Fever of unknown origin (FUO) defined as community- acquired fever of ≥38.3°C lasting ≥3 weeks in immunocompetent individuals generally excludes fever among hospitalized patients or patients with human immunodeficiency virus infection or neutropenia. Due to recent advances in diagnostic technology, including sophisticated imaging tests, improved culture techniques, and molecular diagnostics, there has been increase in cases reported with pathogens previously considered uncommon. At the same time, the realisation that new and subtle forms of immunodeficiency may present in adulthood has prompted practitioners to pursue immunological investigations earlier in the diagnostic workup of infections with unusual pathogens than was done previously. We present a case with FUO in which the isolation of an unexpected pathogen revealed an immunodeficient state that has been recently identified and has long term implications for the patient. We discuss the microbiological findings and laboratory investigations necessary to define the immune deficiency.
CASE REPORT
A 28-year-old Indian man who had been living in Australia for the preceding 3 years, presented to the hospital with a 2-month febrile illness characterized by headaches, abdominal pain, night sweats, and 30kg of weight loss. He reported no significant medical history, medication use, or allergies. At physical examination, he had a fever of 38.3°C and palpable right cervical lymphadenopathy. Initial laboratory testing revealed marked leukocytosis, neutrophilia, and eosinophilia (white blood cell, neutrophil, and eosinophil counts, 32.8 [reference range, 4.0–11.0] × 103/µL, 27.3 [2.0–8.0] × 103/µL, and 1.7 [0.0–0.5] × 103/µL, respectively).
Routine bacterial cultures of blood, urine, sputum and stool were unremarkable. Results of interferon (IFN) γ release assay testing (QuantiFERON-TB Gold) were indeterminate owing to lack of a mitogen response, and results of human immunodeficiency virus serology were negative. Cerebrospinal fluid sampling revealed pleocytosis (cell counts per µL: 693 erythrocytes, 52 polymorphonuclear leukocytes, 87 lymphocytes), an elevated protein level (0.65 mg/dL; reference range, 0.18–0.45 mg/dL), and a normal glucose level (3.0 mmol/L; 2.5–3.5 mmol/L). Computed tomography (CT) of the chest, abdomen, and pelvis revealed a right upper lobe pulmonary infiltrate and widespread lymphadenopathy. Bronchoscopy and bone marrow aspiration revealed neither infection nor cancer.
The patient’s enlarged cervical lymph node was excised for diagnostic testing and empiric treatment for disseminated tuberculosis infection was begun (with isoniazid, rifampicin, pyrazinamide, and ethambutol), including corticosteroids for presumed meningitis. Lymph node culture identified Mycobacterium abscessus complex, but its clinical significance was unclear given a single isolation (Figure 1). After a brief period of stability, the patient’s condition deteriorated, with recrudescence of fever, intractable headaches, and epigastric pain. Whole-body F 18 fluodeoxyglucose (FDG) positron emission tomography/CT scanning revealed an enlarged, mesenteric abdominal lymph node with increased FDG uptake (Figure 2). Histopathology of this sample after surgical excision showed suppurative lymphadenitis without granulomatous inflammation. M. abscessus complex was again cultured, establishing a diagnosis of disseminated M. abscessus infection.
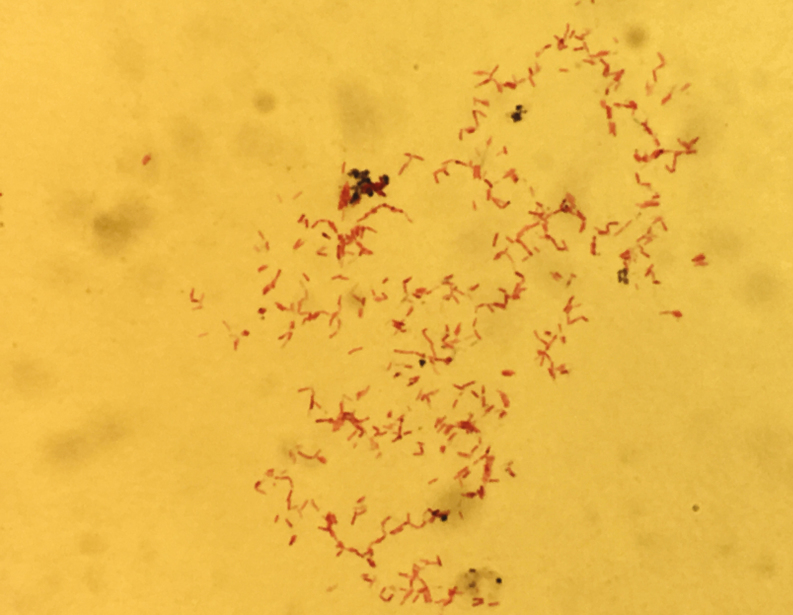
Figure 1.
