Abstract
The interactions among the gut, liver, and immune system play an important role in liver disease. Probiotics have been used for the treatment and prevention of many pathological conditions, including liver diseases. Comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (GC×GC-TOF0020MS) was used herein, in conjunction with chemometric data analysis, to identify metabolites significantly affected by probiotics in mice fed with or without alcohol. The metabolomics analysis indicates that the levels of fatty acids increased in mouse liver and decreased in mouse feces when mice were chronically exposed to alcohol. Supplementing the alcohol-fed mice with culture supernatant from Lactobacillus rhamnosus GG (LGGs) normalized these alcohol-induced abnormalities and prevented alcoholic liver disease (ALD). These results agree well with previous studies. In addition to diet-derived long chain fatty acids (LCFAs), LGGs may positively modify the gut’s bacterial population to stimulate LCFA synthesis, which has been shown to enhance intestinal barrier function, reduce endotoxemia, and prevent ALD. We also found that several amino acids, including L-isoleucine, a branched chain amino acid, were downregulated in the liver and fecal samples from animals exposed to alcohol and that the levels of these amino acids were corrected by LGGs. These results demonstrate that LGGs alleviates alcohol-induced fatty liver by mechanisms involving increasing intestinal and decreasing hepatic fatty acids and increasing amino acid concentration.
Keywords: GC×GC-TOF MS, metabolomics, liver, probiotics, alcohol liver disease
Graphical Abstract
1. INTRODUCTION
Lactobacillus rhamnosus GG (LGG), a strain of the bacterium, Lactobacillus rhamnosus, has great affinity for human intestinal mucosal cells. Previous studies in our laboratory and others demonstrated that LGG is effective in the treatment of alcohol-induced liver injury in rodents.1−4 More recently, we have shown that LGG culture supernatant (LGGs) is effective in the prevention of acute and chronic alcohol exposure-induced fatty liver.5,6 LGGs administration markedly attenuates the effects of alcohol exposure on intestinal barrier dysfunction, by increasing intestinal mucus factors and epithelial tight junction protein expression, leading to decreased circulation endotoxin levels. In addition, LGGs activates hepatic 5′ adenosine monophosphate-activated protein kinase-α (AMPKα) phosphorylation, which is critical for liver lipid degradation.7
L. rhamnosus GG and its health benefits have been studied in genomics and proteomics. For example, Douillard et al. examined the genomes and phenotypes of 100 L. rhamnosus strains and compared them with that of LGG.8 Koskenniemi et al. used gene expression profiling at the transcriptome and proteome levels to investigate the cellular response of LGG toward bile under defined bioreactor conditions.9 Recent technical advances in metabolomics have promoted comprehensive studies to analyze the effect of probiotics on the metabolomic alterations in blood and fecal samples in inflammatory bowel disease10 and to identify potential active ingredients in probiotic preparations for various diseases.11,12 However, comprehensive analysis of metabolite changes in tissue and fecal samples of subjects with alcoholic liver disease (ALD) has not yet been performed.
To gain insight into the metabolic mechanisms by which LGGs exerts its influence in ALD, investigation of metabolome alterations using high-throughput metabolomics analysis is needed. Comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (GC×GC-TOF MS) is a powerful analytical platform in metabolomics. It uses two distinct capillary GC columns of different polarities connected via a thermal modulator.13−15 The analytes co-eluted from the first column are further separated in the second column because of the differences in column temperature and chromatographic polarities. The further-separated analytes are detected by a time-of-flight mass spectrometer. The GC×GC-TOF MS platform has significant advantages for complex sample analysis, including an order-of-magnitude increase in separation capacity, a significant increase in the signal-to-noise ratio and dynamic range, and an improvement in the deconvolution of mass spectra and similarity matches.16−18
The objectives of this study were to determine if the beneficial effects of LGGs administration on the prevention of ALD are associated with alterations in metabolic profiles in the liver and fecal metabolome. Male C57BL/6 mice were pair-fed either an isocaloric control diet (PF) or alcohol-containing diet (AF) for 4 weeks with or without LGGs co-exposure. Liver and fecal samples were collected at the end of the experiments. The metabolite extracts from mouse livers and feces were analyzed on GC×GC-TOF MS for metabolic profiling.
2. EXPERIMENTAL METHODS
2.1. Animals and Diets
C57BL/6 male mice obtained from Harlan (Indianapolis, IN) were fed a modified Lieber–DeCarli liquid diet (Research Diet, New Brunswick, NJ). Mice were fed EtOH-containing diets (35% of total calories, AF) ad libitum for 4 weeks. Control mice were pair-fed an isocaloric diet in which EtOH was replaced with maltose-dextrin. LGG was cultured following the instructions provided by ATCC (Manasses, VA). The culture supernatant was collected when LGG bacterial growth reached 109 CFU/mL. The LGG supernatant (LGGs) was added to the liquid diet at a dose equivalent to 109 CFU/mouse/day. At the end of the experiment, the mice were anesthetized, and the fecal and liver samples were harvested. A portion of the liver samples was fixed in formalin, and the rest of the liver and the fecal samples were frozen immediately in liquid nitrogen and stored at –80 °C for subsequent analysis. The animal protocol was approved by the University of Louisville Institutional Animal Care and Use Committee.
2.2. Liver Oil Red O Staining and Liver Total Free Fatty Acid Analysis
Frozen liver sections were processed for staining with Oil red O and then studied by light microscopy. Hepatic free fatty acid levels were measured using Infinity kits (Thermo Scientific, Waltham, MA).
2.3. Metabolite Sample Preparation
The fecal sample was first weighed and homogenized on ice. Each homogenized fecal sample was added to ice-cold 80% methanol at a ratio of 50 mg of feces in 1 mL of 80% methanol. The mixture was vortexed for 10 min, followed by centrifugation for 10 min at 16 000 rpm. Two-hundred microliters of the supernatant was aspirated into a plastic tube and dried by SpeedVac overnight. The dried metabolite extracts were dissolved in 30 μL of methoxyamine hydrochloride solution (30 mg/mL) and vigorously vortex-mixed for 1 min. Methoxymation was carried out at 70 °C for 1 h. After adding 30 μL of N-(tert-butyldimethylsilyl)-N-methyltrifluoroacetamide (MTBSTFA) mixed with 1% tert-butyldimethyl-chlorosilane (TBDMSCI), derivatization was carried out at 70 °C for 1 h.
To prepare liver samples, a sample of liver tissue was weighed and then homogenized for 2 min after adding water at a ratio of 100 mg of liver tissue/mL of water. The homogenized sample was then stored at –80 °C until use. A 100 μL aliquot of the homogenized liver sample and 400 μL of methanol were mixed and vortexed for 1 min followed by centrifugation at room temperature for 10 min at 15 000 rpm. Four-hundred microliters of the supernatant was aspirated into a plastic tube and dried by N2 flow. The metabolite extracts were then dissolved in 40 μL of methoxyamine hydrochloride solution (30 mg/mL) and vigorously vortex-mixed for 1 min. Methoxymation and derivatization were achieved in the same way as that for the fecal samples.
