Abstract
Background & objectives:
Oxidative stress, lifestyle factors as also exposure to certain environmental factors are known to affect the fertility status in human beings. The aim of this study was to evaluate the role of OS and lifestyle and environmental factors affecting IVF outcome.
Methods:
A total of 253 couples were included, and biological samples such as blood, follicular fluid (FF), cumulus cells and semen were collected. Relevant biochemical parameters and metals namely lead (Pb), cadmium (Cd), copper (Cu) and zinc (Zn) were determined in the biological samples. β-human chorionic gonadotropin levels ≥100 IU/l were considered to predict viable pregnancy on the 15th day of embryo transfer (ET).
Results:
The mean body mass index (BMI) was significantly lower in females with positive IVF outcome compared to those with negative outcome. Couples residing in the residential area showed more percentage of positive IVF outcomes as compared to couples residing in industrial/agricultural area. FF Zn level was significantly higher (P<0.001) among the females’ participants who have undergone ET as compared to those who have not undergone ET. FF MDA and serum Cu levels were significantly higher (P<0.05) in the female participants with negative IVF outcome as compared to positive IVF outcome. Logistic regression revealed that maternal BMI (P=0.034) and FF MDA level (P=0.047) were significantly associated with the IVF outcome.
Interpretation & conclusions:
The success rate of IVF was about 31.8 per cent, and BMI was significantly lower in females with positive outcome. The higher levels of MDA in FF and SP might have a negative impact on IVF outcome, higher Zn level in SP, FF and serum might have a positive role in embryo transfer as well as IVF outcome. The role of stress management and nutrition supplementation during the IVF treatment may be explored.
Keywords: β-human chorionic gonadotropin, DNA damage, embryo transfer, heavy metals and trace metals, in vitro fertilization outcome, oocyte retrieval, oxidative stress
Infertility is caused by the failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse as defined by the World Health Organization1. In most cases infertility is preventable and may be treated. In about eight per cent uses the advance treatment includes assisted reproductive techniques (ARTs). The pregnancy rate per initiated ART cycle and the delivery rate remains around 30 per cent2. Unsuccessful occurrence of ART is a stressful event not only to the couples but also to the family.
The infertility may be due to ovulatory disorders, tubal disease, endometriosis, chromosomal abnormalities, sperm factors and unexplained infertility3. In addition, physical, behavioural, genetic, socio-economic as well as environmental or occupational factors may also contribute. Environmental exposure consists of a wide variety of agents, including metals, organo chlorine compounds, poly-chlorinated biphenyls, dioxin, particulate air pollutants, plasticizers and substances emitted from landfill sites. Lifestyle factors such as psychological stress, advanced age to start a family, nutrition, weight, physical exercise and occupational exposures can also have substantial effects on fertility and outcome. Further, other personal lifestyle factors such as tobacco smoking and chewing, illicit drug use and alcohol and excessive caffeine consumption can also have a negative influence on fertility and outcome4.
Oxidative stress (OS) has been reported to have a significant role in infertility5. This may influence a range of physiological processes such as hormonal homeostasis; sperm, oocyte and embryo quality and spermatogenesis/oogenesis, which ultimately lead to infertility5. Free radicals and OS can also have a significant effect on IVF outcome6. Persistent exposure to certain environmental and industrial chemicals may also cause impairments of reproductive health7. There is a lack of studies on the exposure of couples to environmental/occupational chemicals, lifestyle factors, OS and their association with IVF outcome. Therefore, this study was conducted to find out the role of lifestyle, environmental factors, OS on in vitro fertilization (IVF) outcome.
Material & Methods
This study was a part of a project on investigation of factors affecting in vitro fertilization for which ethical approval was obtained from the Institutional Ethics Committee of National Institute of Occupational Health (NIOH), Ahmedabad, India. A total of 253 couples were enrolled from IVF centre, department of Obstetrics and Gynaecology, Institute of Kidney Diseases and Research Centre, Ahmedabad, India from April 2013 to May 2016. The inclusion criteria of the participants were absence of structural abnormality in both men and women, no spontaneous conception within one year of unprotected intercourse, sperm abnormality, fallopian tube pathology or endometriosis and undergoing 1st IVF cycle. A written consent was obtained from each participant. The predesigned proforma was filled through questionnaire interview that included demographic information (age, height, weight, address, educational status, etc.), personal lifestyle habits (dietary habits, smoking, chewing and alcohol consumption, caffeine consumption, etc.), occupational (employment sector, duration of employment, past employment, nature of work, etc.) and reproductive history. Reproductive history included details of reproduction-related complaints with description, type of infertility and cause of infertility in both male and female, history of abortion, miscarriage, premature delivery, earlier pregnancy, IVF protocol adapted, embryo transfer (ET) and outcome. The biological samples such as blood, follicular fluid (FF) and cumulus cells (CC) from female participants and blood and semen from male participants were collected from the enrolled couples. The FF and seminal plasma (SP) samples were utilized to perform all the biochemical parameters and Zinc estimation. The blood, cumulus and sperm cells were utilized to assess DNA damage. The whole blood and serum of both sexes were utilized for the estimation of heavy and trace metals.
