Abstract
During morphogenesis, cells communicate with each other to shape tissues and organs. Several lines of recent evidence indicate that ion channels play a key role in cellular signaling and tissue morphogenesis. However, little is known about the scope of specific ion-channel types that impinge upon developmental pathways. The Drosophila melanogaster wing is an excellent model in which to address this problem as wing vein patterning is acutely sensitive to changes in developmental pathways. We conducted a screen of 180 ion channels expressed in the wing using loss-of-function mutant and RNAi lines. Here we identify 44 candidates that significantly impacted development of the Drosophila melanogaster wing. Calcium, sodium, potassium, chloride, and ligand-gated cation channels were all identified in our screen, suggesting that a wide variety of ion channel types are important for development. Ion channels belonging to the pickpocket family, the ionotropic receptor family, and the bestrophin family were highly represented among the candidates of our screen. Seven new ion channels with human orthologs that have been implicated in human channelopathies were also identified. Many of the human orthologs of the channels identified in our screen are targets of common general anesthetics, anti-seizure and anti-hypertension drugs, as well as alcohol and nicotine. Our results confirm the importance of ion channels in morphogenesis and identify a number of ion channels that will provide the basis for future studies to understand the role of ion channels in development.
Keywords: Ion channels, channelopathy, bioelectricity, Drosophilia, wing development
Ion channels are well known for their importance in excitable cells such as neurons and muscle cells, but there is also growing evidence that ion channels play a key role in regulating developmental signaling pathways, even in tissues that are non-excitable in adults. Evidence for the importance of ion channels in development can be found in the number of human syndromes associated with morphological defects caused by ion channel mutations. These defects commonly include craniofacial, limb, and digit dysmorphisms. For example, a gain-of-function missense mutation in CACNA1C, a gene encoding an L-type calcium channel, causes Timothy Syndrome (Splawski et al. 2004). Timothy Syndrome is associated with a high incidence of small upper jaw, thin upper lip, low-set ears, syndactyly (fusion of the digits of the hands or feet), and dental defects (Splawski et al. 2004). Similarly, Anderson-Tawil Syndrome, caused by mutations in the gene encoding the inwardly-rectifying potassium channel Kir2.1, leads to syndactyly and clinodactyly (curvature of the fingers or toes) as well as low-set ears, small lower jaw, cleft palate, and dental abnormalities (Plaster et al. 2001). Other channelopathies associated with a high incidence of morphological abnormalities include Temple-Baraitser Syndrome, caused by a gain-of-function mutation in the voltage-gated potassium channel EAG1, Birk-Barel Syndrome, caused by a mutation in the two-pore potassium channel KCNK9, and Keppen-Lubinsky syndrome, caused by disruption of the inwardly-rectifying potassium channel GIRK2 (Barel et al. 2008; Chong et al. 2015; Masotti et al. 2015; Simons et al. 2015).
While the importance of ion channels in development is becoming increasingly apparent, the mechanisms by which ion channel mutations disrupt developmental signaling pathways are not fully understood. Ion channels control the transmembrane potential (Vmem) of cells. Cells within an organism have varying resting potentials creating a “bioelectric” pattern across tissues. This pattern is important for proliferation and migration as well as correct left-right patterning, tissue and organ patterning, and organ size (Levin et al. 2017; Levin 2014). Changes in this transmembrane potential pattern result in significant defects in development across multiple organisms. In planarians, changing the Vmem gradient can cause amputated trunks to regrow heads in place of tails, resulting in two-headed organisms (Durant et al. 2017). In Xenopus laevis, clusters of hyperpolarized cells are found at the locations of eyes during embryogenesis (Pai et al. 2012). Depolarization of these cells results in eye malformation while hyperpolarization of non-eye cells can induce the formation of ectopic eyes (Pai et al. 2012). The Vmem pattern has been found to be important within mammalian systems as well, leading to the proposal of a “bioelectric prepattern” dictating the formation of the face (Adams et al. 2016).
In Drosophila melanogaster, ion channels have been found to play a key role in early development. In Drosophila ovarian follicles during oogenesis the Vmem changes by developmental stage (Krüger and Bohrmann 2015; Woodruff et al. 1988). These changes in transmembrane potentials have been found to influence protein movement and distribution in the oocyte (Woodruff et al. 1988; Cole and Woodruff 2000). Vmem patterns were found to correspond with distribution patterns of calcium channels, sodium channels, proton pumps, and gap junctions (Krüger and Bohrmann 2015). When the gap junction Innexin 2 is inhibited during oogenesis, defects in oocyte development occur, further supporting the importance of these ion channels in early Drosophila development (Bohrmann and Zimmermann 2008). Later on in Drosophila development, proper functioning of the inwardly rectifying potassium channel Irk2 has been found to be essential for wing growth and patterning, suggesting that ion channels continue to influence development in Drosophila beyond oogenesis (Dahal et al. 2012; Dahal et al. 2017).
While it is becoming increasingly evident that ion channels are important for development, it is still not fully known which ion channel types contribute to developmental signaling pathways. Drosophila melanogaster is an excellent model in which to address this question because the Drosophila wing is acutely sensitive to changes in developmental pathways. Disruptions of the BMP/Dpp, Notch, Hedgehog, or Wingless/WNT signaling pathways all cause changes in wing development which are easily observed such as abnormal changes in vein patterning and abnormal wing size or shape (Blair 2007). Disruption of the Drosophila ortholog of the Anderson-Tawil Syndrome associated potassium channel Kir2.1 (Irk2), has been previously found to cause severe wing defects, demonstrating that Drosophila wing development is sensitive to ion channel disruptions (Dahal et al. 2017; Dahal et al. 2012). Disruptions of other channels that play roles in development also cause Drosophila wing defects, making the Drosophila wing a useful system in which to identify ion channels that influence morphogenesis.
In this study, we used the Drosophila wing as a readout to screen for ion channels that impact development. We identified 180 ion channel related genes that are expressed in the Drosophila wing disc and then used loss-of-function Drosophila mutant lines or the UAS-GAL4/RNAi system to individually disrupt or knockdown ion channels. We then examined the wing phenotypes of the adult progeny of these lines. Using this approach, we identified 44 ion channel related genes which cause wing development abnormalities when disrupted or knocked down. In the interest of conducting a broad screen, we only looked at one loss-of-function or RNAi knockdown line per ion channel. While deeper interpretation of any of the candidates identified in this screen will require further confirmation of the phenotypes by CRISPR-knockouts, rescue experiments, and other characterizations in lines with differing genetic backgrounds, the results of our screen provide a starting point for further investigation of the role of ion channels in development.
Materials and Methods
Fly stocks
The majority of the Drosophila melanogaster strains used were obtained from the Bloomington Drosophila Stock Center at Indiana University. We selected RNAi lines that were generated by the Transgenic RNAi project, completed in the lab of Norbert Perrimon at Harvard Medical School (Perkins et al. 2015). The irk1, irk2, and irk3 RNAi lines were obtained from the Vienna Drosophila Resource Center (VDRC, www.vdrc.at) (Dietzl et al. 2007). Flies were raised on standard cornmeal food at 25°. The w1118 strain was used as the wildtype control and MS1096-GAL4 x w1118 as the background control for MS1096-GAL4>UAS-RNAi crosses.