In order to verify compound identification, a total of 61 compound standards were prepared at equal molarity and analyzed via GC×GC-TOF MS. The methoxymation and derivatization of the fecal sample, liver sample, and compound standards were carried out right before GC×GC-TOF MS analysis.
2.4. GC×GC-TOF MS Analysis
A LECO Pegasus 4D GC×GC-TOF MS instrument was equipped with an Agilent 6890 gas chromatograph and a Gerstel MPS2 autosampler (GERSTEL Inc., Linthicum, MD), featuring a LECO two-stage cryogenic modulator and secondary oven. The primary column was a 60 m × 0.25 mm 1dc × 0.25 μm 1df, DB-5 ms GC capillary column (phenyl arylene polymer virtually equivalent to a (5%-phenyl)-methylpolysiloxane). A second GC column of 1 m × 0.25 mm 1dc × 0.25 μm 2df, DB17 ms ((50%-phenyl)-methyl-polysiloxane) was placed inside the secondary GC oven after the thermal modulator. Both columns were obtained from Agilent Technologies (Agilent Technologies J&W, Santa Clara, CA). The helium carrier gas (99.999% purity) flow rate was set to 2.0 mL/min at a corrected constant flow via pressure ramps. The inlet temperature was set at 280 °C. The primary column temperature was programmed with an initial temperature of 60 °C for 0.5 min and then increased at 5 °C/min to 280 °C and kept for 15 min. The secondary column temperature program was set to an initial temperature of 70 °C for 0.5 min and then also increased by the same temperature gradient employed in the first column to 280 °C accordingly. The thermal modulator was set to +15 °C relative to the secondary oven, and a modulation time of PM = 2 s was used. The mass range was set as 29–800 m/z, with an acquisition rate of 200 mass spectra per second. The ion source chamber was set at 230 °C with the transfer line temperature set to 280 °C, and the detector voltage was 1450 V with electron energy of 70 eV. The acceleration voltage was turned on after a solvent delay of 675 s. The split ratio was set at 40:1 for liver samples and 10:1 for fecal samples.
2.5. Data Analysis
The GC×GC-TOF MS data were processed using LECO’s instrument control software, ChromaTOF, for peak picking and tentative metabolite identification, followed by peak merging, retention index filtering, peak list alignment, normalization, and statistical significance testing using MetPP.19 Two-way ANOVA with a threshold p ≤ 0.05 was used to determine whether a metabolite was significantly different in its abundance between sample groups.
For metabolite identification using ChromaTOF, each chromatographic peak was tentatively assigned to a metabolite if its experimental mass spectrum and a NIST11 MS library spectrum have a spectral similarity score higher than 600 (the maximum value of spectral similarity was defined as 1000 in ChromaTOF). If a chromatographic peak was tentatively assigned to a metabolite by mass spectral matching, then the other top four ranked metabolite candidates (regardless of the magnitude of their spectral similarity scores) were also considered as the identification results of mass spectral matching for this chromatographic peak. That is, the top five matched compounds for a chromatographic peak were selected as the tentative identification result of the chromatographic peak giving rise to the mass spectrum. The tentatively assigned metabolites were further filtered by retention index matching using iMatch software with a p-value threshold of <0.001.20 To further verify the identification of metabolites detected with significant abundance difference between sample groups, the authentic standards of these metabolites, if commercially available, were analyzed on GC×GC-TOF MS under the same experimental conditions as the biological samples analyzed. A tentative metabolite assignment was considered to be a correct identification only if the experimental information on the authentic metabolite agrees with the corresponding information on the chromatographic peak in the biological samples, i.e., difference of the first dimension retention time, ≤10 s, difference of the second dimension retention time, ≤0.05 s, and the mass spectral similarity, ≥700.
3. RESULTS
Four biological sample groups were formed in this study: pair-fed control mice without LGGs supplementation (PF-0, n = 5), pair-fed control mice with exposure to LGGs (PF-l, n = 7), alcohol fed mice without LGGs supplementation (AF-0, n = 4), and alcohol fed mice with LGGs supplementation (AF-l, n = 6). Metabolite identification was done in three sequential steps, as described in our previous work, including mass spectral matching, retention index matching, and comparison with authentic standards.21 We compared the metabolic profile differences between PF-0 and AF-0, PF-1 and PF-0, and AF-1 and AF-0 in mouse liver and fecal samples, respectively.
GC×GC-TOF MS instrument data provide four pieces of information for each metabolite: the first dimension retention time 1tR, the second dimension retention 2tR, fragment ion m/z, and peak height of each fragment ion. The information on fragment ion m/z and peak height forms the mass spectrum of the metabolite. Figure 1 is a contour plot of the GC×GC-TOF MS data acquired from a fecal sample randomly selected from sample group PF-0. Each chromatographic peak in Figure 1 is composed of many data points, where the number of data points in each chromatographic peak depends on the size of the chromatographic peak. The color of each data point is the signal intensity, i.e., the total ion current of all fragment ions in the corresponding mass spectrum. The inset in Figure 1 demonstrates the increased separation power of GC×GC-TOF MS system. The two chromatographic peaks have identical retention times in the first dimension GC, but they are completely separated from each other on the second dimension GC. Therefore, these two chromatographic peaks will co-elute on a GC-MS system and generate a mixed-mass spectrum, which may induce false identification and quantification.
Figure 1.
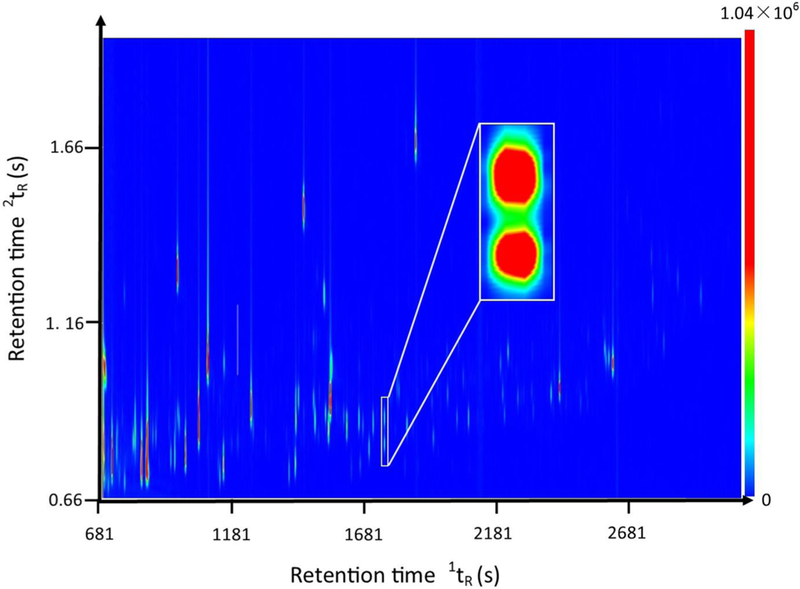
Sample GC×GC-TOF MS chromatogram of metabolites extracted from mouse fecal samples. The x axis is the first dimension retention time 1tR in seconds. The y axis is the second dimension retention time 2tR in seconds. The color bar shows the signal intensity of each peak plotted on the chromatogram in total ion current.
