Abstract
Purpose.
Inborn errors of IFN-γ-mediated immunity underlie Mendelian Susceptibility to Mycobacterial Disease (MSMD), which is characterized by an increased susceptibility to severe and recurrent infections caused by weakly virulent mycobacteria, such as Bacillus Calmette–Guérin (BCG) vaccines and environmental, nontuberculous mycobacteria (NTM).
Methods.
In this study, we investigated four patients from four unrelated consanguineous families from Isfahan, Iran with disseminated BCG disease. We evaluated the patients’ whole blood cell response to IL-12 and IFN-γ, IL-12Rβ1 expression on T-cell blasts, and sequenced candidate genes.
Results.
We reported four patients from Isfahan, Iran, ranging from 3 months to 26 years old, who had impaired IL-12 signaling. All patients suffered from BCG infectious diseases. One of them presented mycobacterial osteomyelitis as a form of infection. By Sanger sequencing, we identified three different types of homozygous mutations in IL12RB1. Expression of IL-12Rβ1 was completely abolished in the four patients with IL12RB1 mutations.
Conclusions.
IL-12Rβ1 deficiency was found in the four MSMD Iranian families tested. It is the first report of an Iranian case with S321X mutant IL-12Rβ1 protein. Mycobacterial osteomyelitis is another type of location of mycobacterial infection in an IL-12Rβ1-deficient patient, notified for the first time in this study.
Keywords: Mendelian susceptibility to mycobacterial disease, Bacillus Calmette-Guérin vaccination, (BCG)-osis, IL-12, interferon
Introduction
Human IFN-gamma (γ) immunity plays a crucial role in host defense against intra-macrophagic pathogens such as Mycobacterium and Salmonella [1]. The importance of this cytokine has been highlighted by the study of Mendelian susceptibility to mycobacterial disease (MSMD), a rare primary immunodeficiency (PID), which was first clinically described in 1951 [2]. Monogenic defects in components of the IFN-γ circuit predispose individuals to severe infections, either disseminated or localized, and recurrent, caused by weakly pathogenic mycobacteria, such as Bacillus Calmette–Guérin (BCG) vaccines and environmental, nontuberculous mycobacteria (NTM), as well as Salmonella species. Furthermore, patients with MSMD also show an increased susceptibility to more virulent mycobacterial species such as Mycobacterium tuberculosis [1, 3, 4]. Different genetic etiologies have been identified in this group of PID [5]. To date, mutations in several autosomal (IFNGR1, IFNGR2, IL12B, IL12RB1, STAT1, ISG15 and IRF8) and two X-linked genes (NEMO and CYBB) have been reported as the cause of MSMD [5-8].
The most common genetic etiology of MSMD is complete AR IL12RB1 deficiency [5, 9]. In patients with deficiency of the IL-12 receptor beta 1 subunit (IL-12Rβ1), the lack of IL-12 and IL-23 signaling leads to abolished or reduced IFN-γ production by NK and T cells. The respective contributions of IL-12 and IL-23 to this process are unknown. These patients typically develop a broader range of non-mycobacterial infections, such as salmonellosis and candidiasis, than patients with other causes of MSMD [10-12]. Defects in IL12RB1 are considered to be the most common cause of MSMD worldwide (44%). IL-12Rβ1-deficient patients usually present recurrent infections due to weakly virulent mycobacteria, while invasive salmonellosis was reported present in up to one third of the patients [5, 9, 13].
A high rate of Iranian patients with IL-12Rβ1 deficiency is already apparent, with 21 patients from 19 kindreds previously reported [9, 14-19]. This highlights the influence of the high parental consanguinity prevalence in Iran leading to an increase in the number of inherited recessive pattern of mutations in genes, particularly in the IL-12/IL-23/IFN-γ axis. This suggests that screening for IL-12Rβ1 deficiency is important in Iranian patients with complications of BCG vaccination. Here, we describe four Iranian patients from Isfahan with MSMD in which cellular and genetic analysis was performed to detect and diagnose IL-12Rβ1 deficiency.
Material and Methods
Patients and their families, including asymptomatic relatives, were enrolled in this study which was held at Isfahan Immunodeficiency Research Center (IIRC), Iran. Informed consent for participation in this study was obtained in accordance with local regulations and with approval from the Institutional Review Board (IRB). All four patients were born to consanguineous parents and all patients displayed unusual mycobacterial infections. The clinical diagnosis of MSMD was made after exclusion of other PID, such as severe combined immunodeficiency (SCID), combined immunodeficiency (CID) including the PID affecting the NF-κB pathway, chronic granulomatous disease (CGD), and the hyper IgE syndrome (HIES).
