Abstract
Alterations in peripheral myelin protein 22 (PMP22) gene expression are associated with a host of heritable demyelinating peripheral neuropathies, yet the function of the protein remains unknown. PMP22 expression is highest in myelinating Schwann cells of peripheral nerves; however, significant levels of PMP22 mRNAs can be detected in a variety of non-neural tissue, including epithelia. To date, PMP22 protein expression and localization in non-neural tissues have not been studied in detail. In adult rat liver and intestine, and cultured epithelial cells, we detected PMP22-like immunoreactivity associated with markers of the tight junctional complex, including zonula occludens 1 (ZO-1) and occludin. Upon disruption of intercellular contacts, PMP22 was internalized into vesicles that were immunoreactive for both anti-occludin and anti-PMP22 antibodies. Nonionic detergent extraction of cultured epithelial cells did not solubilize PMP22, as the majority of the protein remained in the detergent insoluble fraction, as did ZO-1 and occludin. We also observed the targeting of exogenous myc-tagged PMP22 to apical cell junctions in polarized epithelia and to anti-ZO-1 antibody immunoreactive cell contacts of L fibroblasts. These studies support a role for PMP22 at intercellular junctions of epithelia and may indicate a similar function in myelinating Schwann cells. Furthermore, our findings could provide an explanation for certain phenotypes of PMP22 neuropathy mice that cannot be accounted for by dysmyelination.
Peripheral myelin protein 22 (PMP22), also known as gas-3, is a tetraspan glycoprotein with proposed roles in peripheral nerve myelin formation, cell–cell interactions, and cell proliferation (1). PMP22 expression is highest in myelin-forming Schwann cells; however, PMP22 mRNA can be detected in a variety of non-neural tissues. Epithelial cells of the lungs and intestines are known to express the highest levels of PMP22 mRNA outside of the peripheral nervous system (2–4), yet the localization or the role of the protein in these tissues has not been determined. Although the function of PMP22 in Schwann cells and non-neural cells is largely undefined, it is well established that deletions, duplications, or mutations in PMP22 account for the majority of heritable demyelinating peripheral neuropathies, including Charcot-Marie-Tooth disease type IA.
Myelin-forming Schwann cells and epithelial cells, two cell types with high levels of PMP22 mRNA expression, share similarities in that they are both polarized and maintain compositionally unique membrane domains. In addition, similar to the barrier function of epithelia, Schwann cells separate intramyelinic and extramyelinic extracellular space (5). The molecular bases of how Schwann cells attain these functions are not yet understood, although they are likely to involve specialized intercellular junctions, such as adherens and/or tight junctions (TJs). Freeze fracture studies of peripheral nervous system myelin detected rows of TJ-like fibrils within the Schwann cell membrane (6); nevertheless the identities of the proteins forming these structures are unknown. Recent studies revealed the presence of TJ strands in central nervous system myelin (7), which is deposited by oligodendrocytes. A protein component of TJ strands in central nervous system myelin is oligodendrocyte-specific protein/claudin-11, a PMP22-related, tetraspan membrane protein (7, 8).
In addition to oligodendrocyte-specific protein/claudin-11, PMP22 shares significant sequence identity and structural similarity with other claudins, including the first discovered claudin in liver, claudin-1 (9). The claudin protein family now includes more than 20 members with unique, as well as overlapping, tissue distribution (10–12). Claudins appear to have roles in the formation of TJ strands and in the establishment of the ionic selectivity of the junctional barrier (11). The essential function of claudins at TJs is supported by recent reports on claudin missexpression and disease-causing alteration in epithelial physiology (13, 14). Occludin, also a tetraspan protein of TJs, is an adhesive molecule that may have roles in the barrier function of TJs (15–17). These transmembrane junctional proteins form complexes with cytoplasmic molecules, such as zonula occludens-1 and -2 (ZO-1, ZO-2), which link the membrane proteins to cytoskeletal elements (18). As the molecular architecture of intercellular junctions is being uncovered, studies show that in addition to ionic barrier and fence functions, TJs are involved in intracellular vesicle targeting and signaling (19).
Based on the mRNA expression pattern, and the primary and secondary structure of PMP22, we hypothesized that PMP22 might be a component of intercellular junctions in epithelia. Therefore, we examined the expression and localization of PMP22 in cultured epithelia and a variety of tissues with ZO. Using immunochemical, biochemical, and molecular approaches, we found that in epithelial cells PMP22 is coexpressed with occludin and ZO-1 at or near TJs and overexpression of PMP22 in L cell fibroblasts mediated the formation of ZO-1-positive intercellular junctions. These studies suggest that the plasma membrane-associated biological function of PMP22 might involve a role in the establishment and/or maintenance of intercellular junctions and possibly of TJs.
Methods
Cell Culture.
Primary Schwann cell cultures were established from newborn rat pups (20). L cells (American Type Culture Collection) were maintained in 10% horse serum containing DMEM. Madin–Darby canine kidney (MDCK) cells were cultured in 10% FBS containing DMEM on 0.4-μm pore size Transwell filters (Costar), or glass coverslips, with or without type I collagen coating. Highly polarized, confluent MDCK cell monolayers were incubated with 4 mM EGTA for 1–4 h to chelate the calcium from the culture medium (21, 22). EGTA treatment results in the rounding up of the cells and disassembly of intercellular contacts.
