Abstract
Rationale & Objective:
Determining whether a change in estimated glomerular filtration rate (eGFR) or albuminuria is clinically significant requires knowledge of short-term within-person variability of the measurements, which few studies have addressed in the setting of chronic kidney disease.
Study Design:
Cross-sectional study with multiple collections over less than 4 weeks.
Setting & Participants:
Clinically stable outpatients with chronic kidney disease (N = 50; mean age, 56.8 years; median eGFR, 40 mL/min/1.73 m2; median urinary albumin-creatinine ratio (UACR), 173 mg/g).
Exposure:
Repeat measurements from serially collected samples across 3 study visits.
Outcomes:
Measurements of urine albumin concentration (UAC), UACR, and plasma creatinine, cystatin C, β2-microglobulin (B2M), and beta trace protein (BTP).
Analytical Approach:
We calculated within-person coefficients of variation (CVw) values and corresponding reference change positive and negative (RCVpos and RCVneg) values using log-transformed measurements.
Results:
Median CVw (RCVpos; RCVneg) values of filtration markers were 5.4% (+16%; –14%) for serum creatinine, 4.1% (+12%; –11%) for cystatin C, 7.4% (+23%; –18%) for BTP, and 5.6% (+17%; –14%) for B2M. Results for albuminuria were 33.2% (+145%; –59%) for first-morning UAC, 50.6% (+276%; –73%) for random spot UAC, 32.5% (+141%; –58%) for first-morning UACR, and 29.7% (124%; –55%) for random spot UACR. CVw values for filtration markers were comparable across the range of baseline eGFRs. CVw values for UAC and UACR were comparable across the range of baseline albuminuria values.
Limitations:
Small sample size limits the ability to detect differences in variability across markers. Participants were recruited and followed up in a clinical and not research setting, so some preanalytical factors could not be controlled.
Conclusions:
eGFR markers appear to have relatively low short-term within-person variability, whereas variability in albuminuria appears to be high, making it difficult to distinguish random variability from meaningful biologic changes.
Abnormalities in glomerular filtration rate (GFR) and/or albuminuria define chronic kidney disease (CKD), a condition that affects ~ 10% of the US population.1,2 GFR is estimated using endogenous plasma or serum filtration markers, most commonly creatinine.3 Albuminuria is most commonly quantified by measuring urinary albumin concentration (UAC) or urinary albumin-creatinine ratio (UACR), which is UAC divided by urinary creatinine concentration. Reference ranges for estimated GFR (eGFR) and albuminuria in healthy individuals are well established.4
For individuals with CKD, clinicians and researchers measure and follow up serial changes in eGFR and albuminuria to assess disease progression, prognosis, and response to therapy.5–8 Determining whether a change in eGFR or albuminuria is clinically significant requires knowledge of the expected variability in the absence of underlying clinical changes. For example, according to present guidelines, an increase in UACR from the 30–300 mg/g range to >300 mg/g denotes a transition from moderately to severely increased albuminuria, with consequences in clinical decision making. Similarly, an increase in serum creatinine (Scr) level by ≥0.3 mg/dL over 48 hours or ≥50% over 7 days defines acute kidney injury (AKI), a major complication in patients with and without underlying CKD.
Despite the clinical importance of assessing the significance of changes in measurements of kidney function, relatively little is known about the inherent biological variability of eGFR and UACR in the setting of CKD. We therefore conducted this study in clinically stable patients with CKD to provide estimates of the short-term within-person biological variability in measures of kidney function, including albuminuria (UAC and UACR) and plasma eGFR markers (creatinine and the newer markers cystatin C, β2-microglobulin [B2M], and beta trace protein [BTP]).
Methods
Study Cohort
We collected urine and blood samples from individuals with CKD attending a nephrology subspecialty practice at Brigham and Women’s Hospital, a tertiary-care medical center that provides care for a racially and socioeconomically diverse population in eastern Massachusetts and the surrounding region. Patients provided written documentation of informed consent to participate in this study, which was approved by the local Institutional Review Board (2010P002703). Eligible participants had a diagnosis of CKD under the care of a nephrologist and were recruited as a convenience sample during a nephrology clinic visit between February 2011 and February 2014. Participants were excluded for any of the following reasons: inability or unwillingness to return for 2 additional study visits, recent hospitalization or an episode of AKI (>50% increase in Scr over a 1-week period) within the past 3 months, active glomerulonephritis or history of kidney transplantation, reported or suspected urinary tract infection within the past 3 weeks, or a planned change by the attending nephrologist of the dose of a diuretic and/or antihypertensive medication during the study period.
Sample Collection
We collected urine and blood samples shortly after a clinic visit (8 AM to 5 AM) and then at 2 requested follow-up study visits within an approximate 2-week period (range, 1–4 weeks). At each of the 2 subsequent study visits, patients were asked to bring in a refrigerated first-void morning urine sample and also provide a fresh urine sample during the study visit. Thus, up to 3 blood specimens and up to 5 urine specimens were collected. Urine samples were centrifuged at 3,200g for 5 minutes and the supernatants were collected. Trained phleboto-mists at Brigham and Women’s Hospital collected blood samples according to standard clinical protocols. All plasma samples and supernatants of urine samples were frozen at –80°C within 4 hours of collection. We transferred or shipped frozen samples on dry ice to perfomance laboratories at Brigham and Women’s Hospital for the measurement of total urine protein excretion and to the University of Minnesota for the measurement of plasma creatinine, cystatin C, B2M, BTP (as eGFR markers), urine albumin, and urine creatinine.