By setting the spectral similarity threshold to ≥600 in ChromaTOF, 440–1100 and 750–840 metabolites were identified from the liver and fecal samples, respectively. The numbers of metabolites detected in liver samples and fecal samples were further reduced to 390–880 and 435–727, respectively, after retention index matching. After cross-sample alignment and removal of chromatographic peaks detected in blank samples, 236 metabolites presented in more than 75% of liver samples in at least one sample group, whereas 212 were in the fecal samples.
Two-way ANOVA tests were employed to identify metabolites with significant abundance changes between sample groups produced by alcohol, LGGs, or their interaction. The fold-change was defined as the ratio of the large abundance value (peak area) of a metabolite in one group divided by the small abundance value of the same metabolite in the other group. The positive and negative signs indicate an abundance increase or decrease in the test sample group compared to that in the reference sample group, respectively.
Figure 2A is an example of the changes in metabolite abundance detected in the liver samples. The abundance distribution of the metabolite, hexanoic acid, in liver samples was detected in the four sample groups, PF-0, PF-1, AF-0, and AF-1. Hexanoic acid (caproic acid), a natural fatty acid existing in all mammals, has been shown to be beneficial in high-density lipoprotein synthesis in the liver. Compared with the abundance of this metabolite in control group PF-0, the level of this metabolite was increased with a fold change of 1.56 in group AF-0 and was decreased with a fold change of 1.09 in group PF-1. Comparing its abundance between groups AF-1 and AF-0, this metabolite is decreased with a fold change of 1.23 in the AF-1 group. The p-values for the factors of alcohol, LGGs, and their interaction are 0.002, 0.24, and 0.80, respectively. The alcohol factor has the smallest p-value of 0.002, indicating that this metabolite (hexanoic acid) has a significant alteration in its abundance in mouse livers because of the alcohol factor, whereas LGGs alone (p = 0.24) and the interaction of LGGs and alcohol (p = 0.80) did not significantly change the abundance of this metabolite in the liver.
Figure 2.
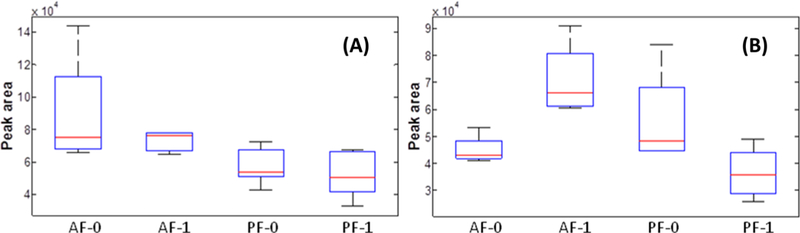
Abundance distribution of metabolite hexanoic acid in four sample groups: (A) liver and (B) fecal samples.
Figure 2B depicts the abundance distribution of the same metabolite (hexanoic acid) in feces among the four sample groups. The trend in the abundance distribution of this metabolite in fecal samples is completely different from that in the liver samples. Compared with the abundance of this metabolite in control group PF-0, the abundance of this metabolite is decreased 1.54-fold in group PF-1 and by 1.25- fold in sample group AF-0. Hexanoic acid is increased with a fold change of 1.58 in group AF-1 compared to AF-0. The p- values of alcohol, LGGs, and their interaction are 0.05, 0.92, and 0.003, respectively. The large p-value of 0.92 indicates that LGGs alone did not significantly alter the abundance of this metabolite in the mouse fecal metabolome, whereas alcohol alone affected the abundance of this metabolite (p = 0.05). Importantly, the smallest magnitude p-value (p = 0.003) for the interaction between alcohol and LGGs indicates that the abundance of this metabolite was significantly changed owing to the synergistic effect of alcohol and LGGs on the mouse fecal metabolome.
Figures S1 and S2 depict the abundance distributions of tetradecanoic acid and irans-9-octadecenoic acid in all sample groups, respectively. Compared with the trend in the abundance changes between sample groups for hexanoic acid, these two metabolites have a similar trend in their abundance changes among the four liver sample groups and the fecal sample groups, respectively.
Table 1 lists all metabolites that were detected with significant abundance changes from liver samples among the four sample groups with a threshold of p ≤ 0.05. These compounds include eight free fatty acids, six amino acids, three other acids, and three other small compounds. Among the eight fatty acids, one is a short chain fatty acid (SCFA, acetic acid), two are medium chain fatty acids (MCFA, hexanoic acid and dodecanoic acid), one is a long chain fatty acid (LCFA, tetradecanoic acid), two are monounsaturated fatty acids (trans-9-octadecenoic acid and cis-9-hexadecenoic acid), and two are polyunsaturated fatty acids (mead acid and arachidonic acid). The abundance of all four saturated fatty acids and cis-9- hexadecenoic acid were increased when the mice were fed alcohol (column FC-2 in Table 1). However, the abundance of all of these acids decreased when the alcohol-fed mice were also exposed to LGGs (column FC-1 in Table 1). Figure 3 depicts the results of total free fatty acid analysis, showing that the total non-esterified fatty acids were increased by alcohol exposure and decreased by LGGs supplementation. Histological examination confirmed our metabolomics finding that hepatic fat accumulation by alcohol was markedly increased and that LGGs supplementation prevented it (Figure 4).
Table 1.