Retroviral Overexpression of PMP22-myc in MDCK and L Cells.
The mouse PMP22 ORF with a myc epitope in the second extracellular loop (23) was directionally inserted into the retroviral plasmid pBMN (24). The resulting pBMN-PMP22myc, or a control pBMN-GFP (green fluorescent protein) plasmid, was transiently transfected into the amphotropic retroviral packaging cell line Phoenix A (obtained from Garry Nolan, Stanford University, Stanford, CA). Retroviral supernatants were collected after 30-h incubation at 32°C and directly applied to 1 × 106 MDCK or L cells (≈40% confluency). Retroviral transductions were performed at 32°C for 24 h in the presence of 5 μg/ml polybrene. Forty-eight hours postinfection, cells were replated and allowed to form confluent monolayers. Estimated from the number of pBMN-GFP-expressing cells, the infection rate in the L cells was ≈99% and ≈15% in MDCK cells.
Immunostaining Procedures.
MDCK cells and 2- or 8-μm thick cryosections of adult rat liver and colon were double immunostained with polyclonal anti-PMP22 (20) and monoclonal anti-tight junction protein antibodies, according to published procedures (25). Primary antibodies included monoclonal anti-occludin and anti-ZO-1 (Zymed), and polyclonal anti-claudin-1 (Zymed) and anti-PMP22 (20). Twelve distinct polyclonal antibodies made against 16-aa peptides of the first (amino acids 27–42) or second (amino acids 117–133) extracellular loops of the mouse, rat, or human PMP22 were used to localize PMP22 in the studied samples. Preimmune and peptide preadsorbed (0.1 mg/ml) rabbit serum and nonspecific mouse IgGs served as controls of antibody binding. Bound primary antibodies were detected with Alexa fluorochrome-conjugated secondary antibodies, including FITC-conjugated anti-mouse IgG and Texas red-conjugated anti-rabbit IgG (Molecular Probes). Nuclei were stained with Hoechst dye. Coverslips were mounted by using a ProLong Antifade kit (Molecular Probes), and images were acquired with a Spot camera attached to a Nikon Eclipse 1000 or an Olympus MRC-1024 confocal microscope. Images were processed for printing by using Adobe PHOTOSHOP 5.0.
To increase the resolution of the immunoreactivity in filter-grown MDCK cells, filters with confluent monolayers were sectioned after freezing and processed for immunostaining (25). For optimal detection of the myc epitope-tagged PMP22, retrovirally infected MDCK and L cells were fixed in 4% paraformaldehyde, followed by permeabilization and immunolabeling with polyclonal or monoclonal anti-myc antibodies (26). These fixation conditions are suboptimal for the detection of endogenous TJ proteins, which is reflected by reduced levels of claudin, ZO-1, and occludin-like immunoreactivities.
RNA Isolation, Northern Analysis, and Reverse Transcriptase–PCR (RT-PCR).
Total RNA was isolated from rat liver and rat Schwann cells by using the TRIzol reagent (GIBCO Life Technologies). The Titan One Tube RT-PCR System (Roche Diagnostic) was used to generate and amplify a 425-bp PMP22 cDNA fragment by using 1 μg of total RNA. Specific primers were synthesized according to the nucleotide sequence of rat PMP22 (sense primer 5′-ACACTTGACCCTGAAGGC-3′ and reverse primer 5′-AGCATCAGAAGGACACCG-3′). Half of each RT-PCR product was digested with a PMP22 sequence-specific restriction enzyme (BsaI), and samples were analyzed on acrylamide gels. Negative controls included samples without the RT enzyme and samples that were RNase-treated.
Biochemical Procedures.
Bile canaliculi-enriched fractions from P70 rat livers were processed according to established procedures (27, 28). Three different membrane fractions were collected (27) and analyzed by Western blotting with anti-PMP22 antiserum.
To confirm that our anti-human, anti-rat, or anti-mouse PMP22 antibodies can detect canine PMP22 in MDCK cells we purchased frozen dog sciatic nerves (Pel-Freez Biologicals). Adult rat, mouse, and canine sciatic nerve lysates were analyzed on 12.5% SDS gels as described (20).
Control and retrovirally infected MDCK cell monolayers were extracted with 0.5% TX-100-containing buffer, and detergent soluble (S) and insoluble (I) fractions were collected (29). Control and retrovirally infected L cells were directly lysed in SDS gel sample buffer, and protein concentrations were determined. Endoglycosidase H and N-glycosidase digestions were performed as described (30). To prevent the aggregation of PMP22, protein samples were heated to 80°C before loading of the gels. Gels were transferred to nitrocellulose membranes and processed for immunoblotting with monoclonal anti-occludin and anti-ZO-1 (Zymed), and polyclonal anti-claudin-1 (Zymed) and anti-PMP22 (20) antibodies. Bound antibodies were detected with horseradish peroxidase-conjugated anti-mouse, or anti-rabbit, secondary antibodies (Sigma) by using ECL chemiluminescent reagents (Amersham Pharmacia).