Identification of ion channel library
To build a library of ion channels for screening we used the Flybase RNA-seq database (flybase.org) to compile a list of ion channels expressed in Drosophila melanogaster (Gramates et al. 2017). To specifically identify ion channels expressed in the wing we overlaid this list with a library of genes expressed in the wing discs of third instar Drosophila larvae (Ibrahim et al. 2013). We chose to only screen ion channels that had loss-of-function mutant lines or RNAi lines readily available from the Bloomington Drosophila Stock Center or the Vienna Drosophila Resource Center, leaving us with a list of 180 ion channels to screen.
Fly crosses and wing phenotype scoring
For screening of UAS-RNAi strains, virgin MS1096-GAL4 females were crossed with males from each UAS-RNAi strain and their progeny were scored for wing phenotypes. MS1096-GAL4 fly wings were examined as controls for all RNAi knock down lines, and for candidates of interest identified using RNAi, the starting UAS-RNAi lines were also screened for wing phenotypes to control for possible background genotype impacts on wing morphology.
If homozygote mutants were viable, they were scored as homozygotes. If homozygotes were not viable, heterozygote mutants were screened directly for wing defects unless the balancer expressed Serate (Ser) or Curly (Cy) which would interfere with identification of wing defects. Mutant strains balanced with Ser or Cy marked chromosomes were crossed with w1118 virgin females, and heterozygous progeny not expressing Ser or Cy were selected for scoring.
The wings of at least 20 males and 20 females were scored under a stereo microscope for each mutant strain and UAS-RNAi cross. We looked for abnormalities in vein patterning, vein thickness, trichome or bristle pattern, wing size, wing shape, or other notable changes when compared to controls. If any abnormality was observed, wings were mounted on a slide and further observed under a histology microscope (Nikon, eclipse 80I).
Candidates of interest were defined differently for those identified using loss-of-function mutant lines and those identified using MS1096 > RNAi knockdown. Heterozygous MS1096-GAL4 expressing flies have mild wing venation defects with variable penetrance up to 100% for males and a lower penetrance for females (averaging 10.8%). For the MS1096 > RNAi knockdown lines we therefore defined candidates of interest as lines in which female progeny had wing defects with a percent penetrance two standard deviations above the mean penetrance of defects in heterozygous MS1096-GAL4 control female flies (at least 29%). We also examined the starting UAS-RNAi lines for wing phenotypes and only identified lines as candidates of interest if the penetrance of phenotypes was at least two standard deviations above both the starting UAS-RNAi line and the MS1096-GAL4 line.
Less than 1% of w1118 (WT) have visible wing defects. For mutant lines we therefore defined candidates of interest as lines with a wing defect penetrance greater than 20%. This threshold was set intentionally high for mutant lines even though wildtype flies have very low penetrance of wing defects to reduce the likelihood of including false positives among the candidates of interest.
Data Availability
A full list of all RNAi lines screened can be found in Supplementary Table 1 and a full list of loss-of-function mutant lines screened can be found in Supplementary Table 2, with their observed phenotypes and percent penetrance. We provided the stock numbers from the Bloomington Drosophila Stock Center at Indiana University so that the same fly lines may be purchased and our studies can be replicated. Supplemental material available at Figshare: https://doi.org/10.25387/g3.7640345.
Results and Discussion
To identify ion channel genes associated with morphological development, we compiled a library of ion-channel related genes expressed in the Drosophila melanogaster third instar wing disc (Ibrahim et al. 2013). We examined wings of flies that harbor loss-of-function alleles of these ion channels. When mutant alleles did not exist, we drove expression of siRNA against ion channels using MS1096-GAL4. MS1094-GAL4 drives expression in the dorsal compartment of the wing pouch throughout the third instar larval stage allowing us to specifically assess the impact of knocking down an ion channel in the wing disc during development (Capdevila and Guerrero 1994; Lunde et al. 1998).
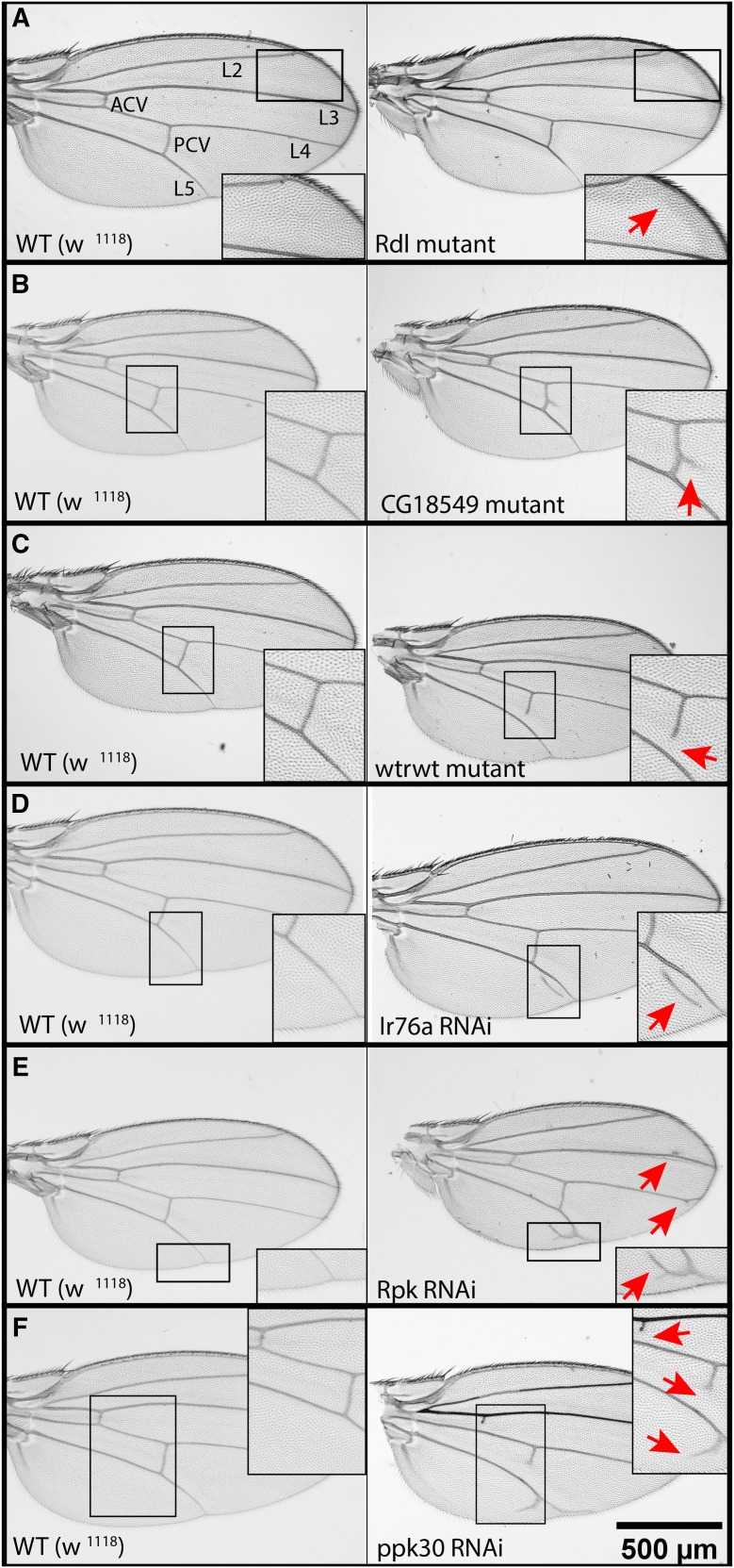
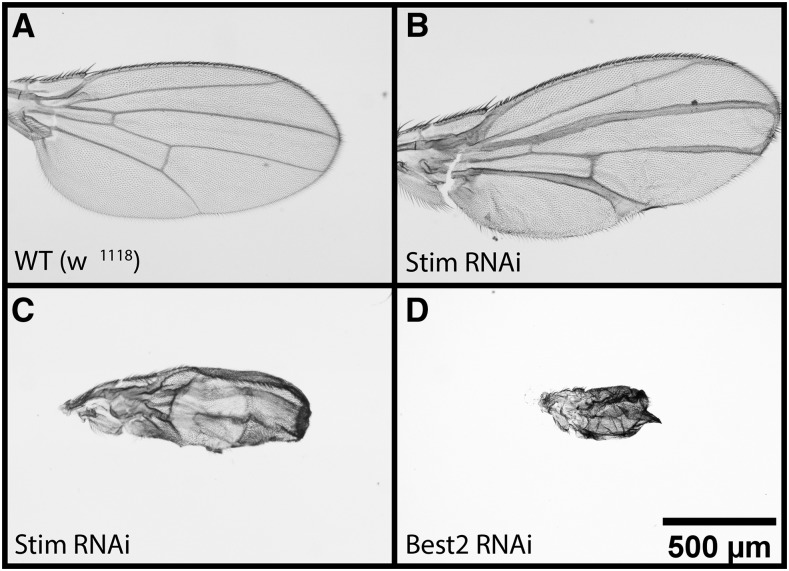