Various biochemical parameters, namely superoxide dismutase (SOD)8, lipid per oxidation (LPO) for determination of Malondialdehyde (MDA)9, reduced Glutathione (GSH)10, total thiols (TT)11, L-ascorbic acid (AA)12, total protein (TP)13, Glutathione S transferase (GST)14 and glutathione reductase (GRD)15 and were carried out in FF and SP.
Estimation of DNA damage was done in blood, cumulus and sperm cells as per method of Singh et al16 with some modifications in lysis buffer (2.5 M NaCl, 100 mM ethylenediaminetetraacetic acid tetrasodium salt (EDTA), 10 mM trihydrochloride, pH 10, 1% Triton X-100 and 10% dimethyl sulphoxide; incubation for 2 h) and electrophoresis buffer (~90 mM Tris base, ~90 mM Boric acid, 20 mM EDTA, pH 10).
Estimation of metals, namely lead (Pb), cadmium (Cd), copper (Cu) and zinc (Zn), was carried out using atomic absorption spectrophotometer (Perkin Elmer, USA). β-human chorionic gonadotropin (β-HCG) levels were also recorded on 15th day of ET. Earlier, Parihar17 has reported that β-HCG level of 100 IU/l predicts viable pregnancy. Thus, the β-HCG levels ≥100 IU/l were considered to predict viable pregnancy (positive IVF outcome) in the present study. The data were analyzed on the basis of participants’ characteristics [lifestyle factors, area of residence, body mass index (BMI), etc.] and on the variables such as β-HCG level, OS parameters and also with respect to exposure history as well as with load of metal, ET and IVF outcome.
Statistical analysis: The statistical analyses were done using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Comparison between the groups was done using Student's unpaired t-test for normally distributed data, Mann-Whitney test for data not following normal distribution and Chi-square test to determine whether there was a significant association between the two categorical variables. A logistic regression model was also applied to assess the odds of IVF outcome with respect to different variables studied. The flow chart of the study is depicted in Fig. 1.
Fig. 1.
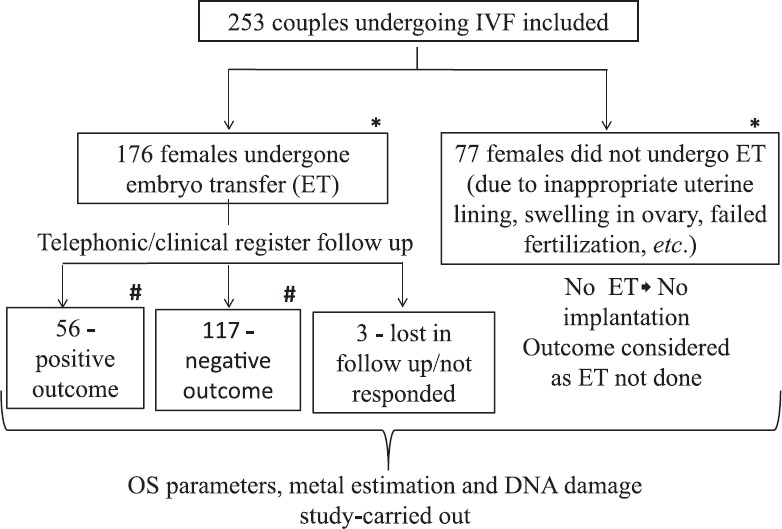
Flow diagram of study population undergone in vitro fertilization (IVF) procedure. *Comparative analysis carried out between these groups; #comparative analysis carried out between these groups.
Results
The mean age of females and males undergone for IVF was 31.86±0.28 and 34.97±0.31 yr, respectively. The mean BMI of female and male participants was 23.97±0.26 and 24.81±0.28 kg/m2, respectively. Overall, of the 253 couples, 176 couples (70%) had undergone the ET and there were 77 (30%) participants who did not undergo ET due to various reasons such as inappropriate uterine lining, swelling in ovary, failed fertilization etc. Of these 176 couples with ET, 56 couples (31.8%) had positive IVF outcome and 117 couples (66.4%) had negative IVF outcome and three couples (1.7%) were lost during follow up (Table I and Fig. 1).
Table I.