Results
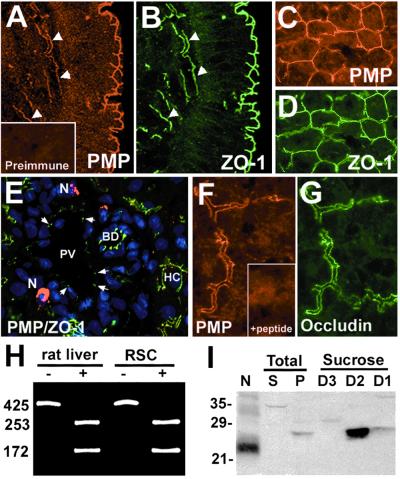
Previous studies have shown high levels of PMP22 mRNA in various non-neural tissues (2–4); however, to date the localization and the expression of protein at these sites has not been determined. Using a TX-100 pre-extraction immunostaining procedure (25), we detected bright PMP22-like immunoreactivity at the surface epithelium of the mucosa in colon (Fig. 1A) and liver bile canaliculi (Fig. 1 C, E, and F). In colon, PMP22 and ZO-1 are found at apical junctions of epithelial cells and in small blood vessels transversing the submucosa (Fig. 1 A and B, arrowheads). In liver, PMP22 and ZO-1 are colocalized to bile canaliculi (Fig. 1 C and D, respectively); however, only ZO-1, but not PMP22, is present at endothelial cell junctions of the portal vein (Fig. 1E, arrows). Nerve terminals show bright PMP22 and no ZO-1 immunoreactivity (Fig. 1E). In addition to ZO-1, PMP22 is colocalized with occludin at bile canaliculi (Fig. 1 F and G, respectively). The lack of PMP22-like immunoreactivities in liver sections incubated with preimmune (Fig. 1A Inset) or antigenic peptide preincubated serum (Fig. 1F Inset) support the specificity of the PMP22-like immunostaining at these novel locations. The localization of PMP22 in colon epithelium and bile canaliculi was verified by eight distinct antibodies, including antisera raised against the first rather than the second extracellular loop of the protein (data not shown).
Figure 1.
PMP22 is coexpressed with ZO-1 and occludin in colon epithelium and bile canaliculi. Frozen sections of normal adult rat colon (A and B) and liver (C–G) were coimmunostained with polyclonal anti-PMP22 (A, C, E, and F) and monoclonal anti-ZO-1 (B, D, and E) or occludin (G) antibodies. (A and B) Confocal images showing the presence of PMP22 at the surface epithelium of the mucosa and in submucosal vasculature (arrowheads). (E) A high-resolution thin section of adult rat liver stained with anti-PMP22 (red), anti-ZO-1 (green) antibodies and nuclear dye (blue). PV, portal vein; N, nerve terminal; BD, bile duct; HC, hepatocyte. Liver sections incubated with preimmune serum (A Inset) or peptide preadsorbed antiserum (F Inset) do not result in TJ-like immunostaining. [Magnifications: ×40 (A–E) and ×60 (F and G).] (H) The expression of PMP22 mRNA in liver was verified by RT-PCR. BsaI undigested (−) and digested (+) PCR-amplified fragments are shown for each sample. The numbers on the left indicate bp. (I) Membrane pellets (P) (75 μg) from adult rat liver homogenates were fractionated and proteins isolated at sucrose densities 1.22 (D3) and 1.18 (D2) and 1.16 (D1) were analyzed (75 μg/lane) for the presence of PMP22. Rat sciatic nerve (N) lysate (4 μg) was used as a positive control for the anti-PMP22 antibody immunoreactivity. S, total liver supernatant (75 μg). Molecular mass, in kDa.
The expression of PMP22 in liver was confirmed by RT-PCR (Fig. 1H) and Western analysis (Fig. 1I). Using specific primers to the rat PMP22 cDNA we detected the identical 425-bp fragment in liver and Schwann cell RNA (Fig. 1H). The identities of the PCR fragments were verified by BsaI restriction enzyme digests. To further corroborate the expression of PMP22 in liver, crude liver membrane pellets were subfractionated by discontinuous sucrose density-gradient ultracentrifugation (Fig. 1I) (27, 28). Although PMP22 is difficult to detect in total liver membrane preparations, in bile canaliculi-enriched fractions [sucrose density fractions 1 (D1) and 2 (D2)] (26), we observed a significant enrichment for PMP22 (Fig. 1I). The majority of PMP22 was concentrated at the interface of sucrose densities 1.22 and 1.18 (D2) and was absent from the highest sucrose density fraction (D3), which contains nuclei, mitochodria, and erythrocyte ghosts (27). Parallel blots incubated with preimmune or antigenic peptide preadsorbed serum were completely blank at the 21-to 35-kDa range (data not shown). The slower migration of PMP22 in bile canaliculi compared with sciatic nerve is likely caused by differential posttranslational modification of PMP22 in myelin and non-neural tissue.
