Abstract
Background
It has been reported that miRNA-125b is associated with carcinogenesis and development of several different kinds of cancers. Nevertheless, there is no clarity regarding the significance and mechanism of action of miR-125b in clinical practice for cervical cancer (CC).
Materials and methods
In the current investigation, the expression of miR-125b in cervical clinical specimens and CC cell lines was analyzed via real-time quantitative PCR, and the relationship of miR-125b with the chromatin-associated protein high mobility group A (HMGA1) expression and clinicopathological parameters of CC patients was explored.
Results
The results indicated that miR-125b expression was remarkably upregulated in CC cell lines as well as in the tissues of humans. miR-125b overexpression was significantly related to a decrease in HMGA1 expression, progression-free survival, overall survival, and prognosis as well. Besides, knockdown of miR-125b inhibited proliferation and colony formation in SW756 and C4-1 cells, where the 3′-UTR of HMGA1 mRNA was directly targeted. Moreover, PI3K/Akt pathway was regulated by miR-125b through suppression of HMGA1.
Conclusion
These findings illustrated that a new regulatory role of HMGA1 is involved in the progression of CC. Our data demonstrated that miR-125b could play a critical role in the carcinogenesis and progression of CC, revealing that miR-125b might serve as a potential new target for treating CC.
Keywords: cervical cancer, CC, miR-125b, high mobility group A, HMGA1, progression-free survival, PFS, overall survival, OS, prognosis
Introduction
Cervical cancer (CC) is one of the common gynecological malignancies encountered globally in females, and has become a prominent public health problem in recent years.1 It was reported that the CC was ranked second in gynecologic malignancies with respect to incidence rate, and was ranked first with respect to mortality rate among the female genital malignancies, thus seriously threatening women’s health.2 In addition, a clinical statistical report showed that of the 61,691 CC patients diagnosed in China, 29,526 were confirmed to have died, accounting for 10% of global cases.3 Currently, the treatment for CC is limited to surgery, radiotherapy, and chemotherapy. These treatments are usually applied in the clinical environment, and the clinical efficacy is not satisfactory due to the occurrence of metastasis.4 However, due to the large population size and significant differences in wealth and medical resources between different regions, the condition of CC in Chinese females may be more serious.
MiRNAs are a class of endogenous, single-stranded, non-coding RNA molecules containing 18–25 nucleotides.5 MiRNAs regulate gene expression levels via direct interaction with the complementary sites at the 3′-UTRs of the target gene, resulting in mRNA degradation and/or inhibition of translation.6 miR-125b was found to be abnormally expressed in several kinds of cancers.7–9 The function of miR-125b is very complicated, and hence could function as a carcinogen or tumor suppressor based on the microenvironment of the cell.10 Abnormal expression profiles of miRNAs were also found in CC cell lines and human tissues.11,12 Nevertheless, as far as we know, only few studies have examined in detail the mechanism of effect of miR-125b in CC. Given the complexity in terms of its function, exploring the functional roles and correlations between miR-125b and high mobility group A (HMGA1) in carcinogenesis and progression of CC is of great significance.
The levels of miR-125b expression within CC cell lines and clinical samples were detected in this investigation, and the involvement of miR-125b in the expression of HMGA1 and clinical pathology data in CC patients was explored. The in vitro experiments have indicated that the cell proliferation and growth can be repressed with the inhibition of miR-125b expression by directly targeting the 3′-UTR HMGA1 mRNA. A new mechanism was identified at a molecular level in these studies, which revealed an association between HMGA1 expression level and CC development. Therefore, targeting of miR-125b could be an effective treatment method for CC.
Materials and methods
Tissue samples and patients
The collection of tissue specimens as well as the related research protocol was approved by the Ethics Committee of Linyi Central Hospital. By signing the informed consent, patients expressed their agreement to donate tissue samples and blood. Hundred and twelve patients were included in the study, for whom clinical and pathological staging was performed in compliance with the standard of International Federation of Obstetrics and Gynecology. All patients signed an informed consent document for diagnosis and research on tissue specimens before being enrolled in the project. All subjects gave written informed consent in accordance with the Declaration of Helsinki principles. The clinicopathological data of the participants are summarized in Table 1.
