-derived IL-25/TSLP can cross-inhibit IL-22 secretion from ILC3 suggesting that a finely tuned equilibrium exists in the maintenance of intestinal barrier immunity [49,50].
In addition to cross-inhibition, ILC subsets may autoregulate their own effector functions. For example, CCR6+ ILC3s control their abundance and the production of IL-17 and IL-22 in response to IL-23 through intercellular interactions between the TNFSF11 (RANKL) and its receptor TNFRSF11 (RANK), suggesting local cell density mediates a feedback mechanism to dampen ILC3 activity, which may represent a form of quorum sensing [51]. Interestingly, a polymorphism in the gene encoding RANKL is associated with Crohn’s disease [52].
ILC regulation is also accomplished through competition for cytokine availability. ILC2 and ILC3 require IL-7 for development, while ILC1 and NK cells mainly depend on IL-15 [1–3]. In an immunocompetent organism, the expansion of ILCs is limited by the presence of adaptive lymphocytes that compete for stroma-derived IL-7 and/or IL-15. In the absence of T cells, ILC3s are overstimulated by intestinal microbiota resulting in sustained IL-22 production, which, in turn, impacts lipid transport by intestinal epithelial cells and impaired lipid metabolism [53]. Conversely, ILC3 can limit homeostatic T cell proliferation by consuming IL-7 [54,55]. Similarly, MHCII+ ILC3s may induce the cell death of commensal bacteria-specific CD4+ T cells through TCR-induction of an apoptotic program in concert with sequestering IL-2 [56].
Uterine NK (uNK) cells are the most abundant ILC population at the feto-maternal interface during early gestation and play a significant role in the establishment and maintenance of pregnancy-related vascularization [57,58]. The interaction between TNFSF12 (also known as TWEAK) and its receptor, TNFRSF12A, (also know as Fn14) helps counterbalance the cytotoxic function of uNK cells to maintain feto-maternal tolerance necessary for successful pregnancy [59] (Figure 3).
Overall, ILCs can form negative feedback loops by sensing cytokines secreted by rival lymphocytic subsets, lymphoid stroma, developing tissues and tumor cells, as well as cell-surface anchored cytokines, such as RANKL, that engage during conditions of increased ILC density.
Cytokine impact on ILC fate
Increasing evidence shows that ILC2 and ILC3 may convert into ILC1-like cells by downregulating either GATA3 or RORγt, respectively, upregulating T-bet and gaining the capacity for IFN-γ production [60,61]. This process, often referred to as ‘ILC plasticity’, may be important to fine tune ILC function to a changing microenvironment (Figure 1). The magnitude and the duration of cytokine stimulation plays an important role in ILC plasticity. For example, IL-23 strongly induces IL-22 secretion by ILC3 [62,63]. However, sustained exposure to IL-23 can facilitate ILC3 to ILC1 conversion. IL-23 activates STAT4 in ILC3, which drives T-bet expression [63]. In humans, ILC3 also develop into ILC1-like cells upon in vitro culture with recombinant IL-2 or IL-15 [62], recombinant IL-12 [64], or IL-12-producing CD14+ intestinal DCs [65]. RA and IL-23-producing CD14− DCs induced the reverse conversion of ILC1s into ILC3s [65], although this reverse conversion has not been established in vivo in mice.
ILC2 also display functional plasticity in response to IL-12. Activation with IL-1β and IL-12 or IL-33 plus TSLP and IL-12 (but not by IL-12 alone) renders ILC2 responsive to type I polarization by upregulating T-bet and IL-12R [66–69]. Consistent with this pathway, patients with Mendelian susceptibility to mycobacterial disease fail to generate IFN-γ-producing ILC1 from ILC2s due to a deficiency in IL-12Rβ1 [67]. The strength of IL-1β signaling has also been shown to influence ILC2 plasticity. Strong IL-1β signaling upregulated the expression of IL-12R and conversion of ILC2 into ILC1, whereas low-dose IL-1β signaling favored type 2 polarization in ILC2 [69]. ILC2 endowed with functional features of ILC3 have also been reported. Systemic injection of IL-25 led to the generation of “inflammatory ILC2” (ilLC2) from a Klrg1Hi progenitor cell that in addition to type 2 cytokines expressed RORγt and the signature ILC3 cytokine IL-17 and contributed to protection against Nippostrongylus brasiliensis and Candida albicans [70] (Figure 1). IL-2 signaling in lung ILC2s promotes cell survival/proliferation and serves as a cofactor for the production of type 2 cytokines [71]. Conversely, IL-2 and RA signaling in combination with other as yet unidentified in vivo factors can enhance the development of a distinct subset of alternatively activated lung ILC2 that produce IL-10 (termed ILC210), which downregulate pro-inflammatory genes and are associated with reduced eosinophil recruitment to the lung [72] (Figure 1). Thus, the magnitude and duration of ILC stimulation by tissue cytokines combined with signals specific to the tissue microenvironment may be a critical factor in regulating the functional plasticity of ILCs in different tissues particularly during inflammatory conditions [14,15].