Variables of study population and in vitro fertilization (IVF) outcome
| Parameters | Variables | n (%) | |
|---|---|---|---|
| Embryo transfer (%) | ET done | 176 (70) | |
| ET not done | 77 (30) | ||
| IVF outcome (%) | Positive outcome | 56 (31.8) | |
| Negative outcome | 117 (66.4) | ||
| Area of residence | Residential (n=191) (ET done~68%, n=129) | ||
| Positive | 43 (33) | ||
| Negative | 83 (65) | ||
| Industrial + Agriculture (n=62) (ET done~76%, n=47) | |||
| Positive | 13 (28) | ||
| Negative | 34 (72) | ||
| Addiction of males (tobacco chewing/smoking/alcohol consumption) | Yes=49% (n=124) (ET done~71%, n=88) | ||
| Positive | 25 (28.4) | ||
| Negative | 63 (71.0) | ||
| No=51% (n=129) (ET done~68%, n=88) | |||
| Positive | 31 (35) | ||
| Negative | 54 (61) | ||
| Male | Female | ||
| BMI (kg/m2) | |||
| Total n (253) | 24.81±0.28 | 23.97±0.26 | |
| IVF positive (56) | 24.67±0.69 | 22.88±0.37* | |
| IVF negative (117) | 24.41±0.37 | 24.38±0.59 | |
| Age (yr) | |||
| Total n (253) | 34.97±0.31 | 31.86±0.28 | |
| IVF positive (56) | 34.36±0.63 | 31.75±0.51 | |
| IVF negative (117) | 35.45±0.49 | 32.16±0.44 | |
| Duration of infertility (yr) in couples | |||
| IVF positive (56) | 8.89±0.56 | ||
| IVF negative (117) | 9.52±0.46 | ||
*P<0.05 compared to negative IVF outcome females. BMI, body mass index; ET, embryo transfer
Demographic study variables and IVF outcome: The BMI was significantly lower in females with the positive IVF outcome as compared to negative IVF outcome (Table I). However, there were no considerable differences in the mean BMI of males with respect to positive or negative IVF outcome. Duration of infertility among participants with negative and positive IVF outcome was 9.52±0.46 and 8.89±0.56 yr, respectively (Table I).
Environmental, lifestyle exposure and IVF outcome: The data pertaining to the area of residence and IVF outcome revealed that, about 68 per cent of participants (n=129) residing in residential areas had undergone ET, of these, 33 per cent couples (n=43) had positive and 65 per cent (n=83) had a negative IVF outcome. While participants residing in the industrial + agricultural area, 76 per cent (n=47) had undergone ET, of whom, 28 (n=13) and 72 (n=34) per cent of couples has positive and negative IVF outcome, respectively (Table I). Regarding personal habits, most of the females did not indulge in any kind of habits (i.e. tobacco chewing, smoking or alcohol consumption) except two with tobacco chewing habit and these two women had negative IVF outcome. Regarding the habits of partners, around 49 per cent of males (n=124) indulged in the habit of smoking, chewing tobacco or alcohol either alone or in combination and female partners of about 71 per cent (n=88) from this group undergone ET. Of these, nearly 28.4 per cent (n=25) had a positive IVF outcome and 71 per cent (n=63) had a negative IVF outcome (Table I). The difference in the proportion of positive and negative IVF outcome with respect to area of residence and lifestyle factors was statistically non-significant.
Factors affecting embryo transfer (ET): Data on OS in FF and SP with respect to ET was presented in Table II. Malondialdehyde (MDA) levels in FF of participants of both the groups (ET done or not done) were almost similar. Non enzymatic antioxidants, i.e. total thiols, reduced glutathione and L-ascorbate were higher in participants who have undergone ET as compared to those who have not undergone ET. No significant difference was found in any of the parameters (Table II).
Table II.
Biochemical assays in male (seminal plasma) and females (follicular fluid) with respect to embryo transfer (ET) status
| Gender | ET status | SOD (U/ml) | Protein (mg/ml) | LPO/MDA (nM/ml) | GSH (μM/ml) | Total thiols (mg/ml) | Ascorbic acid (μg/ml) | GST (nM/min/ml) | GRD (U/Ml) |
|---|---|---|---|---|---|---|---|---|---|
| Female | ET done (n=176) | 4.64±0.38 | 64.34±2.89 | 2.34±0.14 | 0.101±0.008 | 293.82±14.12 | 5.99±0.955 | 8.58±0.68 | 0.076±0.001 |
| ET not done (n=77) | 4.84±0.43 | 52.85±3.61 | 2.34±0.21 | 0.097±0.009 | 255.80±8.60 | 4.50±0.46 | 6.93±0.88 | 0.080±0.01 | |
| Male | ET done (n=176) | 29.16±1.06 | 64.35±3.43 | 10.36±0.54 | 0.52±0.03 | 292.85±17.71 | 50.05±2.86 | 12.22±1.89 | 0.30±0.017 |
| ET not done (n=77) | 27.83±1.25 | 51.83±4.17 | 9.54±0.64 | 0.49±0.044 | 294.89±26.25 | 41.77±2.98 | 9.88±3.08 | 0.25±0.01 |
Values given are mean±SE. SOD, superoxide dismutase; LPO, lipid peroxidation; MDA, malondialdehyde; GSH, glutathione; GST, glutathione S-transferases; GRD, glutathione reductase
The data of metals in females with respect to ET were analyzed. The trace element, Zn, in FF was significantly lower in the group of participants in whom ET was not done as compared to ET-done participants. The levels of Pb and Cd were slightly higher in participants with ET not done as compared to ET-done participants (Table III). No significant difference in metal levels was found in males (Table III).