Table 1.
Association between miR-125b expression and clinicopathological characteristics
| Characteristics | No | miR-125b expression | P-value | |
|---|---|---|---|---|
| Low | High | |||
| Age (years) | 0.574 | |||
| ≤35 | 44 | 20 | 24 | |
| >35 | 68 | 35 | 33 | |
| FIGO stage | 0.018 | |||
| IB | 73 | 42 | 29 | |
| >IB | 39 | 13 | 26 | |
| HR-HPV | 0.097 | |||
| Yes | 99 | 47 | 52 | |
| No | 13 | 8 | 5 | |
| Differentiation | 0.851 | |||
| Well | 51 | 26 | 25 | |
| Moderate/poor | 61 | 29 | 32 | |
| Tumor size | 0.630 | |||
| ≤4 cm | 88 | 41 | 47 | |
| >4 cm | 24 | 14 | 10 | |
| LN metastasis | 0.004 | |||
| Yes | 98 | 44 | 54 | |
| No | 14 | 11 | 3 | |
| Stromal invasion | 0.026 | |||
| <2/3 | 85 | 47 | 38 | |
| ≥2/3 | 27 | 8 | 19 | |
Abbreviations: FIGO, The International Federation of Gynecology and Obstetrics; HR-HPV, high-risk human papilloma virus; LN, lymph node.
Cell viability
The normal cervical epithelial cells (Endl/E6E7) for primary culture were supplied by Shanghai Medical College of Fudan University (Shanghai, China). The use of the cells was approved by the Ethics Committee of Linyi Central Hospital. The cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific, Waltham, MA, USA) medium supplemented with 10% FBS at 5% CO2 and 37°C (Biological Industries, Carlsbad, CA, USA). The CC cell lines, including HeLa, C4-1, Caski, SW756, and SiHA, were cultured in DMEM (Thermo Fisher Scientific). The cells (HeLa, C4-1, Caski, SW756, and SiHA) were cultured in RPMI 1640 medium containing penicillin/streptomycin and 10% FBS at 5% CO2 and 37°C. The viability of these cells was detected by performing the MTT assay (Daochang, Kyushu, Japan), and the absorbance was read at 490 nm (Bio-Rad, Hercules, California, USA). The use of these cell lines was approved by the Ethics Committee of Linyi Central Hospital.
RT-qPCR
Total RNA was extracted with TRIzol reagent (TaKaRa, Dalian, China), which was reverse-transcribed to cDNA by a Prime Script RT Reagent Kit (TaKaRa), in accordance with the manufacturer’s instructions. Real-time quantitative PCR (RT-qPCR) was performed on a CFX96 Real-Time System (Bio-Rad) with SYBR Green (SYBR Premix Ex Taq II; TaKaRa) being used for fluorescent quantification. The following cycling conditions were applied: pre-denaturation at 95°C for 30 seconds, 40 cycles of denaturation at 95°C for 5 seconds, 35 cycles of annealing at 55°C for 40 seconds, extension at 72°C for 1 minute with final extension at 72°C for 10 minutes. The 2−ΔΔCt method was performed to detect the relative expression of mRNA.
Western blotting
The cell protein lysates were separated via SDS-PAGE, and were transferred to polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Then they were incubated at 4°C overnight in the presence of anti-HMGA1 (Cell Signaling Technology, Boston, MA, USA), anti-P21 (Sigma-Aldrich Co., St Louis, MO, USA), and anti-cyclin D1 (Sigma-Aldrich Co.), respectively. After washing, secondary antibody (Pierce, Rockford, IL, USA) was added into the system. Enhanced chemiluminescence chromogenic substrates were used for quantitative measurement by using Quantity One software (Bio-Rad). GAPDH was used as an internal control.
Transfection of oligonucleotide
The inhibitor of miR-125b was chemically produced by Shanghai Jima Pharmaceutical Technology Co., Ltd. (Shanghai, China). When the cell fusion reached 80%, miR-125b inhibitor was transfected into CC cells using Lipofectamine 2000 (Thermo Fisher Scientific) in accordance with the instructions provided by the manufacturer. Nonsense oligonucleotides were transfected into the cells to act as negative control (NC). RT-qPCR was performed to detect the miR-125b expression levels within the osteosarcoma cells which have been transfected.