ILCs engage growth factor pathways
Growth factors (GFs) are important for a variety of cellular and developmental processes and GF signaling pathways are commonly subverted in cancer. NKp44 is an activating immunoreceptor expressed on human activated NK cells, ILC1 and ILC3 (Figure 2). NKp44 was found to bind to PDGF-DD [73], a GF secreted by platelets, endothelial and tumor cells (Figure 3) that can promote angiogenesis, stromal reaction and tumor growth through PDGFRβ signaling. PDGF-DD engagement of NKp44 triggers IFN-γ and TNF-α secretion from NK cells and ILC1 and TNF-α secretion from ILC3 that induced tumor cell growth arrest in vitro and in vivo [73]. The ability of ILCs to engage in GF surveillance through NKp44 is a new immunological paradigm that remains to be fully explored. Interestingly, transforming viruses encode PDGF homologues and a PDGFD polymorphism is associated with IFN-γ levels in humans [74] suggesting that GF surveillance pathways may have been driven by selective pressure imposed by transforming viruses [73].
GF sensing by ILCs may also regulate important developmental processes. For example, human and fetal mouse ILC3s that display LTi activity and express neuropilin receptor 1 (NRP1) are located in near high endothelial venules. VEGF-A binds to NRP1 and serves as a chemotactic factor for lung-resident NRP1+ ILC3, suggesting these cells may play a role in the initiation of ectopic pulmonary lymphoid aggregates in smokers and patients with chronic obstructive pulmonary disease [75].
In pregnancy, maternal uterine spiral artery (SA) remodeling is essential for ensuring efficient blood flow to the developing fetus. A subset of CD49a+Eomes+ decidual NK cells (dNK) that actively secretes GFs, such as pleiotrophin and osteoglycin, have recently been described in humans and in mice. The GF-secreting function of this dNK subset was regulated by trophoblast-expressed HLA-G binding to LILRB1 (also know as ILT2) on dNK and deficiency in this subset impaired fetal development resulting in restricted fetal growth [76]. Thus, GF sensing by ILCs may operate during development, malignancy and possible also during infections with pathogens that have captured GFs or can induce their expression.
ILCs sense complement
The complement system is an evolutionary ancient system of immune defense. Properdin (also known as complement factor P) is a plasma glycoprotein that binds to microbial surfaces and apoptotic cells and triggers the alternate pathway of complement that leads to the formation of the membrane attack complex and target cell lysis. Human and mouse ILC1 and a subset of ILC3 express the activating immunoreceptor NKp46 (Figure 2). Properdin binds to NKp46 and NKp46 and ILC1s were required for resistance to Neisseria meningitidis [77]. Since NKp46 mediates both positive and negative immunoregulation [78], it will be important to delineate the pathways regulated by the NKp46-properdin interaction in different disease models including cancer.
ILCs as chemosensory cells
The Aryl Hydrocarbon Receptor (AHR) is a ligand-activated TF that binds indoles derived from the bacterial degradation of dietary tryptophan, as well as tryptophan metabolites contained in vegetables [79], bacterial toxins [80] and environmental polycyclic hydrocarbons (Figure 3). AHR drives the development of ILC3 and their production of IL-22 [81], providing a mechanism to adapt the intestinal innate immune system to nutrition and intestinal flora. NK cells also express AHR, which was required for optimal NK cell cytotoxicity, IFN-γ production and anti-tumor activity [82].
Studies using genetic mouse models and various diets have shown that sensing of vitamin A metabolites in utero is crucial for the prenatal differentiation of LTi cells, which control the size of secondary lymphoid tissues and the generation of protective immune responses in adults [83]. In adult mice, RA signaling favors the development of ILC3s over ILC2s. Consequently, vitamin-A-deficient mice fail to control C. rodentium infection but are resistant to helminth infection [84]. In contrast to the immunostimulatory functions of vitamin A metabolites, vitamin D is predominantly immunosuppressive for ILCs and downregulates IL-22 expression in ILC3 [85]. Consequently, vitamin D receptor knockout mice have more IL-22-producing ILC3, secrete more antibacterial peptides and are more resistant to C. rodentium infection [86].
In addition to vitamins and metabolites, ILCs sense a range of lipid mediators, such as prostaglandins (PG), leukotrienes, and oxysterols, which are released during inflammation and tissue repair, in addition to the blood borne lipid, sphingosine-1-phosphate (S1P). PGD2 and cysteinyl leukotrienes bind to the CRTH2 and CysLT1R receptors, respectively, to enhance ILC2 cytokine production [87–89], whereas PGE2 inhibited GATA-3 expression and IL-5 and IL-13 production by ILC2s in response to IL-25, IL-33 and TSLP through the PTGER-2 and −4 receptors. [90]. PGE2 also downregulated the expression of IL-2 receptor a (CD25) leading to reduced responsiveness to IL-2 and ILC2 proliferation. In contrast, oxysterols, such as 7α,25-hydroxycholesterol, activate the GPR183 receptor to promote ILC3 migration and localization to cryptopatches and isolated lymphoid follicles [91]. S1P activates S1PR-1, −4 and −5 to promote lymphatic entry, blood circulation and migration of ilLC2s to distal sites [92].
ILCs sense neuronal-derived factors
Recent studies have revealed that ILCs express receptors for neural peptides, thus enabling cross-talk with the peripheral nervous system. ILC3s express RET (Figure 2), which is a receptor for members of the glial cell-derived neurotrophic factor family of ligands (GFL) (Figure 3). Toll-like receptor signaling upregulates GFLs in response to bacterial infections and GFL binding to RET enhances ILC3 secretion of IL-17 and IL-22 [93], ILC2s were found in close contact with enteric neurons that can produce vasoactive intestinal peptide (VIP), which engages VIP receptor to enhance ILC2 secretion of type 2 cytokines in response to helminth infections [94]. Enteric neurons can also release the peptide neuromedin U (NMU), which activates ILC2 cytokine secretion via NMU receptor 1 to stimulate mucus production by goblet cells and control of helminth infection [95–97]. Conversely, ILC2s express adrenergic receptor β2 (ADRB2) that can inhibit cytokine secretion [98].
