Abstract
S100 proteins are calcium (Ca2+)-binding proteins and these have an important function in progression, manifestation and therapeutic aspects of various inflammatory, metabolic and neurodegenerative disorders. Based on their involvement in intracellular or extracellular regulatory effects, S100 proteins are classified into three subgroups: one subgroup is specialized in exerting only intracellular effects, other performs both intracellular and extracellular functions and the third subgroup members only display extracellular regulatory effects. S100 proteins are expressed particularly in vertebrates and have cell-specific expression. Functionally, S100 proteins act through their surface receptors and regulate cell functions in autocrine or paracrine mode. Receptor for advanced glycation end products (RAGEs) and toll-like receptor 4 are the main surface receptors. S100 proteins participate in the regulation of cellular differentiation, proliferation, apoptosis and inflammation along with Ca2+ homeostasis, energy metabolism and cellular migration, and perform the respective functions through their interaction with transcription factors, nucleic acids, enzymes, receptors, cytoskeleton system, etc. Currently, their role in adverse pregnancy outcomes and compromised reproductive health is being explored. These proteins are present in amniotic fluid, endometrium tissue and foetal brain; therefore, it is quite likely that alterations in the expression levels of S100 family members will be affecting the particular function they are involved in and ultimately affecting the pregnancy in adverse manner. The current review discusses about an association of S100 proteins in pregnancy disorders such as endometriosis, intrauterine growth retardation and miscarriage.
Keywords: Calcium signalling, early pregnancy loss, high-risk pregnancy, implantation, inflammation, intrauterine growth retardation
Introduction
Early miscarriage and pregnancy-associated problems are of major concern. The reason behind this is not only genetical or physiological but also environmental and modern lifestyle. Moderate levels of inflammatory reactions are also pre-requisite during the first trimester of pregnancy for implantation and embryo development. These early stages of pregnancy resemble ‘an open wound’1. For invasion and proper blood supply of embryo neovascularization and tissue remodelling occur during early gestational weeks of pregnancy1,2. An appropriate tuning of anti-inflammatory and inflammatory mediators is required for adequate repair of the uterine epithelium and the removal of cellular debris. Thus, this critical period of pregnancy is marked by expression of specific cytokines and adhesion molecules by both foetal and maternal side ensuring successful pregnancy. Any alteration and dysfunction of this balanced inflammatory milieu and any perturbation or disturbance in this during the critical period result in miscarriage or pregnancy-associated complications3.
Earlier studies in mice and human revealed the role of important calcium (Ca2+)-binding S100 proteins in pregnancy-related complications4,5. This group of proteins helps in the recruitment of leucocytes at inflammatory site and functions like cytokines4. These proteins regulate a variety of cellular functions such as cellular differentiation, cell cycle progression and energy intracellular signal transduction by interacting with several other mediatory proteins6. S100 proteins were found to be tumorigenic in function and get elevated in several cancer and melanoma cases6. An earlier study in human also showed elevated level of S100 group proteins in high-risk pregnancy cases, in amniotic fluid and cord blood of foetus with brain damage7. The role of S100 protein in immunomodulation of high-risk pregnancy cases is an active area of research and clinical investigation. This review focuses on new advances regarding the role of S100 protein in diagnosis and treatment of high-risk pregnancies.
S100 protein structure and function
Ca2+ regulates several cellular processes and acts as a messenger8. Many Ca2+-binding proteins, having the EF-hand structural motif, make Ca2+ signalling network in combination with many molecular components9. S100 proteins are the largest subgroup within this family of Ca2+-binding proteins and found to be involved in several diseases such as rheumatoid arthritis, acute inflammatory lesions, cardiomyopathy, Alzheimer's disease and cancer10,11.