Luciferase reporter gene assay
HEK293T cells were seeded in 96-well plates for the promoter luciferase reporter assay. When the cell fusion reached 60%, 100 ng of miR-125b expression vectors or NC was transfected into the cells. A mixture containing 30 ng of 3′-UTR of wild type (WT) or mutant (MT) HMGA1 mRNA was transfected into the cells. Cells were collected 48 hours after the process of transfection. After 48 hours, luciferase activity (Promega, Madison, WI, USA) was determined via a dual luciferase reporter assay system in accordance with the manufacturer’s instructions.
Plate clone formation assay
Briefly, cells, seeded into six-well plates with a density of 500 cells/well, were grown in a culture medium for 24 hours. Then, they were co-cultured with miR-125b inhibitor or NC for 48 hours. Next, the cells were washed in PBS following the removal of the medium, and the cells were cultured continuously for 10 days in a complete medium. The experiments were performed in triplicate. After fixing with 4% formalin, the colonies were stained at room temperature with 0.1% crystal violet, and the colonies were counted.
Flow cytometry analysis
After transfection with NC and miR-125b inhibitors for 48 hours, CC cells were harvested and fixed at −20°C in the presence of 70% ice ethanol for 4 hours. Then, the cells were trypsinized and analyzed for cell cycle distribution. Cells were stained with 50 mg/mL of propidium iodide staining solution containing 0.2% Triton X-100 and 100 mg/mL of RNase A (BD Biologies, San Jose, CA, USA) for 30 minutes at room temperature in a dark room. The proportion of cells in the G1, S, and G2 phases was calculated. FlowJo software was used to analyze the data (TreeStar Inc., Ashland, OR, USA).
Statistical analysis
The data are presented as mean ± SD, and statistical data were analyzed with SPSS, version 21.0 (IBM Corporation, Armonk, NY, USA). The variance regarding the features of the two groups was detected with the chi-squared or Fisher’s exact tests. The Spearman’s correlation was applied to evaluate potential correlations between miR-125b and HMGA1 gene expression in paired CC tissues. P-value <0.05 indicated statistical significance.
Results
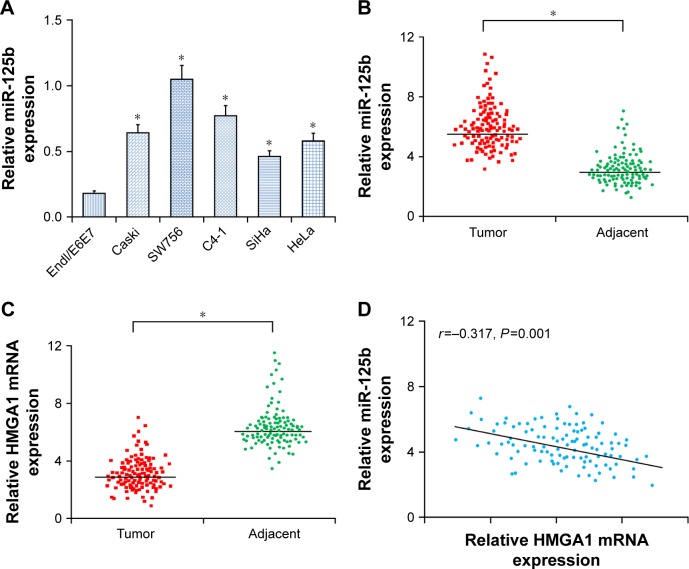
The expression of miR-125b was upregulated within CC cell lines as well as human tissues. RT-qPCR was applied to determine the expression levels of miR-125b in four CC cell lines as well as in Endl/E6E7 and to determine the miR-125b expression levels in CC. The results showed that the expression levels of miR-125b in Caski, Hela, C4-1, SW756, and SiHACC cell lines, particularly C4-1 and SW756, were significantly upregulated in comparison with those in the Endl/E6E7 cells (Figure 1A). The expression levels of miR-125b in the 112 CC tissue samples were remarkably higher relative to those in the surrounding healthy tissues (Figure 1B). In contrast, the expression of HMGA1 in CC tissues was remarkably decreased relative to that in the surrounding normal tissues (Figure 1C). More importantly, a significant inverse correlation between miR-125b and HMGA1 expression levels was found in CC samples by means of Spearman’s correlation analysis (r=−0.317; P=0.001, Figure 1D). Thus, the results of the study revealed that miR-125b played a carcinogenic role in CC, whereas HMGA1 played a tumor suppressive role in CC. In addition, miR-125b was inversely related to HMGA1 in the CC, which indicated that HMGA1 might be a potential target for miR-125b in the CC cells.
