Abstract
Mitochondria play a crucial role in neuronal function, especially in energy production, generation of reactive oxygen species, and calcium signaling. Multiple lines of evidence have suggested possible involvement of mitochondrial deficits in major psychiatric disorders, such as schizophrenia and bipolar disorder. In the first half, this review will outline the current understanding of the physiological role of mitochondria and their dysfunction under pathological conditions, particularly in psychiatric disorders. Nevertheless, the current knowledge about mitochondrial deficits in these disorders is somewhat limited, due to the lack of effective methods to dissect dynamic changes in the functional deficits that are directly associated with psychiatric conditions. Human neuronal cell model systems, which have been dramatically developed in recent years through the use of stem cell technology, may be key tools for overcoming this dilemma and improving our understanding of the dynamic changes in the mitochondrial deficits in patients with psychiatric disorders. We introduce recent discoveries from new experimental models and conclude the discussion by referring to future perspectives. In the perspectives, we emphasize the significance of combining studies of human neuronal cell models with those of other experimental systems, including animal models.
Keywords: Mitochondria, oxidative stress, calcium dysregulation, human neuronal cell models, Schizophrenia, Bipolar disorder
The mitochondrion is an indispensable organelle of eukaryotic cells that plays many important roles required for cell survival and well-being. It is thought to descend from prokaryotic bacteria through endosymbiotic evolution (1). Neurons are especially dependent on mitochondria, partly because of their high energy demands. As a result, mitochondrial dysfunction leads to multiple types of brain disorders (2–4).
Mitochondria-associated cellular dysfunction is dynamic, undergoing constant changes in bioenergetics, oxidative stress, and calcium (Ca2+) homeostasis. Thus, it is important to use living model systems to capture their characteristics, relationships, and mechanisms. In this regard, we also need to pay attention to the relevance of each model system to neuronal and brain conditions.
In this review, we first overview current understanding of the mitochondrial functions, in particular: energy production, reactive oxygen species (ROS) generation, and intracellular Ca2+ signaling. These elements are interconnected through homeostatic regulation. Next, we shed light on altered mitochondrial function and mitochondrial dynamics under pathological conditions, especially in psychiatric diseases. Nevertheless, the current knowledge in mitochondrial deficits in these disorders is somewhat limited by lack of the effective methods to dissect dynamic changes in mitochondrial function directly associated with patients with psychiatric illnesses. Although studies with the postmortem brains have been essential and informative, living model systems that can recapitulate neuron-relevant signatures have been long awaited.
Recent progress in stem cell biology and cell engineering has answered this question. In contrast to the postmortem brains, which are static and cannot be manipulated, human neuronal cell model systems allow us to study dynamic changes involved in mitochondrial dysfunction. Furthermore, these model systems can be manipulated to address the relationship between pathogenesis and pathophysiology, possibly shedding light on critical mechanisms of the disease pathology. We will introduce various neuronal cell model systems by illustrating unique merits of each model and discuss how they are essential for studying dynamic alteration of mitochondrial functions associated with psychiatric diseases. We will then summarize recent discoveries on the association between mitochondrial dysfunction and major psychiatric illnesses through human neuronal cell models. Lastly, we will provide our perspectives on the value of neuronal cell model systems compared with other model systems.
Physiological roles of mitochondria
At baseline condition, cortical neurons are estimated to consume ~4.7 billion adenosine triphosphate (ATP) molecules/second (5). The site of the highest energy demand is around synapses where ATP is needed for synaptic transmission. The majority of ATP is produced by oxidative phosphorylation in mitochondria, making neurons some of the most metabolically active cells in the body; note that the central nervous system consumes 20% of oxygen at rest while accounting for only 2% of body weight (6). The process of ATP production begins in the cytosol where glucose is converted to pyruvate before transport into mitochondria for oxidative phosphorylation. Mitochondria have both an outer mitochondrial membrane and an inner mitochondrial membrane, creating two separate intracellular compartments: the mitochondrial matrix and the intermembrane space. The outer mitochondrial membrane is permeable to solutes less than 5 kDa whereas the inner mitochondrial membrane is largely impermeable. Enzymes of the tricarboxylic acid (TCA) cycle perform a series of oxidation-reduction reactions within the mitochondrial matrix to provide substrates for the electron transport chain in which energy from electron transport is used to pump protons from the matrix into the intermembrane space. The flow of protons back across this gradient is used to drive ATP synthesis by complex V (ATP synthetase). The electrons from the electron transport chain are passed over to O2 to form H2O to complete the process. About 1–5% of the electrons passing through the electron transport chain form superoxide (O2−) instead of O2. The O2− is converted by superoxide dismutase to H2O2, which can form hydroxyl radicals (OH−). These products form ROS.
Mitochondria are thus the major intracellular source of ROS in neurons. Physiological mitochondrial ROS act as signaling molecules playing a crucial role in healthy cell function and providing metabolic adaptation to mild stress (7). For instance, in growth factor signaling, induction of ROS is needed for downstream tyrosine phosphorylation through inactivation of protein tyrosine phosphatases (8–10). In mitochondria, ROS regulate activity of redox sensitive enzymes and ion channels including Ca2+ channels (11). Mitochondria also contain several antioxidant molecules which have been shown to exert neuroprotective effects (12): coenzyme Q10 is a component of the electron transport chain and a potent scavenger of free radicals in the inner mitochondrial membrane; creatine can be converted into phosphocreatine in the mitochondria where it serves as an alternative energy source to ATP and helps maintain the high free energy of ATP; and nicotinamide is the precursor for NADH, the primary substrate for the electron transport chain.
Mitochondria also play a role in Ca2+ homeostasis. The organelles contribute to maintain proper intracellular Ca2+ levels by functioning together with the endoplasmic reticulum, a major reservoir of the intracellular Ca2+ (11,13,14). The outer mitochondrial membrane is Ca2+ permeable, allowing Ca2+ to be transferred across the inner mitochondrial membrane and the electrical gradient by Ca2+ uniporters. High Ca2+ concentrations increase activity of the TCA cycle, allowing for increased ATP production necessary for pumping out the increased intracellular Ca2+. This increased ATP production naturally leads to increased levels of ROS. Ca2+ inside the mitochondrial matrix can be released through the Na+/Ca2+ exchanger (mNCX), the H+/Ca2+ exchanger (mHCX), and possibly the mitochondrial permeability transition pore (mPTP) (15). Together these channels allow for the rapid shuttling of Ca2+ between mitochondria, the endoplasmic reticulum, and the cytosol, coordinating intracellular signaling with energy production. Ca2+ influx into subcellular compartments activates Ca2+/CaM-dependent protein kinases and related molecular cascades, which eventually regulate gene transcription underpinning multiple neuronal events (16).