A total of 128 loss-of-function mutant lines and 61 UAS-RNAi lines were scored. One fourth of the ion channels screened induce significant wing phenotypes upon loss-of-function or knockdown in the wing. These phenotypes range from mild to severe, with mild defects including abnormalities in bristle patterning or wing pigmentation, incomplete wing veins, bifurcations of the wing veins, and the presence of ectopic veins (Figure 1). A few of the ion channel disruptions gave more severe wing defects including vein thickening, blistering, or complete shriveling of the wing (Figure 2).
Figure 1.
Examples of observed vein and pigment defects Disruption of 44 of the ion channel related genes screened using either loss-of-function mutations or RNAi wing-specific knockdown resulted in a wide variety of wing defects. Wild type wings have five longitudinal veins and two cross veins (A, left panel). Disruption of the 44 candidates of interest from the screen commonly resulted in abnormal wing pigment (A), posterior cross vein bifurcations (B), incomplete cross veins (C), ectopic veins (D), or longitudinal vein bifurcations (E). Some channel disruptions resulted in wings with multiple venation defects (F). The left column shows wildtype wings with matching wing sections enlarged for comparison with wing defects in right column. Arrows mark defects. The scale bar in the lower right corner represents 500 μm and applies to all panels in the figure.
Figure 2.
Examples of severe wing phenotypes observed Disruption of a few of the ion channels caused more severe defects. Compared to wildtype wings (A) some ion channel disruptions resulted in thickened veins (B), blistering (C), and smaller, shriveled wings (C, D). The scale bar in the lower right corner represents 500 μm and applies to all panels in the figure.
Candidates of interest from our screen were defined as mutant lines in which more than 20% of scored flies had noticeable wing defects or RNAi knockdown lines in which more than 29% of the scored flies had wing defects (see Methods for details).
Using this approach, we identified 15 RNAi knockdown lines (Table 1) and 29 loss-of-function mutant lines (Table 2) with wing abnormalities. In total, 44 unique ion channels that contribute to wing development were identified. The majority of the identified genes (81.8) have not been previously identified as impacting wing development, and 31 of these genes have human orthologs (Table 3). To further examine the candidates of interest, we divided them into six groups based on ion channel type (calcium, sodium, potassium, chloride, ligand-gated cation channel, and other) (Tables 1 and 2). The majority of the ion channels identified in our screen (29.5%) were ligand-gated cation channels, but channels from several categories were represented (Table 4).
Table 1. Summary of candidates identified in screen of RNAi knockdowns.
| Stock ID# | Gene name | Protein Function | % Penetrance Male | % Penetrance Female | % Penetrance Total | Phenotype |
|---|---|---|---|---|---|---|
| Calcium Channels | ||||||
| BL27263 | Stim | CRAC channel regulator | 100 | 100 | 100 | Wings small and malformed, thick veins, blisters |
| BL31295 | nan | Transient receptor potential channel* | 84 | 32 | 54 | L5 incomplete, L5 bifurcation |
| BL31292 | wtrw | Transient receptor potential channel* | 69 | 39 | 53 | PCV incomplete |
| Sodium Channels | ||||||
| BL25847 | rpk | DEG/epithial sodium channel* | 100 | 85 | 92 | L5 incomplete or bifurcation, L4 bifurcation |
| BL27088 | ppk25 | DEG/epithial sodium channel* | 22 | 37 | 30 | PCV bifurcation, L5 bifurcation |
| BL25810 | ppk30 | DEG/epithial sodium channel* | 73 | 30 | 55 | PCV incomplete, L4 & L5 bifurcations, L5 incomplete, |
| Potassium Channels | ||||||
| VDRC 28430 | Irk1 | Inwardly rectifying K+ channel | 100 | 91 | 95 | L5 & L4 bifurcations, loss of ACV, thick veins |
| VDRC 4341 | Irk2 | Inwardly rectifying K+ channel | 93 | 85 | 89 | L5 & L4 bifurcations, loss of ACV, thick veins |
| VDRC 3886 | Irk3 | Inwardly rectifying K+ channel | 100 | 30 | 47 | L5 & L4 bifurcations, loss of ACV, thick veins |
| Chloride Channels | ||||||
| BL42654 | Best2 | Calcium activated chloride channel* | 100 | 100 | 100 | Wings small and severely malformed |
| BL39040 | Best3 | Chloride channel* | 100 | 40 | 66 | Small narrow wings (male), missing ACV, L2 bifurcation, PCV incomplete |
| Ligand-gated Cation Channels | ||||||
| BL62391 | Ir7b | Ionotropic receptor* | 86 | 97 | 92 | PCV incomplete or bifurcation, L5 bifurcation |
| BL34678 | Ir76a | Ionotropic receptor | 97 | 46 | 74 | PCV incomplete, L5 bifurcation |
| BL53975 | Ir94h | Ionotropic receptor* | 24 | 29 | 27 | Bristle defects |
| Other | ||||||
| BL30501 | Inx3 | Gap junction channel | 98 | 38 | 68 | PCV incomplete, L4 bifurcation, L5 bifurcation, L5 incomplete |
At least 20 female and 20 male flies were scored for each line.
