Abstract
This case report describes the effective use of Saccharomyces boulardii in a boy with autism spectrum disorder, obsessive compulsive disorder (OCD), and self-injurious behavior (SIB). Gastrointestinal dysfunction and OCD are frequent comorbidities in autism, which may share a common etiology resulting from a disturbance in normal gut microbiota. Alterations in microbial diversity influence neuroinflammation and are linked to mood disorders, abdominal pain, and SIB. S boulardii is a nonpathogenic probiotic yeast that supports a healthy microbiome, enhances immune function, and reduces diarrhea. Treatment with S boulardii successfully reduced OCD and SIB symptoms in this child.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by developmental impairments in multiple domains, which may include language, social communication, repetitive or rigid behaviors, cognitive function, and sensory sensitivity. Gastrointestinal dysfunction is a common comorbidity in ASD and is often presented as chronic diarrhea, constipation, and/or abdominal pain.1 Children with ASD have imbalances in normal gut microbiota,2 which are theorized to affect neurologic function via the gut-brain axis3 Heightened pain sensitivity has been identified in persons with ASD, and children with autism with self-injurious behavior (SIB) exhibited significant increases in pain on a nonverbal pain rating scale.4 Increased microglial activation and elevated levels of inflammatory cytokines underscore the role of neuroinflammation in ASD5 and the relation to gut dysfunction. Prevalence of obsessive compulsive disorder (OCD) in ASD has been estimated at 2.6% to 37.2%.6 Neuroinflammation and alterations in gut microbiota are also associated with OCD.7
Saccharomyces boulardii is a safe and effective agent for the prevention and treatment of gastrointestinal diseases and is used most commonly to treat antibiotic associated diarrhea, Clostridium difficile infection, and irritable bowel disease. S boulardii reduces pathogenic adhesion and colonization of the mucosa, acts as a prebiotic and probiotic to support growth of beneficial microbial species, modulates immune responses, and stabilizes gastrointestinal barrier function.8 This case reports on the successful utilization of S boulardii to ameliorate severe OCD and SIB in a child with autism.
Case Presentation
Timeline.
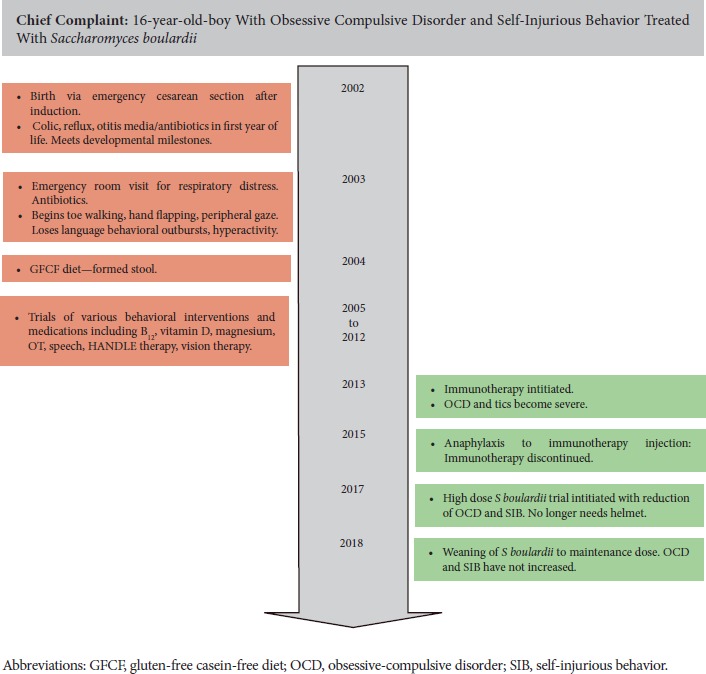
A 16-year-old white male with a diagnosis of ASD was seen for increasingly severe OCD and SIB. Birth history is significant for induction and delivery by Cesarean-section with Apgars 9/9. As an infant, the child was reported to have colic, chronic diarrhea, frequent rashes, and otitis media (OM) treated with antibiotics. Developmental milestones were met until 15 months of age. Shortly after, he was admitted to the emergency department of the local hospital with respiratory distress after taking amoxicillin for OM. At 18 months of age, he began to regress, losing previously acquired language, toe walking, hand flapping, and exhibiting behavioral outbursts and hyperactivity. A gluten-free and casein-free diet was initiated at 26 months, which resulted in his first formed stool, and improved eye contact. He was formally diagnosed with ASD at 2.5 years of age. Bowel movements remained erratic. He had multiple allergies. At age 5 years, he began treatment at the clinic where he currently receives care. OCD, tics, and SIB became severe at age 11 years, at which time he began to wear a helmet to protect his head from constant head banging. Immunotherapy for allergies was initiated at age 11 years but was discontinued due to an anaphylactic response. The patient received supplements directed at gut and immune health including probiotics, vitamin D titrated to levels of 60 to 80 ng/mL, curcumin 600 mg BID, helminthic therapy, and bethanechol titrated for good gut motility. Additional treatment for documented and undocumented but clinically symptomatic yeast infections included fluconazole, itraconazole, nystatin, grape seed extract, and dietary restriction of simple carbohydrates, sugars, and fruits. He received a trial of inositol 4 to 12 grams/day for OCD behaviors. Magnesium for anxiety and neurologic stability was titrated between magnesium citrate and gastrointestinal neutral forms based on bowel movements. He also received trials of lorazepam PRN and clonazepam daily in the past and was taking sertraline 75 mg QD at the time. Prior interventions included valacyclovir, cod liver oil, and methylcobalamin shots. Despite