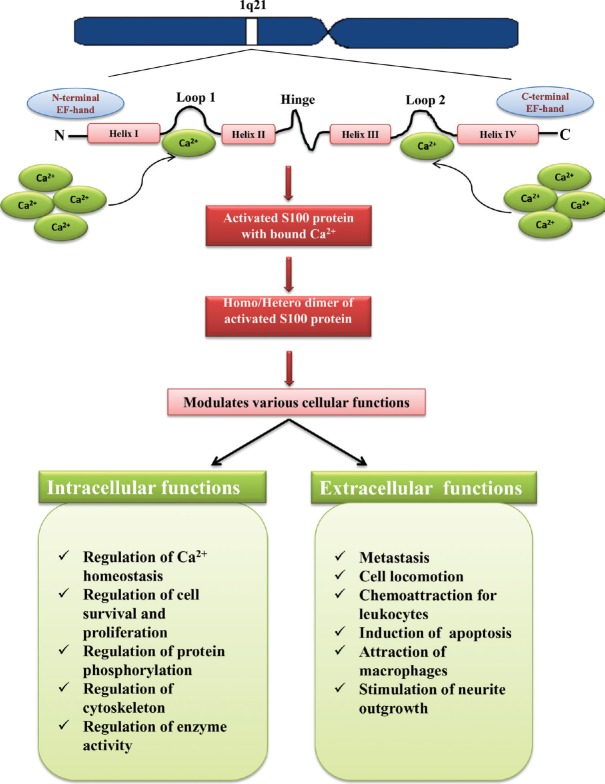
S100 proteins are acidic, Ca2+-binding proteins initially identified in the brain of several mammalian species and called S100 because of their solubility in 100 per cent ammonium sulphate12,13. Genes responsible for the synthesis of most S100 proteins are located on human chromosomes 1q2114. Initially, S100 proteins were found to be located in glial cells and used as a marker of glial cell differentiation and mammalian brain development15,16,17. S100 protein family has 21 members having the same basic structural moiety but entirely different function, and are found in cerebrospinal fluid, urine, serum, seminal plasma and saliva mainly in active disease states. These proteins are found to be present in Ca2+ free (apo); Ca2+-bound and target bound states as a symmetric dimer, with each monomer containing two EF-hand motifs18. The EF-hand motif on N-terminal site contains helix I with pseudo Ca2+-binding site, and the EF-hand of C-terminal is associated with helix III, helix IV and second Ca2+-binding site (Fig. 1).
Fig. 1.
Schematic diagram represents the chromosomal location, structure and various functions of S100 proteins. Source: Refs 14,18.
S100 proteins undergo structural and conformational changes on binding with Ca2+, and this conformational change allows interaction of these proteins with target molecules. Activated S100 proteins perform all cellular functions by both extracellular and intracellular methods (Table I). All S100 proteins function in the form of dimmers, and only S100G protein acts as monomer39. A few hetero-dimmers are also reported: S100A1/B, S100A8/A9, S100A1/A4 and S100A1/P40,41. S100 proteins can also form active tetramers, hexamers or larger oligomers (S100B42, S100A443, S100A8/A944 and S100 A1245).
Table I.
Functions of S100 proteins
| S100 protein | Localization | Functions | References |
|---|---|---|---|
| S100A1 | Skeletal system, neurons and cardiomyocytes | Functioning of cardiomyocytes and skeletal muscle, regulation of energy metabolism | 19 |
| S100A2 | Cancerous cells | Down regulated in many cancers | 20 |
| S100A3 | Localized in root of hair and some cancerous astrocytes | Differentiation of epithelial cell and hair cuticular barrier formation | 21 |
| S100A4 | Tumorous tissue | Stimulating cell proliferation, mobility and migration | 22 |
| S100A5 | Tumorous tissue | Elevated in bladder cancer cases | 23 |
| S100A6 | Tumorous tissue | Cell cycle progression, cytoskeletal movement and tumour formation | 24 |
| S100A7 | Tumorous tissue | Cytoskeletal functions, adhesion and migration, upregulated in breast cancer | 25 |
| S100A8 | Macrophages, dendritic cells, microvascular endothelial cells | Necessary for embryo implantation, promotes myeloid cells differentiation | 5 |
| S100A9 | Neutrophils, dendritic cells | Inhibits myeloid cells differentiation, induces inflammation | 26 |