BL, Bloomington Drosophila Stock Center number, VDRC, Vienna Drosophila Resource Center number.
PCV, posterior cross vein, ACV, anterior cross vein, L, longitudinal vein.
Function predicted by sequence similarity.
Table 2. Summary of candidates identified in screen of loss-of-function mutant lines.
| Stock ID# | Gene Name | Protein Function | % Penetrance Male | % Penetrance Female | % Penetrance Total | Phenotype |
|---|---|---|---|---|---|---|
| Calcium Channels | ||||||
| BL14156 | stj | Voltage-gated Ca2+ channel* | 48 | 60 | 54 | Bristle defects |
| BL13682 | SERCA | Calcium-transporting ATPase* | 67 | 76 | 40 | Ectopic veins, ectopic bristles, pigment defect |
| BL38067 | brv2 | Calcium channel activity* | 20 | 34 | 28 | PCV bifurcation |
| BL19957 | inaF-A; B; C | Calcium channel regulator | 15 | 50 | 31 | PCV bifurcation |
| Sodium Channels | ||||||
| BL38075 | ppk | DEG/epithial sodium channel | 42 | 52 | 47 | PCV bifurcation |
| BL58557 | ppk17 | DEG/epithial sodium channel* | 18 | 31 | 25 | PCV bifurcation, L2 bifurcation, L3 bifurcation |
| BL37430 | NaCP60E | Voltage gated sodium channel | 81 | 100 | 90 | PCV bifurcation, pigment abnormality |
| BL74 | na | Sodium leak channel complex component* | 37 | 5 | 22 | Bristle defects, ectopic vein |
| BL42469 | unc80 | Sodium leak channel complex component* | 62 | 24 | 43 | PCV bifurcation, ectopic vein, pigment defect |
| BL23397 | unc79 | Sodium leak channel complex regulator* | 9 | 36 | 23 | PCV bifurcation |
| BL13221 | Teh1 | Sodium channel regulator | 70 | 91 | 81 | Black spots below L5 |
| Potassium Channels | ||||||
| BL59167 | Task6 | Two-pore domain potassium channel | 100 | 100 | 100 | PCV bifurcation |
| BL59589 | SLO2 | Calcium activated potassium channel | 6 | 37 | 23 | PCV incomplete |
| BL22837 | Shaker | Voltage-gated potassium channel | 21 | 85 | 54 | PCV bifurcation |
| BL37284 | KCNQ | Voltage-gated potassium channel | 20 | 51 | 29 | PCV bifurcation |
| Chloride Channels | ||||||
| BL6879 | Best1 | Calcium activated chloride channel | 70 | 100 | 85 | PCV bifurcation |
| BL1687 | Rdl | GABA-gated chloride channel | 92 | 95 | 94 | L2 incomplete, ectopic bristles, pigment defect |
| BL6353 | GluClα | Glutamate-gated chloride channel | 60 | 100 | 80 | Bristle defects |
| Ligand-gated Cation Channels | ||||||
| BL44812 | Or47a | Olfactory receptor | 32 | 51 | 42 | PCV bifurcation |
| BL56583 | Ir67a | ionotropic receptor* | 80 | 75 | 77 | PCV bifurcation, L3 bifurcation, thick veins |
| BL31033 | Ir84a | Ionotropic receptor | 50 | 34 | 34 | PCV bifurcation, thick veins |
| BL43017 | Ir92a | Ionotropic receptor | 20 | 23 | 22 | Bristle defects, abnormal vein pigment |
| BL25551 | Ir94g | Ionotropic receptor* | 30 | 8.6 | 20 | Bristle defects, abnormal vein pigment |
| BL37066 | GluRIIB | Non-NMDA ionotropic glutamate receptor | 31 | 56 | 44 | PCV bifurcation |
| BL59216 | mAChR-A | G-protein coupled acetylcholine receptor | 21 | 56 | 38 | PCV bifurcation, L4 incomplete |
| BL24880 | nAChRα7 | Nicotinic acetylcholine receptor | 42 | 67 | 52 | PCV bifurcation |
| BL20783 | nAChRα6 | Nicotinic acetylcholine receptor | 26 | 32 | 29 | PCV bifurcation |
| BL41424 | nAChRα5 | Nicotinic acetylcholine receptor | 15 | 43 | 26 | PCV bifurcation |
| Other | ||||||
| BL59187 | CG18549 | Ion channel regulatory protein* | 69 | 97 | 83 | PCV bifurcation |
At least 20 female and 20 male flies were scored for each line.
BL, Bloomington Drosophila Stock Center number.
PCV, posterior cross vein, L, longitudinal vein.
Function predicted by sequence similarity.
Table 3. Human orthologs of ion channel candidates identified in screen.
| Drosophila melanogaster Gene | Human Ortholog* |
|---|---|
| Calcium Channels | |
| stj | CACNA2D3 |
| SERCA | ATP2A1 |
| brv2 | PKD1L2 |
| Stim | STIM1 |
| nan | TRPV6 |
| inaF-A;B;C | none |
| wtrw | none |
| Sodium Channels | |
| NaCP60E | SCN8A |
| narrow abdomen | NALCN |
| unc80 | UNC80 |
| unc79 | UNC79 |
| rpk | ASIC2 |
| ppk | ASIC2 |
| ppk25 | ASIC4 |
| ppk30 | ASIC3 |
| ppk17 | none |
| Teh1 | none |
| Potassium Channels | |
| Task6 | KCNK9 |
| SLO2 | KCNT1 |
| Shaker | KCNA1 |
| KCNQ | KCNQ4 |
| Irk1 | KCNJ2 |
| Irk2 | KCNJ2 |
| Irk3 | KCNJ2 |
| Chloride Channels | |
| Best1 | BEST2 |
| Best2 | BEST4 |
| Best3 | BEST4 |
| Rdl | GLRA4 |
| GluClα | GLRA1 |
| Ligand-gated Cation Channels | |
| GluRIIB | GRIK1 |
| mAChR-A | CHRM1 |
| nAChRα7 | CHRNA7 |
| nAChRα6 | CHRNA7 |
| nAChRα5 | CHRNA7 |
| Or47a | none |
| Ir7b | none |
| Ir67a | none |
| Ir76a | none |
| Ir84a | none |
| Ir92a | none |
| Ir94g | none |
| Ir94h | none |
| Other | |
| CG18549 | MFSD11 |
| Inx3 | none |
Human orthologs were identified using the DRSC Integrative Ortholog Prediction Tool (Version 7.1) (Hu et al. 2011). Only human orthologs with a DIOPT score > 2 are shown.