Finally, mitochondria mediate programmed cell death in both physiological contexts during development and pathological conditions (17–19). Both high levels of Ca2+ and ROS activate intrinsic apoptotic pathways in the mitochondria, by triggering leakage of pro-apoptotic factors like cytochrome c, apoptosis inducing factor from the mitochondria into the cytosol (18,20). Role for mitochondria-associated programmed cell death in mental illnesses remain elusive.
Oxidative stress and calcium deficits in the pathology of major mental illnesses: possible involvement of mitochondria
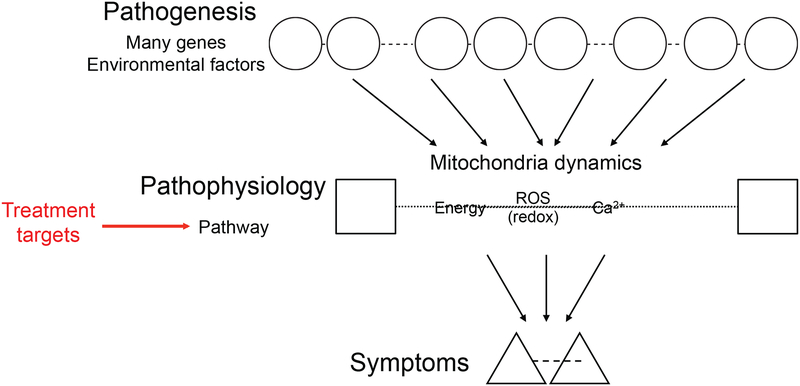
Most psychiatric disorders are caused by multiple etiological factors such as genetic risk factors and environmental stressors (21–25). These multi-factorial etiologies (pathogeneses) likely converge into downstream common pathways in the pathophysiology at the cellular and brain circuitry levels that more directly underlie the symptomatic phenotypes (Figure 1). Within the hierarchical cascades in the pathology, the mitochondrial deficits, such as energy deficits, oxidative stress and redox imbalance, and altered Ca2+ homeostasis, are viewed as common pathways in many but not all cases in the pathophysiology of psychiatric diseases (20,26). Although not discussed in this review article, it is also true that many other non-mitochondrial pathways can also contribute to these disorders (24,27,28).
Figure 1: Multiple etiologies (pathogeneses) converging into common pathways (pathophysiology).
In psychiatric diseases, multi-factorial etiological factors such as genetic risk factors and environmental stressors likely converge into downstream common pathways (e.g., mitochondrial deficits leading to energy deficits, oxidative stress and redox imbalance, and altered Ca2+ homeostasis). These common pathways (pathophysiology) may underlie the symptomatic phenotypes more directly and are potential targets for course-altering intervention.
Major deficits in the mitochondria frequently lead to disturbance of energy maintenance. Given the high energy demand of neurons, these defects underlie many brain disorders, in particular neurological disorders, such as neurodegenerative diseases. Because many review articles have covered this topic (mitochondria-associated energy deficits in brain disorders), we cite several representative articles here (29–31) (see a commentary by Ana C. Andreazza in this special issue), and then the remainder of this article will focus on two other deficits associated with mitochondria (oxidative stress and redox imbalance, as well as altered Ca2+ homeostasis) and their relationship.
Oxidative stress and redox imbalance
Excess oxidative stress has been reported in patients with mental disorders through studies with peripheral and postmortem brain tissues. Specifically, there is evidence of increased lipid and protein oxidation, together with decreased levels of a major antioxidant, glutathione (GSH), in the blood and cerebrospinal fluid (CSF) of patients with schizophrenia (SZ) and mood disorders (32,33). In addition, several studies have identified a redox imbalance in postmortem brains. For example, the levels of GSH were significantly decreased in the prefrontal cortex of psychiatric patients compared to the control group (34), while nitric oxide species were found increased in striatum from schizophrenic patients (35). There are several studies that tried to assess the levels of GSH in the brain of living patients by magnetic resonance spectroscopy, but the data are not fully conclusive at least at present (36–38).
Parvalbumin interneurons and oligodendrocytes/myelin are the main neural substrates targeted by oxidative stress (39). Several lines of evidence in human postmortem tissues and animal models have suggested that alteration of these two substrates are possibly relevant to the pathophysiology of mental conditions (40–42). Fast spiking parvalbumin interneurons are highly susceptible to oxidative stress because they create high metabolic demand and concomitantly high ROS production (39) Myelination is a biological process that is highly susceptible to oxidative stress: first, myelin is dense in ROS-sensitive lipids; and second, oxidative stress can also affect the process of myelination by disrupting oligodendrocyte maturation (43).
Oxidative stress is frequently associated with mitochondrial deficits in the brain. As described above, mitochondria are the major source of ROS generation and also contain several antioxidant mechanisms. Compared to other tissues, the risk of redox imbalance is more robust in the brain because of the high lipid content and high metabolic rate, which can be easily translated into high concentrations of lipid peroxides and free radicals (44). Any tissue damage due to oxidative stress is irreversible since neurons are postmitotic cells with no capacity of regeneration (45). Taking all these concepts into account, we can reasonably assume that mitochondria play a significant role in the oxidative stress-related pathophysiology of mental diseases (46). However, we also acknowledge that there are several distinct hypotheses positing that sources other than mitochondria may contribute to high levels of oxidative stress in the brain (46–49). To address the role of mitochondria-associated oxidative stress in the pathophysiology of mental disease, we would need to introduce better models that allow us to recapitulate in finer detail the dynamic mechanisms that lead to excess oxidative stress in neurons. As described below, human neurons in vitro obtained from living subjects start to offer this opportunity.