It has been shown that the vagus nerve can sense peripheral infections and/or tissue injuries via an afferent arc, which activates efferent neural circuits that modulate the progression of inflammatory responses [99]. Escherichia coli infection in mice triggers the production of acetylcholine (ACh) by the vagal system, which can arouse the cholinergic receptors (CHRM)-1, −2, −4 and −5) and induce ILC3 production of resolution phase lipid mediators, such as the protective immunoresolvent PCTR1 to promote myeloid cell responses, resolution of inflammation, and tissue repair [100]. Future studies are likely to reveal the expression and function of additional receptors for neuronal-derived mediators, expanding the impact of the peripheral nervous system to different aspects of ILC biology.
ILCs sense the endocrine system
The neuroendocrine system is a key player in controlling hyperinflammation and prevention of tissue damage. Inflammatory cytokines stimulate the hypothalamic-pituitary-adrenal (HPA) axis to produce glucocorticoid (GC), which is a critical step in establishing tolerance to septic shock, although the cellular targets remain unclear (Figure 3). NK cells and ILC1 express GC receptor, which was required to limit IFN-γ production, permitting IL-10-dependent tolerance to microbial endotoxins [101] (Figure 2). Moreover, endogenous GC induced the expression of the checkpoint receptor PD-1 on NK cells, which limited IFN-γ production by splenic NK cells and preventing virus-induced immunopathology without compromising viral clearance [102].
Hormones are active at many stages of development. Fetal trophoblast cells express the pregnancy-related peptide hormone adrenomedullin that binds to the Calcitonin receptor-like receptor (CALCRL) to promote the recruitment and activation of maternal uNK cells to the placenta and facilitation of maternal SA remodeling [103]. Sex hormones can regulate many autoimmune and inflammatory diseases, such as asthma. For example, asthma is twice as prevalent in women compared to men. Moreover, administration of testosterone and the downstream active hormone, 5α-dihydrotestosterone, reduced the numbers and IL-5 and IL-13 expression from ILC2 and attenuated allergen-induced airway hypersensitivity in mice. Interestingly, women with asthma have increased ILC2s compared to men [104], suggesting that sexual dimorphism in ILC2 numbers may explain higher susceptibility to asthma in women [104].
Concluding remarks
Collectively, many studies now highlight how ILCs employ a battery of specialized receptors to sense cytokines and other inflammatory mediators, metabolites, cell density, neuronal signals, hormones, complement, damage-associated molecules, and growth factors that are secreted by myeloid cells and specialized epithelial and stromal cells e.g. FRCs and tuft cells. All of these mediators impact the development of ILCs in tissues and establish positive and negative feedback loops that control ILC responses to different pathogens and malignancies, as well as critical developmental process, such as SA remodeling in pregnancy and lymphoid organogenesis. Interestingly, many of the genes encoding products that form vital nodes in these biological circuits are associated with inflammatory diseases. Consequently, a greater understanding of the basic biology underpinning the regulatory feedback loops that ILCs form with their resident tissues, the development and diversity of ILC subsets and their unique functions will provide insights into the mechanisms of immune-mediated diseases and likely lead to the development of the next generation of clinical targets for therapeutic intervention.
Highlights.
Cytokines promote the development, tissue imprinting and effector function of ILCs
Sustained cytokine signaling can change ILC fate
ILCs also express receptors for other tissue-specific immunoregulatory molecules
Polymorphism or deficiency in ILC tissue circuits may predispose to disease
Acknowledgments
Funding
Supported by the NIH grants DE025884, DK103039, and AI120606
Footnotes
Conflicts of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• of special interest
•• of outstanding interest
- 1.Klose CSN, Artis D: Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol 2016, 17:765–774. [DOI] [PubMed] [Google Scholar]
- 2.Diefenbach A, Colonna M, Koyasu S: Development, differentiation, and diversity of innate lymphoid cells. Immunity 2014, 41:354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eberl G, Colonna M, Di Santo JP, McKenzie ANJ: Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 2015, 348:aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weizman O-E, Adams NM, Schuster IS, Krishna C, Pritykin Y, Lau C, Degli-Esposti MA, Leslie CS, Sun JC, O’Sullivan TE: ILC1 Confer Early Host Protection at Initial Sites of Viral Infection. Cell 2017, 171:795–808.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brestoff JR, Kim BS, Saenz SA, Stine RR, Monticelli LA, Sonnenberg GF, Thome JJ, Farber DL, Lutfy K, Seale P, et al. : Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 2015, 519:242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee M-W, Odegaard Jl, Mukundan L, Qiu Y, Molofsky AB, Nussbaum JC, Yun K, Locksley RM, Chawla A: Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 2015, 160:74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulenouar S, Michelet X, Duquette D, Alvarez D, Hogan AE, Dold C, O’Connor D, Stutte S, Tavakkoli A, Winters D, et al. : Adipose Type One Innate Lymphoid Cells Regulate Macrophage Homeostasis through Targeted Cytotoxicity. Immunity 2017, 46:273–286. [DOI] [PubMed] [Google Scholar]