Table 4. Number of candidates identified for each ion channel type.
| Ion Channel Type | Number of Candidates | Percentage of Total Candidates |
|---|---|---|
| Ligand-gated cation | 13 | 29.5% |
| Sodium | 10 | 22.7% |
| Calcium | 7 | 15.9% |
| Potassium | 7 | 15.9% |
| Chloride | 5 | 11.4% |
| Other | 2 | 4.5% |
We found a range in penetrance of defects among the candidates of interest in our screen, with some ion channel disruptions (such as Best2 knockdown) resulting in 100% penetrance of wing defects and other channel disruptions giving much lower penetrance of defects. This variability in penetrance could be because increased expression of other ion channels can compensate for reduced function of one ion channel. For example, when irk2 is deleted or knocked down with RNAi, irk1 and irk3 expression increases (Dahal et al. 2012). Each of the ion channels that affected wing morphology was a member of an ion channel family that similarly affects transmembrane potential. It could be that variability of penetrance reflects the differing abilities of ion channels to compensate for other members of the family. Alternatively, the variability in penetrance could be because ion channel disruptions likely impact development by changing the transmembrane potential pattern. Transmembrane potential is regulated by a large number of channels and ions and thus is likely subject to a fairly large amount of biological noise. It has been found that in the nervous system, transmembrane potential often varies due to sources of cellular and molecular noise (Faisal et al. 2008). Transmembrane potential is likely subject to the same noise in non-nervous system tissue, leading to the variability in penetrance that we found in the results of our screen.
The ppk, IR, and Best families are highly represented among the identified ion channels
Among the 44 ion channels that contribute to morphogenesis identified in our screen, several belonged to three gene families: the pickpocket family, the ionotropic receptor family, and the bestrophin family. Five of the identified ion channels (rpk, ppk, ppk17, ppk25, and ppk30) belong to the pickpocket family. Pickpocket family genes encode Degenerin/epithelial sodium (Na+) channels (DEG/ENaCs). Totaling 31 members, the pickpocket family is one of the largest families of ion channel genes in Drosophila melanogaster. These channels are non-voltage gated, amiloride-sensitive sodium channels, and some have been characterized as ligand or mechanosensory-gated (Zelle et al. 2013). Their functions are not well understood, but they have been implicated in chemosensory and mechanosensory roles, with some members playing roles in pheromone detection required for proper male courtship behavior (Ben-Shahar 2011; Adams et al. 1998; Lu et al. 2012; Starostina et al. 2012). While possible developmental functions of the pickpocket genes in Drosophila melanogaster have not been previously investigated, many of the pickpocket genes exhibit changing expression patterns throughout early development, supporting the hypothesis that they may play roles in morphogenesis (Zelle et al. 2013). Interestingly, in both Drosophila melanogaster and in mammals, DEG/ENaC channels have been recently implicated in neuronal roles, with some studies suggesting that they may directly modulate synaptic processes (Hill and Ben-Shahar 2018; Younger et al. 2013). Our results suggest that some members of the pickpocket families may play roles in developmental signaling, further expanding the diverse functions of this family.
Another gene family highly represented in our screen is the Ionotropic Receptor family. Seven of the candidates of interest (Ir7b, Ir67a, Ir76a, Ir84a, Ir92a, Ir94g, and Ir94h) belong to the Ionotropic Receptor family, including three (Ir76a, Ir84a, Ir92a) belonging to the Antennal Ionotropic Receptor subfamily and four (Ir7b, Ir67a, Ir94g, Ir94h) belonging to the Divergent Ionotropic Receptor subfamily. Ionotropic Receptor family members are similar in sequence to Ionotropic glutamate receptors (iGluRs), but they lack glutamate-interacting residues and are thus thought to be non-responsive to glutamate (Benton et al. 2009). These channels are ligand-gated and primarily thought to play chemosensory roles in taste and odor reception (Rytz et al. 2013). The Antennal Ionotropic Receptors are mostly expressed in the antennae and are thought to play roles in odor reception while the Divergent Ionotropic Receptors are expressed in gustatory neurons and play roles in taste (Rimal and Lee 2018). These receptors are expressed at low levels during development and in the developing wing disc, and our results suggest that they play roles in morphogenesis of the wing in addition to their chemosensory roles.
Three members of the Bestrophin family, Best1, Best2, and Best3, were found to contribute to wing morphogenesis. Bestrophins are non-voltage gated chloride channels. Interestingly, disruption of Best2 resulted in the most severe wing defects of all of our candidates of interest. Wing-specific Best2 RNAi expression (using the MS1096-GAL4 driver) caused the wings to be completely shriveled and malformed (Figure 2D). There is evidence that Best2 may be a calcium activated chloride channel (CaC). Best1 may be both a CaC and a volume regulated anion channel (VRAC) (Chien et al. 2006; Chien and Hartzell 2007). Our results indicate that the Bestrophins play a key role in Drosophila wing development suggesting that the chloride current is important for correct morphogenesis. Indeed, five chloride channels were identified in our screen (Table 3).
Multiple genes identified have human orthologs associated with morphological defects
We found several of the ion channels that impact Drosophila wing development have human orthologs with mutations that are associated with morphological defects. Three of the ion channels from our screen, Irk1, Irk2, and Irk3 are the Drosophila orthologs of Kir2.1, which is a channel associated with Andersen-Tawil syndrome (Tristani-Firouzi et al. 2002; Yoon et al. 2006b; Yoon et al. 2006a). We have previously described the effects of Irk/Kir2.1 disruption on fly and mouse development (Dahal et al. 2012; Dahal et al. 2017; Belus et al. 2018). Here we identify seven additional ion channels that have human orthologs that are associated with morphological defects as part of channelopathies in humans (Table 5). These include Task6 (KCNK9), Nan (TRPV4), unc80 (UNC80), narrow abdomen (NALCN), and the nicotinic acetylcholine receptors nAChRα5, nAChRα6, and nAChRα7 (CHRNA7). Interestingly, the genetic lesions that cause the channelopathies associated with these genes in humans are all loss-of-function mutations (Table 5). The Drosophila lines scored in our screen are also loss-of-function or knockdown lines, but it is important to note that human channelopathies usually occur as a result of heterozygous mutations while the majority of the Drosophila lines we looked at were homozygous, representing a more severe reduction in ion channel function.