Aberrant Ca2+ homeostasis
Pathogenic factors associated with major mental illness could underlie aberrant Ca2+ homeostasis in neurons. These factors include genomic abnormalities in the calcium channel genes, which have been shown by genome wide association studies (GWAS) in major mental illnesses. Specifically, GWAS have shown association of SZ, bipolar disorder (BP), major depressive disorder, autism spectrum disorders, and attention deficit-hyperactivity with CACNA1C (calcium voltage-gated channel subunit alpha1 C) and CACNB2 (calcium voltage-gated channel auxiliary subunit beta 2), encoding voltage-dependent L-type calcium channel subunit alpha-1C and beta-2, respectively (50–53). SZ has also been linked to CACNA1I (calcium voltage-gated channel subunit alpha1 I), encoding voltage-dependent T-type calcium channel subunit alpha-1I, with genome-wide levels of significance (53).
Excess Ca2+ affects both neuronal excitability and signaling cascades regulating gene expression, leading to perturbation of multiple neuronal processes, such as dendrite development, synaptic plasticity, and excitatory/inhibitory balance (16). Altered Ca2+ levels in blood cells (red blood cells, neutrophils, T lymphocytes, lymphoblasts, and platelets) have been reported in patients with both SZ and BP. (54–58), Nevertheless, it is unclear whether and how the disease-associated cellular changes in blood cells also occur in neurons and how such Ca2+-related alterations might impact neuronal functions. As Ca2+ dynamics cannot be measured in postmortem brain tissue, direct evidence showing abnormal intracellular Ca2+ homeostasis is not available within these studies (59).
Ca2+ homeostasis and mitochondrial function are directly linked to each other. Increased intracellular Ca2+ leads to Ca2+ uptake into mitochondria through channels described above. This Ca2+ uptake alters mitochondrial membrane permeability and also the electron transport chain efficiency, leading to oxidative stress (60,61). This process creates a reciprocal interaction between mitochondria function/bioenergetics/ROS generation and Ca2+ homeostasis, (60,61). Because of this relationship, it would be difficult to study causal relationships between aberrant Ca2+ homeostasis and mitochondria dysfunction in a static experimental system like postmortem brains; it requires a dynamic and manipulatable experimental platform.
How to study dynamic alteration in the mitochondrial functions associated with psychiatric illnesses: the significance of the use of human neuronal cell models
As described above, mitochondria-associated cellular dysfunction, such as altered bioenergetics, oxidative stress, and Ca2+ homeostasis, is dynamic. Thus, it is important to use living model systems to capture their characteristics, relationships, and mechanisms. Progress in stem cell biology and cell engineering has answered this need at least to a reasonable extent: recently developed human neuron model systems have the potential to shed light on dynamic changes involved in mitochondrial dysfunction and could also be manipulated to study the relationship between pathogenesis and pathophysiology.
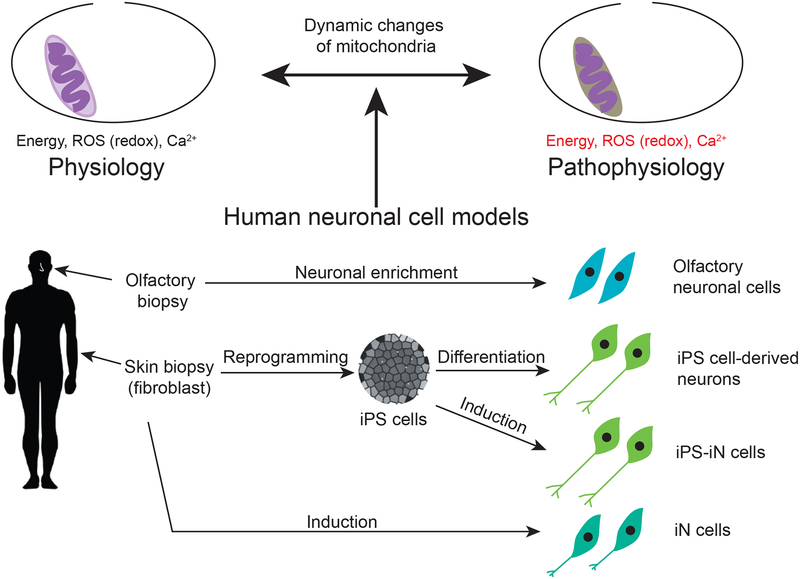
At present there are at least 4 human neuronal cell models, all of which have advantages and potential limitations in a complementary manner. They are (i) induced pluripotent stem (iPS) cell-derived differentiated neurons (62–65); (ii) induced neuronal cells directly induced from peripheral cells (iN cells) (66–69); (iii) iN cells induced from iPS cells (iPS-iN cells) (70); and (iv) olfactory neuronal cells directly biopsied from the nasal cavity (71–73) (Figure 2).
Figure 2: Currently available human neuronal cell models.
Four human neuronal cell models are currently available to study dynamics of mitochondrial function and its abnormality.
iPS cells, iN cells, and iPS-iN cells are genetically engineered cells, in which cell reprogramming and conversion technologies with specific sets of transcription factors are utilized. Generating iPS cells is laborious and expensive: furthermore, it takes many months for iPS cell-derived neurons to differentiate and functionally mature. To overcome these issues, iN cells and iPS-iN cells have been introduced. Skin fibroblasts are directly converted into iN cells, bypassing a stem cell stage to generate neuronal cells. iPS-iN cells are generated from iPS cells by bypassing developmental processes with over-expression of exogenously introduced factors. In contrast to these genetically engineered cells, olfactory neuronal cells are directly obtained via nasal biopsy and avoid non-specific effects induced by exogenous factors. As each model system has some strengths and weaknesses, these cell models can complement each other in answering specific scientific questions (74). We outline how these cell systems can be complementarily utilized in studying mitochondria-associated parameters in Table 1. Bioenergetics, ROS generation, redox reactions, Ca2+ homeostasis, Ca2+ -induced excitability, and gene expression profiles have been studied in iPS cell-derived neural progenitors and/or neurons from patients with SZ or BP (75–78). Given their similar properties, iPS-iN cells can also be used for these assays. However, it would not be easy for almost all institutions to establish iPS cells or iPS-iN cells from a large number of patients due to the laborious and expensive nature of generation and maintenance. This fact limits their utility in research for mental disorders in which the majority of cases are sporadic based on the etiology of multiple genetic factors interacting with the environmental stressors (79). iN cells can be established on a larger scale, but they need a purification step before they can be used for biochemical assays or bulk cell/molecular analysis (68,80). Olfactory neuronal cells are a more homogenous population and can be established from a large number of patients, making them fit for high-throughput screening assays (81). Although olfactory neuronal cells are of doubtful utility for assessments of excitability, they are reasonably suitable for gene expression analysis since they show similar molecular profiles to stem cells and brain tissues (72).