Ziehl-Neelsen staining of the isolate grown on agar plates from excisional biopsy of the right cervical lymph node. Magnification: ×1000, oil immersion.
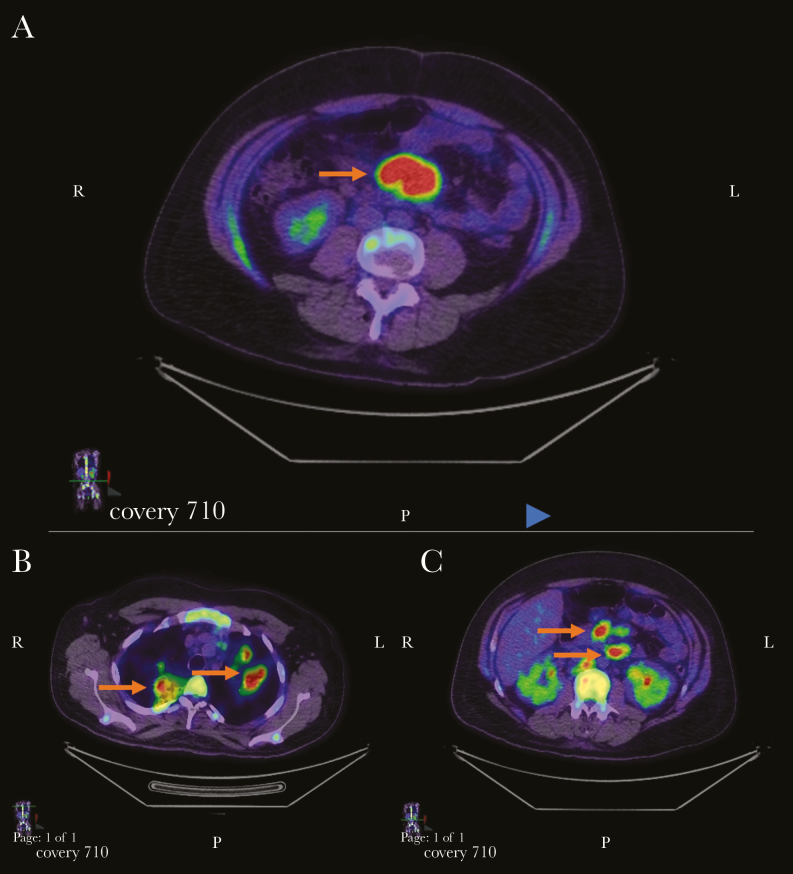
Figure 2.
Axial sections obtained with fludeoxyglucose F 18 (FDG) positron emission tomography/computed tomography, demonstrating lymphadenopathy associated with increased FDG uptake at a mesenteric lymph node (A), pulmonary lymph nodes (B), and upper abdominal lymph nodes (C) (arrows).
Guided by susceptibility testing of the original isolate, an antimicrobial regimen of intravenous cefoxitin, tigecycline, and amikacin was begun. The patient showed clinical improvement with resolution of inflammatory markers and weight gain. Disseminated nontuberculous mycobacterium (NTM) infection in a previously healthy individual prompted investigation for immunodeficiency, the results of which were consistent with serum neutralizing autoantibodies to either IFN-γ (anti–IFN-γ Abs) or its receptor (Figure 3) [1, 2]. Approximately 18 months later, the patient has completed 2 courses of rituximab and 10 months of antimicrobial therapy, with no evidence of disease recurrence.