| S100A10 | Neutrophils, dendritic cells | Interacts with serotonin receptors and control depression like status | 27 |
| S100A11 | Neutrophils, dendritic cells | Controls cell proliferation and survival | 28 |
| S100A12 | In neutrophils and inducible in macrophages | Regulates VSCM functions | 29 |
| S100A13 | Fibroblasts, osteoblasts and melanoma cells | Regulates secretion factor (FGF)- 1 and IL-1α from various cell types | 30 |
| S100A14 | Tumorous tissue | Inhibits cancer progression via suppression of p53 mediated pathway | 31 |
| S100A15 | ‑ | Acts as an extracellular factor | 32 |
| S100A16 | Tumorous tissue | Disrupts insulin sensitivity and promotes obesity | 33 |
| S100B | Astrocytes, certain neuronal populations, Schwann cells, melanocytes, chondrocytes, adipocytes, skeletal myofibers | Stimulator of cell cycle and migration and an inhibits apoptosis | 34 |
| S100G | Neutrophils, dendritic cells | Maintains cytosolic Ca2+ concentration | 35 |
| S100P | Tumorous tissue | Cell migration and potentially metastasis | 36 |
| S100Z | Tumorous tissue | Down regulated in several tumours | 37,38 |
VSCM, vascular smooth muscle cell; FGF, fibroblast growth factor; IL, interleukin
S100 receptors
Function of S100 proteins is determined by their oligomeric forms and their respective binding partners46. Extracellular S100 proteins act via activation of surface receptors such as G protein-coupled receptors, receptor for advanced glycation end products (RAGEs) and toll-like receptors and aid in regulatory processes such as cell proliferation, differentiation and migration in normal as well as different pathological conditions. Intracellular S100 proteins also act via interaction with different target enzymes, cytoskeleton subunits, receptors and transcription factors or nucleic acids regulate Ca2+ homeostasis, energy metabolism and cellular differentiation.
Role of S100 proteins in high-risk pregnancy cases
In maternal endometrium, S100 proteins are expressed by both immune cells and non-immune cells. A few groups of S100 proteins such as S100A8, S100A9 and S100A12 are mainly secreted from myeloid origin of immune cells such as granulocytes, monocytes and early stages of macrophages4. As myeloid origin cells are well known as crucial regulators for other immune cells (T, Treg, uNK and non-inflammatory macrophages and neutrophils cells) in successful pregnancy, any alteration in inflammatory or immunomodulatory stage may change S100 protein levels4 (Fig. 2). Some non-immune cells such as mice placenta and ovaries of cow and pig have been reported to secrete some S100 group proteins such as S100A1, S100A6, S100A9 and S100A85,48.
Fig. 2.
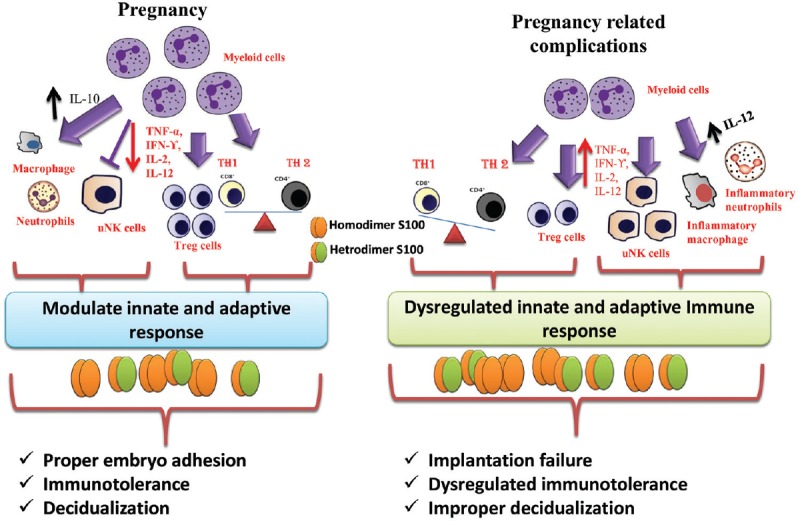
Schematic diagram represents interaction of S100 proteins with immune cells for the regulation of various hallmark processes of pregnancy. IFN-γ, interferon gamma; IL, interleukin; TH, T helper; TNF-α, tumor necrosis factor alpha; uNK, uterine natural killer. Source: Refs 4,47.