Table 1.
Utility of human neuronal cell models in dissecting dynamic alteration in the mitochondrial function associated with psychiatric illnesses
| Bioenergetics | Oxidative stress | Ca2+ homeostasis | ||
|---|---|---|---|---|
| Study parameters | Mitochondrial membrane potential, respiration rate, ATP generation | ROS, cellular oxidative stress | Mitochondrial Ca2+ basal and stimuli-dependent levels | |
| Techniques widely used | (a) Live cell imaging with fluorescent dye labeling; (b) Seahorse assay | Histology | Fluorescent Ca2+ indicators (dyes and genetically encoded indicators) | |
| Reference | 77, 78 | 71, 82, 83, 99, 100 | 78, 84 | |
| Feasibility | iPSC-derived neurons | O | O | O |
| iPS-iN cells | O | O | O | |
| iN cells | Cell enrichment is required for (a) and (b) | O | O | |
| Olfactory neuronal cells | O | O | O | |
o, theoretically and technically feasible
Recent mitochondria-related findings from human neuronal cell models
In this section, we introduce significant findings in studies that used human-derived neuronal model systems and guided our understanding on the direct contribution of mitochondrial dysfunction, oxidative stress, and aberrant Ca2+ homeostasis relevant to the pathophysiology of mental disorders (Table 2). In particular, we highlight studies that showed direct link of the mitochondrial dysfunction and cellular disturbance in patient-derived neuronal cells.
Table 2.
Important findings through human neuronal cell models, that might affect mitochondrial function associated with psychiatric illnesses
| Human neuronal cell models | Major findings | Reference | |
|---|---|---|---|
| Schizophrenia | Hair follicle-originated iPS cell-derived dopaminergic and glutamatergic neurons | Decreased mitochondrial membrane potential; uneven mitochondrial intracellular distribution | 77 |
| iPS cell-derived neural progenitor cells | Decreased mitochondrial membrane potential; increased levels of ROS | 82 | |
| Increased extramitochondrial oxygen consumption and ROS levels. ROS levels reverted by valproic acid | 83 | ||
| Olfactory neuronal cells | Altered expression and regulation of genes involved in cellular protection against oxidative stress | 71 | |
| Bipolar disorder | iPS cell-derived hippocampal dentate gyrus granule-cell-like neurons | Altered mitochondrial gene expression, function, size, and Ca2+ dynamics (hyperexcitability). Hyperexcitability rescued by lithium in lithium responder, but not in non-lithum responder neurons | 78 |
| B lymphoblasts-originated iPS cell-derived hippocampal dentate gyrus granule-cell-like neurons | Ca2+ dynamics (hyperexcitability) rescued by lithium in lithium responder neurons, but not in non-lithum responder neurons | 85 | |
| iPS cell-derived neurons | Altered expression levels of calcium channel genes including CACNA1C and calcium transients after lithium treatment | 101 | |
| Olfactory neuronal cells | Altered Ca2+ signaling | 84 | |
| Subjects with the risk variant for CACNA1C (rs1006737) | iN cells | Increased L-type voltage-gated calcium channel current density and expression of CACNA1C | 102 |
Unless otherwise noted, iPS cells were derived from skin fibroblasts
Brennand et. al. (82) found excess oxidative stress associated with mitochondrial damage in the iPS cell-derived neural progenitor cells obtained from four patients with SZ as compared to six controls. They investigated potential functional deficits by utilizing a mitochondrial membrane potential (MMP) assay in which the voltage difference across the inner mitochondrial membrane was measured. They observed significantly decreased MMP (indicating mitochondrial dysfunction) in SZ neural progenitor cells relative to control neural progenitor cells, which is the first report that depicts dynamic alteration of the mitochondrial function directly in the neuronal context. Immunohistochemical staining for mitochondrial markers revealed that the mitochondria tended to be smaller, disconnected, and distally distributed in SZ neural progenitor cells, whereas mitochondria in control neural progenitors tended to be more connected, tubular, and highly packed near the perinuclear regions. Importantly, they found more ROS-induced oxidized proteins associated with mitochondria damage in SZ neural progenitor cells, compared to controls. Furthermore, proteomic analysis of these cells also identified disturbances in oxidative stress pathways. Although alteration of oxidative stress-associated molecular disposition in neuronal context had been reported by using olfactory neuronal cells (71), the above mentioned study more directly addressed the dynamics of oxidative stress and mitochondrial deficits. Another group made a case report from iPS cell-derived neural progenitor cells generated from a clozapine resistant SZ patient, in which the same conclusion was drawn (83). The neural progenitor cells from the patient presented a two-fold increase in extra-mitochondrial oxygen consumption together with elevated levels of ROS when compared to cells from a control subject. This difference in ROS levels was reverted by the mood stabilizer valproic acid. Although these studies showed dynamic changes in oxidative stress associated with mitochondrial abnormalities in patients’ neuronal cells, the sample size is too small to draw conclusion for mental disorders that are known to be very heterogeneous under the same diagnostic names. Thus, further studies that pay attention to a larger sample size combined with a more sophisticated patient stratification are awaited.