Table 5. Human channelopathies with Drosophila orthologs identified in screen.
| Drosophila Gene | Drosophila Line Screened | Observed wing phenotype | Human Ortholog | Associated Channelopathy | Human channelopathy lesion type | Human morphological phenotypes |
|---|---|---|---|---|---|---|
| Task6 | Mi(MIC) insertion (BL59167) | PCV bifurcation | KCNK9 | Birk-Barel Syndrome | Heterozygous missense mutation causing channel loss-of-function (Barel et al., 2008) | Facial dysmorphism |
| Nanchung | Wing-specific RNAi knockdown (MS1096 driver with BL31295) | L5 incomplete, L5 bifurcation | TRPV4 | TRPV4 skeletal dysplasias | Variety of heterozygous missense mutations causing channel gain-of-function or loss-of-function (Nilius & Voets 2013) | Wide variety of skeletal dysplasias (see Nilius & Voets, 2013 for phenotype summaries) |
| unc80 | Mi(MIC) insertion (BL42469) | PCV bifurcation, ectopic vein, pigment defect | UNC80 | IHPRF2 | Homozygous missense and truncating mutations causing channel loss-of-function (Stray-Pedersen et al., 2016) | Facial dysmorphism, small hands and feet (Stray-Pedersen et al., 2016) |
| narrow abdomen | Hypomorphic allele (BL74) | Bristle defects, ectopic vein | NALCN | IHPRF1 | Homozygous missense and nonsense mutations causing channel loss-of-function (Al-Sayed et al., 2013) | Facial dysmorphism (Al-Sayed et al., 2013) |
| narrow abdomen | Hypomorphic allele (BL74) | Bristle defects, ectopic vein | NALCN | CLIFAHDD | Heterozygous missense mutations in the pore-forming domain, suspected to have a dominant-negative effect (Chong et al., 2015) | Severe facial dysmorphism, limb and digit deformities (Chong et al., 2015) |
| nAChRα5 | Mi(MIC) insertion (BL41424) | PCV bifurcation | CHRNA7 | 15q13.3 microdeletion syndrome | Heterozygous or homozygous deletion of CHRNA7 (Hoppman-Chaney et al., 2013) | Facial dysmorphism (Hoppman-Chaney et al., 2013) |
| nAChRα6 | P-element insertion (BL20783) | PCV bifurcation | ||||
| nAChRα7 | Antimorphic allele (BL24880) | PCV bifurcation |
PCV, posterior cross vein, L, longitudinal vein, BL, Bloomington Drosophila Stock Center number.
Twik related acid-sensitive K+ channel 6 (Task6) encodes a two-pore-domain potassium channel and is the Drosophila ortholog of the human KCNK9 gene. Heterozygous KCNK9 loss-of-function mutations in humans cause Birk-Barel syndrome, a channelopathy associated with craniofacial defects including elongated face, downturned eyelids, protruding ears, and cleft palate (Barel et al. 2008).
Another channel identified in our screen, Nanchung (Nan), a transient receptor potential channel, is the Drosophila ortholog of TRPV4. Both loss-of-function and gain-of-function heterozygousTRPV4 mutations are associated with high number of skeletal dysplasia disorders that cause skeletal defects such as scoliosis and brachydactyly (shortening of the fingers) (Nilius and Voets 2013).
We found that wing morphogenesis was also affected by the reduced function of unc80 and narrow abdomen, the Drosophila orthologs of UNC80 and NALCN, respectively. Together with UNC79, these proteins form a cation channel complex (Lu et al. 2010). Loss-of-function homozygous mutations in NALCN cause infantile hypotonia with psychomotor retardation and characteristic facies-1 (IHPRF1) and loss-of-function homozygous mutations in UNC80 cause infantile hypotonia with psychomotor retardation and characteristic facies-2 (IHPRF2) (Bramswig et al. 2018; Stray-Pedersen et al. 2016; Al-Sayed et al. 2013). These are two closely related channelopathies associated with mild dysmorphic facial features (Bramswig et al. 2018; Stray-Pedersen et al. 2016; Al-Sayed et al. 2013). Some heterozygous mutations in NALCN, speculated to be dominate-negative mutations, cause congenital contractures of the limbs and face, hypotonia, and developmental delay (CLIFAHDD) (Chong et al. 2015). CLIFAHDD is a congenital disorder associated with severe craniofacial defects and limb deformities (Chong et al. 2015). In our screen, homozygous loss-of-function mutations in the Drosophila orthologs unc80 and narrow abdomen both caused wing defects, indicating that these two proteins may play conserved roles in morphogenesis.
Disrupted function of the nicotinic acetylcholine receptors nAChRα5, nAChRα6, and nAChRα7 were also identified in our screen. These three nicotinic acetylcholine receptors are the Drosophila orthologs for the human alpha7 nicotinic acetylcholine receptor (encoded by CHRNA7). A 15q13.3 microdeletion syndrome, in which CHRNA7 and five other genes are deleted, causes facial and digital dysmorphisms (Sharp et al. 2008). Single-gene deletions of CHRNA7 also cause 15q13.3 microdeletion syndrome phenotypes, suggesting that deletion of CHRNA7 is the cause of the syndrome (Hoppman-Chaney et al. 2013). Our screen identified all three of the Drosophila orthologs of CHRNA7 indicating that this nicotinic acetylcholine receptor likely plays a conserved role in development.
Ion channel compensation effects
While we identified 44 ion channels in our screen, it is likely that our results underestimate the true scope of ion channels involved in wing development. Ion channels are often made up of multiple subunits or have multiple family members that are able to compensate for each other when a single channel is disrupted or deleted. In both developmental and non-developmental contexts (such as in cardiac cells) disruption of a single ion channel can cause upregulation of different ion channels to compensate, masking potential phenotypes (Dahal et al. 2012; Rosati and McKinnon 2004). This impact of compensation may be more significant for ion channels that come from large families with many members that could potentially compensate for the loss of one member. It is interesting to note that in the results from our screen, ion channels identified from large families such as the pickpocket family (with 31 members) gave more subtle phenotypes than those from smaller families such as the Bestrophin family (with only four members). This may be a result of ion channel compensation, with other ion channel family members being able to perform the function of the disrupted channels to prevent more severe defects from occurring.
Potential impacts
To confirm the results of the channels in our screen, more experiments will have to be done using rescues and disruptions in other background phenotypes. However, If conserved developmental roles are found for the channels identified in our screen, this would have important implications in human health as ion channels are one of the top targets of known drugs (Overington et al. 2006). We used the drug–gene interaction database (DGIdb, www.dgidb.org) to look for known drugs that act upon the human orthologs of the ion channels identified in our screen (Cotto et al. 2018). We found that many of the human orthologs of the ion channels that we identified interact with common general anesthetics such as halothane, sevoflurane, isoflurane, and desflurane. Other ion channels that impact wing morphogenesis in flies interact with anti-hypertension drugs such as amiloride, nilvadipine, verapamil, mibefradil. Another subset of ion channels that we found to impact morphogenesis interact with anti-seizure drugs such as topiramate, phenacemide, ezogabine, zonisamide. If the ion channels identified in our screen have conserved roles in morphogenesis, the use of drugs like these during pregnancy needs to be examined closely. In addition, alcohol is known to act upon Kir channels, human orthologs of Irk1, Irk2, and Irk3, which were identified as modifiers of wing development (Dahal et al. 2012; Bates 2013). Furthermore, nicotine acts upon nicotinic acetylcholine receptors, three of which were identified as modifiers of development in our screen (nAChRα5, nAChRα6, and nAChRα7). Our results may help to explain the known effects of maternal smoking on fetal development (Hackshaw et al. 2011).
Conclusion
Overall, our screen identified 44 ion channels that impact morphogenesis of the Drosophila melanogaster wing, underscoring the overall importance of ion channels in development. It will be interesting to investigate which specific morphogenic pathways are impacted by the disruption of these channels and the mechanisms by which these ion channels impinge upon these pathways.