DNA extraction and Sanger analysis
Genomic DNA was extracted from whole blood using the QIAamp DNA Blood Mini Kit (QIAGEN, Germany) according to the manufacturer’s instructions. The coding exons and flanking intron regions of IL12RB1 genes were amplified by polymerase chain reaction (PCR) using custom-made primers (sequences available upon request). PCR products were analyzed on a 1% agarose gel and purified using Sephadex G-50 Superfine resin (Amersham GE). The purified DNA was sequenced on a 3700 DNA analyzer (Applied Biosystems) by the dideoxynucleotide termination method using BigDye Terminator chemistry (Applied Biosystems). Results were analyzed with GenalysWin 2.8 software.
Cell culture and whole blood stimulation
Blood samples from patients, their families, and nine healthy controls were diluted 1:4 in RPMI 1640, dispensed at 1 ml/well into a 24-well plate. The following conditions were used for stimulation: with medium alone; with live BCG (Mycobacterium bovis BCG, Pasteur strain) at multiplicity of infection (MOI) = 20; with BCG plus recombinant IFN-γ (5,000 IU/mL, BioLegend, inc) or BCG plus recombinant IL-12p70 (20 ng/mL, R&D Systems); with 100 ng/mL lipopolysaccharide (LPS, from Escherichia coli 026:B6 (L2654-1mg), Sigma USA) alone or in combination with recombinant IFN-γ (100 IU/ml, BioLegend, inc). All cells were incubated at 37 °C and in a 5% CO2 atmosphere. After 48 hours of incubation, supernatants were collected and centrifuged at 1,800xg for 10 minutes.
ELISA and cell surface flow cytometry
Production of cytokines in response to IL-12 and IFN-γ stimulation was evaluated using ELISA assays specific for IFN-γ (LEGEND MAX human IFN-γ kit, BioLegend), TNF (Human TNF Platinum ELISA kit, eBioScience) and IL-12p40 (Human IL-12/23 p40 Platinum ELISA kit, eBioScience) according to the manufacturer’s instructions.
Using heparinized blood samples from patients and healthy controls, peripheral blood mononuclear cells (PBMC) were separated by centrifugation on a Ficoll-Hypaque density gradient as previously described [20]. The PBMCs were cultured (106 cell/well) in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 UI/mL penicillin and 100 μg/mL streptomycin (GibcoBRL). For generation of phytohemagglutinin (PHA)-activated T cells, PBMCs were activated with 20 μg PHA for 72 hours. IL-12Rβ1 expression on the surface of PHA-activated T cells was detected using PE-anti-IL-12Rβ1 mAh (BD) and anti-CD-3-FITC mAh (BD). FITC-anti-mouse IgG2a,κ and PE-anti-mouse IgG1,κ were used as isotype controls. IL-12Rβ1 expression was assessed on CD3+ PHA-activated T cells on a FACS-Calibur flow cytometer using CellQuest Pro software (BD Biosciences).
Results
IL12RB1 genotype in four Iranian kindreds
In this study, we investigated four patients, ranging in age from 3 months to 26 years, from independent consanguineous pedigrees presenting with clinical disease caused by BCG (Supplemental material and Table 1). Sanger sequencing of 17 IL12RB1 coding exons and flanking introns revealed homozygous mutations in all four patients. Sequencing analysis of P1 found a homozygous single nucleotide deletion in exon 10, c.1172delC, which causes a frameshift and is expected to result in a premature stop of translation (p.P391Rfs*62). Her parents were heterozygous for this same mutation (data not shown). P1's sister (1.II) is also a heterozygous carrier for the c.1172delc mutation. This mutation was primarily reported in Iran [16]. In P2 and P3, a known homozygous mutation at the 3’ splice site of exon 15, c.1791+2T>G was identified (data not shown) [9, 16, 21, 22]. P2 and P3's parents were heterozygous for this mutation. In P4, we identified a homozygous nonsense mutation in exon 9, c.962C>A which creates a premature stop codon upstream of the transmembrane domain (TM) [21]; and is likely to result in a truncated protein, p.S321X. He is the first report of this mutation in Iran. P4's parents carried heterozygous c.962C>A allele.
Table 1.