Neuronal hyperexcitability detected by patch-clamp recording and somatic Ca2+ imaging linked with mitochondrial abnormalities were reported in hippocampal dentate gyrus granule-cell-like neurons that were differentiated from fibroblast-derived iPS cells obtained from six BP patients, three of whom were lithium responders and three of whom were non-responders (78). Prior to this study, Hahn et. al. (84) made a pioneering proposal by using olfactory neuronal cells that indicated an alteration in the Ca2+ signaling. By using neurons originating from iPS cells, Mertens et. al. (78) investigated mitochondrial function by measuring the MMP and found that BP neurons showed higher MMP (enhanced mitochondrial function), a change in line with the upregulated mitochondrial gene expression observed in BP neurons, compared to the control neurons. The BP neurons also had smaller mitochondria than controls. RNA-seq analysis revealed that the pathways involving Ca2+ signaling, neuroactive ligand–receptor interaction, and protein kinase PKA/PKC signaling were altered in BP neurons, in addition to changes in the action potential firing system. Using patch-clamp recording and somatic Ca2+ imaging, they also observed neuronal hyperexcitability associated with enhanced mitochondrial function in BP neurons. Interestingly, the hyperexcitability was rescued by lithium application in BP neurons from lithium responders, but not in those from non-responders. Taken all together, these results demonstrated direct links between mitochondria abnormalities, aberrant Ca2+ signaling, and neuronal hyperexcitability in BP pathophysiology. They also acknowledged that further investigations would be necessary to determine whether mitochondrial alterations represent a cause or a consequence of the observed hyperexcitability phenotype, studies now feasible in human neuron model systems. Notably, this same group also reported hyperexcitability in hippocampal dentate gyrus granule-cell-like neurons differentiated from B lymphoblasts-derived iPS cells of BP patients in an independent cohort (85): this study showed that the cellular abnormalities were rescued by lithium only in the cells from the patients who clinically responded to lithium. We should also note that iPS cells can be generated from multiple types of cells, such as fibroblasts and blood cells. There are discussions on which parent cell type is better suited for studies (86–88): for example, Kyttälä et. al. (88) generated iPS cells from two separate sources (fibroblast and blood cells) from multiple individuals, and observed that the individual variability contributes to the iPS cells differentiation potential more than parent cell-type does.
Although they still have some limitations, these studies provide good examples of how human neuronal model systems enable investigation of dynamic changes in mitochondrial function associated with the pathophysiology of the illness. Studies in a larger sample size combined with more sophisticated patient stratification by using multiple, quantitative clinical characteristics are awaited to drive this right direction further.
Perspectives
The study of bioenergetics, oxidative stress, redox signaling, and Ca2+ homeostasis can be challenging as they feedback on each other. Postmortem brain studies, functional brain imaging, and genomic studies have been very useful in providing insights into possible links between mental disorders and mitochondrial dysfunction, oxidative stress, and Ca2+ homeostasis. However, as far as its dynamic changes are involved, in vitro human biopsied neuronal cell models could be a key resource for understanding the mitochondrial dysfunction and its downstream consequences in the patients. Furthermore, by using these models, we can also address whether and how upstream factors including genetic and environmental risk factors affect mitochondrial dysfunction. The potential of using human neuronal cell models in deciphering the hierarchical cascades in the overall pathology is illustrated in Figure 1. Improved techniques using redox sensitive dyes, mitochondrial-targeted probes, and genetically encoded Ca2+ indicators enable investigation of these dynamic changes in the live cells (Table 1). By using these systems, we can test whether pharmacological and genetic manipulations targeted for specific molecules in mitochondrial bioenergetics, oxidative stress, redox signaling, and/or Ca2+ homeostasis could prevent or restore cellular functions (Figure 1). Once we find molecules that could rescue cellular functions, such molecules could be targets for mechanism-based drug discovery research.
Major mental disorders have also been linked to metabolic disorders, which could be intrinsic or developed as a side-effect of medications such as antipsychotics, antidepressants, and mood stabilizers (89–91). Patient-derived cell models could also serve as a valuable resource to tease out the extent of medication or patient-specific factors leading to the pathophysiology, which could ultimately lead to drug discoveries with limited side-effects.
While we have focused primarily on neurons in this review, study of the role of oxidative stress in mental disorders necessarily involves interactions between multiple cell types both within and outside the brain. iPS cell protocols now allow for generation of specific neuronal subtypes as well as astrocytes, oligodendrocytes, and microglia. Human cell lines can thereby be used to study cell autonomous effects in multiple cell types as well as cell-cell interactions between different cell types in co-culture systems. Recently, organoids, miniature functional tissue units, have been generated from human iPS cells through the development of three-dimensional culture systems. Although still in an infant stage of technical development, the experimental system that utilizes organoids would significantly contribute to studying non-cell autonomous effects in normal brain development and brain disorders (92–95).
Nevertheless, in vitro systems are limited in their capacity to study the effects of mitochondrial dysfunction across development or at a circuitry level, which is crucial in understanding complex psychiatric disorders. Thus, we propose to utilize human stem cell biology in parallel with proper animal models (96,97) and human brain imaging studies (98). Each experimental system has limitations: we should be very cautious about potential differences between human and animal phenotypes at the circuitry and behavioral levels, and about low spatial and temporal resolution in human brain imaging. However, we optimistically and positively propose that these systems will be able to further identify non-cell autonomous interactions which affect neuronal function and integrate those findings into models of human brain circuitry. In particular, recent studies have highlighted the roles of the immune system, endocrine system, and microbiome in influencing levels of oxidative stress within the brain (28). In summary, studying human neuron cell models complementarily with studying animal models and human brain imaging will clarify dynamic changes in the mitochondrial functions and their mechanisms underlying manifestations of major mental disorders (Table 3).
Table 3:
Future perspectives: a multifaceted approach that centers the study of human neuronal cell models
| Research domains | Research targets | Suitable research model system/s |
|---|---|---|
| Cell autonomous | Cell-specific dynamic changes in mitochondrial bioenergetics, oxidative stress, and Ca2+ homeostasis under genetic/environmental manipulations and/or drug treatment | Human neuronal cell models |
| Non-cell autonomous | Effects of dynamic mitochondrial deficits on interactions among multiple cell types both within and outside the brain | Human neuronal cell models applied to co-culture systems and organoid culture |
| Circuit | Brain structure/connectivity and clinical manifestations associated with dynamic mitochondrial deficits affected by alterations in the immune system, endocrine system, and microbiome | Human neuronal cell models combined with human brain imaging and/or animal models |
| Behavior | Human neuronal cell models combined with human clinical evaluations and/or animal models |
Acknowledgements
We thank Mr. Brian Lee and Ms. Alejandra Patino for critical reading of this manuscript, and Ms. Yukiko Lema for organizing the figures and manuscript. This work was supported by the National Institute of Mental Health MH-105660 (A.S. and K.I.), MH-094268 Silvio O. Conte center (A.S.), MH-092443 (A.S.), as well as foundation grants from Stanley (A.S.), S-R/RUSK (A.S.), BBRF (A.S., K.I.) and Maryland Stem Cell Research Fund (A.S. and K.I.). R.S. is supported by the International Bipolar Foundation through the BBRF young investigator award.