- 8.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, et al. : Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol 2013, 13:145–149. [DOI] [PubMed] [Google Scholar]
- 9.Koyasu S, Moro K, Tanabe M, Takeuchi T: Natural helper cells: a new player in the innate immune response against helminth infection. Adv Immunol 2010, 108:21–44. [DOI] [PubMed] [Google Scholar]
- 10.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TKA, Bucks C, Kane CM, Fallon PG, Pannell R, et al. : Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010, 464:1367–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price AE, Liang H-E, Sullivan BM, Reinhardt RL, Eisley CJ, Erie DJ, Locksley RM: Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA 2010, 107:11489–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rauber S, Luber M, Weber S, Maul L, Soare A, Wohlfahrt T, Lin N-Y, Dietel K, Bozec A, Herrmann M, et al. : Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat Med 2017, 23:938–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishizuka IE, Chea S, Gudjonson H, Constantinides MG, Dinner AR, Bendelac A, Golub R: Single-cell analysis defines the divergence between the innate lymphoid cell lineage and lymphoid tissue-inducer cell lineage. Nat Immunol 2016, 17:269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simoni Y, Fehlings M, Kløverpris HN, McGovern N, Koo S-L, Loh CY, Lim S, Kurioka A, Fergusson JR, Tang C-L, et al. : Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency. Immunity 2017, 46:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricardo-Gonzalez RR, Van Dyken SJ, Schneider C, Lee J, Nussbaum JC, Liang H-E, Vaka D, Eckalbar WL, Molofsky AB, Erie DJ, et al. : Tissue signals imprint ILC2 identity with anticipatory function. Nat Immunol 2018, doi: 10.1038/s41590-018-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim Al, Li Y, Lopez-Lastra S, Stadhouders R, Paul F, Casrouge A, Serafini N, Puel A, Bustamante J, Surace L, et al. : Systemic Human ILC Precursors Provide a Substrate for Tissue ILC Differentiation. Cell 2017, 168:1086–1100.e10. [DOI] [PubMed] [Google Scholar]
- 17.Nadjsombati MS, McGinty JW, Lyons-Cohen MR, Jaffe JB, DiPeso L, Schneider C, Miller CN, Pollack JL, Nagana Gowda GA, Fontana MF, et al. : Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity 2018, 49:33–41.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider C, O’Leary CE, von Moltke J, Liang H-E, Ang QY, Turnbaugh PJ, Radhakrishnan S, Pellizzon M, Ma A, Locksley RM: A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell 2018, 174:271–284.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilen CB, Lee S, Hsieh LL, Orchard RC, Desai C, Hykes BL, McAllaster MR, Balce DR, Feehley T, Brestoff JR, et al. : Tropism for tuft cells determines immune promotion of norovirus pathogenesis. Science 2018, 360:204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalmas E, Lehmann FM, Dror E, Wueest S, Thienel C, Borsigova M, Stawiski M, Traunecker E, Lucchini FC, Dapito DH, et al. : Interleukin-33-Activated Islet-Resident Innate Lymphoid Cells Promote Insulin Secretion through Myeloid Cell Retinoic Acid Production. Immunity 2017, 47:928–942.e7. [DOI] [PubMed] [Google Scholar]; •• This manuscript describes how ILC2 can sense islet mesenchymal cell-derived IL-33 to promote insulin secretion showing that ILCs can regulate the endocrine system.
- 21.Hernández PP, Mahlakoiv T, Yang I, Schwierzeck V, Nguyen N, Guendel F, Gronke K, Ryffel B, Hoelscher C, Dumoutier L, et al. : Interferon-λ and interleukin-22 cooperate for the induction of interferon-stimulated genes and control of rotavirus infection. Nat Immunol 2015, 16:698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gil-Cruz C, Perez-Shibayama C, Onder L, Chai Q, Cupovic J, Cheng H-W, Novkovic M, Lang PA, Geuking MB, McCoy KD, et al. : Fibroblastic reticular cells regulate intestinal inflammation via IL-15-mediated control of group 1 ILCs. Nat Immunol 2016, 17:1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gebhardt T, Palendira U, Tscharke DC, Bedoui S: Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol Rev 2018, 283:54–76. [DOI] [PubMed] [Google Scholar]
- 24.Cortez VS, Cervantes-Barragan L, Robinette ML, Bando JK, Wang Y, Geiger TL, Gilfillan S, Fuchs A, Vivier E, Sun JC, et al. : Transforming Growth Factor-β Signaling Guides the Differentiation of Innate Lymphoid Cells in Salivary Glands. Immunity 2016, 44:1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Upadhyay V, Fu Y-X: Lymphotoxin organizes contributions to host defense and metabolic illness from innate lymphoid cells. Cytokine Growth Factor Rev 2014. 25:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bando JK, Liang H-E, Locksley RM: Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine. Nat Immunol 2015. 16:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoorweg K, Narang P, Li Z, Thuery A, Papazian N, Withers DR, Coles MC, Cupedo T: A Stromal Cell Niche for Human and Mouse Type 3 Innate Lymphoid Cells. J Immunol 2015, 195:4257–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koga S, Hozumi K, Hirano K-I, Yazawa M, Terooatea T, Minoda A, Nagasawa T, Koyasu S, Moro K: Peripheral PDGFRα+gp38+ mesenchymal cells support the differentiation of fetal liver-derived ILC2. J Exp Med 2018, 215:1609–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longman RS, Diehl GE, Victorio DA, Huh JR, Galan C, Miraldi ER, Swaminath A, Bonneau R, Scherl EJ, Littman DR: CX3CR1+ mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J Exp Med 2014, 211:1571–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Victor AR, Nalin AP, Dong W, McClory S, Wei M, Mao C, Kladney RD, Youssef Y, Chan WK, Briercheck EL, et al. : IL-18 Drives ILC3 Proliferation and Promotes IL-22 Production via NF-κB. J Immunol 2017, 199:2333–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, Merad M: Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014, 343:1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu X, Pappu R, Ramirez-Carrozzi V, Ota N, Caplazi P, Zhang J, Yan D, Xu M, Lee WP, Grogan JL: TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol 2014, 7:730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo G-Y, Shui J-W, Takahashi D, Song C, Wang Q, Kim K, Mikulski Z, Chandra S, Giles DA, Zahner S, et al. : LIGHT-HVEM Signaling in Innate Lymphoid Cell Subsets Protects Against Enteric Bacterial Infection. Cell Host Microbe 2018, 24:249–260.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savage AK, Liang H-E, Locksley RM: The Development of Steady-State Activation Hubs between Adult LTi ILC3s and Primed Macrophages in Small Intestine. J Immunol 2017. 199:1912–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tumanov AV, Koroleva EP, Guo X, Wang Y, Kruglov A, Nedospasov S, Fu Y-X: Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe 2011, 10:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ota N, Wong K, Valdez PA, Zheng Y, Crellin NK, Diehl L, Ouyang W: IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nat Immunol 2011, 12:941–948. [DOI] [PubMed] [Google Scholar]
- 37.Xiong H, Keith JW, Samilo DW, Carter RA, Leiner IM, Pamer EG: Innate Lymphocyte/Ly6C(hi) Monocyte Crosstalk Promotes Klebsiella Pneumoniae Clearance. Cell 2016, 165:679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper shows that ILCs can also engage in cross-talk with inflammatory monocytes recruited to resident tissues to control pathogens.
- 38.Kubo M: Mast cells and basophils in allergic inflammation. Curr Opln Immunol 2018, 54:74–79. [DOI] [PubMed] [Google Scholar]
- 39.Motomura Y, Morita H, Moro K, Nakae S, Artis D, Endo TA, Kuroki Y, Ohara O, Koyasu S, Kubo M: Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity 2014, 40:758–771. [DOI] [PubMed] [Google Scholar]
- 40.Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA, Noti M, Tait Wojno ED, Fung TC, Kubo M, et al. : Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol 2014, 193:3717–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Zhang Y, Zhang L: Discovering susceptibility genes for allergic rhinitis and allergy using a genome-wide association study strategy. Curr Opin Allergy Clin Immunol 2015, 15:33–40. [DOI] [PubMed] [Google Scholar]
- 42.Gao Y, Souza-Fonseca-Guimaraes F, Bald T, Ng SS, Young A, Ngiow SF, Rautela J, Straube J, Waddell N, Blake SJ, et al. : Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat Immunol 2017, 18:1004–1015. [DOI] [PubMed] [Google Scholar]; •• This paper shows that TGF-β secreted by tumor cells can influence intra-tumoral ILC plasticity by promoting NK cell differentiation into pro-tumor ILC1.
- 43.Cortez VS, Ulland TK, Cervantes-Barragan L, Bando JK, Robinette ML, Wang Q, White AJ, Gilfillan S, Cella M, Colonna M: SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-β signaling. Nat Immunol 2017, 18:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viant C, Rankin LC, Girard-Madoux MJH, Seillet C, Shi W, Smyth MJ, Bartholin L, Walzer T, Huntington ND, Vivier E, et al. : Transforming growth factor-β and Notch ligands act as opposing environmental cues in regulating the plasticity of type 3 innate lymphoid cells. Sci Signal 2016, 9:ra46. [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Xia P, Chen Y, Qu Y, Xiong Z, Ye B, Du Y, Tian Y, Yin Z, Xu Z, et al. : Regulatory Innate Lymphoid Cells Control Innate Intestinal Inflammation. Cell 2017, 171:201–216.e18. [DOI] [PubMed] [Google Scholar]
- 46.Duerr CU, McCarthy CDA, Mindt BC, Rubio M, Meli AP, Pothlichet J, Eva MM, Gauchat J-F, Qureshi ST, Mazer BD, et al. : Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol 2016, 17:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molofsky AB, Van Gool F, Liang H-E, Van Dyken SJ, Nussbaum JC, Lee J, Bluestone JA, Locksley RM: Interleukin-33 and lnterferon-γ Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity 2015, 43:161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moro K, Kabata H, Tanabe M, Koga S, Takeno N, Mochizuki M, Fukunaga K, Asano K, Betsuyaku T, Koyasu S: Interferon and IL-27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol 2016, 17:76–86. [DOI] [PubMed] [Google Scholar]
- 49.Giacomin PR, Moy RH, Noti M, Osborne LC, Siracusa MC, Alenghat T, Liu B, McCorkell KA, Troy AE, Rak GD, et al. : Epithelial-intrinsic IKKα expression regulates group 3 innate lymphoid cell responses and antibacterial immunity. J Exp Med 2015, 212:1513–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Bérard M, Kleinschek M, Cua D, Di Santo JP, Eberl G: RORγt+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol 2011, 12:320–326. [DOI] [PubMed] [Google Scholar]
- 51.Bando JK, Gilfillan S, Song C, McDonald KG, Huang SC-C, Newberry RD, Kobayashi Y, Allan DSJ, Carlyle JR, Cella M, et al. : The Tumor Necrosis Factor Superfamily Member RANKL Suppresses Effector Cytokine Production in Group 3 Innate Lymphoid Cells. Immunity 2018, 48:1208–1219.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper shows that CCR6+ ILC3s can autoregulate themselves by sensing cell densities via RANKL-RANK interactions: the gene for RANKL (TNFSF11) is associated with Crohn’s disease (see reference 52).