Genetic and clinical manifestations of patients with complete IL-12Rβ1 deficiency
| Case | Age of investigation |
Age of onset |
Candida infection |
Mycobacterial osteomyelitis |
Treatment | Outcome | Mutation |
|---|---|---|---|---|---|---|---|
| P1 | 7 years | 4 months: BCG-itis | + | − | INH, RIF, CLM, ETB, INF-γ | alive | c.1172delC/1172delC |
| P2 | 5 years | 4 months: BCG-itis | + | − | RIF,CLM,INH, IFN-γ | alive | c.1791+2T>G/1791+2T>G |
| P3 | 26 years | 2 years: BCG-itis | − | + | RIF, CLM,INH | alive | c.1791+2T>G/1791+2T>G |
| P4 | 3 months | 3 months: BCG-itis | − | − | INH, CLM,RIF | dead | c.962C>A /962C>A |
Isoniazid (INH), Rifampicin (RIF), Clindamycin (CLM), Ethambutol (ETB)
Abolished IL-12Rβ1 expression on T-cell PHA blasts
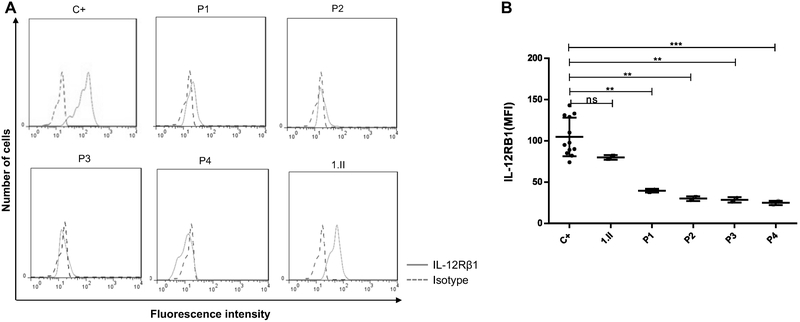
Surface expression of IL-12Rβ1 was evaluated by flow cytometry analysis of T-cell PHA blasts from patients and healthy controls using different antibodies specific to IL-12Rβ1 protein. We found that expression of IL-12Rβ1 was completely abolished on the surface of cells from the patient with c.1172delC mutation (P1), as indicated by very weak specific fluorescence compared to healthy controls, while her sister (1.II) and her parents (data not shown for her parents), who are heterozygous for the same mutation, had normal IL-12Rβ1 expression (Figure 1A). Surface expression of IL-12Rβ1 was also undetectable in patients with c.1791+2T>G and c.962C>A mutations (P2, P3 and P4), while their parents showed normal expression (Figure 1 A and B, data not shown for their parents).
Figure 1: IL-12Rβ1 expression in the cells of IL-12Rβ1 patients.
A. T-cell blasts from c.1172delC (P1), c.1791+2T>G (P2,3) and C.C962A (P4) patients and from healthy control (C+) were stained with IL-12Rβ1-specific mAbs (solid line) and isotype control antibodies (dashed line).
B. Expression of IL-12Rβ1 protein in patients were indicated by fluorescence intensity (MFI). A p-value <0.05, <0.01 and <0.001 in one way Anowa is indicated by *, ** and ** Respectively. ns, not significant. NS, not stimulated
Impaired cellular responses to IL-12
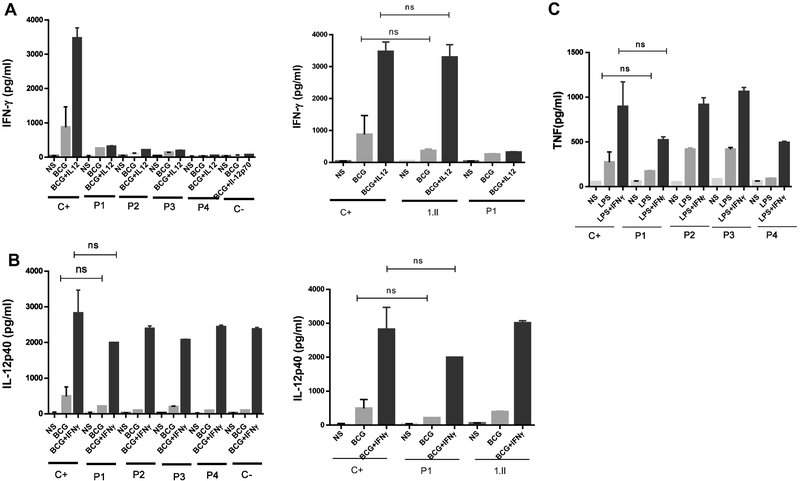
Next, we investigated the response of patients’ cells to IFN-γ and IL-12 cytokines. Using whole blood, we assessed IFN-γ production in response to BCG alone and BCG + IL-12 after 48 hours of stimulation. As shown in Figure 2A, IL-12Rβ1-deficient patients (P1, P2, P3 and P4), failed to mount a response to IL-12 (no increase of the IFN-γ production following addition of IL-12 to BCG). IFN-γ secretion by BCG+IL-12-stimulated cells from 1.II, P1's sister, who is heterozygous for the c.1172delC mutation, was comparable to healthy controls (Figure 2A). Production of the IL-12 p40 subunit, in the absence of stimulation and in response to BCG alone or BCG+IFN-γ, was not significantly different across patients (P1,P2,P3,P4) with IL12RB1 mutations compared to healthy controls (Figure 2B). We also generally assessed TNF production in response to LPS and LPS plus IFN-γ. All patients produced TNF levels that were similar to those of healthy controls (Figure 2C).