Figure 1.
The expression levels of miR-125b and HMGA1 were upregulated in CC tissues and cell lines. (A) The expression levels of miR-125b in the Endl/E6E7 and CC cell lines (Caski, HeLa, C4-1, SW756, and SiHa). (B) The expression levels of miR-125b in the 112 pairs of CC tissues and adjacent normal tissues. (C) The expression levels of HMGA1 mRNA in the 112 pairs of CC tissues and adjacent normal tissues. (D) Correlation of expression levels between miR-125b and HMGA1 mRNA in the CC tissues; *P<0.05.
Abbreviation: CC, cervical cancer.
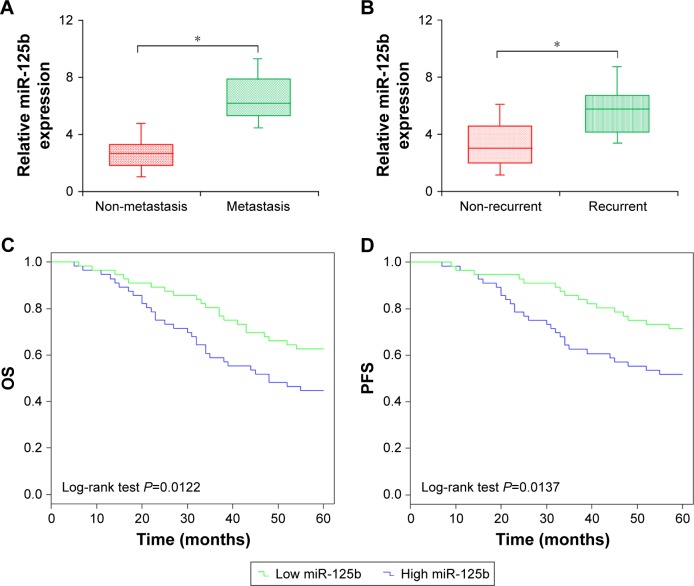
miR-125b upregulation is involved in the metastasis and recurrence of CC
To find out the association of miR-125b with CC, correlation analysis between miR-125b expression levels and metastasis, as well as the recurrence, of the CC was performed. Relative to the non-metastatic CC samples, there was a significant upregulation in the miR-125b expression level in the metastatic CC tissues (Figure 2A). Furthermore, the miR-125b expression levels were remarkably upregulated in relapsed patients with CC (Figure 2B). The results indicated that a significant upregulation in the expression of miR-125b was involved in the recurrence and metastasis of CC in these patients.
Figure 2.
Clinical significance of miR-125b in patients with CC. (A) Comparison of miR-125b expression levels between patients with metastatic and non-metastatic CC. (B) Comparison of miR-125b expression levels between patients with relapsed and non-relapsed CC. (C) The OS of the CC patients with low and high expression levels of miR-125b. (D) The PFS of the CC patients with low and high expression levels of miR-125b; *P<0.05.
Abbreviations: CC, cervical cancer; OS, overall survival; PFS, progression-free survival.