Financial Disclosures
This work was supported by the National Institute of Mental Health MH-105660 (A.S. and K.I.), MH-094268 Silvio O. Conte center (A.S.), MH-092443 (A.S.), as well as foundation grants from Stanley (A.S.), S-R/RUSK (A.S.), NARSAD (A.S., K.I., and R.S.), and Maryland Stem Cell Research Fund (A.S. and K.I.). The authors report no biomedical financial interests or potential conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gray MW. (2017): Lynn Margulis and the endosymbiont hypothesis: 50 years later. Mol Biol Cell 28:1285–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goh S, Dong Z, Zhang Y, DiMauro S, Peterson BS. (2014): Mitochondrial dysfunction as a neurobiological subtype of autism spectrum disorder: evidence from brain imaging. JAMA Psychiatry 71:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, et al. (2015): Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother 74:101–110. [DOI] [PubMed] [Google Scholar]
- 4.Sorrentino V, Menzies KJ, Auwerx J (2018): Repairing Mitochondrial Dysfunction in Disease. Annu Rev Pharmacol Toxicol 58:9.1–9.37. [DOI] [PubMed] [Google Scholar]
- 5.Zhu XH, Qiao H, Du F, Xiong Q, Liu X, Zhang X, et al. (2012): Quantitative imaging of energy expenditure in human brain. Neuroimage 60:2107–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erecinska M, Silver IA. (2001): Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol 128:263–276. [DOI] [PubMed] [Google Scholar]
- 7.Sena LA, Chandel NS. (2012): Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T (1995): Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270:296–299. [DOI] [PubMed] [Google Scholar]
- 9.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, et al. (1997): Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem 272:217–221. [PubMed] [Google Scholar]
- 10.Meng TC, Fukada T, Tonks NK. (2002): Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell 9:387–399. [DOI] [PubMed] [Google Scholar]
- 11.Rizzuto R, De Stefani D, Raffaello A, Mammucari C (2012): Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13:566–578. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi RK, Beal MF. (2008): Mitochondrial approaches for neuroprotection. Ann N Y Acad Sci 1147:395–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kornmann B (2013): The molecular hug between the ER and the mitochondria. Curr Opin Cell Biol 25:443–448. [DOI] [PubMed] [Google Scholar]
- 14.Bagur R, Hajnoczky G (2017): Intracellular Ca(2+) Sensing: Its Role in Calcium Homeostasis and Signaling. Mol Cell 66:780–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seidlmayer LK, Juettner VV, Kettlewell S, Pavlov EV, Blatter LA, Dedkova EN. (2015): Distinct mPTP activation mechanisms in ischaemia-reperfusion: contributions of Ca2+, ROS, pH, and inorganic polyphosphate. Cardiovasc Res 106:237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greer PL, Greenberg ME. (2008): From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron 59:846–860. [DOI] [PubMed] [Google Scholar]
- 17.Czabotar PE, Lessene G, Strasser A, Adams JM. (2014): Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol 15:49–63. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs Y, Steller H (2015): Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol 16:329–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhola PD, Letai A (2016): Mitochondria-Judges and Executioners of Cell Death Sentences. Mol Cell 61:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, et al. (2012): Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci 13:293–307. [DOI] [PubMed] [Google Scholar]
- 21.Sawa A, Snyder SH. (2002): Schizophrenia: diverse approaches to a complex disease. Science 296:692–695. [DOI] [PubMed] [Google Scholar]
- 22.van Os J, Kenis G, Rutten BP. (2010): The environment and schizophrenia. Nature 468:203–212. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan PF, Daly MJ, O’Donovan M (2012): Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet 13:537–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandal MJ, Leppa V, Won H, Parikshak NN, Geschwind DH. (2016): The road to precision psychiatry: translating genetics into disease mechanisms. Nat Neurosci 19:1397–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owen MJ, Sawa A, Mortensen PB. (2016): Schizophrenia. Lancet 388:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park C, Park SK. (2012): Molecular links between mitochondrial dysfunctions and schizophrenia. Mol Cells 33:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Issler O, Chen A (2015): Determining the role of microRNAs in psychiatric disorders. Nat Rev Neurosci 16:201–212. [DOI] [PubMed] [Google Scholar]
- 28.Landek-Salgado MA, Faust TE, Sawa A (2016): Molecular substrates of schizophrenia: homeostatic signaling to connectivity. Mol Psychiatry 21:10–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salminen A, Haapasalo A, Kauppinen A, Kaarniranta K, Soininen H, Hiltunen M (2015): Impaired mitochondrial energy metabolism in Alzheimer’s disease: Impact on pathogenesis via disturbed epigenetic regulation of chromatin landscape. Prog Neurobiol 131:1–20. [DOI] [PubMed] [Google Scholar]
- 30.Swerdlow RH. (2016): Bioenergetics and metabolism: a bench to bedside perspective. J Neurochem 139 Suppl 2:126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace DC. (2011): Bioenergetic origins of complexity and disease. Cold Spring Harb Symp Quant Biol 76:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raffa M, Mechri A, Othman LB, Fendri C, Gaha L, Kerkeni A (2009): Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry 33:1178–1183. [DOI] [PubMed] [Google Scholar]
- 33.Flatow J, Buckley P, Miller BJ. (2013): Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry 74:400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. (2011): Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 14:123–130. [DOI] [PubMed] [Google Scholar]
- 35.Yao JK, Leonard S, Reddy RD. (2004): Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophr Bull 30:923–934. [DOI] [PubMed] [Google Scholar]
- 36.Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, et al. (2000): Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci 12:3721–3728. [DOI] [PubMed] [Google Scholar]
- 37.Matsuzawa D, Obata T, Shirayama Y, Nonaka H, Kanazawa Y, Yoshitome E, et al. (2008): Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS study. PLoS One 3:e1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wood SJ, Berger GE, Wellard RM, Proffitt TM, McConchie M, Berk M, et al. (2009): Medial temporal lobe glutathione concentration in first episode psychosis: a 1H-MRS investigation. Neurobiol Dis 33:354–357. [DOI] [PubMed] [Google Scholar]