all of these interventions, OCD and SIB remained severe enough that he was often homebound. Laboratory evaluation was positive for elevated eosinophil counts and immunoglobulin E 4300 UI/m: (Centra lab, reference range 5 to 127 IU/mL), consistent with allergies and immune dysregulation. At age 15 years, a trial of S boulardii was initiated because it has the unique benefits of promoting normalization of gut flora, improving immunomodulation, and managing recurrent diarrhea. S Boulardii (Klaire Labs, 3 billion CFU per capsule) was initiated at 6 capsules daily: 2 at breakfast, 2 at lunch, 1 at dinner, and 1 at bedtime. Initial response was an exacerbation of symptoms attributed to Jarisch-Herxheimer reaction and treated with activated charcoal (Bulletproof) 1 capsule QID. After 1 week, S boulardii was increased to 2 capsules QID, and the patient was able to discontinue use of the helmet for 2 days. He continued weekly increases of S boulardii, reaching a final dose of 12 capsules BID equivalent to 72 billion CFU daily. With each increase there was a transient uptick in OCD and SIB, which was addressed with activated charcoal. During this time, use of the helmet diminished markedly and OCD symptoms decreased to a parent rating of 2/10. After 3 months at 24 capsules of S boulardii, the patient began weaning down by 4 capsules per week to a dose of 3 capsules BID. SIB occurs once or twice per week for approximately 10 minutes each, whereas prior to the S boulardii treatment, SIB occurred multiple times per day and would last for more than 1 hour each time. He has maintained all gains to date.
Discussion
Although the exact etiology of ASD had yet to be elucidated, it is recognized that both genetic and environmental influences play a role, and that perturbations of the bidirectional gut-brain axis are an important area of exploration. GI dysfunction, frequently presenting as diarrhea, constipation, or abdominal pain has been identified in up to 70% of children with autism with increased symptoms correlated with ASD severity.9 Increased pain is correlated with SIB. Both mouse and human models of autism identify alterations in the normal composition of gut microbiota in ASD. Antibiotic use disrupts the normal microbial balance, and decreased diversity in gut flora has been associated with C-section birth.10
Gut microbiota have immunoregulatory effects on the production of inflammatory cytokines such as tumor necrosis factor α, immunoglobulin 6, and immunoglobulin 1. Elevations in these cytokines are linked to mood disorders such as anxiety and depression. Research regarding the role of imbalanced gut ecology and related immune activation in OCD provides parallels to autism and may be a factor in the high frequency of comorbidity. The immunological alterations in OCD that reduce serotonin (5-HT) synthesis via activation of indolamine-2, 3-dehydrogenase (IDO) have been described by Marzetti et al.11 Increased IDO will shunt tryptophan to kynurenic and quinolinic acids which have excitotoxic properties. An abrupt onset of OCD is a hallmark of pediatric acute-onset neuropsychiatric syndrome (PANS), a disease in which neuroinflammation is suspected to play a role in the majority of cases.12 Fungal gut pathogens are also implicated in mental health disorders. Levels of Candida albicans IgG were significantly elevated in male patients with schizophrenia and bipolar disorder as compared with controls.13
S boulardii is a nonpathogenic, transient yeast that increases the activities of intestinal brush border enzymes, enhances stool concentrations of short-chain fatty acids that nourish colonic mucosal cells, raises intestinal secretion of secretory IgA and immunoglobulin receptors, and reduces diarrhea. It functions as both a prebiotic and probiotic and has the ability to reduce adhesion of pathogenic species such as C albicans in the intestinal tract. S boulardii survives gastric acidity and exposure to antibiotics. It safe for use in adult and pediatric populations, however, some instances of fungemia has been documented in severely immunocompromised patients.14
Conclusion
There are currently no widely recognized effective treatments for the often debilitating behavioral and gastrointestinal manifestations of ASDs. S boulardii offers great promise due to its ability to potentiate desirable microbial balance in the gut, positively modulate immune response, and control chronic diarrhea, while maintaining a strong safety profile. More studies of S Boulardii in autism and OCD are warranted.