S100 proteins regulate embryo implantation, intrauterine growth and normal foetal brain development during pregnancy. S100 family proteins have been found to be dysregulated in various endometrial diseases (Table II). S100A8 proteins are found to be down regulated in receptive phase of endometrium57. S100A8 protein recruits mouse and human neutrophils and macrophages at the site of inflammation58. Endometrial epithelium and stromal cells also showed expression of S100A10 protein during the implantation window and found to play an important role in endometrial receptivity54. The expression of these proteins have been found to be down regulated in the endometrium of infertile patients54,55. This is the reason behind the failure of 30 per cent of embryo implantation in assisted reproduction.
Table II.
Altered expression profile of S100 proteins in various human pregnancy-related diseases
| Pregnancy-related diseases | S100 family | References |
|---|---|---|
| Pregnancy-associated with Down syndrome | Upregulated S100ß | 49 |
| IUGR | Upregulated S100ß | 50 |
| SGA babies SGA foetus | No change in S100ß | 51 |
| Pre eclampsia+IUGR | Upregulated S100ß | 52 |
| Miscarriage | Down regulated S100A11 | 53 |
| Up regulated S100A8, S100A9 | 4,47 | |
| Pre eclampsia | Up regulated S100ß | 52 |
| Pre-term labour | Up regulated S100ß | 51 |
| IVF failure | Down regulated S100A11, S100A10 | 54 |
| PCOS | Up regulated S100A12 | 55 |
| Endometriosis | Up regulated S100A4 | 56 |
| Endometriosis associated with infertility | Up regulated S100P | 56 |
| Gestational diabetes | Up regulated S100A9 | 57 |
IUGR, intrauterine growth restriction; SGA, small for gestational age; IVF, in vitro fertilization; PCOS, polycystic ovary syndrome
A study conducted by Passey et al5 showed that S100A8 knockout gene in mice caused a late embryonic lethality and suggested its role in fetomaternal tolerance. A study on transcriptome-based analysis in equine pregnancy revealed that S100A6 protein was expressed in conceptus side, and S100A2, S100A4, S100A6 and S100A8 were present in maternal endometrium on day 16 in mare59. A key role of these proteins has been suggested in epidermal growth factor-stimulated embryo adhesion, acquisition of endometrial receptivity, immunotolerance, apoptosis of dead endometrial epithelial cells and prolactin secretion, a marker for onset of decidualization60,61. S100 β protein is also found to be up regulated in trisomy cases, and their upregulation is an indicator of a brain lesion in developing foetus58. Thus, monitoring of S100 protein could be helpful in the detection of brain distress in intrauterine growth-retarded (IUGR) foetuses50. In preeclampsia and IUGR cases, amniotic fluid S100B protein concentration was found to be elevated23.
Conclusion
The present review summarizes the role of S100 proteins in high-risk pregnancy cases along with its structure and mechanism of action. This also covers the importance of S100 proteins as a main player of successful implantation, embryonic growth and birth of physically and mentally healthy child. The optimal expression and signalling of S100 proteins, at particular stages of pregnancy is a pre-requisite for avoiding high-risk pregnancy cases and can serve as therapeutic target and prognostic biomarker in pregnancy-related complications.
Financial support & sponsorship:
The first author (RV) and the second author (PV) acknowledge the Department of Biotechnology, New Delhi, India, for providing financial assistance in the form of Research Associateship and Senior Research Fellowship, respectively. The third author (SB) thanks the University Grant Commission, New Delhi, India, for providing financial assistance in the form of Senior Research Fellowship.
Conflicts of Interest:
None.