The level of miR-125b expression is related to clinicopathological features as well as prognosis in CC patients
To evaluate the distinctiveness of miR-125b within CC in clinical practice, 112 volunteers were divided into two teams in accordance with miR-125b expression levels, and the median expression level was used as the cut-off point. It was shown via Kaplan–Meier method that upregulation of miR-125b expression was remarkably associated with overall survival (OS) and progression-free survival (PFS) in the 112 CC patients being investigated (log-rank test: P=0.0122 and P=0.0137, respectively; Figure 2C and D). Relative to patients with low expression levels of miR-125b, OS and PFS were shorter in those exhibiting high expression levels of miR-125b. Additionally, upregulation of miR-125b was remarkably involved in the metastasis to lymph node, the International Federation of Gynecology and Obstetrics (FIGO) stage, and deep interstitial invasion, but not with additional clinicopathological parameters (Table 1). It was shown through univariate analysis that the OS and PFS were related to FIGO stage, high-risk human papilloma virus (HR-HPV), lymph node status, as well as miR-125b expression in patients with CC (Tables 2 and 3). The Cox proportional hazards regression model was used via the multivariate analysis to detect whether the miR-125b prognostic value was related to other clinicopathological indicators in CC patients with poor OS and PFS. The indicators for analysis included: miR-125b expression, FIGO stage, age, differentiation status, HR-HPV, lymph node metastasis, and tumor size. Multivariate analysis indicated that high expression of miR-125b was an independent prognostic biomarker for poor OS and PFS in CC patients (Tables 2 and 3; HR =3.093, 95% CI=1.164-5.033, P=0.014; HR =4.573, 95% CI=2.634-10.015, P<0.001). There were critical differences for lymph node metastasis and FIGO stage, and other indicators were dependent prognostic biomarkers for OS and PFS in patients with CC. Taken together, it was indicated in this study that the upregulation of miR-125b was remarkably related to poor clinical outcome, which was also associated with progression of CC.
Table 2.
Univariate and multivariate analyses of correlations between clinicopathological parameters and OS
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| miR-125b expression | 4.215 (1.653–6.442) | 0.007* | 3.093 (1.164–5.033) | 0.014* |
| Age (years) | 2.083 (0.579–2.683) | 0.569 | 1.695 (0.867–2.281) | 0.882 |
| FIGO stage | 4.164 (2.127–5.231) | 0.013* | 3.405 (2.133–5.417) | 0.004* |
| HR-HPV | 3.084 (1.307–4.365) | 0.032* | 2.877 (1.141–4.255) | 0.020* |
| Differentiation | 1.704 (0.657–2.901) | 0.316 | 2.136 (0.605–2.537) | 0.383 |
| Tumor size | 1.391 (0.519–1.894) | 0.554 | 1.606 (0.747–2.175) | 0.447 |
| LN metastasis | 3.668 (1.725–5.682) | 0.013* | 4.091 (1.465–6.352) | 0.006* |
| Stromal invasion | 1.152 (0.523–2.034) | 0.127 | 1.556 (0.884–2.273) | 0.394 |
Note:
Significant correlation of clinicopathological parameters with OS.
Abbreviations: FIGO, The International Federation of Gynecology and Obstetrics; HR-HPV, high-risk human papilloma virus; LN, lymph node; OS, overall survival.
Table 3.
Univariate and multivariate analyses of clinicopathological parameters in relation to PFS
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| miR-125b expression | 4.698 (2.326–6.547) | 0.006* | 4.573 (2.634–10.015) | <0.001* |
| Age (years) | 1.509 (0.647–2.265) | 0.368 | 1. 763 (0.936–2.217) | 0.508 |
| FIGO stage | 3.623 (1.684–4.374) | 0.031* | 3.017 (1.980–4.226) | 0.017* |
| HR-HPV | 3.632 (1.476–5.381) | 0.012* | 2.873 (1.560–4.735) | 0.006* |
| Differentiation | 1.780 (0.664–2.603) | 0.285 | 2.248 (0.905–3.144) | 0.483 |
| Tumor size | 1.682 (0.742–2.724) | 0.406 | 1.739 (0.804–3.225) | 0.452 |
| LN metastasis | 5.003 (2.052–10.024) | 0.037* | 4.108 (2.437–7.523) | 0.029* |
| Stromal invasion | 1.416 (0.725–2.683) | 0.285 | 1.436 (0.572–2.938) | 0.271 |
Note:
Significant correlation of clinicopathological parameters with PFS.
Abbreviations: FIGO, The International Federation of Gynecology and Obstetrics; HR-HPV, high-risk human papilloma virus; LN, lymph node; PFS, progression-free survival.