- 39.Hardingham GE, Do KQ. (2016): Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat Rev Neurosci 17:125–134. [DOI] [PubMed] [Google Scholar]
- 40.Stedehouder J, Kushner SA. (2017): Myelination of parvalbumin interneurons: a parsimonious locus of pathophysiological convergence in schizophrenia. Mol Psychiatry 22:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steullet P, Cabungcal JH, Coyle J, Didriksen M, Gill K, Grace AA, et al. (2017): Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Mol Psychiatry 22:936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maas DA, Vallès A, Martens GJM. (2017): Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Translational Psychiatry 7:e1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.French HM, Reid M, Mamontov P, Simmons RA, Grinspan JB. (2009): Oxidative stress disrupts oligodendrocyte maturation. J Neurosci Res 87:3076–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massaad CA, Klann E (2011): Reactive oxygen species in the regulation of synaptic plasticity and memory. Antioxid Redox Signal 14:2013–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uttara B, Singh AV, Zamboni P, Mahajan RT. (2009): Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koga M, Serritella AV, Sawa A, Sedlak TW. (2016): Implications for reactive oxygen species in schizophrenia pathogenesis. Schizophr Res 176:52–71. [DOI] [PubMed] [Google Scholar]
- 47.Holmstrom KM, Finkel T (2014): Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 15:411–421. [DOI] [PubMed] [Google Scholar]
- 48.Cao SS, Kaufman RJ. (2014): Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 21:396–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel S, Sharma D, Kalia K, Tiwari V (2017): Crosstalk between Endoplasmic Reticulum Stress and Oxidative Stress in Schizophrenia: The Dawn of New Therapeutic Approaches. Neurosci Biobehav Rev pii: S0149–7634(17)30356–1. doi: 10.1016/j.neubiorev.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 50.Psychiatric GCBDWG. (2011): Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet 43:977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schizophrenia Psychiatric Genome-Wide Association Study, C. (2011): Genome-wide association study identifies five new schizophrenia loci. Nat Genet 43:969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cross-Disorder Group of the Psychiatric Genomics, C. (2013): Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schizophrenia Working Group of the Psychiatric Genomics, C. (2014): Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dubovsky SL, Daurignac E, Leonard KE. (2014): Increased platelet intracellular calcium ion concentration is specific to bipolar disorder. J Affect Disord 164:38–42. [DOI] [PubMed] [Google Scholar]
- 55.Dubovsky SL, Murphy J, Thomas M, Rademacher J (1992): Abnormal intracellular calcium ion concentration in platelets and lymphocytes of bipolar patients. Am J Psychiatry 149:118–120. [DOI] [PubMed] [Google Scholar]
- 56.Linnoila M, MacDonald E, Reinila M, Leroy A, Rubinow DR, Goodwin FK. (1983): RBC membrane adenosine triphosphatase activities in patients with major affective disorders. Arch Gen Psychiatry 40:1021–1026. [DOI] [PubMed] [Google Scholar]
- 57.Ripova D, Strunecka A, Nemcova V, Farska I (1997): Phospholipids and calcium alterations in platelets of schizophrenic patients. Physiol Res 46:59–68. [PubMed] [Google Scholar]
- 58.Harrison PJ. (2016): Molecular neurobiological clues to the pathogenesis of bipolar disorder. Curr Opin Neurobiol 36:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warsh JJ, Andreopoulos S, Li PP. (2004): Role of intracellular calcium signaling in the pathophysiology and pharmacotherapy of bipolar disorder: current status. Clinical Neuroscience Research 4:201–213. [Google Scholar]
- 60.Peng TI, Jou MJ. (2010): Oxidative stress caused by mitochondrial calcium overload. Ann N Y Acad Sci 1201:183–188. [DOI] [PubMed] [Google Scholar]
- 61.Feissner RF, Skalska J, Gaum WE, Sheu SS. (2009): Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci (Landmark Ed) 14:1197–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, et al. (2011): Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oki K, Tatarishvili J, Wood J, Koch P, Wattananit S, Mine Y, et al. (2012): Human-induced pluripotent stem cells form functional neurons and improve recovery after grafting in stroke-damaged brain. Stem Cells 30:1120–1133. [DOI] [PubMed] [Google Scholar]
- 64.Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. (2012): Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci 15:477–486, S471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haggarty SJ, Silva MC, Cross A, Brandon NJ, Perlis RH. (2016): Advancing drug discovery for neuropsychiatric disorders using patient-specific stem cell models. Mol Cell Neurosci 73:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ladewig J, Mertens J, Kesavan J, Doerr J, Poppe D, Glaue F, et al. (2012): Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods 9:575–578. [DOI] [PubMed] [Google Scholar]
- 67.Passeri E, Wilson AM, Primerano A, Kondo MA, Sengupta S, Srivastava R, et al. (2015): Enhanced conversion of induced neuronal cells (iN cells) from human fibroblasts: Utility in uncovering cellular deficits in mental illness-associated chromosomal abnormalities. Neurosci Res 101:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, et al. (2011): Induction of human neuronal cells by defined transcription factors. Nature 476:220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kano S, Yuan M, Cardarelli RA, Maegawa G, Higurashi N, Gaval-Cruz M, et al. (2015): Clinical utility of neuronal cells directly converted from fibroblasts of patients for neuropsychiatric disorders: studies of lysosomal storage diseases and channelopathy. Curr Mol Med 15:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, et al. (2013): Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78:785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kano S, Colantuoni C, Han F, Zhou Z, Yuan Q, Wilson A, et al. (2013): Genome-wide profiling of multiple histone methylations in olfactory cells: further implications for cellular susceptibility to oxidative stress in schizophrenia. Mol Psychiatry 18:740–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horiuchi Y, Kano S, Ishizuka K, Cascella NG, Ishii S, Talbot CC Jr., et al. (2013): Olfactory cells via nasal biopsy reflect the developing brain in gene expression profiles: utility and limitation of the surrogate tissues in research for brain disorders. Neurosci Res 77:247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mackay-Sim A (2012): Concise review: Patient-derived olfactory stem cells: new models for brain diseases. Stem Cells 30:2361–2365. [DOI] [PubMed] [Google Scholar]