Metabolites with Significant Changes in Their Abundance among the Four Liver Sample Groups
| name | CAS | 1tR (s) | 2tR (s) | FC-1a | FC-2b | FC-3c | p-1d | p-2e | p-3f |
|---|---|---|---|---|---|---|---|---|---|
| Fatty Acids | |||||||||
| acetic acidg | 67226-76-0 | 1443 | 1.01 | −1.05 | 2.05 | 1.22 | 1.3 × 10–3 | 9.0 × 10−1 | 2.9 × 10–1 |
| dodecanoic acid | 143-07-7 | 1961 | 1.04 | −1.41 | 1.59 | −1.09 | 8.9 × 10–3 | 7.6 × 10–2 | 4.7 × 10–1 |
| hexanoic acid | 142-62-1 | 1113 | 1.00 | −1.23 | 1.56 | −1.09 | 2.1 × 10–3 | 2.4 × 10–1 | 8.1 × 10–1 |
| tetradecanoic acid | 4544-63-8 | 2196 | 1.06 | −1.62 | 1.42 | 1.20 | 9.0 × 10–1 | 4.4 × 10–1 | 4.4 × 10–2 |
| cis-9-hexadecenoic acidg | 373-49-9 | 2385 | 1.11 | −1.40 | 2.25 | 1.26 | 8.2 × 10–3 | 9.9 × 10–1 | 1.0 × 10–1 |
| trans-9-octadecenoic acidg | 112-79-8 | 2594 | 1.17 | −2.44 | −1.28 | −2.28 | 3.6 × 10–1 | 3.1 × 10–2 | 8.5 × 10–1 |
| mead acidg | 20590-32-3 | 2793 | 1.51 | 1.01 | 1.82 | 1.59 | 3.5 × 10–2 | 2.7 × 10–1 | 1.7 × 10–1 |
| arachidonic acidg | 506-32-1 | 2762 | 1.51 | 1.35 | −1.55 | 1.10 | 1.7 × 10–2 | 7.9 × 10–2 | 4.0 × 10–1 |
| Amino Acids | |||||||||
| L-isoleucine | 73-32-5 | 1748 | 0.99 | 1.43 | −1.09 | 1.20 | 1.0 × 1000 | 3.9 × 10–3 | 2.8 × 10–1 |
| L-proline | 147-85-3 | 1803 | 1.08 | 1.33 | −1.46 | 1.13 | 1.7 × 10–3 | 2.2 × 10–2 | 2.5 × 10–1 |
| L-threonine | 72-19-5 | 1769 | 1.05 | 1.20 | −1.08 | −1.18 | 7.1 × 10–5 | 1.4 × 10–1 | 1.3 × 10–2 |
| L-phenylalanine | 63-91-2 | 2189 | 1.16 | 1.28 | −1.10 | 1.31 | 3.2 × 10–1 | 2.8 × 10–2 | 9.9 × 10–1 |
| L-tyrosine | 60-18-4 | 2768 | 1.38 | 1.22 | 1.02 | 1.20 | 6.6 × 10–1 | 1.6 × 10–2 | 8.8 × 10–1 |
| L-valine | 72-18-4 | 1652 | 0.99 | 1.34 | −1.06 | 1.16 | 9.7 × 10–1 | 3.0 × 10–2 | 4.8 × 10–1 |
| Other Acids | |||||||||
| 2-aminobutanoic acid | 2835-81-6 | 1585 | 0.99 | −2.16 | 3.57 | −1.14 | 2.5 × 10–4 | 1.3 × 10–1 | 8.0 × 10–2 |
| glycolic acidg | 79-14-1 | 1443 | 1.01 | −1.05 | 2.05 | 1.22 | 1.4 × 10–3 | 9.0 × 10–1 | 2.9 × 10–1 |
| α-ethylphenylacetic acidg | 90-27-7 | 2499 | 1.29 | 1.30 | −2.39 | 1.04 | 9.6 × 10–4 | 3.7 × 10–1 | 7.2 × 10–1 |
| Others | |||||||||
| 3-pyridinolg | 109-00-2 | 1245 | 1.20 | −1.18 | 1.43 | 1.46 | 3.3 × 10–1 | 2.8 × 10–1 | 1.2 × 10–2 |
| hypoxanthineg | 68-94-0 | 2345 | 1.43 | −1.09 | 1.55 | 1.26 | 3.2 × 10–3 | 5.1 × 10–1 | 9.4 × 10–2 |
| 2-(2-(2-ethoxyethoxy)ethoxy)acetic acidg | 16024-58-1 | 2456 | 1.05 | 4.20 | −4.04 | 1.14 | 4.3 × 10–3 | 3.9 × 10–3 | 1.5 × 10–2 |
Fold change for AF-1 to AF-0.
Fold change for AF-0 to PF-0
Fold change for PF-1 to PF-0.
p-value of factor alcohol.
p-value of factor LGGs.
p-value of the interaction of alcohol and LGGs.
Tentative identification without using authentic standards.
Figure 3.
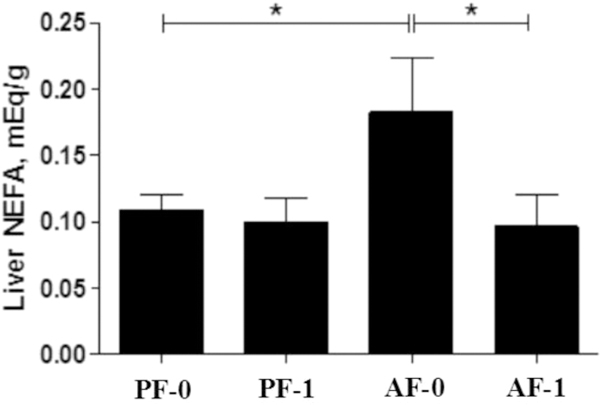
Effect of LGGs on hepatic free fatty acid levels. Asterisks (*) indicate that the amount of total free fatty acids between two sample groups is significantly different (p < 0.05).
Figure 4.
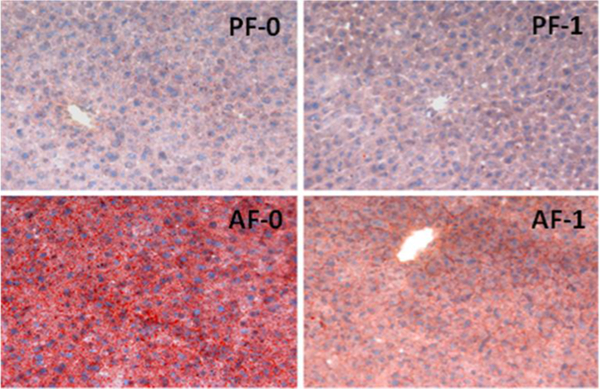
Effect of LGGs on liver fat accumulation. Hepatic cryosections were Oil red O stained, and images were acquired by light microscopy. The blue dots denote nuclei, and the red stain represents fat droplets in the liver.
Among six amino acids, the abundance of five amino acids, including L-isolucine, L-proline, L-threonine, L-phenylalanine, and L-valine, decreased when mice were exposed to alcohol alone (column FC-2 in Table 1), whereas they all increased when the alcohol-fed mice were exposed to LGGs (column FC- 1 in Table 1).
Table 2 lists metabolites with significant abundance changes among sample groups detected from fecal samples with a threshold of p ≤ 0.05. A total of nine free fatty acids, nine amino acids, two alcohols, six other acids, and nine other small compounds have significant changes in abundance due to alcohol, LGGs, or their synergetic interaction. Among these nine fatty acids, two are short chain fatty acids (SCFA, isovaleric acid and pentanoic acid), one is a MCFA (hexanoic acid), four are LCFA (heptadecanoic acid, hexadecanoic acid, nonadecanoic acid, tetradecanoic acid), one is a polyunsaturated omega-6 fatty acid (9,12-octadecadienoic acid), and one is an omega-7 monounsaturated fatty acid (cis-9-hexadecenoic acid). Compared with their abundance in sample group AF-0, the abundance of these SCFAs, MCFAs, and LCFAs in sample group AF-l were significantly increased, whereas that of omega-7 monounsaturated fatty acid and polyunsaturated omega-6 fatty acid decreased (column FC-1 in Table 2). Even though the fold changes of most of the fatty acids are moderate, the abundance of hexadecanoic acid increased 20.6-fold in group AF-1. Among the nine amino acids, the abundance of seven decreased in group AF-0 compared to that in group PF-0 (column FC-2 in Table 2). Among these seven amino acids, six amino acids increased in the alcohol and LGGs treated group, AF-1, compared to that in group AF-0 (column FC-1 in Table 2). It is interesting that the abundance of L-proline decreased in group AF-1 compared to that in group AF-0, decreased in group AF-0 compared to that in group PF-0, and decreased in group PF-1 compared to that in group PF-0.