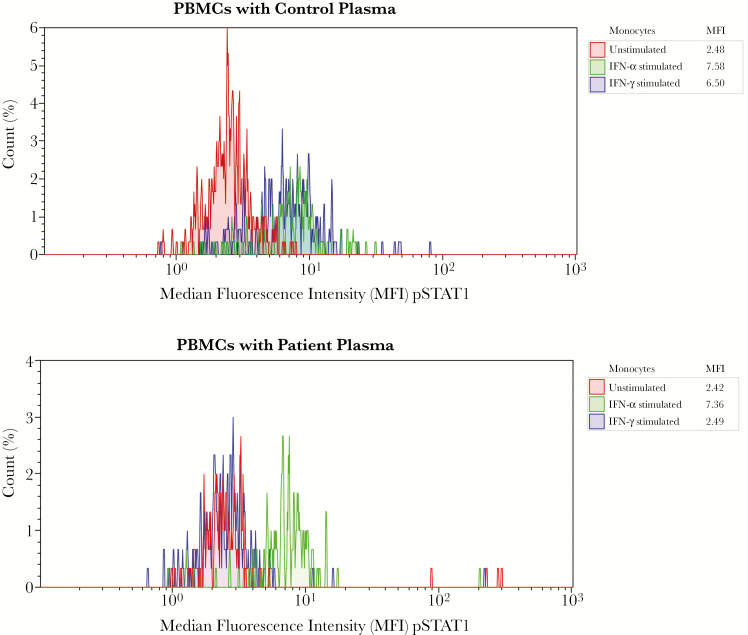
Figure 3.
Flow cytometry of a functional assay for detecting anti–interferon (IFN) γ autoantibodies. Healthy control peripheral blood mononuclear cells were surface stained with CD14-PC5 to identify monocytes and incubated with control (autologous) plasma and patient plasma in 3 conditions: unstimulated, IFN-α stimulated (as an internal control), and IFN-γ stimulated. Cells were stained for intracellular phosphorylated STAT1 (pSTAT1) with anti-STAT1 conjugated to Alexa Fluor 488 (Becton Dickinson), fixed, and then analyzed with flow cytometry. Median fluorescence intensities (MFIs) for pSTAT1 in CD14+ gated monocytes for each condition are compared between unstimulated and stimulated cells cultured in control and patient plasma. Red peak represents unstimulated cells; green peak, IFN-α–stimulated cells; and blue peak, IFN-γ–stimulated cells. Top plot (control plasma) shows increase in MFI, tabled under “MFI,” after stimulation with IFN-α and IFN-γ (blue and green peaks), and bottom plot (patient plasma) shows absence of pSTAT1 when stimulated with IFN-γ (blue peak), suggesting an inhibitor to IFN-γ in the patient’s plasma. This functional assay does not determine whether autoantibodies in the patient’s serum are targeting IFN-γ itself or IFN-γ receptors. Target-specific enzyme-linked immunosorbent assay was not performed for confirmation.
DISCUSSION
The clinical entity of adult-onset disseminated NTM infection resulting from anti–IFN-γ Abs is described in adults of Southeast or East Asian descent with a median age of 48 years [1, 3, 4]. Here we report the first case, to our knowledge, of anti–IFN-γ Abs arising in an individual of Indian origin. Genetic factors include a strong association with the HLA class II alleles DRB1*16 and DQB1*5, initially reported in a Taiwanese cohort, and DRB1*15, later reported in Thai patients [5–7]. We did not perform HLA analysis in our patient, but previous large-scale population studies have shown DRB1*15 as a common allele among individuals from Asian countries, including India, Taiwan, and Thailand [8].
Disseminated NTM infection is the most common infection in individuals with anti –IFN-γ Abs [5]. Frequently isolated organisms include Mycobacterium avium complex, M. abscessus complex, and Mycobacterium fortuitum [3]. Herpesvirus reactivations and certain bacterial infections, such as nontyphoidal Salmonella, have also been described [3]. M. abscessus complex comprises a group of rapidly growing and multidrug-resistant NTM, found widespread in the environment. Infection in humans can result in a range of different clinical manifestations, although skin and soft-tissue and pulmonary infections seem most common [9].
At present, there are no evidence-based guidelines regarding the specific treatment of this type of immunodeficiency. Previous uncontrolled case series include reports of treatment with intravenous immunoglobulin, plasmapheresis, cyclophosphamide, and exogenous IFN-γ to diminish the activity of anti–IFN-γ Abs [3]. Other case series have shown therapeutic benefit with the anti-CD20 monoclonal antibody rituximab [10, 11]. Our patient received 2 doses of rituximab over a 1-month period with continued antimicrobial therapy (10 months in total), after an initial period of 6 months of antimicrobial therapy alone to minimize the chance of recurrence.