- 74.Gamo NJ, Sawa A (2014): Human stem cells and surrogate tissues for basic and translational study of mental disorders. Biol Psychiatry 75:918–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Brien LC, Keeney PM, Bennett JP Jr. (2015): Differentiation of Human Neural Stem Cells into Motor Neurons Stimulates Mitochondrial Biogenesis and Decreases Glycolytic Flux. Stem Cells Dev 24:1984–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saporta MA, Dang V, Volfson D, Zou B, Xie XS, Adebola A, et al. (2015): Axonal Charcot-Marie-Tooth disease patient-derived motor neurons demonstrate disease-specific phenotypes including abnormal electrophysiological properties. Exp Neurol 263:190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robicsek O, Karry R, Petit I, Salman-Kesner N, Muller FJ, Klein E, et al. (2013): Abnormal neuronal differentiation and mitochondrial dysfunction in hair follicle-derived induced pluripotent stem cells of schizophrenia patients. Mol Psychiatry 18:1067–1076. [DOI] [PubMed] [Google Scholar]
- 78.Mertens J, Wang QW, Kim Y, Yu DX, Pham S, Yang B, et al. (2015): Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature 527:95–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Os J, Rutten BP, Poulton R (2008): Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull 34:1066–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang N, Ng YH, Pang ZP, Sudhof TC, Wernig M (2011): Induced neuronal cells: how to make and define a neuron. Cell Stem Cell 9:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lavoie J, Gasso Astorga P, Segal-Gavish H, Wu YC, Chung Y, Cascella NG, et al. (2017): The Olfactory Neural Epithelium As a Tool in Neuroscience. Trends Mol Med 23:100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brennand K, Savas JN, Kim Y, Tran N, Simone A, Hashimoto-Torii K, et al. (2015): Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol Psychiatry 20:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paulsen Bda S, de Moraes Maciel R, Galina A, Souza da Silveira M, dos Santos Souza C, Drummond H, et al. (2012): Altered oxygen metabolism associated to neurogenesis of induced pluripotent stem cells derived from a schizophrenic patient. Cell Transplant 21:1547–1559. [DOI] [PubMed] [Google Scholar]
- 84.Hahn CG, Gomez G, Restrepo D, Friedman E, Josiassen R, Pribitkin EA, et al. (2005): Aberrant intracellular calcium signaling in olfactory neurons from patients with bipolar disorder. Am J Psychiatry 162:616–618. [DOI] [PubMed] [Google Scholar]
- 85.Stern S, Santos R, Marchetto MC, Mendes AP, Rouleau GA, Biesmans S, et al. (2017): Neurons derived from patients with bipolar disorder divide into intrinsically different sub-populations of neurons, predicting the patients’ responsiveness to lithium. Mol Psychiatry doi: 10.1038/mp.2016.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shtrichman R, Germanguz I, Itskovitz-Eldor J (2013): Induced pluripotent stem cells (iPSCs) derived from different cell sources and their potential for regenerative and personalized medicine. Curr Mol Med 13:792–805. [DOI] [PubMed] [Google Scholar]
- 87.Sanchez-Freire V, Lee AS, Hu S, Abilez OJ, Liang P, Lan F, et al. (2014): Effect of human donor cell source on differentiation and function of cardiac induced pluripotent stem cells. J Am Coll Cardiol 64:436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kyttala A, Moraghebi R, Valensisi C, Kettunen J, Andrus C, Pasumarthy KK, et al. (2016): Genetic Variability Overrides the Impact of Parental Cell Type and Determines iPSC Differentiation Potential. Stem Cell Reports 6:200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fagiolini A, Chengappa KN, Soreca I, Chang J (2008): Bipolar disorder and the metabolic syndrome: causal factors, psychiatric outcomes and economic burden. CNS Drugs 22:655–669. [DOI] [PubMed] [Google Scholar]
- 90.Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, et al. (2015): Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry 14:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Correll CU, Joffe BI, Rosen LM, Sullivan TB, Joffe RT. (2015): Cardiovascular and cerebrovascular risk factors and events associated with second-generation antipsychotic compared to antidepressant use in a non-elderly adult sample: results from a claims-based inception cohort study. World Psychiatry 14:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lancaster MA, Knoblich JA. (2014): Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345:1247125. [DOI] [PubMed] [Google Scholar]
- 93.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. (2013): Cerebral organoids model human brain development and microcephaly. Nature 501:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, et al. (2016): Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165:1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Di Lullo E, Kriegstein AR. (2017): The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci 18:573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nestler EJ, Hyman SE. (2010): Animal models of neuropsychiatric disorders. Nat Neurosci 13:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaiser T, Feng G (2015): Modeling psychiatric disorders for developing effective treatments. Nat Med 21:979–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ugurbil K (2016): What is feasible with imaging human brain function and connectivity using functional magnetic resonance imaging. Philos Trans R Soc Lond B Biol Sci 371:20150361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nguyen HN, Byers B, Cord B, Shcheglovitov A, Byrne J, Gujar P, et al. (2011): LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 8:267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Suzuki S, Akamatsu W, Kisa F, Sone T, Ishikawa KI, Kuzumaki N et al. (2017): Efficient induction of dopaminergic neuron differentiation from induced pluripotent stem cells reveals impaired mitophagy in PARK2 neurons. Biochem Biophys Res Commun 483:88–93. [DOI] [PubMed] [Google Scholar]
- 101.Chen HM, DeLong CJ, Bame M, Rajapakse I, Herron TJ, McInnis MG, et al. (2014): Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients. Transl Psychiatry 4, e375. doi: 10.1038/tp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yoshimizu T, Pan JQ, Mungenast AE, Madison JM, Su S, Ketterman J, et al. (2015): Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol Psychiatry 20:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]