Acknowledgments
We would like to thank students from the High School Science and Technology Research program including America Salas, Santia Gomez, Zenetta Zepeda, and Jalalludin Besharat for their contributions in the screen. We would like to thank undergraduate research interns Alana Karat and Abhinav Shrestha for contributions to the effort of screening fly wings. We would like to thank the National Science Foundation, grant number NSF-IOS 1354282 for funds to start this project. We could not have done this project without the Bloomington Drosophila Stock Center providing us with fly stocks that they maintain.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25387/g3.7640345.
Communicating editor: E. Gavis
Literature Cited
- Adams C. M., Anderson M. G., Motto D. G., Price M. P., Johnson W. A., et al. , 1998. Ripped pocket and pickpocket, novel Drosophila DEG/ENaC subunits expressed in early development and in mechanosensory neurons. J. Cell Biol. 140: 143–152. 10.1083/jcb.140.1.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. S., Uzel S. G., Akagi J., Wlodkowic D., Andreeva V., et al. , 2016. Bioelectric signalling via potassium channels: a mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome. J. Physiol. 594: 3245–3270. 10.1113/JP271930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sayed M. D., Al-Zaidan H., Albakheet A., Hakami H., Kenana R., et al. , 2013. Mutations in NALCN cause an autosomal-recessive syndrome with severe hypotonia, speech impairment, and cognitive delay. Am. J. Hum. Genet. 93: 721–726. 10.1016/j.ajhg.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barel O., Shalev S. A., Ofir R., Cohen A., Zlotogora J., et al. , 2008. Maternally inherited Birk Barel mental retardation dysmorphism syndrome caused by a mutation in the genomically imprinted potassium channel KCNK9. Am. J. Hum. Genet. 83: 193–199. 10.1016/j.ajhg.2008.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E. A., 2013. A potential molecular target for morphological defects of fetal alcohol syndrome: Kir2.1. Curr. Opin. Genet. Dev. 23: 324–329. 10.1016/j.gde.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Belus M. T., Rogers M. A., Elzubeir A., Josey M., Rose S., et al. , 2018. Kir2.1 is important for efficient BMP signaling in mammalian face development. Dev. Biol. Mar 20. pii: S0012-1606(17)30829-1. 10.1016/j.ydbio.2018.02.012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar Y., 2011. Sensory functions for degenerin/epithelial sodium channels (DEG/ENaC). Adv. Genet. 76: 1–26. 10.1016/B978-0-12-386481-9.00001-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R., Vannice K. S., Gomez-Diaz C., Vosshall L. B., 2009. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136: 149–162. 10.1016/j.cell.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair S. S., 2007. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu. Rev. Cell Dev. Biol. 23: 293–319. 10.1146/annurev.cellbio.23.090506.123606 [DOI] [PubMed] [Google Scholar]
- Bohrmann J., Zimmermann J., 2008. Gap junctions in the ovary of Drosophila melanogaster: localization of innexins 1, 2, 3 and 4 and evidence for intercellular communication via innexin-2 containing channels. BMC Dev. Biol. 8: 111 10.1186/1471-213X-8-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramswig N. C., Bertoli-Avella A. M., Albrecht B., Al Aqeel A. I., Alhashem A., et al. , 2018. Genetic variants in components of the NALCN-UNC80–UNC79 ion channel complex cause a broad clinical phenotype (NALCN channelopathies). Hum. Genet. 137: 753–768. 10.1007/s00439-018-1929-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdevila J., Guerrero I., 1994. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 13: 4459–4468. 10.1002/j.1460-2075.1994.tb06768.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien L. T., Hartzell H. C., 2007. Drosophila bestrophin-1 chloride current is dually regulated by calcium and cell volume. J. Gen. Physiol. 130: 513–524. 10.1085/jgp.200709795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien L. T., Zhang Z. R., Hartzell H. C., 2006. Single Cl- channels activated by Ca2+ in Drosophila S2 cells are mediated by bestrophins. J. Gen. Physiol. 128: 247–259. 10.1085/jgp.200609581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J. X., McMillin M. J., Shively K. M., Beck A. E., Marvin C. T., et al. , 2015. De novo mutations in NALCN cause a syndrome characterized by congenital contractures of the limbs and face, hypotonia, and developmental delay. Am. J. Hum. Genet. 96: 462–473. 10.1016/j.ajhg.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. W., Woodruff R. I., 2000. Vitellogenic ovarian follicles of Drosophila exhibit a charge-dependent distribution of endogenous soluble proteins. J. Insect Physiol. 46: 1239–1248. 10.1016/S0022-1910(00)00044-5 [DOI] [PubMed] [Google Scholar]
- Cotto K. C., Wagner A. H., Feng Y. Y., Kiwala S., Coffman A. C., et al. , 2018. DGIdb 3.0: a redesign and expansion of the drug-gene interaction database. Nucleic Acids Res. 46: D1068–D1073. 10.1093/nar/gkx1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal G. R., Pradhan S. J., Bates E. A., 2017. Inwardly rectifying potassium channels influence Drosophila wing morphogenesis by regulating Dpp release. Development 144: 2771–2783. 10.1242/dev.146647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal G. R., Rawson J., Gassaway B., Kwok B., Tong Y., et al. , 2012. An inwardly rectifying K+ channel is required for patterning. Development 139: 3653–3664. 10.1242/dev.078592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y., et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156. 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- Durant F., Morokuma J., Fields C., Williams K., Adams D. S., et al. , 2017. Long-Term, Stochastic Editing of Regenerative Anatomy via Targeting Endogenous Bioelectric Gradients. Biophys. J. 112: 2231–2243. 10.1016/j.bpj.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisal A. A., Selen L. P., Wolpert D. M., 2008. Noise in the nervous system. Nat. Rev. Neurosci. 9: 292–303. 10.1038/nrn2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramates L. S., Marygold S. J., Santos G. D., Urbano J. M., Antonazzo G., et al. , 2017. FlyBase at 25: looking to the future. Nucleic Acids Res. 45: D663–D671. 10.1093/nar/gkw1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackshaw A., Rodeck C., Boniface S., 2011. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum. Reprod. Update 17: 589–604. 10.1093/humupd/dmr022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. S., Ben-Shahar Y., 2018. The synaptic action of Degenerin/Epithelial sodium channels. Channels (Austin) 12: 262–275. 10.1080/19336950.2018.1495006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppman-Chaney N., Wain K., Seger P. R., Superneau D. W., Hodge J. C., 2013. Identification of single gene deletions at 15q13.3: further evidence that CHRNA7 causes the 15q13.3 microdeletion syndrome phenotype. Clin. Genet. 83: 345–351. 10.1111/j.1399-0004.2012.01925.x [DOI] [PubMed] [Google Scholar]