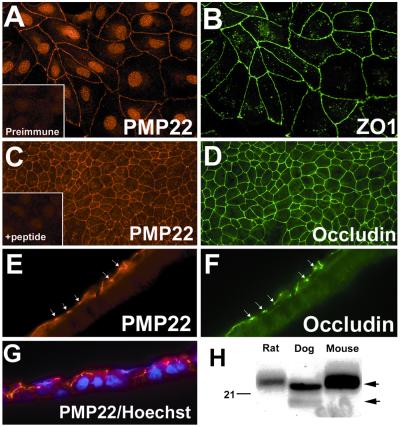
The in vivo tissue localization studies suggest that PMP22 is a component of intercellular junctions in epithelia, therefore we examined the distribution of PMP22 in MDCK cell monolayers. In subconfluent MDCK cell cultures, PMP22 (Fig. 2A) is found at anti-ZO-1 antibody (Fig. 2B) immunoreactive intercellular junctions. The nuclear staining observed with the anti-PMP22 antibody has been described before (30) and, in part, is caused by nonspecific immunoreactivity of the antiserum (Fig. 2 A and C Insets). The distribution of PMP22 in confluent filter-grown MDCK monolayers also was determined (Fig. 2 C–G). Similar to the subconfluent cultures (Fig. 2 A and B), PMP22-like immunoreactivity (Fig. 2 C and E) is colocalized with ZO-1 (not shown) and occludin (Fig. 2 D and F) at intercellular junctions. Sectioned filters, double-stained with anti-occludin and anti-PMP22 antibodies, demonstrate that PMP22 (Fig. 2E) is present at apical cell–cell contacts, similar to occludin (Fig. 2F). The colocalization of PMP22 and occludin at apical intercellular contacts was confirmed by confocal microscopy and rotated three-dimensional images of deconvolution microscopy (not shown). Because MDCK cells are of canine origin, we verified by Western analysis that our anti-PMP22 antibodies raised against human, rat, or mouse peptides can recognize the dog PMP22 (Fig. 2H). As the anti-PMP22 immunoblot shows, we positively identified the dog PMP22 in total sciatic nerve lysates, using an anti-human PMP22 antibody that stains intercellular junctions in MDCK cells (Fig. 2H). On SDS gels, the canine nerve PMP22 has a similar mobility as the rat or the mouse protein (≈22 kDa) and shifts ≈4 kDa upon N-glycosidase treatment (not shown) (30).
Figure 2.
PMP22 is coexpressed with ZO-1 and occludin at cell–cell contacts in MDCK cells. Subconfluent (A and B) and filter-grown (C–G) MDCK cells were immunostained with polyclonal anti-PMP22 (A, C, E, and G) and monoclonal anti-ZO-1 (B), or anti-occludin (D and F) antibodies. (A Inset) Cells stained with preimmune rabbit serum. In filter-grown MDCK cultures PMP22 (C) is codistributed with occludin (D) at apical cell contacts. PMP22 antigenic peptide preadsorbed antiserum does not stain intercellular contacts of MDCK cells (C Inset). On sectioned (8 μm) filters (Z plane) PMP22-like immunoreactivity (E and G) is associated with the apical border of the monolayer, which is also reactive with the anti-occludin (F) antibodies (arrows in E and F). (G) Anti-PMP22 (red) and Hoechst nuclear dye (blue)-stained MDCK cell monolayer is shown. [Magnifications: ×60 (A, B, and E–G) and ×40 (C and D)]. (H) Protein blots of (18 μg/lane) normal adult rat, canine, and mouse sciatic nerves were reacted with anti-PMP22 antiserum. The upper arrow on the right indicates the glycosylated 22-kDa PMP22, while the lower arrow points to the newly synthesized 18-kDa, endoglycosidase H-sensitive protein. Molecular mass, in kDa.
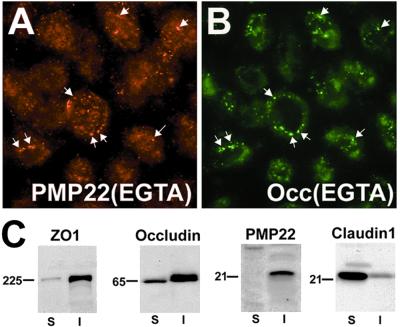
To begin to elucidate the relationship of PMP22 to known tight junctional proteins, filter-grown MDCK cell monolayers were treated with EGTA to disrupt intercellular contacts (Fig. 3). Previous studies showed that such treatment leads to the internalization of proteins found at adherens and TJs (31). After a 1-h EGTA treatment of the cultures, the majority of anti-ZO-1 and anti-occludin antibody immunoreactive intercellular contacts disappeared, and both occludin (Fig. 3B) and ZO-1 (not shown) were internalized in vesicles. Using double immunolabeling, PMP22 and occludin were detected together in a subpopulation of vesicles (arrows in Fig. 3 A and B). These results strongly support that PMP22 is a protein component of apical intercellular junctions in epithelial cells.
Figure 3.
PMP22 is internalized with occludin in EGTA-treated MDCK cells. Confluent MDCK monolayers were cultured in the presence of 4 mM EGTA for 1 h followed by immunostaining with polyclonal anti-PMP22 (A) and monoclonal anti-occludin (B) antibodies. Arrows point to vesicles that contain both PMP22 (A) and occludin (B). (Magnification: ×60.) (C) Confluent MDCK cell monolayers were extracted with 0.5% TX-100 containing buffer and detergent soluble (S), and detergent-insoluble (I) fractions were immunoblotted with antibodies against ZO-1, occludin, PMP22, and claudin-1. Molecular mass, in kDa.
Tight junctional proteins are insoluble in TX-100 (29); therefore we compared the solubility properties of PMP22 to ZO-1, occludin, and claudin-1 (Fig. 3C). Confluent MDCK monolayers were incubated with 0.5% TX-100 containing buffer, and detergent soluble and insoluble fractions (29) were analyzed for the four antigens (Fig. 3C). In agreement with previous studies, we found that the greater portion of ZO-1 and occludin remain in the TX-100 insoluble fraction (29), whereas the majority of claudin-1 is extracted in the detergent (S). The greater solubility of claudin-1 correlates with its high intracellular levels in MDCK cells (Fig. 4B). In contrast to claudin-1, PMP22 is largely insoluble in 0.5% TX-100 containing buffer (I) (Fig. 3C).