Assays
Plasma and urine creatinine were measured using the Roche enzymatic method on a Roche cobas 6000 chemistry analyzer, calibrated using the isotope-dilution mass spectrometry standard traceable to the fresh-frozen serum-based National Institute of Standards and Technology Standard Reference Material SRM 967. Plasma cystatin C and urinary albumin were measured on the Roche chemistry analyzer using a turbidimetric assay. Plasma B2M was measured using a latex agglutination assay. Plasma BTP was measured on a Siemens ProSpec nephelometer. All assays were performed over a 2-day period. Interassay coefficients of variation (CVs) of all plasma and urine markers were assessed using blind split-replicate samples from individuals with CKD and were < 3% for each marker. Published equations were used to estimate GFR from concentrations of filtration markers.9–11
Statistical Analysis
All biomarker measurements and GFR estimates were transformed onto the natural log-scale for analyses of within-person variability. For each participant, we calculated within-person CV (CVw) values across repeat sample measurements (up to 5 plasma and up to 3 urine; missing values were not imputed) using the equation where var refers to within-participant variance. We then used median CVw values to estimate 95% reference change values (RCVs) as:
RCVpos and RCVneg refer to the increase or decrease that must be exceeded between 2 sequential results for a change to be considered different at a statistical significance level of 0.05.12 For example, RCVpos of +20% means that an increase from a 1.0 to 1.2 (arbitrary measurement) may be within the expected range of values.
Bootstrap with 1,000 iterations was used to calculate 95% confidence intervals (CIs) for median CV and differences in median CVs. Comparisons of median CVs between subgroups were conducted using Mann-Whitney tests and generalized Hodges-Lehmann median difference test, with Bonferroni-corrected P < 0.005 regarded as significant.
Results
Clinical Characteristics
The study cohort consisted of 50 individuals who provided a total of 139 plasma samples and 227 urine samples during the 4-week study period (Table 1). Mean age was 56.8 ± 15.8 (SD) years. The cohort was composed of 44% women, 32% African Americans, and 32% with diabetes mellitus. At enrollment, 36 (72%) were taking an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and 42% were taking a diuretic. From samples obtained at the first timepoint, median eGFR using the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation was 33 (range, 11–97) mL/min/1.73 m2 and median random spot UACR was 143 (range, < lower limit of detection for the assay – 4,408) mg/g.
Table 1.
Demographic and Clinical Characteristics
| CKD Cohort (N = 50) | |
|---|---|
| Age, y | 57.2 ± 16.1 |
| Female sex | 22 (44%) |
| Black | 16 (32%) |
| SBP, mm Hg | 132.6 ± 18.9 |
| Diabetes | 17 (34%) |
| ACEi/ARB use | 36 (72%) |
| Diuretic use | 21 (42%) |
| CKD stage (GFR category)a | |
| G1 (≥90 mL/min/1.73 m2) | 3 (6%) |
| G2 (60–89 mL/min/1.73 m2) | 4 (8%) |
| G3a (45–59 mL/min/1.73 m2) | 9 (18%) |
| G3b (30–44 mL/min/1.73 m2) | 16 (32%) |
| G4 (15–29 mL/min/1.73 m2) | 10 (20%) |
| G5 (<15 mL/min/1.73 m2) | 8 (16%) |
| CKD stage (albuminuria category) | |
| A1 (<30 mg/g) | 1 7 (34%) |
| A2 (30–300 mg/g) | 11 (22%) |
| A3 (>300 mg/g) | 22 (44%) |
| Presumed cause of CKD | |
| Diabetic nephropathy | 16 (32%) |
| Vascular/HTN | 14 (28%) |
| Lupus/glomerulonephritis | 6 (12%) |
| Nephrectomy | 5 (10%) |
| CAKUT/PKD | 3 (6%) |
| CNI toxicity | 2 (4%) |
| Idiopathic NS from MN, FSGS | 2 (4%) |
| Lithium toxicity | 1 (2%) |
| Unknown/otherb | 7 (14%) |
Note: Values for continuous variables given as mean ± standard deviation; values for categorical variables given as count (percentage).
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAKUT, congenital anomalies of the kidney and urinary tract; CKD, chronic kidney disease; CNI, calcineurin inhibitor; FSGS, focal segmental glomerulosclerosis; GFR, glomerular filtration rate; HTN, hypertension; MN, membranous nephropathy; NS, nephrotic syndrome; PKD, polycystic kidney disease; SBP, systolic blood pressure.
Staging based on serum creatinine level, CKD Epidemiology Collaboration equation from the first sample collection.
Includes chronic interstitial nephritis, Alport disease, and chronic lithium toxicity.
Within-Person Variability of eGFR Markers
Table 2 shows within-person variability results for the 4 filtration markers and eGFRs. Values for filtration markers at each time point from all participants (without exclusion of potential outliers) are listed in Table S1. Figure 1A to D shows scatterplots of CVw values for each filtration marker against mean values for each marker. The 2 highest CVw values for each of the 4 eGFRs were from the same 2 individuals. There were no statistically significant differences in pairwise comparisons of median CVw values. Rank ordering of CVw values for eGFRs using the 4 markers did not correspond to the rank ordering of CVw values across the filtration markers themselves due to the different exponents used in the estimating equations. For example, filtration markers with the lowest and highest CVw values were cystatin C (median CVw = 4.1%) and BTP (median CVw = 7.4%), whereas eGFRs with the lowest and highest CVw values were eGFRB2M (median CVw = 4.7%) and eGFRScr (median CVw = 6.5%).
Table 2.