Table 2.
Metabolites with Significant Changes in Their Abundance among Four Fecal Sample Groups
| name | CAS | 1tR (s) | 2tR (s) | FC-1a | FC-2b | FC-3c | p-1d | p-2e | p-3f |
|---|---|---|---|---|---|---|---|---|---|
| Fatty Acids | |||||||||
| isovaleric acidg | 503-74-2 | 882 | 0.98 | 1.43 | −2.22 | 1.02 | 1.7 × 10–2 | 4.1 X 10–1 | 5.5 × 10–1 |
| pentanoic acid | 109-52-4 | 957 | 1.00 | 1.67 | 1.26 | 1.96 | 5.7 × 10–1 | 1.7 X 10–2 | 5.9 × 10–1 |
| hexanoic acid | 142-62-1 | 1114 | 1.02 | 1.58 | −1.25 | −1.54 | 5.5 × 10–2 | 9.2 X 10–1 | 3.2 × 10–3 |
| heptadecanoic acid | 506-12-7 | 2511 | 1.12 | 1.25 | −2.41 | 1.44 | 1.5 × 10–2 | 1.5 X 10–1 | 5.5 × 10–1 |
| hexadecanoic acid | 57-10-3 | 2396 | 1.10 | 20.6 | 2.04 | 8.74 | 1.3 × 10–2 | 1.5 X 10–2 | 6.5 × 10–2 |
| nonadecanoic acid | 646-30-0 | 2728 | 1.31 | 1.06 | 1.69 | −1.06 | 1.2 × 10–3 | 9.6 X 10–1 | 6.2 × 10–1 |
| tetradecanoic acid | 544-63-8 | 2197 | 1.09 | 1.81 | −1.41 | −1.01 | 4.5 × 10–1 | 1.6 X 10–4 | 1.4 × 10–4 |
| cis-9-hexadecenoic acidg | 373-49-9 | 2385 | 1.14 | −2.84 | 1.88 | 1.67 | 4.7 × 10–1 | 5.1 X 10–1 | 4.8 × 10–2 |
| 9,12-octadecadienoic acidg | 60-33-3 | 2687 | 1.37 | −1.82 | 1.02 | −1.88 | 8.8 × 10–1 | 4.1 X 10–3 | 6.5 × 10–1 |
| Amino Acids | |||||||||
| L-methionine | 63-68-3 | 2047 | 1.15 | 1.61 | −2.82 | 1.13 | 1.4 × 10–4 | 4.0 X 10–2 | 3.1 × 10–1 |
| L-phenylalanine | 63-91-2 | 2189 | 1.20 | 1.99 | −2.57 | −1.13 | 3.6 × 10–3 | 1.4 X 10–1 | 4.4 × 10–2 |
| L-ornithineg | 70-26-8 | 2389 | 1.05 | 8.34 | −1.68 | 1.13 | 3.4 × 10–1 | 1.1 × 10–2 | 4.5 × 10–2 |
| L-alanine | 56-41-7 | 789 | 0.97 | 1.66 | −1.18 | 1.47 | 3.9 × 10–1 | 2.0 × 10–2 | 9.1 × 10–1 |
| L-threonine | 72-19-5 | 2101 | 1.01 | 1.04 | −7.26 | 1.15 | 6.6 × 10–6 | 7.9 × 10–1 | 6.2 × 10–1 |
| L-aspartic acid | 56-84-8 | 2255 | 1.08 | 1.58 | −1.51 | −1.68 | 6.1 × 10–1 | 9.4 × 10–1 | 4.8 × 10–2 |
| L-valine | 72-18-4 | 1667 | 0.99 | 1.17 | 27.6 | 2.33 | 2.6 × 10–6 | 4.4 × 10–3 | 2.9 × 10–2 |
| L-proline | 147-85-3 | 1803 | 1.11 | −1.10 | −1.33 | −1.11 | 3.7 × 10–3 | 2.7 × 10–1 | 9.6 × 10–1 |
| L-glutamic acid | 56-86-0 | 2381 | 1.10 | −1.10 | 1.76 | −1.22 | 3.2 × 10–2 | 7.1 × 10–1 | 6.6 × 10–1 |
| Other Acids | |||||||||
| 4-hydroxybenzoic acidg | 99-96-7 | 2231 | 1.19 | 3.12 | −2.83 | −1.10 | 1.5 × 10–1 | 4.9 × 10–2 | 6.0 × 10–2 |
| trans-crotonic acidg | 107-93-7 | 888 | 1.05 | 1.59 | −1.31 | −1.01 | 6.1 × 10–1 | 8.9 × 10–3 | 5.9 × 10–3 |
| p-coumaric acidg | 501-98-4 | 2579 | 1.32 | 1.46 | 2.34 | −1.43 | 7.0 × 10–3 | 7.5 × 10–1 | 2.6 × 10–1 |
| 2-ketobutyric acidg | 600-18-0 | 1735 | 1.07 | −1.11 | 3.37 | 1.72 | 2.6 × 10–3 | 3.3 X 10–1 | 2.4 × 10–1 |
| nicotinic acidg | 59-67-6 | 1509 | 1.28 | −1.07 | 6.29 | 1.12 | 1.6 × 10–5 | 6.7 × 10–1 | 8.6 × 10–1 |
| nonanedioic acidg | 123-99-9 | 2391 | 1.17 | 1.89 | 1.42 | 1.14 | 3.0 × 10–3 | 5.2 × 10–2 | 1.6 × 10–1 |
| Alcohols | |||||||||
| propylene glycolg | 57-55-6 | 1311 | 0.93 | 1.94 | −3.15 | −1.10 | 7.9 × 10–5 | 4.2 × 10–2 | 1.1 × 10–2 |
| 2-hydroxybenzyl alcoholg | 90-01-7 | 1961 | 1.13 | 1.46 | 2.36 | −1.36 | 6.6 × 10–6 | 6.5 × 10–1 | 1.3 × 10–2 |
| Others | |||||||||
| phenol | 108-95-2 | 1100 | 1.13 | 1.51 | −2.88 | −1.04 | 2.4 × 10–5 | 1.0 × 10–1 | 3.9 × 10–2 |
| D,L-glyceraldehydeg | 56-82-6 | 2622 | 1.18 | −1.60 | −2.31 | 1.25 | 2.2 × 10–2 | 7.7 × 10–1 | 8.1 × 10–1 |
| hydroxylamineg | 7803-49-8 | 1076 | 0.94 | 1.58 | −2.12 | −1.23 | 1.4 × 10–3 | 2.2 × 10–1 | 7.1 × 10–3 |
| ethanolamine, N-acetyl-g | 142-26-7 | 965 | 1.04 | 1.58 | −1.68 | −1.02 | 2.9 × 10–2 | 7.1 × 10–2 | 5.0 × 10–2 |
| 2-(2-(2-ethoxyethoxy)ethoxy)acetic acidg | 16024-58-1 | 2455 | 1.07 | −2.49 | −1.13 | −1.07 | 2.9 × 10–2 | 4.3 × 10–1 | 6.1 × 10–1 |
| 1,2-pyrrolidinedicarboxylic acid, (2R)-g | 1067236-75-2 | 2139 | 1.29 | 1.70 | −1.08 | 1.22 | 5.3 × 10–1 | 2.1 × 10–2 | 4.0 × 10–1 |
| urea | 57-13-6 | 1657 | 1.17 | 1.86 | 2.00 | 1.77 | 8.7 × 10–3 | 4.9 × 10–2 | 9.0 × 10–1 |
| 3-pyridinolg | 109-00-2 | 1061 | 1.14 | 1.94 | 1.83 | 1.32 | 1.1 × 10–4 | 2.5 × 10–3 | 8.6 × 10–2 |
| D,L-homoserineg | 1927-25-9 | 2187 | 1.02 | −1.67 | 2.31 | −1.94 | 2.2 × 10–2 | 1.5 × 10–1 | 4.3 × 10–1 |
Fold change for AF-1 to AF-0.