Table III.
Metal levels in males with respect to embryo transfer (ET) status
| Gender | Parameters | ET done (n=176) | ET not done (n=77) |
|---|---|---|---|
| Female | Serum Zn (mg/l) | 3.52±0.14 | 3.99±0.29 |
| FF Zn (mg/l) | 0.53±0.015 | 0.44±0.018*** | |
| Serum Cu (mg/l) | 1.63±0.06 | 1.47±0.06 | |
| Blood Cd (μg/dl) | 0.10±0.006 | 0.15±0.03 | |
| Blood Pb (μg/dl) | 3.67±0.30 | 3.91±0.43 | |
| Male | Serum Zn (mg/l) | 2.28±0.09 | 1.95±0.11 |
| SP Zn (mg/l) | 78.63±4.79 | 65.25±5.22 | |
| Serum Cu (mg/l) | 1.21±0.03 | 1.20±0.03 | |
| Blood Cd (μg/dl) | 0.092±0.007 | 0.093±0.007 | |
| Blood Pb (μg/dl) | 4.17±0.41 | 4.64±0.83 |
Values are mean±SE. ***P<0.001 compared to ET done group. Zn, Zinc; FF, follicular fluid; SP, seminal plasma; Cu, copper; Pb, lead; Cd, cadmium
Factors affecting IVF outcomes: It was noted that the activities of all enzymatic antioxidants, i.e. SOD, GST and GRD were slightly higher in the participants with positive IVF outcome with respect to negative outcome (Table IV). SOD and GRD were higher, but GST activity was significantly lower in males with positive outcome as compared to negative IVF outcome. LPO activity was significantly lower in females with positive IVF outcome compared to those with negative outcome (Table IV).
Table IV.
Biochemical assays in participants with respect to in vitro fertilization (IVF) outcome
| Gender | IVF outcome | SOD (U/ml) | Protein (mg/ml) | LPO/MDA (nM/ml) | GSH (μM/ml) | Total thiols (mg/ml) | Ascorbic acid (μg/ml) | GST (nM/min/ml) | GRD (U/Ml) |
|---|---|---|---|---|---|---|---|---|---|
| Female | Positive (n=56) | 4.89±0.88 | 66.81±4.95 | 1.77±0.20* | 0.095±0.007 | 289.68±19.11 | 5.37±0.91 | 8.57±1.19 | 0.078±0.002 |
| Negative (n=117) | 4.46±0.40 | 61.53±3.55 | 2.50±0.18 | 0.104±0.012 | 292.48±19.24 | 6.37±1.34 | 8.54±0.84 | 0.076±0.002 | |
| Male | Positive (n=56) | 30.33±2.16 | 69.08±6.22 | 9.52±0.73 | 0.53±0.05 | 313.88±28.33 | 48.38±3.82 | 7.85±1.22* | 0.33±0.024 |
| Negative (n=117) | 28.23±1.17 | 61.31±4.15 | 10.96±0.72 | 0.51±0.05 | 279.31±23.02 | 52.12±3.88 | 14.85±3.12 | 0.29±0.023 |
Values are mean±SE. *P<0.05 compared to those with negative outcome in the respective group. SOD, superoxide dismutase; LPO, lipid peroxidation; MDA, malondialdehyde; GSH, glutathione; GST, glutathione S-transferases; GRD, glutathione reductase
Further, no significant alterations were found in blood Pb, Cd and serum Zn level among females with respect to positive and negative IVF outcome. The data indicated that the level of Zn in SP and Serum Zn in males was higher in positive IVF outcome couples. The Cu level was significantly (P<0.05) higher in female serum of negative IVF outcome participants as compared to the positive IVF outcome (Table V).
Table V.