Estimates of Variability for Markers of eGFR
| Filtration Marker Concentration | eGFR Based on Filtration Marker, mL/min/1.73 m2a | |||||||
|---|---|---|---|---|---|---|---|---|
| Scr, mg/dL | CysC, mg/L | BTP, mg/L | B2M, mg/L | eGFRcr | eGFRcys | eGFRBTP | eGFRB2M | |
| Median valueb |
1.80 [0.62–9.41] |
1.88 [0.82–4.39] |
1.26 [0.28–4.91] |
4.54 [1.62–15.54] |
40 [6–135] |
33 [11–97] |
40 [17–106] |
37 [13–88] |
| Median CVw, %c | 5.4(3.2–7.5) | 4.1 (3.0–5.2) | 7.4 (5.2–9.6) | 5.6 (3.7–7.4) | 6.5 (3.9–9.1) | 5.5 (4.0–6.9) | 5.1 (3.6–6.7) | 4.7 (3.2–6.3) |
| Median RCVpos | + 16% | + 12% | +23% | + 17% | +20% | + 16% | + 15% | + 14% |
| Median RCVneg | –14% | –11% | –18% | –14% | –16% | –14% | –13% | –12% |
Abbreviations and definitions: B2M, β2-microglobulin; BTP, beta trace protein; CysC, cystatin C; CVw, within-person coefficient of variation; eGFR, estimated glomerular filtration rate; RCVpos and RCVneg, reference change values positive and negative, which refer to the fold increase or decrease that must be exceeded between 2 sequential results for a change to be considered different at a significance level of 0.05; Scr, serum creatinine.
Equations used for estimation of GFR:
eGFRcr =141 × min(Scr/K, 1)a × max(Scr/K, 1)–1.209 × 0.9 93Age x 1.018 [if female] x 1.159 [if black]; K = 0.7 (females) or 0.9 (males); α = –0.329 (females) or –0.411 (males); min = indicates the minimum of Scr/K or 1; max = indicates the maximum of Scr/K or 1.
eGFRcys = 133 × min(CysC/0.8,1)−0.499 x max (CysC/0.8, 1)–1.328 × 0.9 9 6Age x 0.932 [if female]; min = indicates the minimum of CysC/0.8 or 1; max = indicates the maximum of CysC/0.8 or 1.
eGFRBTP: GFR = 55 × BTP–0694 × 0.9 9 8age x 0.897 if female
eGFRB2M: GFR = 96 × BTP–0278 × B2M–0586
Based on first collected sample; values in brackets indicate range.
Values in parentheses are 95% confidence intervals.
Figure 1.
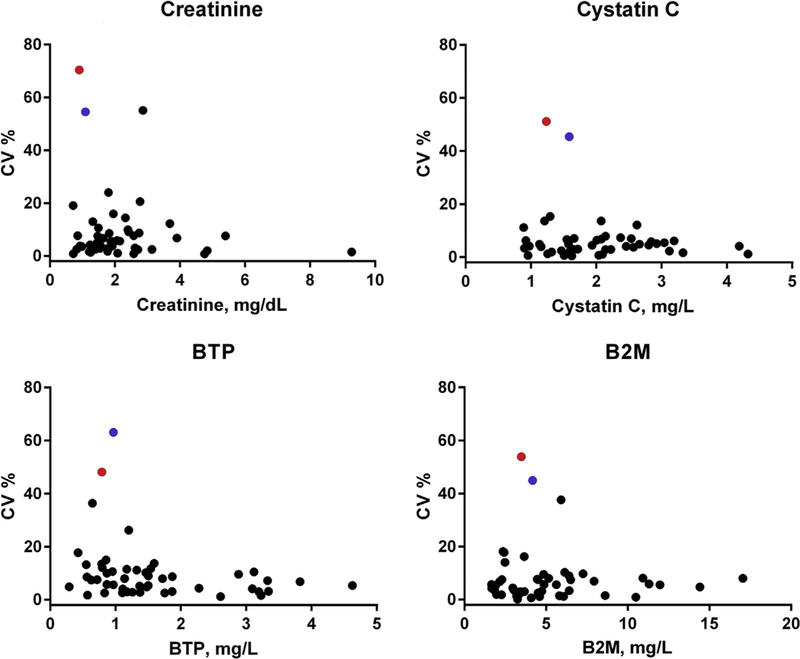
Scatterplots of within-person coefficients of variation (CVs) for filtration markers (creatinine, cystatin C, beta trace protein [BTP], and β2-microglobulin [B2M]) against mean values for each marker. Two individuals (highlighted in red and blue circles) had high levels of variability across all four makers.
Within-Person Variability of Albuminuria
Median CVw values for first-morning UAC and UACR were 33.2% (95% CI, 14.4%−52.0%) and 32.5% (95% CI, 23.2%−41.9%), respectively. Median CVw values for random UAC and UACR were 50.6% (95% CI, 39.0%−62.3%) and 29.7% (95% CI, 19.5%−40.0%; Table 3). UACR and UAC values at each time point for all participants are listed in Table S2. Median CVw values were not significantly different in first-morning versus random-void specimens for UACR or UAC. Figure 2A to D shows scatterplots of CVw values for random and first-void albuminuria (UAC and UACR) against mean UAC and UACR. Table 4 shows results for within-person variability of UACR according to baseline UACR level. Variability in random UACRs was sufficient to qualify as a change in albuminuria categorization in only 6 of 50 participants, all between moderately increased (30–300 mg/g) and severely increased (> 300 mg/g) categories.
Table 3.
Estimates of Within-Person Variability for Albuminuria
| Albumin, mg/L |
UACR, mg/g |
|||
|---|---|---|---|---|
| First-Morning | Random | First-Morning | Random | |
| Median concentrationa | 68 [0.5–3813] | 108 [0.5–4,407] | 125 [1.3–3,557] | 1 73 [0.8–4,006] |
| Median CVw, %b | 33.2 (14.4–52.0) | 50.6 (39.0–62.3) | 32.5 (23.2–41.9) | 29.7 (19.5–40.0) |
| Median RCVpos | + 145% | +276% | + 141% | + 124% |
| Median RCVneg | –59% | –73% | –58% | –55% |
| Difference in median CVw vs random sampleb | –11.6 (–34.9 to 11.8) | — | +4.7 (–7.7 to 17.0) | — |
Abbreviations and definitions: CVw, within-person coefficient of variation; RCVpos and RCVneg, reference change values positive and negative, which refer to the percentage increase or decrease that must be exceeded between two sequential results for a change to be considered different at a significance level of 0.05; UACR, urinary albumin-creatinine ratio.