References
- 1.Mor G, Cardenas I. The immune system in pregnancy: A unique complexity. Am J Reprod Immunol. 2010;63:425–33. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–7. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwak-Kim J, Yang KM, Gilman-Sachs A. Recurrent pregnancy loss: A disease of inflammation and coagulation. J Obstet Gynaecol Res. 2009;35:609–22. doi: 10.1111/j.1447-0756.2009.01079.x. [DOI] [PubMed] [Google Scholar]
- 4.Nair RR, Khanna A, Singh K. Association of increased S100A8 serum protein with early pregnancy loss. Am J Reprod Immunol. 2015;73:91–4. doi: 10.1111/aji.12318. [DOI] [PubMed] [Google Scholar]
- 5.Passey RJ, Williams E, Lichanska AM, Wells C, Hu S, Geczy CL, et al. A null mutation in the inflammation-associated S100 protein S100A8 causes early resorption of the mouse embryo. J Immunol. 1999;163:2209–16. [PubMed] [Google Scholar]
- 6.Kanamori T, Takakura K, Mandai M, Kariya M, Fukuhara K, Sakaguchi M, et al. Increased expression of calcium-binding protein S100 in human uterine smooth muscle tumours. Mol Hum Reprod. 2004;10:735–42. doi: 10.1093/molehr/gah100. [DOI] [PubMed] [Google Scholar]
- 7.Michetti F, Gazzolo D. S100B testing in pregnancy. Clin Chim Acta. 2003;335:1–7. doi: 10.1016/s0009-8981(03)00243-2. [DOI] [PubMed] [Google Scholar]
- 8.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki H, Nakayama S, Kretsinger RH. Classification and evolution of EF-hand proteins. Biometals. 1998;11:277–95. doi: 10.1023/a:1009282307967. [DOI] [PubMed] [Google Scholar]
- 10.Van Eldik LJ, Griffin WS. S100 beta expression in Alzheimer's disease: Relation to neuropathology in brain regions. Biochim Biophys Acta. 1994;1223:398–403. doi: 10.1016/0167-4889(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 11.Heizmann CW, Cox JA. New perspectives on S100 proteins: A multi-functional Ca(2+)-, Zn(2+)- and Cu(2+)-binding protein family. Biometals. 1998;11:383–97. doi: 10.1023/a:1009212521172. [DOI] [PubMed] [Google Scholar]
- 12.Kessler D, Levine L, Fasman G. Some conformational and immunological properties of a bovine brain acidic protein (S-100) Biochemistry. 1968;7:758–64. doi: 10.1021/bi00842a034. [DOI] [PubMed] [Google Scholar]
- 13.Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965;19:739–44. doi: 10.1016/0006-291x(65)90320-7. [DOI] [PubMed] [Google Scholar]
- 14.Heizmann CW, Fritz G, Schäfer BW. S100 proteins: Structure, functions and pathology. Front Biosci. 2002;7:d1356–68. doi: 10.2741/A846. [DOI] [PubMed] [Google Scholar]
- 15.Cicero TJ, Ferrendelli JA, Suntzeff V, Moore BW. Regional changes in CNS levels of the S-100 and 14-3-2 proteins during development and aging of the mouse. J Neurochem. 1972;19:2119–25. doi: 10.1111/j.1471-4159.1972.tb05121.x. [DOI] [PubMed] [Google Scholar]
- 16.De Vitry F, Picart R, Jacque C, Legault L, Dupouey P, Tixier-Vidal A, et al. Presumptive common precursor for neuronal and glial cell lineages in mouse hypothalamus. Proc Natl Acad Sci U S A. 1980;77:4165–9. doi: 10.1073/pnas.77.7.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herschman HR, Levine L, De Vellis J. Appearance of a brain-specific antigen (S-100 protein) in the developing rat brain. J Neurochem. 1971;18:629–33. doi: 10.1111/j.1471-4159.1971.tb11993.x. [DOI] [PubMed] [Google Scholar]