Metal levels in participants with respect to in vitro fertilization (IVF) outcome
| Gender | Parameters | Positive IVF outcome (n=56) | Negative IVF outcome (n=117) |
|---|---|---|---|
| Female | Serum Zn (mg/l) | 3.38±0.19 | 3.6±0.19 |
| FF Zn (mg/l) | 0.52±0.02 | 0.54±0.02 | |
| Serum Cu (mg/l) | 1.41±0.10 | 1.74±0.076* | |
| Blood Cd (μg/dl) | 0.115±0.01 | 0.102±0.006 | |
| Blood Pb (μg/dl) | 4.44±0.76 | 3.37±0.27 | |
| Male | Serum Zn (mg/l) | 2.36±0.16 | 2.25±0.11 |
| SP Zn (mg/l) | 90.23±8.40 | 72.85±5.93 | |
| Serum Cu (mg/l) | 1.15±0.04 | 1.23±0.04 | |
| Blood Cd (μg/dl) | 0.094±0.019 | 0.09±0.006 | |
| Blood Pb (μg/dl) | 3.87±0.45 | 3.79±0.27 |
Values are mean±SE, *P<0.05. Zn, Zinc; FF, follicular fluid; Cu, copper; Pb, lead; Cd, cadmium
DNA damage in blood, cumulus and sperm cells and IVF outcome: The data on DNA damage in sperm and blood cells in male participants with respect to habits indicated that a higher % of head DNA and lower level of tail DNA % among the participants without any habits (tobacco smoking, chewing or alcohol consumption) as compared those with such habits. The data on DNA damage in blood, sperm and cumulus cells with respect to area of residence indicated that higher % of head DNA and lower level of tail DNA % in cumulus and sperm cells of participants residing in the residential area as compared to participants residing in industrial/ agricultural area. However, all these alterations were non-significant (Tables VI and VII). In addition, the DNA damage data were further analyzed with respect to IVF outcome which revealed that the head DNA % was slightly higher and tail DNA % was slightly lower in blood and cumulus cells of females with positive IVF outcome (Table VIII). These alterations were non-significant. Representative images of intact and fragmented DNA in blood, cumulus and sperm cells are depicted in Figs. 2–4.
Table VI.
DNA damage among male partners with respect to habits
| Male habits | Sperm cells | Blood cells | ||||
|---|---|---|---|---|---|---|
| Head DNA (%) | Tail DNA (%) | Olive tail moment | Head DNA (%) | Tail DNA (%) | Olive tail moment | |
| Any habit (n=26) | 97.02±0.68 | 2.98±0.68 | 3.25±0.86 | 86.82±1.66 | 13.18±1.66 | 17.31±2.47 |
| No habit (n=34) | 97.33±1.01 | 2.67±1.01 | 4.02±2.09 | 88.13±2.17 | 11.87±2.17 | 17.16±3.58 |
Values are mean±SE
Table VII.
DNA damage among blood cells, cumulus cells and sperm cells with respect to area of residence
| Area of residence | Sperm cells | Cumulus cells | ||||
|---|---|---|---|---|---|---|
| Head DNA (%) | Tail DNA (%) | Olive tail moment | Head DNA (%) | Tail DNA (%) | Olive tail moment | |
| Residential (n=29) | 97.30±0.69 | 2.70±0.69 | 3.15±0.94 | 92.27±0.86 | 7.73±0.86 | 15.25±2.44 |
| Industrial+agriculture (n=21) | 96.86±0.93 | 3.14±0.93 | 3.96±1.66 | 90.81±1.37 | 9.19±1.37 | 17.35±3.36 |
| Blood cells-male | Blood cells-female | |||||
| Residential (n=29) | 87.12±1.58 | 12.88±1.58 | 17.47±2.46 | 92.07±0.74 | 8.14±0.75 | 14.24±1.83 |
| Industrial+agriculture (n=21) | 87.95±2.46 | 12.05±2.46 | 16.75±3.91 | 93.01±1.19 | 6.99±1.19 | 12.99±2.59 |
Values are mean±SE
Table VIII.
DNA damage in females with respect to in vitro fertilization (IVF) outcome
| IVF outcome | Cumulus cells | Blood cells | ||||
|---|---|---|---|---|---|---|
| Head DNA (%) | Tail DNA (%) | Olive tail moment | Head DNA (%) | Tail DNA (%) | Olive tail moment | |
| Positive (n=16) | 91.20±1.39 | 8.79±1.39 | 17.23±3.73 | 92.99±1.59 | 7.28±1.62 | 13.67±4.23 |
| Negative (n=49) | 91.17±1.01 | 8.82±1.01 | 17.06±2.72 | 91.94±0.76 | 8.17±0.77 | 14.00±1.70 |
Values are mean±SE
Fig. 2.
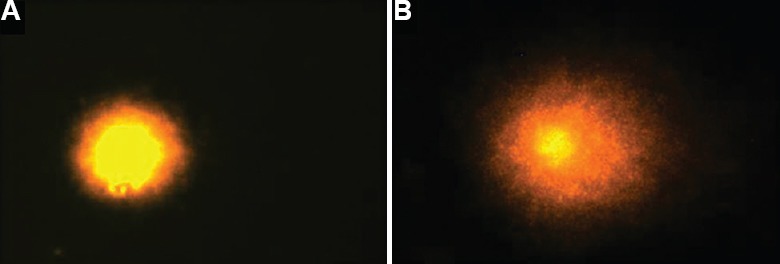
Comet assay of blood cells: (A) intact and (B) fragmented comet tail DNA.
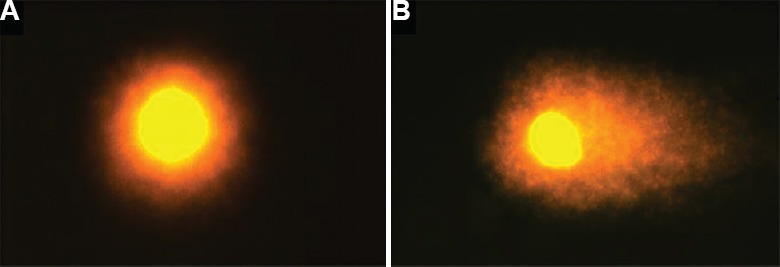
Fig. 4.