Based on first collected sample; values in brackets indicate range.
Values in parentheses are 95% confidence intervals.
Figure 2.
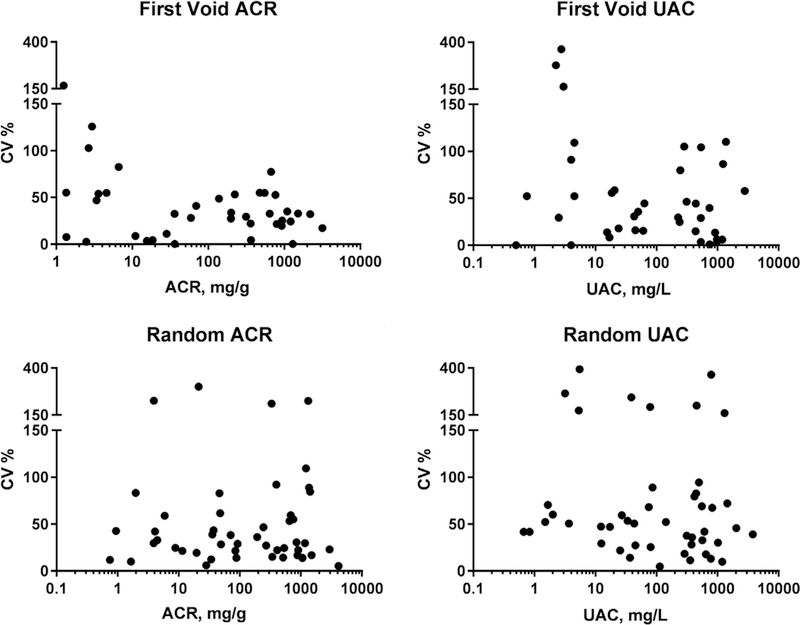
Scatterplots of within-person coefficients of variation (CVs) for random and first-void albuminuria (urine albumin concentration [UAC] and urinary albumin-creatinine ratio [UACR]) against mean values of UAC and UACR.
Table 4.
Estimates of Within-Person Variability for Albuminuria, According to Baselinea Albuminuria Level
| UACR < 30 mg/g (n = 13) |
UACR 30–300 mg/g (n = 13) |
UACR 301–3,000 mg/g (n = 20) |
UACR > 3,000 mg/g (n = 3) |
|||||
|---|---|---|---|---|---|---|---|---|
| First-Morning | Random | First-Morning | Random | First-Morning | Random | First-Morning | Random | |
| Median CVw, %b | 54.5 (11.0 to 97.9) |
29.7 (13.8 to 45.6) |
28.1 (5.2 to 50.9) |
38.2 (25.4 to 51.1) |
32.6 (21.0 to 44.3) |
28.5 (5.2 to 51.8) |
27.2 (16.6 to 37.9) |
23.0 (–157.9 to 203.8) |
| Median RCVpos |
+311 % | + 124% | + 114% | +178% | + 142% | + 117% | + 110% | +87% |
| Median RCVneg |
–76% | –55% | –53% | –64% | –59% | –54% | –52% | –47% |
Abbreviations and definitions: CVw, within-person coefficient of variation; RCVpos and RCVneg, reference change values positive and negative, which refer to the percentage increase or decrease that must be exceeded between 2 sequential results for a change to be considered different at a significance level of 0.05; UACR, urinary albumin-creatinine ratio.
Based on first collected sample.
Values in parentheses are 95% confidence intervals.
Clinical Correlates of Within-Person Variability
Table 5 shows median CVw values according to clinical characteristics. There were no statistically significant differences in median CVw values for each of the markers in individuals with versus without a given characteristic (eg, diabetes vs no diabetes, blacks vs nonblacks, or eGFR or UACR at median or less vs more than median).
Table 5.