- 18.Santamaria-Kisiel L, Rintala-Dempsey AC, Shaw GS. Calcium-dependent and -independent interactions of the S100 protein family. Biochem J. 2006;396:201–14. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donato R. S100: A multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–68. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 20.Wolf S, Haase-Kohn C, Pietzsch J. S100A2 in cancerogenesis: A friend or a foe? Amino Acids. 2011;41:849–61. doi: 10.1007/s00726-010-0623-2. [DOI] [PubMed] [Google Scholar]
- 21.Kizawa K, Takahara H, Troxler H, Kleinert P, Mochida U, Heizmann CW, et al. Specific citrullination causes assembly of a globular S100A3 homotetramer: A putative Ca2+ modulator matures human hair cuticle. J Biol Chem. 2008;283:5004–13. doi: 10.1074/jbc.M709357200. [DOI] [PubMed] [Google Scholar]
- 22.Ismail TM, Fernig DG, Rudland PS, Terry CJ, Wang G, Barraclough R, et al. The basic C-terminal amino acids of calcium-binding protein S100A4 promote metastasis. Carcinogenesis. 2008;29:2259–66. doi: 10.1093/carcin/bgn217. [DOI] [PubMed] [Google Scholar]
- 23.Hancq S, Salmon I, Brotchi J, De Witte O, Gabius HJ, Heizmann CW, et al. S100A5: A marker of recurrence in WHO grade I meningiomas. Neuropathol Appl Neurobiol. 2004;30:178–87. doi: 10.1046/j.0305-1846.2003.00525.x. [DOI] [PubMed] [Google Scholar]
- 24.Leśniak W, Słomnicki ŁP, Filipek A. S100A6 – New facts and features. Biochem Biophys Res Commun. 2009;390:1087–92. doi: 10.1016/j.bbrc.2009.10.150. [DOI] [PubMed] [Google Scholar]
- 25.Deol YS, Nasser MW, Yu L, Zou X, Ganju RK. Tumor-suppressive effects of psoriasin (S100A7) are mediated through the β-catenin/T cell factor 4 protein pathway in estrogen receptor-positive breast cancer cells. J Biol Chem. 2011;286:44845–54. doi: 10.1074/jbc.M111.225466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svenningsson P, Greengard P. P11 (S100A10) – An inducible adaptor protein that modulates neuronal functions. Curr Opin Pharmacol. 2007;7:27–32. doi: 10.1016/j.coph.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Sakaguchi M, Miyazaki M, Sonegawa H, Kashiwagi M, Ohba M, Kuroki T, et al. PKCalpha mediates TGFbeta-induced growth inhibition of human keratinocytes via phosphorylation of S100C/A11. J Cell Biol. 2004;164:979–84. doi: 10.1083/jcb.200312041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goyette J, Geczy CL. Inflammation-associated S100 proteins: New mechanisms that regulate function. Amino Acids. 2011;41:821–42. doi: 10.1007/s00726-010-0528-0. [DOI] [PubMed] [Google Scholar]
- 30.Prudovsky I, Donato R. S100a13. UCSD- Nature Molecule Pages. 2009 Doi: 10.1038/mp.a002115.01. [Google Scholar]
- 31.Chen H, Yu D, Luo A, Tan W, Zhang C, Zhao D, et al. Functional role of S100A14 genetic variants and their association with esophageal squamous cell carcinoma. Cancer Res. 2009;69:3451–7. doi: 10.1158/0008-5472.CAN-08-4231. [DOI] [PubMed] [Google Scholar]
- 32.Wolf R, Ruzicka T, Yuspa SH. Novel S100A7 (psoriasin)/S100A15 (koebnerisin) subfamily: Highly homologous but distinct in regulation and function. Amino Acids. 2011;41:789–96. doi: 10.1007/s00726-010-0666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sturchler E, Cox JA, Durussel I, Weibel M, Heizmann CW. S100A16, a novel calcium-binding protein of the EF-hand superfamily. J Biol Chem. 2006;281:38905–17. doi: 10.1074/jbc.M605798200. [DOI] [PubMed] [Google Scholar]