Comet assay of sperm cells: (A) intact and (B) fragmented comet tail DNA.
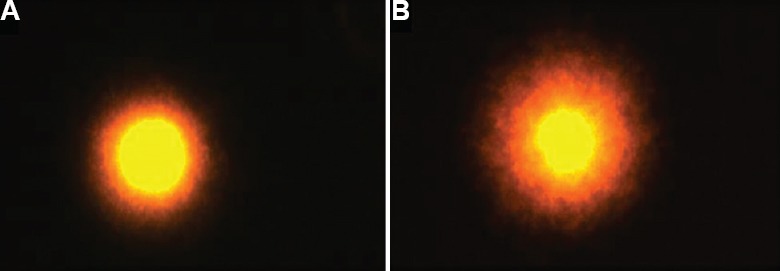
Fig. 3.
Comet assay of cumulus cells: (A) intact and (B) fragmented comet tail DNA.
Logistic regression model applied for continuous and dichotomous variables: Based upon logistic regression models applied with different variables associated with IVF (Table IX), it was found that maternal BMI and FF LPO were significantly associated with IVF outcome. One-unit elevation in the level of LPO in serum, decreased the odds for positive outcome by approximately 1.41 times [P=0.047; 95% confidence interval (CI) - 0.499-0.996]. By one unit increase in maternal BMI, the odds for positive outcome reduced by 8.4 per cent (P=0.034; 95% CI - 0.845-0.993) (Table IX).
Table IX.
Logistic regression analysis of demographic, reproductive history, anthropometric, oxidative stress and metal variables with respect to in vitro fertilization positive outcome
| Demographic and reproductive history tested for positive outcome | ||||
|---|---|---|---|---|
| Variables | Exp(B)/OR | Significance (P) | 95% CI for Exp(B)/OR | |
| Lower | Upper | |||
| Industrial area of residence | 0.678 | 0.461 | 0.242 | 1.903 |
| Agricultural area of residence | 0.780 | 0.611 | 0.299 | 2.033 |
| Occupation | 1.021 | 0.956 | 0.480 | 2.173 |
| History of spontaneous abortion (SAb) | 0.897 | 0.848 | 0.296 | 2.722 |
| Earlier pregnancy | 0.696 | 0.326 | 0.337 | 1.435 |
| Previous IUI attempts | 1.351 | 0.400 | 0.671 | 2.722 |
| Fresh/frozen embryo transferred | 0.875 | 0.706 | 0.438 | 1.749 |
| Variable (s) entered on Step 1: Area of residence, occupation, history of SAb, earlier pregnancy, previous IUI attempts, fresh/frozen embryo transferred | ||||
| Anthropometric parameters tested for positive outcome | ||||
| Female age | 0.996 | 0.918 | 0.926 | 1.072 |
| Female BMI | 0.916 | 0.034 | 0.845 | 0.993 |
| Variable (s) entered on Step 1: Female age and BMI | ||||
| Oxidative stress parameters tested for positive outcome | ||||
| SOD | 1.030 | 0.498 | 0.945 | 1.124 |
| TP | 1.006 | 0.355 | 0.993 | 1.019 |
| LPO/MDA | 0.705 | 0.047 | 0.499 | 0.996 |
| GSH | 0.209 | 0.640 | 0.000 | 145.730 |
| TT | 1.000 | 0.771 | 0.997 | 1.002 |
| Ascorbate | 0.978 | 0.634 | 0.890 | 1.073 |
| GST | 0.995 | 0.836 | 0.950 | 1.042 |
| Variable (s) entered on Step 1: SOD, TP, LPO/MDA, reduced GSH, TT, ascorbate, GST, GRD | ||||
| Heavy metals tested for positive outcome | ||||
| Blood cadmium | 5.198 | 0.425 | 0.091 | 298.445 |
| Blood lead | 1.080 | 0.102 | 0.985 | 1.184 |
| Variable (s) entered on Step 1: Blood cadmium and lead | ||||
BMI, body mass index; OR, odd ratio; SAb, spontaneous abortion; TT, total thiols; GST, glutathione S-transferases; GRD, glutathione reductase; GST, glutathione S-transferases; LPO, lipid peroxidation; MDA, malondialdehyde; CI, confidence interval; SOD, superoxide dismutase; TP, total protein; IUI, intrauterine insemination
Discussion
Despite extensive progress made in the area of ART, the pregnancy rate per initiated ART cycle and the delivery rate are still around 30-33 per cent18,19,20. The data indicated that the couples residing in an industrial/agricultural area and male partners indulged in habits of tobacco chewing, smoking or alcohol consumption had less success rate of IVF outcome. This suggests that toxicants from such exposures may have some role, at least in part in the adverse IVF outcome. These observations are in agreement with the study of Zitzmann et al21; they also reported that male smokers had decreased success rates of ARTs, not only in IVF, but also in intracytoplasmic sperm injection (ICSI). Marginal DNA damage was also observed in sperm and blood cells of male partners with any habits, i.e. tobacco chewing/smoking or alcohol consumption in the present study. Recently, Heger et al22 showed that smoking had a negative effect on endometrial thickness on the day of ET. This explains the detrimental influence of tobacco smoke on implantation. Earlier, Klemetti et al23 retrieved information on IVF women's background characteristics and suggested that no remarkable regional differences were observed according to the urbanity (rural, semi urban and urban) of the living area with regards to IVF success rates. However, in the present study, couples residing in residential areas had more positive outcomes. This might be due to the possible low level of environmental toxicants in this area.