Median Within-Person Coefficients of Variation According to Clinical Characteristics
| Characteristic | Scr | CysC | BTP | B2M | UACR, Random | UACR, First am | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No diabetes | 4.0 | 2.0–6.1) | 4.5 | (3.0–6.0) | 5.5 | (3.2–78) | 5.7 | (3.6–78) | 31.7 | (18.3–45.1) | 32.5 | (22.0–43.0) |
| Diabetes | 7.6 | 4.9–10.3) | 3.7 | (1.6–5.8) | 10.0 | (7.3–12.7) | 3.4 | (0.6–6.2) | 24.3 | (10.5–38.2) | 33.4 | 11.5–55.4) |
| No ACEi/ARB | 4.0 | 1.1–7.0) | 4.5 | (2.2–6.8) | 8.3 | (5.3–11.4) | 5.3 | (1.5–9.1) | 35.6 | (13.9–57.2) | 30.0 | 8.6–51.3) |
| ACEi/ARB | 5.5 | 3.0–8.0) | 4.1 | (2.7–5.5) | 6.8 | (4.3–9.3) | 5.6 | (3.5–76) | 29.7 | (1 7.3–42.2) | 32.5 | 19.2–45.8) |
| No diuretic use | 5.4 | 2.6–8.2) | 3.3 | (2.0–4.7) | 8.7 | (5.3–12.1) | 5.4 | (3.5–72) | 36.2 | (23.8–48.5) | 32.6 | 21.9–43.4) |
| Diuretic use | 5.2 | 2.1–8.3) | 5.6 | (4.1–7.2) | 6.2 | (3.7–8.7) | 6.6 | (2.8–10.3) | 25.9 | (10.4–41.5) | 32.0 | 4.5–59.4) |
| Male sex | 4.4 | 2.3–6.5) | 2.9 | (1.7–4.0) | 8.0 | (5.2–10.7) | 3.4 | (1.1–5.7) | 28.9 | (20.1–37.7) | 32.0 | 15.2–48.7) |
| Female sex | 6.3 | 1.7–11.0) | 5.0 | (3.4–6.5) | 5.3 | (2.4–8.1) | 6.2 | (4.4–8.1) | 41.4 | (19.0–63.7) | 32.6 | 18.9–46.3) |
| Nonblack | 4.0 | 2.3–5.8) | 4.0 | (2.6–5.4) | 8.3 | (6.0–10.5) | 5.1 | (2.6–7.6) | 31.7 | (19.7–43.7) | 32.6 | 15.4–49.9) |
| Black | 7.6 | 3.1–12.1) | 4.9 | (2.8–6.9) | 4.9 | (2.1–77) | 5.7 | (3.0–8.4) | 28.3 | (11.1–45.5) | 32.2 | 20.0–44.4) |
| Age ≤ median | 3.8 | 1.2–6.4) | 5.1 | (3.6–6.6) | 5.3 | (3.0–77) | 6.8 | (4.5–9.0) | 27.2 | (13.2–41.2) | 28.1 | 1 7.3–38.8) |
| Age > median | 6.8 | 4.1–9.4) | 2.9 | (1.4–4.4) | 8.8 | (5.9–11.7) | 3.2 | (1.1–5.4) | 37.2 | (25.3–49.0) | 48.7 | 31.6–65.8) |
| SBP ≤ median | 4.9 | 1.5–8.3) | 3.8 | (2.3–5.4) | 7.7 | (3.9–11.4) | 5.8 | (3.2–8.4) | 31.3 | (19.0–43.6) | 33.0 | 11.8–54.2) |
| SBP > median | 5.5 | 3.2–78) | 4.9 | (3.1–6.6) | 7.2 | (4.7–9.7) | 5.4 | (3.0–78) | 29.7 | (11.8–47.7) | 32.3 | 22.3–42.3) |
| eGFR ≤ median | 5.7 | 2.3–9.0) | 4.3 | (2.9–5.7) | 7.0 | (4.0–10.0) | 5.6 | (3.1–8.1) | 26.4 | (12.9–39.9) | 27.2 | 13.6–40.9) |
| eGFR > median | 4.3 | 1.7–6.9) | 4.6 | (2.6–6.7) | 7.5 | (3.8–11.1) | 5.5 | (2.4–8.7) | 37.2 | (24.5–49.8) | 46.7 | 28.8–64.7) |
| Random UACR ≤ median | 6.1 | 3.2–9.0) | 3.8 | (2.1–5.5) | 7.7 | (5.1–10.4) | 4.6 | (1.5–76) | 31.3 | (19.7–42.9) | 43.8 | 13.7–73.9) |
| Random UACR > median | 4.3 | 1.3–73) | 4.9 | (3.4–6.3) | 5.1 | (1.6–8.7) | 5.8 | (3.8–78) | 28.5 | (11.4–45.5) | 30.6 | 24.7–36.6) |
| First AM UACR ≤ median | 4.9 | 2.6–71) | 3.8 | (2.5–5.1) | 7.7 | (5.2–10.2) | 3.5 | (0.7–6.3) | 31.3 | (20.1–42.5) | 36.7 | 9.8–63.6) |
| First AM UACR > median | 5.6 | 2.3–8.9) | 4.9 | (3.3–6.4) | 6.8 | (3.2–10.3) | 5.6 | (3.7–74) | 29.7 | (9.1–50.3) | 32.3 | 23.9–40.6) |
Note: Results are expressed as percentages (95% confidence interval). Median values were age 60 years, SBP of 131 mm Hg, eGFR of 40 mL/min/1.73 m2, random UACR of 142 mg/g, and first am UACR of 125 mg/g.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; B2M, β2-microglobulin; BTP, beta trace protein; CysC, cystatin C; eGFR, estimated glomerular filtration rate; Scr, serum creatinine; SBP, systolic blood pressure; UACR, urinary albumin-creatinine ratio.
Discussion
The major findings of this study of the biological variability of diagnostic and prognostic laboratory tests in CKD were that: (1) filtration markers used to estimate GFR have relatively low short-term (< 4 weeks) within-person variability, with CVw values < 10%; and (2) albuminuria has relatively high short-term within-person variability, with CVw values exceeding ~30%, whether measured as first-morning voids, random spot urine samples, or normalized to creatinine level. Our estimates of CVw for filtration markers are comparable to those reported by other investigators in healthy individuals and CKD populations.13–15 Our estimates of CVw for albuminuria are also consistent with those reported previously in diabetic nephropathy and CKD16 and somewhat higher than that reported by Selvin et al14 in healthy individuals.
CVw can be directly translated into the more clinically relevant parameters RCVpos and RCVneg, which are thresholds for an increase or decrease between successive measurements in the same individual to be considered statistically significant at a given probability level (in our case, P < 0.05).12 Based on our results for plasma creatinine (median CVw = 5.4%), an increase from baseline of 16% or a decrease of 23% can be expected by chance. For random UACR (median CVw = 29.7%), an increase from baseline of 124% or a decrease of 55% can be expected by chance. It should be kept in mind that CVw values may be different from one individual to the next and that our results are summary measures based on median values from the individuals enrolled in this study. Measurements from some individuals in our study had relatively little variability, whereas others had substantially higher variability, as shown in the supplementary tables.