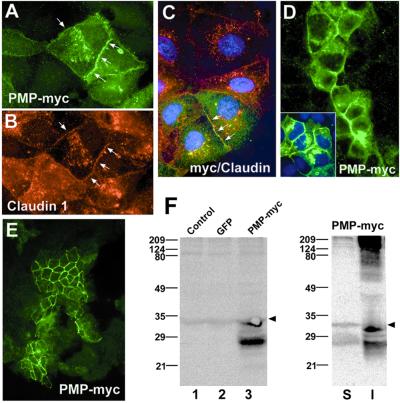
Figure 4.
The exogenously expressed PMP22-myc is targeted to TJs in MDCK cells. pBMN-PMP22-myc-infected cells were cultured on coverslips (A–D) or Transwell filters (E) and immunostained with monoclonal anti-myc (A and C–E), and polyclonal anti-claudin-1 (B and C) antibodies. Note, only ≈15% of the cells (green cells) were infected with the PMP22-myc construct (A and C–E). PMP22-myc is targeted to anti-claudin-1 immunoreactive intercellular contacts (arrows in A–C) and PMP22-myc-expressing cells form contacts with noninfected cells (D). Nuclei were stained with Hoechst dye (C and D Inset). [Magnifications: ×60 (A–C), and ×40 (D and E).] (F) The expression of PMP22-myc was verified by anti-myc Western analysis. Lysates of PMP22-myc-infected cells (lane 3) show expression of a ≈27-kDa and a ≈33-kDa PMP22-myc protein. A nonspecific ≈34-kDa band is present in all samples, including uninfected control (lane 1) and pBMN-GFP (lane 2)-infected cell lysates (arrowheads). The majority of PMP22-myc protein is insoluble in TX-100 (I). S, TX-100 soluble. Molecular mass, in kDa.
To further validate our findings on the apical junctional localization of PMP22 in MDCK cells, we studied the targeting of myc-tagged mouse PMP22 (Fig. 4). A myc epitope tag in the second extracellular loop of PMP22 does not interfere with the normal processing and trafficking of the protein (23, 26). MDCK cells infected with the pBMN-PMP22-myc construct were plated on coverslips or filters and allowed to proliferate. In subconfluent cultures we detected PMP22-myc (Fig. 4 A and C) and claudin-1 (Fig. 4 B and C) at intercellular junctions. Cells that are overexpressing the mouse PMP22-myc are able to integrate and establish intercellular contacts with parental cells (Fig. 4 A, C, and D). Furthermore, in filter-grown MDCK cell monolayers, the exogenous PMP22-myc protein is correctly targeted to apical cell contacts (Fig. 4E). The apical junctional targeting of PMP22-myc also was established by Western analysis (Fig. 4F). Anti-myc immunoblots of control, pBMN-GFP, and PMP22-myc-infected MDCK cells specifically detects an ≈27-kDa and a less abundant ≈33-kDa band in the PMP22-myc sample (Fig. 4F, lane 3). Both ≈27-kDa and ≈33-kDa bands shift upon deglycosylation with N-glycosidase (data not shown) (30). The arrowheads at ≈34 kDa indicate a nonspecific protein that is immunoreactive with the myc antibody in control (Fig. 4F, lane 1) and GFP-infected (Fig. 4F, lane 2) cells. Significantly, similarly to the endogenous canine PMP22 (Fig. 3C), the majority of PMP22-myc is also insoluble in 0.5% TX-100 (Fig. 4F, lane I). In addition to the ≈27-kDa and ≈33-kDa bands, the detergent-insoluble fraction contains a range of high molecular mass anti-myc antibody immunoreactive proteins, which likely represent aggregates of PMP22 multimers (23). Together, these overexpression experiments strongly support that PMP22 is a component of the apical intercellular junctional complex in epithelia.
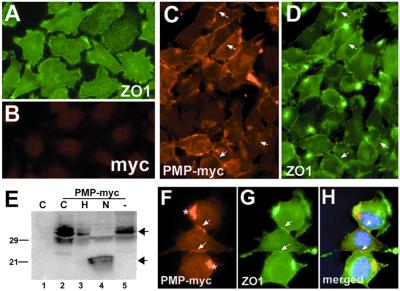
Overexpression of claudins or occludin in nonadherent L fibroblasts has been shown to induce the formation of intercellular contacts, including well-organized TJs (32). Therefore, we examined the targeting of mouse PMP22-myc in mouse L fibroblasts (Fig. 5). Parental L cells express low levels of PMP22 mRNA and undetectable PMP22 protein (data not shown). In agreement with previous studies in parental L cells, ZO-1 is diffusely distributed over cell bodies and concentrated in puncta at processes (Fig. 5A). In cells overexpressing PMP22-myc, we detected anti-myc (Fig. 5 C and F) and anti-ZO-1 (Fig. 5 D and G) immunoreactivity at cell–cell contacts. Well-defined intercellular junctions can be seen, which are immunoreactive with both anti-myc and anti-ZO-1 antibodies (arrows in Fig. 5 C, D, and F–H). Significantly, overexpression of PMP22 alters the distribution of ZO-1 and appears to cause the recruitment of ZO-1 to intercellular contacts (compare Fig. 5 A and D). Although the formation of intermittent intercellular contacts is consistently observed in PMP22-myc infected L cells, overexpression of PMP22 does not appear to induce long strands involving multiple cells, such as described for claudins (32). Cells that have integrated multiple copies of PMP22-myc often contain intracellular PMP22 aggregates, termed aggresomes (Fig. 5F *) (33). Uninfected (Fig. 5B) or pBMN-GFP-infected (not shown) L cells do not adhere together and exhibit low levels of nonspecific immunoreactivity with the myc antibody.