The age of males and females did not differ considerably in the positive and negative IVF outcome. Earlier, it has been reported that advanced age of male affects the likelihood of conception, abnormalities in sperm chromosomes. In addition, a significant decrease in blastocyst embryo formation was also reported with increased paternal age, probably reflecting male genomic activation within the embryo24. Laopaiboon et al25 reported that advanced maternal age significantly elevated the risk of maternal adverse outcomes, including maternal near miss, maternal death, severe maternal outcome and stillbirths and perinatal mortalities.
The present study revealed that BMI was significantly higher among the females with negative IVF outcome as compared to those with positive outcome. Provost et al26 reported a progressive and significant worsening of pregnancy outcomes in groups with higher BMIs. Earlier, Pinborg et al27 reported that the number of positive pregnancy per treatment declined with increasing BMI and the rate of clinical pregnancies and live-birth rate per cycle were also lower in obese women.
Insufficient antioxidant enzymes and elevated OS may contribute to the risk of waning semen quality28. Yildizfer et al29 found that the serum LPO level was higher in non-pregnant females as compared to pregnant females undergone IVF. Earlier study conducted by Das et al30 concluded that ROS levels in FF appeared to play a significant role in embryo formation and quality.
Excessive ROS levels have been associated with lipid peroxidation in the sperm plasma membrane which may affect fluidity, structure and function31. Thus, OS in SP of male may also have some role in IVF and its outcome. The data on OS status in male with respect to IVF outcome revealed that the level of MDA was slightly higher in participants with negative IVF outcome indicating the role of male at least in part in IVF outcome.
The limitations of this study were that the oestrogen and progesterone levels were measured at the time of stimulation and not on the day of HCG estimation, controlled ovarian stimulation was done as per the requirement of the participants by the clinician and demographic and lifestyle factors data based upon recall of the participants.
The present study indicated the success rate of IVF was about 31.8 per cent and BMI was significantly lower in females with positive IVF outcome. The higher levels of MDA in FF and SP might have negative impact on IVF outcome; however, the impact was more pronounced with respect to FF MDA level. No conclusion can be drawn for relationship between maternal and paternal blood Cd, Pb level and IVF outcome whereas Zn level was slightly higher in SP and serum of IVF positive outcome participants. Significant positive role of FF Zn was also observed with respect to ET. Environmental and lifestyle factors can also play some role in inducing the DNA damage, which in turn would affect IVF outcome even though the data are statistically non-significant. Further studies can be planned to explore the possibilities of use of antioxidants in the management of IVF.
Financial support & sponsorship:
Financial support in the form of Ad-hoc research grant (BT/PR14616/BRB/10/860/2010) from Department of Biotechnology, New Delhi, to the first author (SK) is acknowledged.
Conflicts of Interest:
None.
Acknowledgment
Authors thank the nursing staff of the IVF centre, IKD and project staffs (Ms. P. Kapadia, Ms. K. Prajapati, Ms. P. Sawant, Ms. D. Jaiswal) who worked in the project at different time period, and to all the participants.