- 52.Franke A, McGovern DPB, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, et al. : Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet 2010, 42:1118–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mao K, Baptista AP, Tamoutounour S, Zhuang L, Bouladoux N, Martins AJ, Huang Y, Gerner MY, Belkaid Y, Germain RN: Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature 2018, 554:255–259. [DOI] [PubMed] [Google Scholar]
- 54.Martin CE, Spasova DS, Frimpong-Boateng K, Kim H-O, Lee M, Kim KS, Surh CD: Interleukin-7 Availability Is Maintained by a Hematopoietic Cytokine Sink Comprising Innate Lymphoid Cells and T Cells. Immunity 2017, 47:171–182.e4. [DOI] [PubMed] [Google Scholar]
- 55.Bank U, Deiser K, Finke D, Hämmerling GJ, Arnold B, Schüler T: Cutting Edge: Innate Lymphoid Cells Suppress Homeostatic T Cell Expansion in Neonatal Mice. J Immunol 2016, 196:3532–3536. [DOI] [PubMed] [Google Scholar]
- 56.Hepworth MR, Fung TC, Masur SH, Kelsen JR, McConnell FM, Dubrot J, Withers DR, Hugues S, Farrar MA, Reith W, et al. : Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science 2015, 348:1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Collectively, references 54 – 56 show that ILCs can regulate adaptive immune responses by sequestering T cell growth factors.
- 57.Doisne J-M, Balmas E, Boulenouar S, Gaynor LM, Kieckbusch J, Gardner L, Hawkes DA, Barbara CF, Sharkey AM, Brady HJM, et al. : Composition, Development, and Function of Uterine Innate Lymphoid Cells. J Immunol 2015, 195:3937–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boulenouar S, Doisne J-M, Sferruzzi-Perri A, Gaynor LM, Kieckbusch J, Balmas E, Yung HW, Javadzadeh S, Volmer L, Hawkes DA, et al. : The Residual Innate Lymphoid Cells in NFIL3-Deficient Mice Support Suboptimal Maternal Adaptations to Pregnancy. Front Immunol 2016, 7:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qi X, Lei M, Qin L, Xie M, Zhao D, Wang J: Endogenous TWEAK is critical for regulating the function of mouse uterine natural killer cells in an immunological model of pregnancy loss. Immunology 2016, 148:70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, Flach M, Bengsch B, Thimme R, Hölscher C, et al. : Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity 2010, 33:736–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klose CSN, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d’Hargues Y, Göppert N, Croxford AL, Waisman A, Tanriver Y, et al. : A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature 2013, 494:261–265. [DOI] [PubMed] [Google Scholar]
- 62.Cella M, Otero K, Colonna M: Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Scl USA 2010, 107:10961–10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mikami Y, Scarno G, Zitti B, Shih H-Y, Kanno Y, Santoni A, O’Shea J JJ, Sciumè G: NCR+ ILC3 maintain larger STAT4 reservoir via T-BET to regulate type 1 features upon IL-23 stimulation in mice. Eur J Immunol 2018, 48:1174–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, Hreggvidsdottir HS, Heinsbroek SE, Legrand N, Buskens CJ, et al. : Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol 2013, 14:221–229. [DOI] [PubMed] [Google Scholar]
- 65.Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, Kofoed-Nielsen M, Munneke JM, Hazenberg MD, Villaudy J, Buskens CJ, et al. : Interleukin-12 and −23 Control Plasticity of CD127(+) Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity 2015, 43:146–160. [DOI] [PubMed] [Google Scholar]
- 66.Bal SM, Bernink JH, Nagasawa M, Groot J, Shikhagaie MM, Golebski K, van Drunen CM, Lutter R, Jonkers RE, Hombrink P, et al. : IL-1β, IL-4 and IL-12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol 2016, 17:636–645. [DOI] [PubMed] [Google Scholar]; • Collectively, references 62 – 66 show that sustained cytokine signaling can control ILC fate, which may be important during conditions of chronic inflammation.