Figure 2: The IL-12 and IFN-γ responses on whole blood activation.
A. Production of IFN-γ (pg/mL) in response to Bacille Calmette–Guérin (BCG) and BCG+IL-12 in whole blood cells was quantified in patients with IL-12Rβ1 deficiency (P1, P2, P3 and P4).
B. Production of IL-12 was assessed in supernants of whole blood activation by BCG and BCG+IFN-γ activation.
C. TNF production was indicated in response to Lipopolysacharide (LPS) and LPS + IFN-γ. 1.II is the sister of P.1, C- is an IL-12Rβ1 deficient, ns, not significant. NS, not stimulated
Discussion
We reported here four Iranian patients with complete IL-12Rβ1 deficiency from unrelated consanguineous families from Isfahan. The present study reports three homozygous mutant alleles of IL12RB1 as following: one patient (P1) with a single nucleotide deletion in exon 10, c.1172delC, who is only the second child reported [16]; two patients (P2, P3) with a splice site mutation affecting exon 15, c.1791+2T>G; and the c.962C>A mutation in exon 9 identified in P4. This last mutation has been previously reported in only one child from Pakistan [21]. To our knowledge, one of the most common IL12RB1 mutations is c.1791+2T>G, found in 30 cases from 20 kindreds in Iran, Spain, Sri Lanka, China, Turkey, Mexico, Ukraine, Saudi Arabia, and France [9, 16, 21-27]. To date, the most frequent c.1791+2T>G mutation is detected in Spain with 6 patients, followed by 5 patients in Iran and in Mexico [9, 16, 21, 22, 25, 28]. If we take our patients (P2 and P3) into account, Iran ranks as the country with the most frequency of IL-12Rβ1 deficient patients (7 patients), caused by c.1791+2T>G mutation. It was first described in an Iranian case affected by disseminated Salmonella enteritidis without any mycobacterial diseases due to birth in the USA and lack of BCG vaccination [22]. Clinically, all four patients reported here received BCG vaccination during the first days of life, a standard procedure in Iran, resulting later in a BCG infectious disease. In general, this infection can affect lymph nodes, the liver, or the spleen. We also described infection by Candida in two of our patients (P1, P2). Candidiasis is a frequent infection among IL-12Rβ1 deficient patients [9], previously reported in cases of those who carried the c.1791+2T>G mutation in Mexico, Turkey, and Spain [9, 24, 25, 29]. This report is the first Iranian IL-12Rβ1-deficient case presenting candidiasis with c.1791+2T>G mutation. Additionally, this study is the first report of the c.962C>A mutation in Iran, described only in an 18-year-old man from Pakistan who complained from salmonella infection [21].