- 34.Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, et al. S100B's double life: Intracellular regulator and extracellular signal. Biochim Biophys Acta. 2009;1793:1008–22. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Luu KC, Nie GY, Salamonsen LA. Endometrial calbindins are critical for embryo implantation: Evidence from in vivo use of morpholino antisense oligonucleotides. Proc Natl Acad Sci U S A. 2004;101:8028–33. doi: 10.1073/pnas.0401069101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Austermann J, Nazmi AR, Müller-Tidow C, Gerke V. Characterization of the Ca2+ -regulated ezrin-S100P interaction and its role in tumor cell migration. J Biol Chem. 2008;283:29331–40. doi: 10.1074/jbc.M806145200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gribenko AV, Hopper JE, Makhatadze GI. Molecular characterization and tissue distribution of a novel member of the S100 family of EF-hand proteins. Biochemistry. 2001;40:15538–48. doi: 10.1021/bi0114731. [DOI] [PubMed] [Google Scholar]
- 38.Bresnick AR, Weber DJ, Zimmer DB. S100 proteins in cancer. Nat Rev Cancer. 2015;15:96–109. doi: 10.1038/nrc3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skelton NJ, Kördel J, Akke M, Forsén S, Chazin WJ. Signal transduction versus buffering activity in Ca2+–binding proteins. Nat Struct Mol Biol. 1994;1:239. doi: 10.1038/nsb0494-239. [DOI] [PubMed] [Google Scholar]
- 40.Wang G, Zhang S, Fernig DG, Spiller D, Martin-Fernandez M, Zhang H, et al. Heterodimeric interaction and interfaces of S100A1 and S100P. Biochem J. 2004;382:375–83. doi: 10.1042/BJ20040142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lügering N, Stoll R, Schmid KW, Kucharzik T, Stein H, Burmeister G, et al. The myeloic related protein MRP8/14 (27E10 antigen) – Usefulness as a potential marker for disease activity in ulcerative colitis and putative biological function. Eur J Clin Invest. 1995;25:659–64. doi: 10.1111/j.1365-2362.1995.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 42.Ostendorp T, Leclerc E, Galichet A, Koch M, Demling N, Weigle B, et al. Structural and functional insights into RAGE activation by multimeric S100B. EMBO J. 2007;26:3868–78. doi: 10.1038/sj.emboj.7601805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kiryushko D, Novitskaya V, Soroka V, Klingelhofer J, Lukanidin E, Berezin V, et al. Molecular mechanisms of Ca(2+) signaling in neurons induced by the S100A4 protein. Mol Cell Biol. 2006;26:3625–38. doi: 10.1128/MCB.26.9.3625-3638.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leukert N, Vogl T, Strupat K, Reichelt R, Sorg C, Roth J, et al. Calcium-dependent tetramer formation of S100A8 and S100A9 is essential for biological activity. J Mol Biol. 2006;359:961–72. doi: 10.1016/j.jmb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Moroz OV, Antson AA, Dodson EJ, Burrell HJ, Grist SJ, Lloyd RM, et al. The structure of S100A12 in a hexameric form and its proposed role in receptor signalling. Acta Crystallogr D Biol Crystallogr. 2002;58:407–13. doi: 10.1107/s0907444901021278. [DOI] [PubMed] [Google Scholar]
- 46.Desamero MJ, Delgado RA, de Ocampo GD, Bariuan JV, Collantes TM, Estacio MA. Immunohistochemical demonstration of S100 protein in the ovary of the Philippine water buffalo (Bubalus bubalis Linnaeus, 1758) (Artiodactyla: Bovidae) Philipp J Vet Med 2015. 52 [Google Scholar]
- 47.Nair RR, Khanna A, Singh K. Role of inflammatory proteins S100A8 and S100A9 in pathophysiology of recurrent early pregnancy loss. Placenta. 2013;34:824–7. doi: 10.1016/j.placenta.2013.06.307. [DOI] [PubMed] [Google Scholar]
- 48.Fritz G, Botelho HM, Morozova-Roche LA, Gomes CM. Natural and amyloid self-assembly of S100 proteins: Structural basis of functional diversity. FEBS J. 2010;277:4578–90. doi: 10.1111/j.1742-4658.2010.07887.x. [DOI] [PubMed] [Google Scholar]