Figure 5.
PMP22-myc is colocalized with ZO-1 at intercellular junctions of L fibroblasts. Uninfected control L cells (A and B) show diffuse ZO-1-like membrane staining with focal concentration at cell processes (A) and low levels of nonspecific immunoreactivity with polyclonal anti-myc (B) antibodies. In PMP22-myc-infected cells, PMP22-myc is detected at cell–cell contacts (arrows in C and F), which are costained with the anti-ZO-1 antibody (arrows in D and G). PMP22-myc is detected in aggresome-like structures in some cells (* in F). Nuclei are stained with Hoechst dye (H). (Magnification: ×60.) (E) The expression and processing of PMP22-myc was studied in lysates of control, uninfected (lane 1), and PMP22-myc-infected L cells (lanes 2–5) by anti-myc Western blotting. Untreated (lane 2), endoglycosidase H (lane 3), N-glycosidase (lane 4), and no enzyme (lane 5) PMP22-myc samples are shown. N-glycosidase (N) treatment of PMP22-myc cell lysates results in a characteristic shift of PMP22, from a mature high molecular mass, endoglycosidase H-resistant form (upper arrow) to a deglycosylated, lower molecular mass core protein (lower arrow). The N-glycosidase resistant, anti-myc immunoreactive ≈29-kDa band likely represents a mono-ubiquitinylated PMP22-myc (lane 4). Molecular mass, in kDa.
The expression and processing of PMP22-myc in L cells was studied by anti-myc Western analysis of endoglycosidase H-treated and N-glycosidase-treated cell lysates (Fig. 5E). Overexpression of PMP22-myc in L cells yields several bands with apparent mobilities ≈30 kDa. A portion of the overexpressed protein is resistant to endoglycosidase H treatment (Fig. 5E, lane 3) and likely represents the membrane fraction of the protein (30). N-glycosidase treatment shifts the majority of these bands to ≈22 kDa, which corresponds to the peptide backbone of PMP22-myc (Fig. 5E, lane 4). The anti-myc immunoreactive ≈29-kDa band in the N-glycosidase-treated sample might represent ubiquitinylated PMP22-myc, which is suggested by the presence of PMP22-myc aggregates in PMP22-myc infected L cells (Fig. 5F *) (33). These L cell overexpression studies suggest that PMP22 might serve an adhesive role at intercellular junctions and through indirect protein interactions it affects the localization of ZO-1.
Discussion
Because of its well-established disease association, PMP22 has received considerable attention during the last decade (1, 34). Although we have gained significant insight into the genetics of PMP22-associated peripheral neuropathies, as well as the intracellular turnover and processing of PMP22 in normal and neuropathy Schwann cells, we still do not understand the function of the protein. Here, we present data on intercellular junctional localization of PMP22 in epithelial cells, suggesting that PMP22 plays a role in cell–cell interactions.
Given that PMP22 is primarily known as a peripheral nervous system myelin component, the expression of PMP22 at epithelial cell junctions may seem unexpected. Nonetheless, our results are in complete agreement with previous studies on the tissue distribution of PMP22 mRNA (2–4). One of the tissues with reported highest levels of PMP22 mRNA is the gastrointestinal tract (3), where we detected bright PMP22-like immunoreactivity at intercellular junctions of absorptive colonic epithelium. In addition to the epithelial cells of the gastrointestinal tract, PMP22 is present at TJs of the liver. Previously, PMP22 mRNA expression was shown to be high in embryonic liver; however, message levels decreased significantly during postnatal development (3). These findings are in agreement with reports describing that the turnover rate of junctional proteins at established membrane contacts is fairly slow (48 h) (36, 37), therefore the rate of PMP22 mRNA and protein synthesis in postembryonic liver is expected to be low. Nonetheless, by RT-PCR we detected PMP22 mRNA in the adult rat liver; and by using an established membrane subfractionation procedure (27), we identified PMP22 in bile canaliculi-enriched liver membrane preparations.
Although PMP22 is expressed in a variety of tissues, the gross pathological findings in PMP22 mutant animals are limited to myelinated peripheral nerves. These data may indicate that Schwann cells are particularly sensitive to PMP22 missexpression and/or that PMP22-related proteins compensate for the normal function of PMP22 in other tissues. A similar compensatory mechanism might operate in occludin-deficient mice, which form morphologically and functionally intact TJs (38). Our results on the epithelial localization of PMP22 warrant a closer examination of PMP22 neuropathy animals, as it is known that certain PMP22 mutant mice display nonglial abnormalities, which are difficult to explain by myelination defects alone. For example, during early postnatal development homozygous trembler J animals exhibit ≈35% reduction in weight compared with wild-type littermates (unpublished data) and die at around postnatal day 18 (39). The homozygous trembler J condition is the only known lethal phenotype associated with PMP22 missexpression and it cannot be explained by peripheral myelination defects alone, as several other peripheral myelin-deficient animals live normal life spans (40). These paradoxes regarding the phenotypes of PMP22 neuropathy animals have puzzled investigators of the field for many years; however, to date possible explanations have not previously been put forth.