Fold change for AF-0 to PF-0.
Fold change for PF-1 to PF-0.
p-value of factor alcohol.
p-value of factor LGGs.
p-value of the interaction of alcohol and LGGs.
Tentative identification without verification using authentic standards.
4. DISCUSSION
Four sample groups were formed in this study to investigate the effects of alcohol, LGGs, and their interaction on the mouse liver and fecal metabolomes. Figure 5 depicts clustering results of the metabolite profiles of the four liver sample groups (Figure 5A) and four fecal sample groups (Figure 5B), using partial least-squares discriminant analysis (PLSDA). The cross-validation demonstrates good predictive ability of the models, with relatively high Q2 values of 0.54 for fecal samples and 0.67 for liver samples (Figure S3). The clear separation among sample groups indicates that there are significant metabolic profiling differences among the sample groups.
Figure 5.
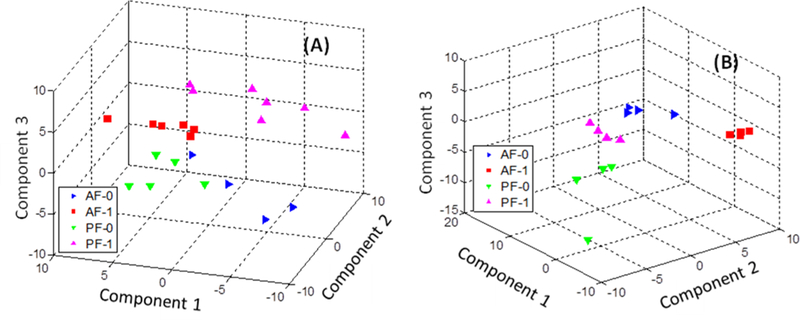
Clustering of metabolite profiles using partial least-squares discriminant analysis (PLSDA): (A) liver samples and (B) fecal samples.
Figure S4 shows the clustering results using the k-means clustering method, during which principal component analysis (PCA) was used for data dimensionality reduction. The clustering accuracies of fecal samples and liver samples are only 0.55 and 0.52, respectively. While such different clustering results were mainly caused by the nature of the clustering algorithms (i.e., PLSDA is a supervised clustering method, but k-means is not), the poor clustering results of k-means reflect moderate differences in the metabolite profiles among the sample groups.
4.1. Analysis of Liver Samples
Excess alcohol consumption is one of the major factors in the development of fatty liver, which is characterized by hepatic accumulation of triglyceride, cholesterol, and other types of lipid. A total of eight fatty acids were detected with significant alterations in their abundance among the four sample groups. The four saturated fatty acids and cis-9-hexadecenoic acid have the same trend in their alteration of abundance, whereas the polyunsaturated fatty acid arachidonic acid has the opposite direction of its abundance changes. The abundance of all four saturated fatty acids and cis-9-hexadecenoic acid are increased in the liver of the mice fed alcohol. While SCFAs are metabolized in the mitochondria for energy production, MCFAs and LCFAs are mainly oxidized in the peroxisome.22 The higher levels of MCFAs and LCFAs in the liver of mice fed alcohol strongly suggest that alcohol feeding interferes with lipid peroxisomal oxidation, which is critical for hepatic fat elimination. However, hepatic levels of these fatty acids were significantly decreased in liver when LGGs was supplemented to alcohol-fed mice. This agrees with our total non-esterified fatty acids analysis and the results of the histological examination of liver sections (Figures 3 and 4). Taken together, the beneficial effects of LGGs on liver fat accumulation are mediated, at least in part, by increasing fatty acid peroxisomal oxidation.
Another finding in the current study is the decrease in five amino acids, L-isoleucine, L-proline, L-threonine, L-phenylalanine, and L-valine, by alcohol feeding and the normalization of their abundance by LGGs supplementation. L-Threonine is an essential amino acid that is not synthesized in mice. L-Threonine deficiency causes fat buildup in the liver, whereas supplementation with L-threonine lowers the hepatic fat concentration.23 L-Proline is a nonessential amino acid that can be synthesized from glutamate. Importantly, L-isoleucine is a branched-chain amino acid (BCAA), which is an essential amino acid and plays an important role in many aspects of hepatic physiology.24 Clinical studies have shown that BCAA-enriched nutritional supplementation is beneficial for the treatment of ALD.25 Our results agree with these studies and demonstrate that LGGs supplementation increases the concentration of L-isoleucine to reduce the fat buildup induced by alcohol, likely through improved intestinal absorption. Altogether, our studies demonstrate that LGGs helps to remedy the amino acid depletion-mediated liver damage caused by alcohol consumption.