Remarkably, in this study, we presented for the first time one IL-12Rβ1-deficient patient (P3) with mycobacterial osteomyelitis manifestation who had no deficiency in IFN-γ signaling pathway [3, 6]. P3 displayed multi-focal and recurrent mycobacterial osteomyelitis. In general, mycobacterial osteomyelitis (MOM) is a typical feature of patients with impaired response to IFN-γ, sometimes only involving the bone [13, 30-33]. Clinical presentation is in most of the cases multifocal MOM caused by environmental mycobacteria (EM), mainly M. avium or BCG. MOM is a frequent manifestation in AD IFN-γR1 or AD STAT1-deficient patients, all but one as disseminated osteomyelitis [34-38]. Additionally, patients with autosomal recessive (AR) IFN-γR1 deficiency have been described with MOM as part of disseminated mycobacterial disease. This has been reported in half of the patients with AR partial IFN-γR1 deficiency; in 10 of the 20 patients described [39-42]; less frequently in patients with AR complete IFN-γR1 deficiency; and in 16 of the 59 (27,1%) cases published [13, 43-53]. Moreover, a patient with AR partial IFN-γR1 deficiency has been reported with osteomyelitis caused by Coccidioidomycosis [54]. These patients have an impaired cellular response to IFN-γ compared to healthy controls and complete AR IFN-γR deficiency that failed completely to respond to IFN-γ. Inversely, the IL-12p40 production in response to IFN-γ in P3 was similar to those in healthy controls. Interestingly, no mycobacterial osteomyelitis has been previously described in the 205 reported patients with IL-12Rβ1 deficiency [5, 9, 14, 16, 17, 57-60]. Osteomyelitis has however been described in these patients as caused by Salmonella spp [61], Cryptococcus. neoformans [62], and Coccidioides spp [63]. Thus, mycobacterial osteomyelitis is another type of location in the defect of IFN-γ production and it expands the spectrum of clinical IL-12Rβ1-deficient patients.
Supplementary Material
Acknowledgements
We would like to thank the patients and their families for their collaboration and their participation in the present study. Finally, we would like to thank Dr Hamid Zarkesh for her great help and invaluable advice.
Financial support
This study was supported by Isfahan University of Medical Sciences. The Laboratory of Human Genetics of Infectious Diseases is supported by the National Institute of Allergy and Infectious Diseases (grant number 5R01AI089970); the National Center for Research Resources and the National Center for Advancing Sciences of the National Institutes of Health (grant number 8UL1TR000043); The Rockefeller University; the St. Giles Foundation; the Institut National de la Santé et de la Recherche Médicale (INSERM); Paris Descartes University; Laboratoire d’Excellence Integrative Biology of Emerging Infectious Diseases (ANR-10-LABX-62-IBEID); the French National Research Agency under the “Investments for the future” (grant number ANR-10-IAHU-01), ANR-GENMSMD (ANR-16-CE17-0005-01 for JB); and the German Academic Exchange Service (DAAD).
Footnotes
Potential conflicts of interest
The authors have no conflict of interest to declare.
Research involving Human Participants
Informed consent for participation in this study was obtained in accordance with local regulations, with approval from the IRB. The experiments described here were performed in Iran and in France, in accordance with local regulations, and with the approval of the IRB for Isfahan Immunodeficiency Research Center (IIRC), Iran, and for Necker Hospital for Sick Children, France.
Informed consent
Written informed consent was obtained from the patients.
References
- 1.Casanova J-L, Mendelian susceptibility to mycobacterial infection in man. Swiss medical weekly, 2001. 131(31-32): p. 445–454. [DOI] [PubMed] [Google Scholar]
- 2.Flynn JL and Chan J, Immunology of tuberculosis. Annual review of immunology, 2001. 19(1): p. 93–129. [DOI] [PubMed] [Google Scholar]
- 3.Feinberg J, et al. , Bacillus Calmette Guérin triggers the IL-12/IFN-γ axis by an IRAK-4- and NEMO-dependent, non-cognate interaction between monocytes, NK, and T lymphocytes. European journal of immunology, 2004. 34(11): p. 3276–3284. [DOI] [PubMed] [Google Scholar]
- 4.Lee W-I, et al. , Immune defects in active mycobacterial diseases in patients with primary immunodeficiency diseases (PIDs). Journal of the Formosan Medical Association, 2011. 110(12): p. 750–758. [DOI] [PubMed] [Google Scholar]