- Hu Y., Flockhart I., Vinayagam A., Bergwitz C., Berger B., et al. , 2011. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinformatics 12: 357 10.1186/1471-2105-12-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim D. M., Biehs B., Kornberg T. B., Klebes A., 2013. Microarray comparison of anterior and posterior Drosophila wing imaginal disc cells identifies novel wing genes. G3 (Bethesda) 3: 1353–1362. 10.1534/g3.113.006569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger J., Bohrmann J., 2015. Bioelectric patterning during oogenesis: stage-specific distribution of membrane potentials, intracellular pH and ion-transport mechanisms in Drosophila ovarian follicles. BMC Dev. Biol. 15: 1 10.1186/s12861-015-0051-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., 2014. Molecular bioelectricity: how endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol. Biol. Cell 25: 3835–3850. 10.1091/mbc.e13-12-0708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Pezzulo G., Finkelstein J. M., 2017. Endogenous Bioelectric Signaling Networks: Exploiting Voltage Gradients for Control of Growth and Form. Annu. Rev. Biomed. Eng. 19: 353–387. 10.1146/annurev-bioeng-071114-040647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., LaMora A., Sun Y., Welsh M. J., Ben-Shahar Y., 2012. ppk23-Dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS Genet. 8: e1002587 10.1371/journal.pgen.1002587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Zhang Q., Wang H., Wang Y., Nakayama M., et al. , 2010. Extracellular calcium controls background current and neuronal excitability via an UNC79–UNC80-NALCN cation channel complex. Neuron 68: 488–499. 10.1016/j.neuron.2010.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde K., Biehs B., Nauber U., Bier E., 1998. The knirps and knirps-related genes organize development of the second wing vein in Drosophila. Development 125: 4145–4154. [DOI] [PubMed] [Google Scholar]
- Masotti A., Uva P., Davis-Keppen L., Basel-Vanagaite L., Cohen L., et al. , 2015. Keppen-Lubinsky syndrome is caused by mutations in the inwardly rectifying K+ channel encoded by KCNJ6. Am. J. Hum. Genet. 96: 295–300. 10.1016/j.ajhg.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Voets T., 2013. The puzzle of TRPV4 channelopathies. EMBO Rep. 14: 152–163. 10.1038/embor.2012.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overington J. P., Al-Lazikani B., Hopkins A. L., 2006. How many drug targets are there? Nat. Rev. Drug Discov. 5: 993–996. 10.1038/nrd2199 [DOI] [PubMed] [Google Scholar]
- Pai V. P., Aw S., Shomrat T., Lemire J. M., Levin M., 2012. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development 139: 313–323. Erratum: 139: 623. 10.1242/dev.073759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins L. A., Holderbaum L., Tao R., Hu Y., Sopko R., et al. , 2015. The Transgenic RNAi Project at Harvard Medical School: Resources and Validation. Genetics 201: 843–852. 10.1534/genetics.115.180208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaster N. M., Tawil R., Tristani-Firouzi M., Canun S., Bendahhou S., et al. , 2001. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell 105: 511–519. 10.1016/S0092-8674(01)00342-7 [DOI] [PubMed] [Google Scholar]
- Rimal S., Lee Y., 2018. The multidimensional ionotropic receptors of Drosophila melanogaster. Insect Mol. Biol. 27: 1–7. 10.1111/imb.12347 [DOI] [PubMed] [Google Scholar]
- Rosati B., McKinnon D., 2004. Regulation of ion channel expression. Circ. Res. 94: 874–883. 10.1161/01.RES.0000124921.81025.1F [DOI] [PubMed] [Google Scholar]
- Rytz R., Croset V., Benton R., 2013. Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem. Mol. Biol. 43: 888–897. 10.1016/j.ibmb.2013.02.007 [DOI] [PubMed] [Google Scholar]
- Sharp A. J., Mefford H. C., Li K., Baker C., Skinner C., et al. , 2008. A recurrent 15q13.3 microdeletion syndrome associated with mental retardation and seizures. Nat. Genet. 40: 322–328. 10.1038/ng.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons C., Rash L. D., Crawford J., Ma L., Cristofori-Armstrong B., et al. , 2015. Mutations in the voltage-gated potassium channel gene KCNH1 cause Temple-Baraitser syndrome and epilepsy. Nat. Genet. 47: 73–77. Erratum: Nat Genet. 47:304. 10.1038/ng.3153 [DOI] [PubMed] [Google Scholar]
- Splawski I., Timothy K. W., Sharpe L. M., Decher N., Kumar P., et al. , 2004. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 119: 19–31. 10.1016/j.cell.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Starostina E., Liu T., Vijayan V., Zheng Z., Siwicki K. K., et al. , 2012. A Drosophila DEG/ENaC subunit functions specifically in gustatory neurons required for male courtship behavior. J. Neurosci. 32: 4665–4674. 10.1523/JNEUROSCI.6178-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stray-Pedersen A., Cobben J. M., Prescott T. E., Lee S., Cang C., et al. , 2016. Biallelic Mutations in UNC80 Cause Persistent Hypotonia, Encephalopathy, Growth Retardation, and Severe Intellectual Disability. Am. J. Hum. Genet. 98: 202–209. 10.1016/j.ajhg.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristani-Firouzi M., Jensen J. L., Donaldson M. R., Sansone V., Meola G., et al. , 2002. Functional and clinical characterization of KCNJ2 mutations associated with LQT7 (Andersen syndrome). J. Clin. Invest. 110: 381–388. 10.1172/JCI15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff R. I., Kulp J. H., LaGaccia E. D., 1988. Electrically mediated protein movement inDrosophila follicles. Rouxs Arch. Dev. Biol. 197: 231–238. 10.1007/BF02439430 [DOI] [PubMed] [Google Scholar]
- Yoon G., Oberoi S., Tristani-Firouzi M., Etheridge S. P., Quitania L., et al. , 2006a Andersen-Tawil syndrome: prospective cohort analysis and expansion of the phenotype. Am. J. Med. Genet. A. 140: 312–321. 10.1002/ajmg.a.31092 [DOI] [PubMed] [Google Scholar]
- Yoon G., Quitania L., Kramer J. H., Fu Y. H., Miller B. L., et al. , 2006b Andersen-Tawil syndrome: definition of a neurocognitive phenotype. Neurology 66: 1703–1710. 10.1212/01.wnl.0000218214.64942.64 [DOI] [PubMed] [Google Scholar]
- Younger M. A., Muller M., Tong A., Pym E. C., Davis G. W., 2013. A presynaptic ENaC channel drives homeostatic plasticity. Neuron 79: 1183–1196. 10.1016/j.neuron.2013.06.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelle K. M., Lu B., Pyfrom S. C., Ben-Shahar Y., 2013. The genetic architecture of degenerin/epithelial sodium channels in Drosophila. G3 (Bethesda) 3: 441–450. 10.1534/g3.112.005272 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A full list of all RNAi lines screened can be found in Supplementary Table 1 and a full list of loss-of-function mutant lines screened can be found in Supplementary Table 2, with their observed phenotypes and percent penetrance. We provided the stock numbers from the Bloomington Drosophila Stock Center at Indiana University so that the same fly lines may be purchased and our studies can be replicated. Supplemental material available at Figshare: https://doi.org/10.25387/g3.7640345.