Threshold changes from RCV have clinical implications. AKI when defined as a 50% increase over 7 days17 in Scr level exceeds the RCVpos substantially, even in patients with CKD, and therefore would not be expected to be a false-positive diagnosis from biological variability alone. However, another definition of AKI incorporates smaller absolute changes, such as 0.3 mg/dL over 48 hours.18 An individual with a baseline Scr level of 2.0 mg/dL could be expected to have values up to 2.32 mg/dL on the basis of random variability alone. Such an individual could be misclassified as having AKI despite having normal fluctuations in Scr values from expected short-term within-person biological variability. Clinical context needs to be taken into consideration when considering changes in Scr levels. The issue of false-positive designations of AKI on the basis of small changes in Scr levels in the setting of CKD has been investigated by us19 and others previously20 and can also be inferred on the basis of the inverse relationship between filtration markers and actual GFR21: compared with healthy individuals with higher GFRs, individuals with CKD and lower GFRs will have larger absolute increases in filtration marker values for a given absolute change in GFR.
GFR itself exhibits diurnal and day-to-day variability,22,23 although teasing apart analytical from biological variability for a physiologic measure such as GFR is difficult. Two individuals in this study had the highest CVw values for all 4 filtration markers, suggesting that the underlying GFR was the parameter that was changing, rather than reflecting analytical variation in the measurement of the filtration marker. Although we excluded participants on the basis of known factors that change GFR (such as adjustment of antihypertensive or diuretic medication doses), we cannot account for other clinical variables such as medication adherence or other nonrenal determinants of filtration markers that could have led to the observed variability.
Our findings on albuminuria are also clinically relevant. An individual with a random spot UACR of 200 mg/g, for example, might be expected to have repeat measurements as high as 448 mg/g or as low as 90mg/g, even in the absence of a change in clinical status. We found that the highest variability in albuminuria was at the lower end of the distribution curve, which is consistent with findings by Naresh et al,24 who estimated RCVs of 467% for UACRs < 27 mg/g, 170% for UACRs of 27 to 265 mg/g, and 83% for UACRs > 265 mg/g. Differences in the methodology to estimate CVw values make it difficult to compare directly our numeric results with those of Naresh et al. The CVw values for random UACR of the 3 individuals with the highest degrees of albuminuria were 5.3% (mean UACR over 3 measurements, 4,151 mg/g), 23.0% (mean UACR, 2,962 mg/g), and 225% (mean UACR, 1,319 mg/g). The applicability of our findings for heavily proteinuric patients is limited given the small numbers of such individuals we studied.
Thresholds for expected variability should be taken into account when monitoring patients serially for changes in disease status such as nephrotic syndrome. Intensifying immunosuppressive therapy or angiotensin blockade for proteinuric kidney disease, for example, is sometimes done clinically on the basis of changes in albuminuria. Our findings suggest that a doubling of albuminuria might be within the limits of normal biological variability, which should be taken into account when making therapeutic decisions as important therapeutic effects are also within this range. The other practical implications of our findings on albuminuria relate to defining end points for clinical trials in proteinuric kidney disease. The definition for partial remission in lupus nephritis, membranous nephropathy, and other kidney diseases includes proteinuria or albuminuria reduction ≥ 50%.7,25,26 Some individuals with a 50% decline in albuminuria may not have a true improvement in their clinical status and would be mis-classified as having a partial remission simply due to expected biological variability. Similar considerations apply for the cutoffs used to classify diabetic nephropathy. Progression (or regression) from one albuminuria stage to the other can occur on the basis of simple biological variability, without progression or remission of the underlying disease process. Again, as with any biological marker, clinical context needs to be taken into consideration when evaluating the significance of longitudinal changes. Repeat measurements can also increase confidence in the significance of changes in eGFR or albuminuria and have been recommended for albuminuria assessments in clinical trials in diabetic nephropathy.27 In clinical trials, it is also important to recognize that average effects in a group and their consequences can be estimated much more reliably than effects in individual patients.
A number of factors can influence UAC and UACR and contribute to short-term within-person variability. Urinary albumin and creatinine excretion both exhibit diurnal variability.28 However, we did not find a significant difference in the CVw values for random versus first-morning void UACR, unlike the report by Witte et al,29 who reported CVw values of 19.1% for first-morning and 35.8% for random UACR. Variability in albuminuria can also come from variability in blood pressure (and hence filtration pressure across the glomerulus) and short-term variability in other physiologic factors, such as glomerular permeability and tubular functions of secretion, reabsorption, and catabolism.23,30–34 Variability in water intake also influences the absolute UAC. By dividing UAC by urinary creatinine concentration for UACR, the effects of dilution are minimized, and hence UACR would be expected to have less within-person variability than UAC (neglecting the variability of creatinine excretion and its covariance with albumin excretion). We found that random UACR had significantly lower CVs than random UAC. Lower CVs with normalization to urinary creatinine was also reported by Carter et al13 for a number of urinary biomarkers. Lower within-person variability from urinary normalization by creatinine is another reason to use normalized rather than absolute concentrations of biomarkers such as albumin in clinical practice and epidemiology studies.
The strengths of the present study include the simultaneous assessment of short-term within-person variability of 4 plasma filtration markers and albuminuria in both first-morning and random-void samples in a cohort of individuals with CKD.