- 5.Bustamante J, et al. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. in Seminars in immunology. Seminars in Immunology, 2014. 26(6): p. 454–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casanova J-L and Abel L, Genetic dissection of immunity to mycobacteria: the human model. Annual review of immunology, 2002. 20(1): p. 581–620. [DOI] [PubMed] [Google Scholar]
- 7.Fieschi C, et al. , A novel form of complete IL-12/IL-23 receptor β1 deficiency with cell surface-expressed nonfunctional receptors. Blood, 2004. 104(7): p. 2095–2101. [DOI] [PubMed] [Google Scholar]
- 8.Bustamante J, et al. , A novel X-linked recessive form of Mendelian susceptibility to mycobaterial disease. Journal of medical genetics, 2007. 44(2): p. e65–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Beaucoudrey L, et al. , Revisiting human IL-l2Rβ1 deficiency: A survey of 141 patients from 30 countries. Medicine. 2010. 86(6): p. 381–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoeve MA, et al. , IL-12 receptor deficiency revisited: IL-23-mediated signaling is also impaired in human genetic IL-12 receptor β1 deficiency. European journal of immunology, 2003. 33(12): p. 3393–3397. [DOI] [PubMed] [Google Scholar]
- 11.Oppmann B, et al. , Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity, 2000. 13: p. 715–25. [DOI] [PubMed] [Google Scholar]
- 12.Parham C, et al. , A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. Journal of immunology (Baltimore, Md. : 1950), 2002. 168: p. 5699–708. [DOI] [PubMed] [Google Scholar]
- 13.Dorman SE, et al. , Clinical features of dominant and recessive interferon γ receptor 1 deficiencies. The Lancet, 2004. 364(9451): p. 2113–2121. [DOI] [PubMed] [Google Scholar]
- 14.Dizaj MA, et al. , Susceptibility to mycobacterial disease due to mutations in II-12Rβ1 in three Iranian patients. Immunogenetics, 2018. 70(6): p. 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boisson-Dupuis S, et al. , II-12Rβ1 deficiency in two of fifty children with severe tuberculosis from Iran, Morocco, and Turkey. PloS one, 2011. 6(4): p. e18524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarrafzadeh SA, et al. , Case Report Mendelian Susceptibility to Mycobacterial Disease due to II-12Rβ1 Deficiency in Three Iranian Children. Iran J Public Health, 2016. 45: p. 370–375. [PMC free article] [PubMed] [Google Scholar]
- 17.Parvaneh N, et al. , Visceral leishmaniasis in two patients with IL-12p40 and IL-12Rβ1 deficiencies. Pediatric blood & cancer, 2017. 64(6): p. e26362. [DOI] [PubMed] [Google Scholar]
- 18.Rosain J, et al. , A Variety of Alu-Mediated Copy Number Variations Can Underlie IL-12Rβ1 Deficiency. Journal of clinical immunology, 2018: p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabarsi P, et al. , Lethal Tuberculosis in a Previously Healthy Adult with IL-12 Receptor Deficiency. Journal of Clinical Immunology, 2011. 31: p. 537–539. [DOI] [PubMed] [Google Scholar]
- 20.Germann A, et al. , Temperature fluctuations during deep temperature cryopreservation reduce PBMC recovery, viability and T-cell function. Cryobiology, 2013. 67(2): p. 193–200. [DOI] [PubMed] [Google Scholar]
- 21.Fieschi C, et al. , Low Penetrance, Broad Resistance, and Favorable Outcome of Interleukin 12 Receptor β 1 Deficiency: Medical and Immunological Implications. The Journal of Experimental Medicine J. Exp. Med, 2003. 00: p. 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleary AM, et al. , Impaired accumulation and function of memory CD4 T cells in human IL-12 receptor β1 deficiency. The Journal of Immunology, 2003. 170(1): p. 597–603. [DOI] [PubMed] [Google Scholar]
- 23.Lee PP, et al. , Severe mycobacterial infections in two pairs of Chinese siblings with interleukin-12 receptor β1 deficiency. European journal of pediatrics, 2008. 167(2): p. 231–232. [DOI] [PubMed] [Google Scholar]
- 24.Pedraza-Sanchez S, et al. , Bacille Calmette–Guérin infection and disease with fatal outcome associated with a point mutation in the interleukin-12/interleukin-22 receptor beta-1 chain in two Mexican families. International Journal of Infectious Diseases, 2010. 14: p. e256–e260. [DOI] [PubMed] [Google Scholar]