- 67.Lim AI, Menegatti S, Bustamante J, Le Bourhis L, Allez M, Rogge L, Casanova J-L, Yssel H, Di Santo JP: IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J Exp Med 2016, 213:569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silver JS, Kearley J, Copenhaver AM, Sanden C, Mori M, Yu L, Pritchard GH, Berlin AA, Hunter CA, Bowler R, et al. : Inflammatory triggers associated with exacerbations of COPD orchestrate plasticity of group 2 innate lymphoid cells in the lungs. Nat Immunol 2016, 17:626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ohne Y, Silver JS, Thompson-Snipes L, Collet MA, Blanck JP, Cantarel BL, Copenhaver AM, Humbles AA, Liu Y-J: IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat Immunol 2016. 17:646–655. [DOI] [PubMed] [Google Scholar]
- 70.Huang Y, Guo L, Qiu J, Chen X, Hu-Li J, Siebenlist U, Williamson PR, Urban JF, Paul WE: IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential “inflammatory” type 2 innate lymphoid cells. Nat Immunol 2015. 16:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roediger B, Kyle R, Tay SS, Mitchell AJ, Bolton HA, Guy TV, Tan S-Y, Forbes-Blom E, Tong PL, Köller Y, et al. : IL-2 is a critical regulator of group 2 innate lymphoid cell function during pulmonary inflammation. J Allergy Clin Immunol 2015, 136:1653–1663.e7. [DOI] [PubMed] [Google Scholar]
- 72.Seehus CR, Kadavallore A, Torre B de la, Yeckes AR, Wang Y, Tang J, Kaye J: Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nat Commun 2017, 8:1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barrow AD, Edeling MA, Trifonov V, Luo J, Goyal P, Bohl B, Bando JK, Kim AH, Walker J, Andahazy M, et al. : Natural Killer Cells Control Tumor Growth by Sensing a Growth Factor. Cell 2018, 172:534–548.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This manuscript shows that ILCs express an activating immunoreceptor for a growth factor expressed by cancers cells that promotes control of tumor expansion.
- 74.Ahola-Olli AV, Würtz P, Havulinna AS, Aalto K, Pitkånen N, Lehtimåki T, Kåhönen M, Lyytikåinen L-P, Raitoharju E, Seppålå I, et al. : Genome-wide Association Study Identifies 27 Loci Influencing Concentrations of Circulating Cytokines and Growth Factors. Am J Hum Genet 2017, 100:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shikhagaie MM, Björklund ÅK, Mjösberg J, Erjefålt JS, Cornelissen AS, Ros XR, Bal SM, Koning JJ, Mebius RE, Mori M, et al. : Neuropilin-1 Is Expressed on Lymphoid Tissue Residing LTi-like Group 3 Innate Lymphoid Cells and Associated with Ectopic Lymphoid Aggregates. Cell Rep 2017, 18:1761–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu B, Zhou Y, Ni X, Tong X, Xu X, Dong Z, Sun R, Tian Z, Wei H: Natural Killer Cells Promote Fetal Development through the Secretion of Growth-Promoting Factors. Immunity 2017, 47:1100–1113.e6. [DOI] [PubMed] [Google Scholar]
- 77.Narni-Mancinelli E, Gauthier L, Baratin M, Guia S, Fenis A, Deghmane A-E, Rossi B, Fourquet P, Escalière B, Kerdiles YM, et al. : Complement factor P is a ligand for the natural killer cell-activating receptor NKp46. Sci Immunol 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This manuscript shows that ILCs express an activating immunoreceptor for complement factor P that promotes control of lethal bacterial infection.
- 78.Narni-Mancinelli E, Jaeger BN, Bernat C, Fenis A, Kung S, De Gassart A, Mahmood S, Gut M, Heath SC, Estellée J, et al. : Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science 2012, 335:344–348. [DOI] [PubMed] [Google Scholar]
- 79.Stockinger B, Di Meglio P, Gialitakis M, Duarte JH: The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol 2014, 32:403–432. [DOI] [PubMed] [Google Scholar]
- 80.Moura-Alves P, Faé K, Houthuys E, Dorhoi A, Kreuchwig A, Furkert J, Barison N, Diehl A, Munder A, Constant P, et al. : AhR sensing of bacterial pigments regulates antibacterial defence. Nature 2014, 512:387–392. [DOI] [PubMed] [Google Scholar]; •• This manuscript shows that AhR evolved to sense not only environmental pollutants but also microbial insults.
- 81.Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A: Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011, 334:1561–1565. [DOI] [PubMed] [Google Scholar]
- 82.Shin JH, Zhang L, Murillo-Sauca O, Kim J, Kohrt HEK, Bui JD, Sunwoo JB: Modulation of natural killer cell antitumor activity by the aryl hydrocarbon receptor. Proc Natl Acad Sci USA 2013, 110:12391–12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, et al. : Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 2014, 508:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This manuscript shows that fetal LTi sense maternal retinoid intake to control the size of secondary lymphoid organs and the efficiency of immune responses in the adult offspring.
- 84.Spencer SP, Wilhelm C, Yang Q, Hall JA, Bouladoux N, Boyd A, Nutman TB, Urban JF, Wang J, Ramalingam TR, et al. : Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 2014, 343:432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This manuscript shows that ILCs can sense changes in dietary nutrients, such as vitamin A, that influence ILC subset expansion and barrier immunity.