In our patient, disseminated M. abscessus infection mimicked the typical clinical manifestations of disseminated tuberculosis and was initially treated as such. There is evidence suggesting that the QuantiFERON-TB Gold assay, which in this setting results in subnormal responses to mitogens, may serve as a screening tool for anti–IFN-γ Ab activity in similar presentations [12, 13]. The test can be especially useful in resource-poor settings where specialized assays to measure IFN-γ activity are not available. Collecting suitable tissue samples for diagnostic testing is also critical, and FDG positron emission tomography/CT allowed for a targeted biopsy to better direct management [14, 15].
Acknowledgments
We would like to thank the Victorian Infectious Diseases References Laboratory (VIDRL) and The Royal Melbourne Hospital (RMH) Microbiology Department for identification and susceptibility testing of the first and second isolates respectively, as well as providing the accompanying micrograph for this article (RMH Microbiology Department). We also thank the Victorian Infectious Diseases Service (VIDS), including nursing and allied health staff, and the Immunology, Haematology, Radiology and Pathology units at RMH for helpful contributions to this case.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med 2012; 367:725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shima K, Sakagami T, Tanabe Y, et al. Novel assay to detect increased level of neutralizing anti-interferon gamma autoantibodies in non-tuberculous mycobacterial patients. J Infect Chemother 2014; 20:52–6. [DOI] [PubMed] [Google Scholar]
- 3. Valour F, Perpoint T, Sénéchal A, et al. ; Lyon TB study group Interferon-γ autoantibodies as predisposing factor for nontuberculous mycobacterial infection. Emerg Infect Dis 2016; 22:1124–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aoki A, Sakagami T, Yoshizawa K, et al. Clinical significance of interferon-γ neutralizing autoantibodies against disseminated nontuberculous mycobacterial disease. Clin Infect Dis 2018; 66:1239–45. [DOI] [PubMed] [Google Scholar]
- 5. Chi CY, Chu CC, Liu JP, et al. Anti-IFN-γ autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood 2013; 121:1357–66. [DOI] [PubMed] [Google Scholar]
- 6. Ku CL, Lin CH, Chang SW, et al. Anti-IFN-gamma autoantibodies are strongly associated with HLA-DR*15:02/16:02 and HLA-DQ*05:01/05:02 across Southeast Asia. J Allergy Clin Immunol 2016; 137:945–8.e8. [DOI] [PubMed] [Google Scholar]
- 7. Phoompoung P, Ankasekwinai N, Pithukpakorn M, et al. Factors associated with acquired anti IFN-γ autoantibody in patients with nontuberculous mycobacterial infection. PLoS One 2017; 12:e0176342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shankarkumar U. Complexities and similarities of HLA antigen distribution in Asian subcontinent. Indian J Hum Genet 2010; 16:108–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee MR, Sheng WH, Hung CC, et al. Mycobacterium abscessus complex infections in humans. Emerg Infect Dis 2015; 21:1638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Browne SK, Zaman R, Sampaio EP, et al. Anti-CD20 (rituximab) therapy for anti-IFN-γ autoantibody-associated nontuberculous mycobacterial infection. Blood 2012; 119:3933–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Czaja CA, Merkel PA, Chan ED, et al. Rituximab as successful adjunct treatment in a patient with disseminated nontuberculous mycobacterial infection due to acquired anti-interferon-gamma autoantibody. Clin Infect Dis 2014; 58:e115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suárez I, Lehmann C, Gruell H, et al. Repurposing QuantiFERON for detection of neutralizing interferon-γ autoantibodies in patients with nontuberculous mycobacterial infections. Clin Infect Dis 2017; 65:518–21. [DOI] [PubMed] [Google Scholar]
- 13. Wu UI, Chuang YC, Sheng WH, et al. Use of QuantiFERON-TB Gold In-tube assay in screening for neutralizing anti-interferon-γ autoantibodies in patients with disseminated nontuberculous mycobacterial infection. Clin Microbiol Infect 2018; 24:159–65. [DOI] [PubMed] [Google Scholar]
- 14. Lin KH, Wang JH, Peng NJ. Disseminated nontuberculous mycobacterial infection mimic metastases on PET/CT scan. Clin Nucl Med 2008; 33:276–7. [DOI] [PubMed] [Google Scholar]
- 15. Meller J, Sahlmann CO, Scheel AK. 18F-FDG PET and PET/CT in fever of unknown origin. J Nucl Med 2007; 48:35–45. [PubMed] [Google Scholar]