Acknowledgements
Victoria Kobliner, MS, RDN, conducted the literature review and was the primary contributor to the creation of the manuscript. Elizabeth Mumper, MD, conducted the chart review, provided feedback, revisions, and editing of the manuscript. Sidney M. Baker, MD, conducted chart review and provided feedback, revisions, and editing of the manuscript. Victoria Kobliner, MS, RDN, is the owner of Holcare Nutrition, a private practice specializing in functional nutrition therapy. It is located at 3 Hollyhock Road, Wilton, CT, USA 06897. Elizabeth Mumper MD, FAAP, IFMCP, is a pediatrician and chief executive officer of Advocates for Children, Advocates for Families, and the Rimland Center for Integrative Medicine at 2919 Confederate Avenue, Lynchburg, VA, USA 24501. Sidney M Baker, MD, is located at 71 Ferry Road, Sag Harbor, NY, USA 11963. The authors would like to acknowledge the patient and his family for their willingness to share their story.
Biographies
Victoria Kobliner, MS, RDN, is the owner of Holcare Nutrition located in Wilton, Connecticut.
Elizabeth Mumper, MD, FAAP, IFMCP, is pediatrician and chief executive officer of Advocates for Children, Advocates for Families, and the Rimland Center for Integrative Medicine located in Lynchburg, Virginia.
Sidney M. Baker, MD, is located in Sag Harbor, New York.
Footnotes
Author Disclosure Statement
Written informed consent was obtained from the parent of the minor child for publication of this case report. The authors declare no competing interest/financial disclosures.
References
- 1.McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics. 2014;133(5):872-883. [DOI] [PubMed] [Google Scholar]
- 2.Grossi E, Terruzzi V. The role of intestinal dysbiosis in the pathogenesis of autism: Minireview.” Int J Microbiol Adv Immunol. 2014;2:41-44. [Google Scholar]
- 3.Li Q, Zhou J-M. The microbiota–gut–brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience. 2016;324:131-139. [DOI] [PubMed] [Google Scholar]
- 4.Courtemanche AB, Black WR, Matthew Reese R. The relationship between pain, self-injury, and other problem behaviors in young children with autism and other developmental disabilities. Am J Intellect Development Disabil. 2016;121(3);194-203. [DOI] [PubMed] [Google Scholar]
- 5.Vuong HE, Hsiao EY. Emerging roles for the gut microbiome in autism spectrum disorder. Biologic Psychiatr. 2017;81(5):411-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postorino V. Anxiety disorders and obsessive-compulsive disorder in individuals with autism spectrum disorder. Curr Psychiatr Rep. 2017;19(12):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turna J. “What’s Bugging the Gut in OCD?” A review of the gut microbiome in obsessive–compulsive disorder. Depress Anx. 2016;33(3):171-178. [DOI] [PubMed] [Google Scholar]
- 8.Tomičić ZM. Beneficial properties of probiotic yeast Saccharomyces boulardii. Food Feed Res. 2016;43(2):103-110. [Google Scholar]
- 9.Adams JB, Johansen LJ, Powell LD, Quig D, Rubin RA. Gastrointestinal flora and gastrointestinal status in children with autism: Comparisons to typical children and correlation with autism severity. BMC Castroenterol. 2011;11(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakobsson HR, Abrahamsson TR, Jenmalm MC, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 201;gutjnl-2012. [DOI] [PubMed] [Google Scholar]
- 11.Marazziti D, Mucci M, Fontenelle LF. Immune system and obsessive-compulsive disorder. Psychoneuroendocrinology. 2018;93:39-44. [DOI] [PubMed] [Google Scholar]
- 12.Frankovich J, Swedo S, Murphy T, et al. Clinical management of pediatric acute-onset neuropsychiatric syndrome: part II: Use of immunomodulatory therapies. J Child Adolescent Psychopharmacol. 2017;7(27):574-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Severance EG. Candida albicans exposures, sex specificity and cognitive deficits in schizophrenia and bipolar disorder. NPJ Schizophrenia. 2016;2:16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomičić Zorica M. Beneficial properties of probiotic yeast Saccharomyces boulardii. Food Feed Res. 2016;43(2):103-110. [Google Scholar]