- 49.Abraha HD, Noble PL, Nicolaides KH, Sherwood RA. Maternal serum S100 protein in normal and down syndrome pregnancies. Prenat Diagn. 1999;19:334–6. [PubMed] [Google Scholar]
- 50.Gazzolo D, Marinoni E, di Iorio R, Lituania M, Bruschettini PL, Michetti F, et al. Circulating S100beta protein is increased in intrauterine growth-retarded fetuses. Pediatr Res. 2002;51:215–9. doi: 10.1203/00006450-200202000-00015. [DOI] [PubMed] [Google Scholar]
- 51.Morales-Roselló J, Khalil A, Alba-Redondo A, Martinez-Triguero L, Akhoundova F, Perales-Marín A, et al. Protein S100β in late-pregnancy fetuses with low birth weight and abnormal cerebroplacental ratio. Fetal Diagn Ther. 2017;41:15–22. doi: 10.1159/000445114. [DOI] [PubMed] [Google Scholar]
- 52.Tskitishvili E, Komoto Y, Temma-Asano K, Hayashi S, Kinugasa Y, Tsubouchi H, et al. S100B protein expression in the amnion and amniotic fluid in pregnancies complicated by pre-eclampsia. Mol Hum Reprod. 2006;12:755–61. doi: 10.1093/molehr/gal083. [DOI] [PubMed] [Google Scholar]
- 53.Liu AX, Jin F, Zhang WW, Zhou TH, Zhou CY, Yao WM, et al. Proteomic analysis on the alteration of protein expression in the placental villous tissue of early pregnancy loss. Biol Reprod. 2006;75:414–20. doi: 10.1095/biolreprod.105.049379. [DOI] [PubMed] [Google Scholar]
- 54.Liu XM, Ding GL, Jiang Y, Pan HJ, Zhang D, Wang TT, et al. Down-regulation of S100A11, a calcium-binding protein, in human endometrium may cause reproductive failure. J Clin Endocrinol Metab. 2012;97:3672–83. doi: 10.1210/jc.2012-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su NJ, Ma J, Feng DF, Zhou S, Li ZT, Zhou WP, et al. The peripheral blood transcriptome identifies dysregulation of inflammatory response genes in polycystic ovary syndrome. Gynecol Endocrinol. 2018;34:584–8. doi: 10.1080/09513590.2017.1418851. [DOI] [PubMed] [Google Scholar]
- 56.Hapangama DK, Raju RS, Valentijn AJ, Barraclough D, Hart A, Turner MA, et al. Aberrant expression of metastasis-inducing proteins in ectopic and matched eutopic endometrium of women with endometriosis: Implications for the pathogenesis of endometriosis. Hum Reprod. 2012;27:394–407. doi: 10.1093/humrep/der412. [DOI] [PubMed] [Google Scholar]
- 57.Oliva K, Barker G, Rice GE, Bailey MJ, Lappas M. 2D-DIGE to identify proteins associated with gestational diabetes in omental adipose tissue. J Endocrinol. 2013;218:165–78. doi: 10.1530/JOE-13-0010. [DOI] [PubMed] [Google Scholar]
- 58.Portela LC, Tort AB, Neto EC, Kessler RG, Penchaszadeh V, Souza DO, et al. High immunocontent of S100 beta protein in amniotic fluid of pregnancies with down syndrome. Ultrasound Obstet Gynecol. 2000;16:590–2. doi: 10.1046/j.1469-0705.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 59.Klein C. Novel equine conceptus-endometrial interactions on day 16 of pregnancy based on RNA sequencing. Reprod Fertil Dev. 2016;28:1712–20. doi: 10.1071/RD14489. [DOI] [PubMed] [Google Scholar]
- 60.Bissonnette L, Drissennek L, Antoine Y, Tiers L, Hirtz C, Lehmann S, et al. Human S100A10 plays a crucial role in the acquisition of the endometrial receptivity phenotype. Cell Adh Migr. 2016;10:282–98. doi: 10.1080/19336918.2015.1128623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhagwat SR, Chandrashekar DS, Kakar R, Davuluri S, Bajpai AK, Nayak S, et al. Endometrial receptivity: A revisit to functional genomics studies on human endometrium and creation of HGEx-ERdb. PLoS One. 2013;8:e58419. doi: 10.1371/journal.pone.0058419. [DOI] [PMC free article] [PubMed] [Google Scholar]