4.2. Analysis of Fecal Samples
We have previously demonstrated that saturated fat supplementation protects the liver from alcohol-induced injury through stabilizing intestinal tight junctions, leading to reduced levels of circulating lipopolysaccharide (LPS).26 Although the underlying mechanisms of this are still unclear, recent studies have shown that saturated fatty acids are metabolized by commensal Lactobacilli to promote their expansion.27 Fatty acids can be synthesized by bacteria in the host in addition to being derived from fat in the diet. In the current studies, we fed mice with the same amount of fat in an isocaloric diet in all animal groups. Therefore, the changes in fatty acids that we observed are likely from bacterial production. The concentration of five out of nine detected fatty acids (i.e., isovaleric acid, hexanoic acid, heptadecanoic acid, tetradecanoic acid, and cis-9-hexadecenoic acid) was significantly reduced in the feces of mice fed alcohol. Of particular interest, hepatadecanoic acid (C17:0), which is a LCFA produced only by bacteria,28 was reduced 2.41-fold by alcohol. Importantly, LGGs supplementation to both alcohol-fed and pair-fed mice significantly increased luminal levels of hepatadecanoic acid.
Chronic alcohol exposure significantly changes the gut’s bacterial population.29 Administration of probiotics to alcohol-exposed mice normalized gut microflora, restored intestinal barrier tight junctions, reduced endotoxin translocation, and attenuated liver steatosis and inflammation.2 The increased abundance of fatty acids (i.e., isovaleric acid, pentanoic acid, hexanoic acid, heptadecanoic acid, hexadecanoic acid, non- adecanoic acid, and tetradecanoic acid), therefore, contributes to the beneficial effects of LGGs, namely, reduced fatty liver and liver injury, in response to alcohol exposure.
Similar to our findings in the liver, fecal concentrations of six amino acids (L-methionine, L-phenylalanine, L-omithine, L- alanine, L-threonine, and L-aspartic acid) were reduced by alcohol feeding and increased by LGGs supplementation. Proteins in the diet are degraded in the intestine to amino acids, which serve as nutrients for intestinal cells and extraenteric tissues. Previous work demonstrated that gut microflora potentially plays a major role in proteolysis in the large intestine. Amino acid metabolism in host cells depends on the type of bacteria and amino acids. Currently, it is still unclear how a specific amino acid is metabolized in the gut in response to alcohol exposure. The fact that the decreased amino acid concentration of six amino acids was increased by LGGs supplementation suggests that the effects of LGGs on amino acid anabolism could potentially contribute to the beneficial effects in ALD.
While differences in metabolite regulation among the sample groups observed in this study are consistent with our histological study, our current work still has some limitations. Metabolites in mouse liver and mouse feces can have very diverse chemical characteristics. In this work, metabolites were extracted by water and methanol. Most nonpolar metabolites were lost during this analytical step. The extracted metabolites were then analyzed by GC×GC-TOF MS. Even though GC×GC-TOF MS has better separation power compared to that of conventional one-dimensional GC-MS, it may not be enough to resolve all metabolites and may result in overlapping chromatographic peaks, which would introduce significant challenges for metabolite identification and quantification.
In this work, metabolite identification was first achieved by matching the experimental mass spectra to the mass spectra recorded in the NIST11 MS library. The incompleteness of the existing mass spectra library not only introduces a certain degree of false-positive identifications but also leaves a number of chromatographic peaks without any compound identification. Besides the MS library, metabolite identification in the analysis of GC×GC-TOF MS data can also be affected by the spectral similarity measure and the optimal weight factor used during mass spectral matching.30,31 It is impossible to estimate the rate of false-positive identifications induced by the incompleteness of the NIST11 MS library. However, analysis of the NIST11 MS library demonstrated that the accuracy of mass spectral matching-based metabolite identification can be improved from about 80 to 96% if the top five ranked metabolite candidates are considered as the identification results.30 For this reason, we used the top five ranked metabolite candidates of each chromatographic peak as the results of mass spectral matching and further subjected the mass spectral matching results to retention index matching. The metabolite candidate with the best retention index matching was kept for further analysis.
All of these technical limitations in the current study prevent us from seeing the entire picture of the metabolite profile in mouse liver and feces. Further studies, such as using different extraction methods and combining both GC×GC-TOF MS with two-dimensional liquid chromatography high-resolution mass spectrometry (2D LC-MS), may provide more comprehensive results.
5. CONCLUSIONS
GC×GC-TOF MS was used to identify metabolites that have significant alterations in their abundance in mouse liver and fecal samples collected from mice fed with and without alcohol and with and without co-exposure to LGGs. Our results show that the abundance of saturated fatty acids increased in liver samples but that the abundance of a fraction of these fatty acids decreased in fecal samples when the mice were fed alcohol. However, the abundance of all saturated fatty acids decreased in mouse livers but increased in mouse feces when the mice were co-exposed to LGGs. Also, we found that alcohol consumption depletes some amino acids in mouse livers and that such liver damage can be remedied by supplementing the mice with LGGs. These results demonstrate that LGGs alleviates alcohol- induced fatty liver by mechanisms involving increasing intestinal and decreasing hepatic fatty acids and increasing amino acid concentration.
Supplementary Material
Figure S1: Abundance distribution of metabolite tetradecanoic acid. Figure S2: Abundance distribution of metabolite trans-9-octadecenoic acid. Figure S3: Using R2 and Q2 to evaluate the quality of the principal component models. Figure S4: Clustering plots using k-means method for fecal samples and liver samples. This material is available free of charge via the Internet at http://pubs.acs.org.
ACKNOWLEDGMENTS
The authors thank Marion McClain for review of this manuscript. This work was supported by NIH grant nos. R01GM087735, R01AA023681, R21AA020848, and R21AA022416.
Footnotes
ASSOCIATED CONTENT
Supporting Information
Notes
The authors declare no competing financial interest.