- 25.Pedraza S, et al. , Clinical disease caused by Klebsiella in 2 unrelated patients with interleukin 12 receptor β1 deficiency. Pediatrics, 2010: p. peds. 2009–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahid MF, et al. , Recurrent Salmonellosis in a Child with Complete IL-12Rβ1 Deficiency. Journal of immunodeficiency & disorders, 2014. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khamassi I, et al. , Salmonella enteriditis inducing cutaneous leucocytoclasic vasculitis: An unusual complication in a patient with an interleukine-12 receptor beta-1 deficiency. La Tunisie medicale, 2015. 93(5): p. 328. [PubMed] [Google Scholar]
- 28.Caragol I, et al. , Clinical tuberculosis in 2 of 3 siblings with interleukin-12 receptor β1 deficiency. Clinical infectious diseases, 2003. 37(2): p. 302–306. [DOI] [PubMed] [Google Scholar]
- 29.Ouederni M, et al. , Clinical Features of Candidiasis in Patients With Inherited Interleukin 12 Receptor β1 Deficiency. Clinical Infectious Diseases, 2014. 58: p. 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arend SM, et al. , Multifocal osteomyelitis caused by nontuberculous mycobacteria in patients with a genetic defect of the interferon-γ receptor. The Netherlands journal of medicine, 2001. 59(3): p. 140–151. [DOI] [PubMed] [Google Scholar]
- 31.Glosli H, et al. , Infections due to various atypical mycobacteria in a Norwegian multiplex family with dominant interferon-γ receptor deficiency. Clinical Infectious Diseases, 2008. 46(3): p. e23–e27. [DOI] [PubMed] [Google Scholar]
- 32.Rose DM, et al. , A novel mutation in IFN-[gamma] receptor 1 presenting as multisystem Mycobacterium intracellulare infection. Journal of Allergy and Clinical Immunology, 2014. 133(2): p. 591. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki Y, et al. , Genetic basis of patients with bacille Calmette-Guerin osteomyelitis in Japan: identification of dominant partial interferon-γ receptor 1 deficiency as a predominant type. The Journal of infectious diseases, 2002. 185(5): p. 706–709. [DOI] [PubMed] [Google Scholar]
- 34.Hirata O, et al. , Heterozygosity for the Y701C STAT1 mutation in a multiplex kindred with multifocal osteomyelitis. Haematologica, 2013. 98(10): p. 1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsumura M, et al. , Dominant-negative STAT1 SH2 domain mutations in unrelated patients with mendelian susceptibility to mycobacterial disease. Human mutation, 2012. 33(9): p. 1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapgier A, et al. , Novel STAT1 alleles in otherwise healthy patients with mycobacterial disease. PLoS genetics, 2006. 2(8): p. e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueki M, et al. , A heterozygous dominant-negative mutation in the coiled-coil domain of STAT1 is the cause of autosomal-dominant Mendelian susceptibility to mycobacterial diseases. Clinical Immunology, 2017. 174: p. 24–31. [DOI] [PubMed] [Google Scholar]
- 38.Kagawa R, et al. , Alanine-scanning mutagenesis of human signal transducer and activator of transcription 1 to estimate loss-or gain-of-function variants. Journal of Allergy and Clinical Immunology, 2017. 140(1): p. 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allende LM, et al. , A point mutation in a domain of gamma interferon receptor 1 provokes severe immunodeficiency. Clinical and diagnostic laboratory immunology, 2001. 8(1): p. 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong X-F, et al. , A novel form of cell type-specific partial IFN-γR1 deficiency caused by a germ line mutation of the IFNGR1 initiation codon. Human molecular genetics, 2009. 19(3): p. 434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Remiszewski P, et al. , Disseminated Mycobacterium avium infection in a 20-year-old female with partial recessive IFNγR1 deficiency. Respiration, 2006. 73(3): p. 375–378. [DOI] [PubMed] [Google Scholar]
- 42.Sologuren I, et al. , Partial recessive IFN-γR1 deficiency: genetic, immunological and clinical features of 14 patients from 11 kindreds. Human molecular genetics, 2011. 20(8): p. 1509–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bax H, et al. , Interferon alpha treatment of patients with impaired interferon gamma signaling. Journal of clinical immunology, 2013. 33(5): p. 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jouanguy E, et al. , Interferon-γ–receptor deficiency in an infant with fatal bacille Calmette–Guérin infection. New England Journal of Medicine, 1996. 335(26): p. 1956–1962. [DOI] [PubMed] [Google Scholar]