There are several limitations, including a relatively small sample size with numerous causes of CKD that may have limited the ability to detect differences in variability across markers or across clinical conditions. We did not collect and measure albuminuria in 24-hour samples, which could have lower within-person variability than spot samples.35 Our estimates of variability may be conservative because samples were batch measured in a single laboratory over a 2-day period, which could minimize technical variability compared with clinical measurements that are performed on different days and potentially in different laboratories. We did not measure urine total protein and also included few individuals with severely increased albuminuria, which limits the generalizability of our results to individuals with heavy or nephrotic-range proteinuria or albuminuria. We enrolled participants as a convenience sample from individuals attending a CKD clinic and not in a research setting in which clinical variables such as food and water intake, timing of sample collection, and adherence to medications could be better controlled. We also report median CV values for the entire cohort, but recognize that there may be disease-specific differences as has been reported in lupus nephritis compared with other forms of CKD.36 Furthermore, individuals may have their own individual degree of within-person variability, as has been suggested by others for albuminuria and other biomarkers.16
In conclusion, assessment of the clinical significance of changes in kidney disease markers such as eGFR and albuminuria needs to take into account the expected within-person variability of these measurements in CKD. For filtration markers for GFR estimation, within-person variability appears to be low (CVw < 10%), but sufficient to cause potential false-positive designations of AKI in individuals with more advanced CKD. For albuminuria, within-person variability may be substantial, and increases or decreases of 50% in an individual patient should not be taken as sole evidence of disease progression or remission without repeat testing and consideration of the clinical context.
Supplementary Material
Support:
This work was supported by the CKD Biomarker Consortium (funded by National Institute of Diabetes and Digestive and Kidney Diseases U01DK85649, U01DK085673, U01DK085660, U01DK085688, U01DK085651, and U01DK085689) by DK107782 (to CMR) and by R37 DK039773 (to JVB). The funders of this study had no role in study design; data collection, analysis, or reporting; or the decision to submit for publication.
Chronic Kidney Disease Biomarkers Consortium Investigators:
Boston University School of Medicine: Vasan S. Ramachandran, MD (Chair, Steering Committee); Brigham and Women’s Hospital, Harvard University (Regulatory and Assay Center): Joseph Bonventre, MD, PhD (co-Principal Investigator [PI]), Sushrut Waikar, MD, MPH (co-PI), Venkata Sabbisetti, PhD; Cedars Sinai Medical Center: Jennifer Van Eyk, PhD, Dawn Chen, PhD, Qin Fu, PhD; Cincinnati Children’s Hospital Medical Center: Hermine Brunner, MD; Columbia University College of Physicians and Surgeons: Vivette D’Agati, MD, Jonathan Barasch, MD; Johns Hopkins University: Josef Coresh, MD, PhD (PI), Casey Rebholz, PhD; Division of Research, Kaiser Permanente Northern California: Alan S. Go, MD; Icahn School of Medicine at Mount Sinai: Erwin Bottinger, MD (PI), Avelino Teixeira, PhD, Ilse Daehn, PhD; Northwestern University: Mark Molitch, MD (PI), Daniel Batlle, MD; Ohio State University: Brad Rovin, MD (PI), Haifeng Wu, MD; Tufts Medical Center: Andrew S. Levey, MD, Lesley A. Inker, MD, MS, Meredith Foster, PhD; University of California, San Francisco: Chi-yuan Hsu, MD, MSc (PI), Kathleen Liu, MD, PhD (PI); University of Louisville: Jon Klein, MD, PhD; University of Minnesota: Michael Mauer, MD (PI), Paola Fioretto, MD, PhD, Gary Nelsestuen, PhD, John H. Eckfeldt, MD, PhD, Amy Karger, MD, PhD; University of Padova: Paola Fioretto, MD, PhD; University of Pennsylvania (Coordinating Center): Harold I. Feldman, MD, MSCE (PI), Shawn Ballard, MS, Krista Whitehead, MS, Dawei Xie, PhD, Phyllis Gimotty, PhD, Haochang Shou, PhD, Xiaoming Zhang, Kellie Ryan, Theodore E. Mifflin, PhD, DABCC (Laboratory); University of Utah: Tom Greene, PhD; NIDDK Phoenix: Robert G. Nelson, MD, PhD; NIDDK: Paul L. Kimmel, MD, John W. Kusek, PhD.
Footnotes
Financial Disclosure:
JHE has been a consultant for Gentian and Siemens, which have provided free or steeply discounted reagents to his research laboratory for measurement of B2M and BTP. The other authors declare that they have no relevant financial interests.
Peer Review:
Received December 22, 2017. Evaluated by 2 external peer reviewers and a statistician, with editorial input from an Acting Editor-in-Chief (Editorial Board Member Bradley A. Warady, MD). Accepted in revised form April 30, 2018. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Correction Notice:
This article was amended on August 8, 2018 to remedy sporadic errors in units.
Contributor Information
Sushrut S. Waikar, Brigham and Women’s Hospital, Boston, MA
Casey M. Rebholz, Johns Hopkins University, Baltimore
Zihe Zheng, Johns Hopkins University, Baltimore, MD.
Shelley Hurwitz, Brigham and Women’s Hospital, Boston, MA.
Chi-yuan Hsu, University of California, San Francisco, San Francisco, CA.
Harold I. Feldman, University of Pennsylvania, Philadelphia, PA
Dawei Xie, University of Pennsylvania, Philadelphia, PA.