References
- 1.World Health Organization. Infertility definitions and terminology. Geneva: WHO; 2016. [Google Scholar]
- 2.National Guidelines for Accreditation, Supervision and Regulation of ART Clinics in India Drafting Committee. New Delhi: ICMR/ NAMS; 2005. Ministry of Health & Family Welfare, Indian Council of Medical Research/National Academy of Medical Sciences. [Google Scholar]
- 3.Nazni P. Association of western diet & lifestyle with decreased fertility. Indian J Med Res. 2014;140(Suppl 1):S78–81. [PMC free article] [PubMed] [Google Scholar]
- 4.Acharya S, Gowda CR. Lifestyle factors associated with infertility in a rural area: A cross-sectional study. Int J Med Sci Public Health. 2017;6:502. [Google Scholar]
- 5.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: A review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du Plessis SS, Makker K, Desai NR, Agarwal A. Impacts of oxidative stress on IVF. Expert Rev Obstet Gynecol. 2008;3:539–54. [Google Scholar]
- 7.Kumar S, Mishra VV. Toxicants in reproductive fluid and in vitro fertilization (IVF) outcome. Toxicol Ind Health. 2010;26:505–11. doi: 10.1177/0748233710373081. [DOI] [PubMed] [Google Scholar]
- 8.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 9.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 10.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 11.Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994;233:380–5. doi: 10.1016/s0076-6879(94)33044-1. [DOI] [PubMed] [Google Scholar]
- 12.Roe JH, Kuether CA. The determination of ascorbic acid in whole blood and urine through the 2,4-dinitrophenylhydrazine derivative of dehydroascorbic acid. J Biol Chem. 1943;147:399–407. [Google Scholar]
- 13.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 14.Carlberg I, Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem. 1975;250:5475–80. [PubMed] [Google Scholar]
- 15.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 16.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 17.Parihar M. Can early hCG levels be a marker for pregnancy outcome in ART cycles? South Asian Fed Obstet Gynecol. 2009;1:33–9. [Google Scholar]
- 18.Malhotra N, Shah D, Pai R, Pai HD, Bankar M. Assisted reproductive technology in India: A 3-year retrospective data analysis. J Hum Reprod Sci. 2013;66:235–40. doi: 10.4103/0974-1208.126286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Society for Assisted Reproductive Technology, American Society for Reproductive Medicine. Assisted reproductive technology in the United States: 2000 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil Steril. 2004;81:1207–20. doi: 10.1016/j.fertnstert.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Society for Assisted Reproductive Technology, American Society for Reproductive Medicine. Assisted reproductive technology in the United States: 2001 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology Registry. Fertil Steril. 2007;87:1253–66. doi: 10.1016/j.fertnstert.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 21.Zitzmann M, Rolf C, Nordhoff V, Schräder G, Rickert-Föhring M, Gassner P, et al. Male smokers have a decreased success rate for in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2003;79(Suppl 3):1550–4. doi: 10.1016/s0015-0282(03)00339-x. [DOI] [PubMed] [Google Scholar]
- 22.Heger A, Sator M, Walch K, Pietrowski D. Smoking decreases endometrial thickness in IVF/ICSI patients. Geburtshilfe Frauenheilkd. 2018;78:78–82. doi: 10.1055/s-0043-123762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klemetti R, Gissler M, Sevón T, Hemminki E. Resource allocation of in vitro fertilization: A nationwide register-based cohort study. BMC Health Serv Res. 2007;77:210. doi: 10.1186/1472-6963-7-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dain L, Auslander R, Dirnfeld M. The effect of paternal age on assisted reproduction outcome. Fertil Steril. 2011;95:1–8. doi: 10.1016/j.fertnstert.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 25.Laopaiboon M, Lumbiganon P, Intarut N, Mori R, Ganchimeg T, Vogel JP, et al. WHO Multicountry Survey on Maternal Newborn Health Research Network. Advanced maternal age and pregnancy outcomes: A multicountry assessment. BJOG. 2014;121(Suppl 1):49–56. doi: 10.1111/1471-0528.12659. [DOI] [PubMed] [Google Scholar]
- 26.Provost MP, Acharya KS, Acharya CR, Yeh JS, Steward RG, Eaton JL, et al. Pregnancy outcomes decline with increasing body mass index: Analysis of 239,127 fresh autologous in vitro fertilization cycles from the 2008-2010 society for assisted reproductive technology registry. Fertil Steril. 2016;105:663–9. doi: 10.1016/j.fertnstert.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Pinborg A, Gaarslev C, Hougaard CO, Nyboe Andersen A, Andersen PK, Boivin J, et al. Influence of female bodyweight on IVF outcome: A longitudinal multicentre cohort study of 487 infertile couples. Reprod Biomed Online. 2011;23:490–9. doi: 10.1016/j.rbmo.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Shiva M, Gautam AK, Verma Y, Shivgotra V, Doshi H, Kumar S. Association between sperm quality, oxidative stress, and seminal antioxidant activity. Clin Biochem. 2011;44:319–24. doi: 10.1016/j.clinbiochem.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Yildizfer F, Donma O, Yen M, Ekmekci O, Karatas Kul ZA, Keser Z, et al. In vitro fertilization, levels of pro-inflammatory factors and lipid peroxidation. Int J Fertil Steril. 2015;9:277–84. doi: 10.22074/ijfs.2015.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das S, Chattopadhyay R, Ghosh S, Ghosh S, Goswami SK, Chakravarty BN, et al. Reactive oxygen species level in follicular fluid - Embryo quality marker in IVF? Hum Reprod. 2006;21:2403–7. doi: 10.1093/humrep/del156. [DOI] [PubMed] [Google Scholar]
- 31.Sharma A. Investigation on the effects of exogenous H2O2 on sperm motility, LPO, catalase and SOD levels in seminal plasma. Health Sci J. 2015;10:1. [Google Scholar]