- 45.Cecilia Martínez-Morales M, et al. , Disseminated infection by M. tuberculosis complex in patient with IFN-γ receptor 1 complete deficiency. Revista Alergia de Mexico, 2017. 64(4). [DOI] [PubMed] [Google Scholar]
- 46.Newport MJ, et al. , A mutation in the interferon-γ–receptor gene and susceptibility to mycobacterial infection. New England Journal of Medicine, 1996. 335(26): p. 1941–1949. [DOI] [PubMed] [Google Scholar]
- 47.Reuter U, et al. , Correction of complete interferon-γ receptor 1 deficiency by bone marrow transplantation. Blood, 2002. 100(12): p. 4234–4235. [DOI] [PubMed] [Google Scholar]
- 48.Dorman SE, et al. , Viral infections in interferon-γ receptor deficiency. The Journal of pediatrics, 1999. 135(5): p. 640–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koscielniak E, et al. , Disseminated Mycobacterium peregrinum infection in a child with complete interferon-gamma receptor-1 deficiency. The Pediatric infectious disease journal, 2003. 22(4): p. 378–380. [PubMed] [Google Scholar]
- 50.Lee W-I, et al. , Chinese patients with defective IL-12/23-interferon-γ circuit in Taiwan: partial dominant interferon-γ receptor 1 mutation presenting as cutaneous granuloma and IL-12 receptor β1 mutation as pneumatocele. Journal of clinical immunology, 2009. 29(2): p. 238. [DOI] [PubMed] [Google Scholar]
- 51.Marazzi MG, et al. , Disseminated Mycobacterium scrofulaceum infection in a child with interferon-γ receptor 1 deficiency. International Journal of Infectious Diseases, 2010. 14(2): p. e167–e170. [DOI] [PubMed] [Google Scholar]
- 52.Noordzij JG, et al. , Two patients with complete defects in interferon gamma receptor-dependent signaling. Journal of clinical immunology, 2007. 27(5): p. 490–496. [DOI] [PubMed] [Google Scholar]
- 53.Roesler J, et al. , Listeria monocytogenes and recurrent mycobacterial infections in a child with complete interferon-γ-receptor (IFNγR1) deficiency: mutational analysis and evaluation of therapeutic options. Experimental hematology, 1999. 27(9): p. 1368–1374. [DOI] [PubMed] [Google Scholar]
- 54.Vinh DC, et al. , Refractory disseminated coccidioidomycosis and mycobacteriosis in interferon-γ receptor 1 deficiency. Clinical Infectious Diseases, 2009. 49(6): p. e62–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasaki Y, et al. , Genetic Basis of Patients with Bacille Calmette-Guérin Osteomyelitis in Japan: Identification of Dominant Partial Interferon-γ Receptor 1 Deficiency as a Predominant Type. The Journal of Infectious Diseases, 2002. 185: p. 706–709. [DOI] [PubMed] [Google Scholar]
- 56.Ueki M, et al. , A heterozygous dominant-negative mutation in the coiled-coil domain of STAT1 is the cause of autosomal-dominant Mendelian susceptibility to mycobacterial diseases. Clinical immunology (Orlando, Fla.), 2017. 174: p. 24–31. [DOI] [PubMed] [Google Scholar]
- 57.Nabhani S, et al. , DEREGULATION OF FAS LIGAND EXPRESSION AS A NOVEL CAUSE OF AUTOIMMUNE LYMPHOPROLIFERATIVE SYNDROME LIKE DISEASE. haematologica, 2015: p. 100(9): p. 1189–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hatipoglu N, et al. , Inherited IL-12Rβ1 Deficiency in a Child With BCG Adenitis and Oral Candidiasis: A Case Report. Pediatrics, 2017. 140: p. e20161668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Göktürk B, et al. , Infectious diseases, autoimmunity and midline defect in a patient with a novel bi-allelic mutation in IL12RB1 gene. Turk J Pediatr, 2016. 58(3): p. 331–6. [DOI] [PubMed] [Google Scholar]
- 60.Dhalla F, et al. , Chronic mucocutaneous candidiasis: characterization of a family with STAT-1 gain-of-function and development of an ex-vivo assay for Th17 deficiency of diagnostic utility. Clinical & Experimental Immunology, 2016. 184: p. 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cárdenes M, et al. , Oesophageal squamous cell carcinoma in a young adult with IL-12Rβ1 deficiency. Journal of medical genetics, 2010. 47(9): p. 635–637. [DOI] [PubMed] [Google Scholar]
- 62.Jirapongsananuruk O, et al. , Cryptococcal osteomyelitis in a child with a novel compound mutation of the IL12RB1 gene. Asian Pacific journal of allergy and immunology, 2012. 30(1): p. 79. [PubMed] [Google Scholar]
- 63.Vinh DC, Insights into human antifungal immunity from primary immunodeficiencies. The Lancet infectious diseases, 2011. 11(10): p. 780–792. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.