The in vivo protein expression studies suggest that PMP22 is a constituent of membrane junctions in epithelia; however, they provide limited information on the relationship of PMP22 to established TJ membrane proteins. The internalization of PMP22 with occludin, in EGTA-treated MDCK cells, supports the notion that PMP22 is a constituent of the apical junctional complex. Ongoing coimmunoprecipitation experiments of MDCK cell lysates indicate that PMP22 interacts with 2–3 as yet to be identified proteins (unpublished data). The detergent solubility properties of PMP22 imply that PMP22 might be associated with occludin and/or ZO-1, or other TX-100-insoluble proteins, rather than with claudin-1. Because PMP22 shares 28% amino acid sequence identity with claudin-1 (41), it is important to note that in this assay the two proteins segregate differently. These results strongly argue against any cross-reactivity of our anti-PMP22 antibodies with claudin-1, or other claudins, as the two proteins are detected in opposite fractions of the cell lysates. Nonetheless, because PMP22 shares significant sequence identity with several members of the claudin gene family (41), our studies raise the question of whether PMP22 may be another claudin. It has recently been established that all well-characterized claudins are able to reconstitute long TJ strands in fibroblasts (11). Previous studies performed in HeLa cells (42) and our results in L cells suggest that PMP22 has adhesive properties, as it can mediate intercellular contacts between nonadherent cells and is able to recruit ZO-1 to newly formed cell junctions (Fig. 5). In comparison to L cells, PMP22 overexpression does not appear to induce intercellular adhesion in C6 glioma cells (41), a central nervous system-derived tumor cell line. These differences in response to PMP22 overexpression are likely the results of cell specificities in endogenous junctional molecules and/or differences in the processing and trafficking of the overexpressed PMP22. Nonetheless, further studies will be necessary to examine the ultrastructure of these newly formed membrane junctions, as claudins are known to mediate the assembly of long fibrils, in comparison to short strands that are formed by occludin overexpression (16, 32).
Besides structural similarities, PMP22 shares functional properties with some of the claudins. Recent reports revealed that single point mutations in claudin-16 and claudin-14 cause kidney and hearing abnormalities, respectively (13, 14). Of the known claudins, claudin-15 is the most homologous to PMP22, sharing 30% identity and 54% similarity in their amino acid sequences (unpublished data). Although the total molecular mass of the claudins and PMP22 is identical (22 kDa), 4 kDa of the total molecular mass of PMP22 is comprised of carbohydrate that is attached to Asn-41 in the first extracellular loop. This carbohydrate motif has a role in the homodimerization of PMP22 (23, 26) and is required for the cell spreading effect observed in PMP22-overexpressing fibroblasts (35). The carbohydrate modification of PMP22 in epithelial cells is unknown, but it could have a role in mediating homophilic interaction between neighboring cells.
The studies described here provide insights into the potential function of PMP22 in membrane physiology. Our results demonstrate that PMP22 is a protein component of intercellular junctions, where it might mediate the formation of cell-to-cell contacts and/or stabilize membrane contacts. A similar role for PMP22 in the Schwann cells membrane could explain the demyelinating phenotypes associated with various forms of PMP22 misexpression.
Acknowledgments
We thank Drs. Steven Sugrue and Toby Ferguson for critical reading of the manuscript. These studies were supported by the Howard Hughes Medical Institute's Biomedical Research Support Program for Medical Schools and the Muscular Dystrophy Association (to L.N.).
Abbreviations
- PMP22
peripheral myelin protein 22
- ZO
zonula occludens
- TJ
tight junction
- MDCK
Madin–Darby canine kidney
- GFP
green fluorescent protein
- RT-PCR
reverse transcriptase–PCR
References
- 1.Suter U, Snipes G J. Annu Rev Neurosci. 1995;18:45–75. doi: 10.1146/annurev.ne.18.030195.000401. [DOI] [PubMed] [Google Scholar]
- 2.Taylor V, Welcher A, Suter U. J Biol Chem. 1995;270:28824–28883. doi: 10.1074/jbc.270.48.28824. [DOI] [PubMed] [Google Scholar]
- 3.Baechner D, Liehr T, Hameister H, Altenberger H, Grehl H. J Neurosci Res. 1995;42:733–741. doi: 10.1002/jnr.490420602. [DOI] [PubMed] [Google Scholar]
- 4.Wulf P, Bernhardt R, Suter U. J Neurosci Res. 1999;57:467–478. [PubMed] [Google Scholar]
- 5.Mugnaini E, Schnapp B. Nature (London) 1974;251:725–727. doi: 10.1038/251725a0. [DOI] [PubMed] [Google Scholar]
- 6.Shinowara N L, Beutel W B, Revel J P. J Neurocytol. 1980;9:15–38. doi: 10.1007/BF01205225. [DOI] [PubMed] [Google Scholar]