- 85.Konya V, Czarnewski P, Forkel M, Rao A, Kokkinou E, Villablanca EJ, Aimer S, Lindforss U, Friberg D, Höög C, et al. : Vitamin D downregulates the IL-23 receptor pathway in human mucosal group 3 innate lymphoid cells. J Allergy Clin Immunol 2018, 141:279–292. [DOI] [PubMed] [Google Scholar]
- 86.Chen J, Waddell A, Lin Y-D, Cantorna MT: Dysbiosis caused by vitamin D receptor deficiency confers colonization resistance to Citrobacter rodentium through modulation of innate lymphoid cells. Mucosal Immunol 2015, 8:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH: Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol 2013, 132:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.von Moltke J, O’Leary CE, Barrett NA, Kanaoka Y, Austen KF, Locksley RM: Leukotrienes provide an NFAT-dependent signal that synergizes with IL-33 to activate ILC2s. J Exp Med 2017, 214:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xue L, Salimi M, Panse I, Mjösberg JM, McKenzie ANJ, Spits H, Klenerman P, Ogg G: Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J Allergy Clin Immunol 2014, 133:1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maric J, Ravindran A, Mazzurana L, Björklund ÅK, Van Acker A, Rao A, Friberg D, Dahlén S-E, Heinemann A, Konya V, et al. : Prostaglandin E2 suppresses human group 2 innate lymphoid cell function. J Allergy Clin Immunol 2018, 141:1761–1773.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Emgård J, Kammoun H, García-Cassani B, Chesnée J, Parigi SM, Jacob J-M, Cheng H-W, Evren E, Das S, Czarnewski P, et al. : Oxysterol Sensing through the Receptor GPR183 Promotes the Lymphoid-Tissue-Inducing Function of Innate Lymphoid Cells and Colonic Inflammation. Immunity 2018, 48:120–132.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This manuscript shows that ILC3 with an LTi-like phenotype express GPR183 to sense oxysterols expressed by FRCs for colonic lymphoid tissue development.
- 92.Huang Y, Mao K, Chen X, Sun M-A, Kawabe T, Li W, Usher N, Zhu J, Urban JF, Paul WE, et al. : S1P-dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 2018, 359:114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ibiza S, García-Cassani B, Ribeiro H, Carvalho T, Almeida L, Marques R, Misic AM, Bartow-McKenney C, Larson DM, Pavan WJ, et al. : Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 2016, 535:440–443. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This manuscript show that ILC3 can sense neurotrophic factors in their environment to control gut defense as part of a glial—ILC3—epithelial cell unit.
- 94.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang H-E, et al. : Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013, 502:245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cardoso V, Chesné J, Ribeiro H, Garcéia-Cassani B, Carvalho T, Bouchery T, Shah K, Barbosa-Morais NL, Harris N, Veiga-Fernandes H: Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 2017, 549:277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klose CSN, Mahlakõiv T, Moeller JB, Rankin LC, Flamar A-L, Kabata H, Monticelli LA, Moriyama S, Putzel GG, Rakhilin N, et al. : The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 2017, 549:282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wallrapp A, Riesenfeld SJ, Burkett PR, Abdulnour R-EE, Nyman J, Dionne D, Hofree M, Cuoco MS, Rodman C, Farouq D, et al. : The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature 2017, 549:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moriyama S, Brestoff JR, Flamar A-L, Moeller JB, Klose CSN, Rankin LC, Yudanin NA, Monticelli LA, Putzel GG, Rodewald H-R, et al. : β2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science 2018, 359:1056–1061. [DOI] [PubMed] [Google Scholar]; •• Collectively, references 95 – 98 show that ILC2s express receptors for neuronal-derived factors that can promote ILC2 function and highlights the importance of neuro-immune cross-talk in regulating tissue protection.
- 99.Pavlov VA, Tracey KJ: Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci 2017, 20:156–166. [DOI] [PubMed] [Google Scholar]
- 100.Dalli J, Colas RA, Arnardottir H, Serhan CN: Vagal Regulation of Group 3 Innate Lymphoid Cells and the Immunoresolvent PCTR1 Controls Infection Resolution. Immunity 2017, 46:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This manuscript shows that ILC3 sense acetylcholine produced by the vagus nerve to promote tissue repair, highlighting the importance of neuro-immune cross-talk in regulating tissue protection.
- 101.Quatrini L, Wieduwild E, Guia S, Bernat C, Glaichenhaus N, Vivier E, Ugolini S: Host resistance to endotoxic shock requires the neuroendocrine regulation of group 1 innate lymphoid cells. J Exp Med 2017, 214:3531–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quatrini L, Wieduwild E, Escaliere B, Filtjens J, Chasson L, Laprie C, Vivier E, Ugolini S: Endogenous glucocorticoids control host resistance to viral infection through the tissue-specific regulation of PD-1 expression on NK cells. Nat Immunol 2018, 19:954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This manuscript shows ILC1 express the receptor for glucocorticoid that can mediate resistance to viral infection and highlights the importance of ILC cross-talk with the neuroendocrine system in regulating tissue protection.
- 103.Li M, Schwerbrock NMJ, Lenhart PM, Fritz-Six KL, Kadmiel M, Christine KS, Kraus DM, Espenschied ST, Willcockson HH, Mack CP, et al. : Fetal-derived adrenomedullin mediates the innate immune milieu of the placenta. J Clin Invest 2013, 123:2408–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cephus J-Y, Stier MT, Fuseini H, Yung JA, Toki S, Bloodworth MH, Zhou W, Goleniewska K, Zhang J, Garon SL, et al. : Testosterone Attenuates Group 2 Innate Lymphoid Cell-Mediated Airway Inflammation. Cell Rep 2017, 21:2487–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This manuscript shows ILC2 can sense sex hormones, thus providing a basis for understanding sexual dimorphism in ILC function.
- 105.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A: A committed precursor to innate lymphoid cells. Nature 2014, 508:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klose CSN, Flach M, Möhle L, Rogell L, Floyler T, Ebert K, Fabiunke C, Pfeifer D, Sexl V, Fonseca-Pereira D, et al. : Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 2014, 157:340–356. [DOI] [PubMed] [Google Scholar]