REFERENCES
- (1).Kirpich IA; McClain CJ Probiotics in the treatment of the liver diseases. J. Am. Coll. Nutr. 2012, 31, 14–23. [DOI] [PubMed] [Google Scholar]
- (2).Wang Y; Kirpich I; Liu Y; Ma Z; Barve S; McClain CJ; Feng W Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am. J. Pathol. 2011, 179, 2866–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Forsyth CB; Farhadi A; Jakate SM; Tang Y; Shaikh M; Keshavarzian A Lactobacillus GG treatment ameliorates alcohol- induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol (N.Y. NY, U.S.) 2009, 43, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kelishadi R; Farajian S; Mirlohi M Probiotics as a novel treatment for non-alcoholic fatty liver disease; a systematic review on the current evidences. Hepatitis Mon. 2013, 13, e7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Wang Y; Liu Y; Kirpich I; Ma Z; Wang C; Zhang M; Suttles J; McClain C; Feng W Lactobacillus rhamnosus GG reduces hepatic TNFa production and inflammation in chronic alcohol- induced liver injury. J. Nutr. Biochem. 2013, 24, 1609–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Wang Y; Liu Y; Sidhu A; Ma Z; McClain C; Feng W Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am. J. Physiol. 2012, 303, G32–G41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Zhang M; Wang C; Wang C; Zhao H; Zhao C; Wang Y; McClain C; Feng W Enhanced AMPK phosphorylation contributes to the beneficial effects of Lactobacillus rhamnosus GG supernatant on chronic alcohol-induced fatty liver disease. J. Nutr. Biochem 2014, DOI: 10.1016/j.jnutbio.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Douillard FP; Ribbera A; Kant R; Pietila TE; Jarvinen HM; Messing M; Randazzo CL; Paulin L; Laine P; Ritari J; Caggia C; Lahteinen T; Brouns SJJ; Satokari R; von Ossowski I; Reunanen J; Palva A; de Vos WM Comparative genomic and functional analysis of 100 Lactobacillus rhamnosus strains and their comparison with strain GG. PLoS Genet. 2013, 9, e1003683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Koskenniemi K; Laakso K; Koponen J; Kankainen M; Greco D; Auvinen P; Savijoki K; Nyman TA; Surakka A; Salusjarvi T; de Vos WM; Tynkkynen S; Kalkkinen N; Varmanen P Proteomics and transcriptomics characterization of bile stress response in probiotic Lactobacillus rhamnosus GG. Mol Cell. Proteomics 2011, 10, M110.002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hong YS; Hong KS; Park MH; Ahn YT; Lee JH; Huh CS; Lee J; Kim IK; Hwang GS; Kim JS Metabonomic understanding of probiotic effects in humans with irritable bowel syndrome. J. Clin. Gastroenterol. 2011, 45, 415–425. [DOI] [PubMed] [Google Scholar]
- (11).Thomas CM; Hong T; van Pijkeren JP; Hemarajata P; Trinh DV; Hu WD; Britton RA; Kalkum M; Versalovic J Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 2012, 7, e31951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ewaschuk JB; Walker JW; Diaz H; Madsen KL Bioproduction of conjugated linoleic acid by probiotic bacteria occurs in vitro and in vivo in mice. J. Nutr. 2006, 136, 1483–1487. [DOI] [PubMed] [Google Scholar]
- (13).Ralston-Hooper K; Hopf A; Oh C; Zhang X; Adamec J; Sepulveda MS Development of GCxGC/TOF-MS metabolomics for use in ecotoxicological studies with invertebrates. Aquat. Toxicol. 2008, 88, 48–52. [DOI] [PubMed] [Google Scholar]
- (14).Mohler RE; Dombek KM; Hoggard JC; Pierce KM; Young ET; Synovec RE Comprehensive analysis of yeast metabolite GCxGC-TOFMS data: combining discovery-mode and deconvolution chemometric software. Analyst 2007, 132, 756–67. [DOI] [PubMed] [Google Scholar]
- (15).Huang X; Regnier FE Differential metabolomics using stable isotope labeling and two-dimensional gas chromatography with time- of-flight mass spectrometry. Anal. Chem. 2008, 80, 107–14. [DOI] [PubMed] [Google Scholar]
- (16).Liu SM; Miller DM; Roberts RF Cloning of genes encoding colicin E2 in Lactococcus lactis subspecies lactis and evaluation of the colicin-producing transformants as inhibitors of Escherichia coli O157:H7 during milk fermentation. J. Dairy Sci. 2011, 94, 1146–1154. [DOI] [PubMed] [Google Scholar]
- (17).Godsey HS; Oviatt CG; Miller DM; Chan MA Stratigraphy and chronology of offshore to nearshore deposits associated with the Provo shoreline, Pleistocene Lake Bonneville, Utah. Palaeogeogr., Palaeoclimatol., Palaeoecol. 2011, 310, 442–450. [Google Scholar]
- (18).Koo I; Zhang X; Kim S Wavelet- and Fourier-transform- based spectrum similarity approaches to compound identification in gas chromatography/mass spectrometry. Anal. Chem. 2011, 83, 5631–5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Wei X; Shi X; Koo I; Kim S; Schmidt RH; Arteel GE; Watson WH; McClain C; Zhang X MetPP: a computational platform for comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry-based metabolomics. Bioinformatics 2013, 29, 1786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Zhang J; Fang AQ; Wang B; Kim SH; Bogdanov B; Zhou ZX; McClain C; Zhang X iMatch: a retention index tool for analysis of gas chromatography-mass spectrometry data. J. Chromatogr. A 2011, 1218, 6522–6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Shi X; Wei X; Koo I; Schmidt RH; Yin X; Vaughn A; Kim SH; McClain CJ; Arteel GE; Zhang X; Watson WH Metabolomic analysis of the effects of chronic arsenic exposure in a mouse model of diet-induced fatty liver disease. J. Proteome Res. 2014, 13, 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Rao MS; Reddy JK Peroxisomal β-oxidation and steatohepatitis. Semin. Liver Dis. 2001, 21, 43–55. [DOI] [PubMed] [Google Scholar]
- (23).Morris L; Arata D; Cederquist DC Fatty livers in weanling rats fed a low protein, threonine-deficient diet. I. Effect of various diet fats. J. Nutr. 1965, 85, 362–366. [DOI] [PubMed] [Google Scholar]
- (24).Charlton M Branched-chain amino acid enriched supplements as therapy for liver disease. J. Nutr. 2006, 136, 295S–298S. [DOI] [PubMed] [Google Scholar]
- (25).Calvey H; Davis M; Williams R Controlled trial of nutritional supplementation, with and without branched chain amino acid enrichment, in treatment of acute alcoholic hepatitis. J. Hepatol. 1985, 1, 141–51. [DOI] [PubMed] [Google Scholar]
- (26).Kirpich IA; Feng W; Wang Y; Liu Y; Barker DF; Barve SS; McClain CJ The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol.: Clin. Exp. Res. 2012, 36, 835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Chen P; Loomba R; Torralba M; DePew J; Nelson KE; Tan J; Embree M; Zengler K; Starkel P; Pijkeren J.-P.v.; Ho SB; Bajaj JS; Mutlu EA; Keshavarzian A; Tsukamoto H; Fouts DE; Schnabl B Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology 2015, 148, 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Aggarwal S; Suzuki T; Taylor WL; Bhargava A; Rao RK Contrasting effects of ERK on tight junction integrity in differentiated and under-differentiated Caco-2 cell monolayers. Biochem. J. 2011, 433, 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Bull-Otterson L; Feng W; Kirpich I; Wang Y; Qin X; Liu Y; Gobejishvili L; Joshi-Barve S; Ayvaz T; Petrosino J; Kong M; Barker D; McClain C; Barve S Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One 2013, 8, e53028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Koo I; Kim S; Zhang X Comparative analysis of mass spectral matching-based compound identification in gas chromatog¬raphy mass spectrometry. J. Chromatogr. A 2013, 1298, 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Kim S; Koo I; Jeong J; Wu S; Shi X; Zhang X Compound identification using partial and semipartial correlations for gas chromatography-mass spectrometry data. Anal. Chem. 2012, 84, 6477–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Abundance distribution of metabolite tetradecanoic acid. Figure S2: Abundance distribution of metabolite trans-9-octadecenoic acid. Figure S3: Using R2 and Q2 to evaluate the quality of the principal component models. Figure S4: Clustering plots using k-means method for fecal samples and liver samples. This material is available free of charge via the Internet at http://pubs.acs.org.