- 7.Morita K, Furuse M, Fujimoto D, Tsukita S. Proc Natl Acad Sci USA. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bronstein J M, Popper P, Micevych P E, Farber D B. Neurology. 1996;47:772–778. doi: 10.1212/wnl.47.3.772. [DOI] [PubMed] [Google Scholar]
- 9.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitic L L, Van Itallie C M, Anderson J M. Am J Physiol. 2000;279:G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 11.Tsukita S, Furuse M, Itoh M. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 12.Rahner C, Mitic L L, Anderson J M. Gastroenterology. 2001;120:411–422. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 13.Simon D, Lu Y, Choate K, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, et al. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 14.Wilcox E, Burton Q, Naz S, Riazuddin S, Smith T, Ploplis B, Belyantseva I, Ben-Yosef T, Liburd N, Morell R, et al. Cell. 2001;104:165–172. doi: 10.1016/s0092-8674(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 15.Furuse M, Fujimoto K, Sato N, Hirase T, Tsukita S, Tsukita S. J Cell Sci. 1996;109:429–435. doi: 10.1242/jcs.109.2.429. [DOI] [PubMed] [Google Scholar]
- 16.Van Itallie C, Anderson J M. J Cell Sci. 1997;110:1113–1121. doi: 10.1242/jcs.110.9.1113. [DOI] [PubMed] [Google Scholar]
- 17.Wong V, Gumbiner B M. J Cell Biol. 1997;139:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fanning A S, Jameson B J, Jesaitis L A, Anderson J M. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 19.Zahraoui A, Louvard D, Galli T. J Cell Biol. 2000;151:F31–F36. doi: 10.1083/jcb.151.5.f31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Notterpek L, Snipes G J, Shooter E M. Glia. 1999;25:358–369. [PubMed] [Google Scholar]
- 21.Gumbiner B, Simons K. J Cell Biol. 1986;102:457–468. doi: 10.1083/jcb.102.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kartenbeck J, Schmelz M, Franke W W, Geiger B. J Cell Biol. 1991;113:881–892. doi: 10.1083/jcb.113.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tobler A R, Notterpek L, Naef R, Taylor V, Suter U, Shooter E M. J Neurosci. 1999;19:2027–2036. doi: 10.1523/JNEUROSCI.19-06-02027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hitoshi Y, Lorens J, Kitada S I, Fisher J, LaBarge M, Ring H Z, Francke U, Reed J C, Kinoshita S, Nolan G P. Immunity. 1998;8:461–471. doi: 10.1016/s1074-7613(00)80551-8. [DOI] [PubMed] [Google Scholar]
- 25.Itoh M, Nagafuchi A, Moroi S, Tsukita S. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan M C, Notterpek L, Tobler A R, Liu N, Shooter E M. J Neurochem. 2000;75:1465–1474. doi: 10.1046/j.1471-4159.2000.0751465.x. [DOI] [PubMed] [Google Scholar]
- 27.Song C S, Rubin W, Rifkind A B, Kappas A. J Cell Biol. 1969;41:124–132. doi: 10.1083/jcb.41.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsukita S, Tsukita S. J Cell Biol. 1989;108:31–41. doi: 10.1083/jcb.108.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jou T S, Schneeberger E E, Nelson W J. J Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pareek S, Notterpek L, Snipes G J, Naef R, Sossin W, Laliberté J, Iacampo S, Suter U, Shooter E M, Murphy R A. J Neurosci. 1997;17:7754–7762. doi: 10.1523/JNEUROSCI.17-20-07754.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cereijido M, Shoshani L, Contreras R G. Am J Physiol. 2000;279:G477–G482. doi: 10.1152/ajpgi.2000.279.3.G477. [DOI] [PubMed] [Google Scholar]
- 32.Furuse M, Sasaki H, Fujimoto D, Tsukita S. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Notterpek L, Ryan M C, Tobler A T, Shooter E M. Neurobiol Dis. 1999;6:450–460. doi: 10.1006/nbdi.1999.0274. [DOI] [PubMed] [Google Scholar]
- 34.Naef R, Suter U. Micros Res Tech. 1998;41:359–371. doi: 10.1002/(SICI)1097-0029(19980601)41:5<359::AID-JEMT3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Brancolini C, Edomi P, Marzinotto S, Schneider C. Mol Biol Cell. 2000;11:2901–2914. doi: 10.1091/mbc.11.9.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasdar M, Nelson W J. J Cell Biol. 1989;109:163–177. doi: 10.1083/jcb.109.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy K M, Francis S A, McCormack J M, Lai J, Rogers R A, Skare I B, Lynch R D, Schneeberger E E. J Cell Sci. 2000;113:3387–3398. doi: 10.1242/jcs.113.19.3387. [DOI] [PubMed] [Google Scholar]
- 38.Saitou M, Furuse M, Sasaki H, Schulzke J, Fromm M, Takano H, Noda T, Tsukita S. Mol Biol Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henry E, Cowen J, Sidman R. J Neuropathol Exp Neurol. 1983;42:688–706. doi: 10.1097/00005072-198311000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Martini R, Schachner M. Glia. 1997;19:298–310. [PubMed] [Google Scholar]
- 41.Takeda Y, Notsu T, Kitamura K, Uyemura K. Neurochem Res. 2001;26:599–607. doi: 10.1023/a:1010927001378. [DOI] [PubMed] [Google Scholar]
- 42.D'Urso D, Ehrhardt P, Muller H W. J Neurosci. 1999;19:3396–3403. doi: 10.1523/JNEUROSCI.19-09-03396.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]