Kathleen D. Liu, University of California, San Francisco, San Francisco, CA
Theodore E. Mifflin, University of Pennsylvania, Philadelphia, PA
John H. Eckfeldt, University of Minnesota
Paul L. Kimmel, NIDDK
Ramachandran S. Vasan, Boston University School of Medicine, Boston, MA
Joseph V. Bonventre, Brigham and Women’s Hospital, Boston, MA (SSW, JVB, SH)
Lesley A. Inker, Tufts Medical Center, Boston, MA
Josef Coresh, Johns Hopkins University, Baltimore, MD.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–204. [DOI] [PubMed] [Google Scholar]
- 2.Murphy D, McCulloch CE, Lin F, et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016;165(7):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis. 2014;63(5):820–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 5.Inker LA, Levey AS, Pandya K, Stoycheff N, Okparavero A, Greene T. Early change in proteinuria as a surrogate end point for kidney disease progression: an individual patient metaanalysis. Am J Kidney Dis. 2014;64(1):74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebholz CM, Grams ME, Matsushita K, Selvin E, Coresh J. Change in novel filtration markers and risk of ESRD. Am J Kidney Dis. 2015;66(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson A, Cattran DC, Blank M, Nachman PH. Complete and partial remission as surrogate end points in membranous nephropathy. J Am Soc Nephrol. 2015;26(12):2930–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45(2): 281–28. [DOI] [PubMed] [Google Scholar]
- 9.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inker LA, Tighiouart H, Coresh J, et al. GFR estimation using beta-trace protein and beta2-microglobulin in CKD. Am J Kidney Dis. 2016;67(1):40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris EK, Yasaka T. On the calculation of a “reference change” for comparing two consecutive measurements. Clin Chem. 1983;29(1):25–30. [PubMed] [Google Scholar]
- 13.Carter JL, Parker CT, Stevens PE, et al. Biological variation of plasma and urinary markers of acute kidney injury in patients with chronic kidney disease. Clin Chem. 2016;62(6):876–883. [DOI] [PubMed] [Google Scholar]
- 14.Selvin E, Juraschek SP, Eckfeldt J, Levey AS, Inker LA, Coresh J. Within-person variability in kidney measures. Am J Kidney Dis. 2013;61(5):716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckfeldt JH, Chambless LE, Shen YL. Short-term, within-person variability in clinical chemistry test results. Experience from the Atherosclerosis Risk in Communities Study. Arch Pathol Lab Med. 1994;118(5):496–500. [PubMed] [Google Scholar]
- 16.Petrykiv SI, de Zeeuw D, Persson F, et al. Variability in response to albuminuria-lowering drugs: true or random? Br J Clin Pharmacol. 2016;83(6):1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellum JA, Bellomo R, Ronco C. Definition and classification of acute kidney injury. Nephron Clin Pract. 2008;109(4): c182–c18. [DOI] [PubMed] [Google Scholar]
- 18.Ronco C, Levin A, Warnock DG, et al. Improving outcomes from acute kidney injury (AKI): report on an initiative. Int J Artif Organs. 2007;30(5):373–376. [PubMed] [Google Scholar]
- 19.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20(3):672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J, Fernandez H, Shashaty MG, et al. False-positive rate of AKI using consensus creatinine-based criteria. J Am Soc Nephrol. 2015;10(10):1723–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Perrone RD, Madias NE. Serum creatinine and renal function. Ann Rev Med. 1988;39:465–490. [DOI] [PubMed] [Google Scholar]
- 22.Kwong YT, Stevens LA, Selvin E, et al. Imprecision of urinary iothalamate clearance as a gold-standard measure of GFR decreases the diagnostic accuracy of kidney function estimating equations. Am J Kidney Dis. 2010;56(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koopman MG, Koomen GC, Krediet RT, de Moor EA, Hoek FJ, Arisz L. Circadian rhythm of glomerular filtration rate in normal individuals. Clin Sci (Lond). 1989;77(1):105–111. [DOI] [PubMed] [Google Scholar]
- 24.Naresh CN, Hayen A, Weening A, Craig JC, Chadban SJ. Day-to-day variability in spot urine albumin-creatinine ratio. Am J Kidney Dis. 2013;62(6):1095–1101. [DOI] [PubMed] [Google Scholar]
- 25.Troyanov S, Wall CA, Miller JA, Scholey JW, Cattran DC. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16(4): 1061–1068. [DOI] [PubMed] [Google Scholar]
- 26.Chen YE, Korbet SM, Katz RS, Schwartz MM, Lewis EJ. Value of a complete or partial remission in severe lupus nephritis. Clin J Am Soc Nephrol. 2008;3(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kropelin TF, de Zeeuw D, Andress DL, et al. Number and frequency of albuminuria measurements in clinical trials in diabetic nephropathy. Clin J Am Soc Nephrol. 2015;10(3):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenblatt DJ, Ransil BJ, Harmatz JS, Smith TW, Duhme DW, Koch-Weser J. Variability of 24-hour urinary creatinine excretion by normal subjects. J Clin Pharmacol. 1976;16(7):321–328. [DOI] [PubMed] [Google Scholar]
- 29.Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R. First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol. 2009;20(2):436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voogel AJ, Koopman MG, Hart AA, van Montfrans GA, Arisz L. Circadian rhythms in systemic hemodynamics and renal function in healthy subjects and patients with nephrotic syndrome. Kidney Int. 2001;59(5):1873–1880. [DOI] [PubMed] [Google Scholar]
- 31.van Acker BA, Koomen GC, Koopman MG, Krediet RT, Arisz L. Discrepancy between circadian rhythms of inulin and creatinine clearance. J Lab Clin Med. 1992;120(3):400–410. [PubMed] [Google Scholar]
- 32.Koopman MG, Koomen GC, van Acker BA, Arisz L. Circadian rhythm in glomerular transport of macromolecules through large pores and shunt pathway. Kidney Int. 1996;49(5):1242–1249. [DOI] [PubMed] [Google Scholar]
- 33.Koopman MG, Koomen GC, van Acker BA, Arisz L. Urinary sodium excretion in patients with nephrotic syndrome, and its circadian variation. Q J Med. 1994;87(2):109–11. [PubMed] [Google Scholar]
- 34.Koopman MG, Arisz L. Spectrum of diurnal rhythms in glomerular permeability in patients with membranous nephropathy. Nephrol Dial Transplant. 1997;12(suppl 2):47–52. [PubMed] [Google Scholar]
- 35.Miller WG, Bruns DE, Hortin GL, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009;55(1):24–38. [DOI] [PubMed] [Google Scholar]
- 36.Birmingham DJ, Shidham G, Perna A, et al. Spot PC ratio estimates of 24-hour proteinuria are more unreliable in lupus nephritis than in other forms of chronic glomerular disease. Ann Rheum Dis. 2014;73(2):475–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