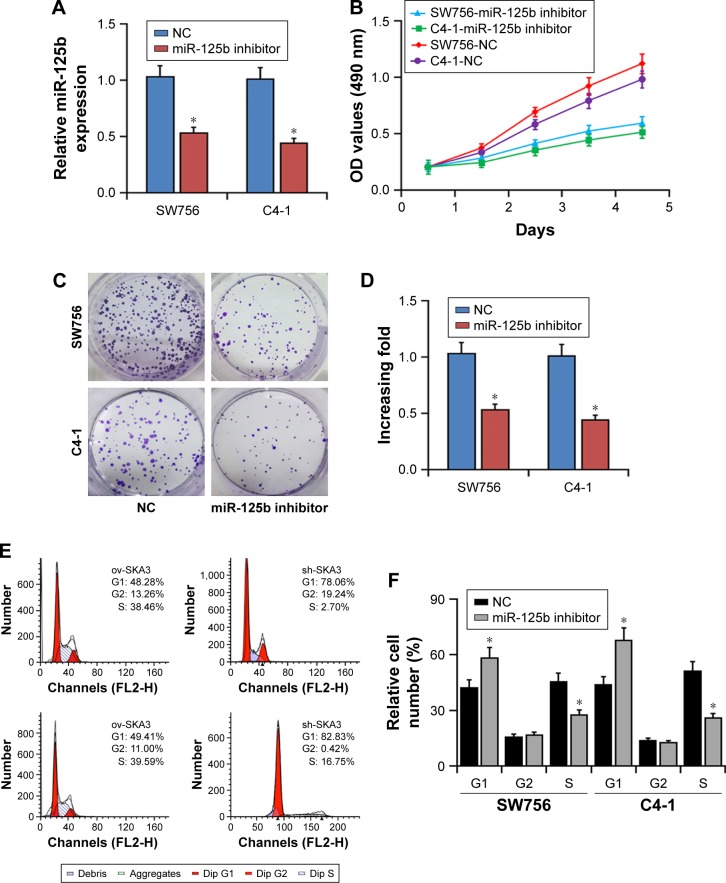
Proliferation of CC cells is promoted by miR-125b
Since the expression levels of miR-125b were relatively high in the SW756 and C4-1 cell lines relative to those in the SiHa and Caski cell lines, we selected SW756 and C4-1 cells to investigate the physiological function of miR-125b in the cells affected with CC. The inhibitors of miR-125b were transfected into the SW756 and C4-1 cell lines to determine the impact of miR-125b on the CC cell proliferation. Transfection with miR-125b inhibitors downregulated the expression levels of miR-125b in the SW756 and C4-1 cells (Figure 3A). Following confirming the efficiency of miR-125b inhibitors, the impact of miR-125b regarding cell viability was detected by MTT assay. Compared with that of the control, the viability of the CC cells, which were transfected with the miR-125b inhibitors, was significantly reduced (Figure 3B). The plate colony formation experiments and soft agar colony formation assays were applied to evaluate the effects of miR-125b on the proliferation of the cells. Compared with the controls, the growth rate of both cervical cell lines was significantly reduced by the inhibition of miR-125b (Figure 3C and D). The findings of this study also showed that the proliferation of CC cells can be inhibited via reduction of miR-125b expression.
Figure 3.
Effects of miR-125b knockdown on proliferation and cell cycle of the CC cells. (A) Expression levels of miR-125b in SW756 and C4-1 cells transfected with miR-125b inhibitors. (B) Effects of miR-125b knockdown on the viability of SW756 and C4-1 CC cells. (C, D) Effects of miR-125b knockdown on the colony formation abilities of SW756 and C4-1 CC cells. (E, F) Effects of miR-125b knockdown on the cell cycle progression of SW756 and C4-1 CC cells; *P<0.05.
Abbreviations: CC, cervical cancer; NC, negative control.
Effects of miR-125b on cell cycle in vitro
Since miR-125b remarkably affected the proliferation of the SW756 and C4-1 cells, we hypothesized that miR-125b functioned by regulating the cell cycle process of the CC cells. Therefore, the impact of miR-125b on the cell cycle was investigated through flow cytometry. The results indicated that overexpression of the miR-125b inhibitors significantly enhanced the cell number at the G1 peak and reduced the cell number at the S peak (Figure 3E and F). The results showed that the proliferation of cells could be inhibited by miR-125b through inducing cell cycle arrest.
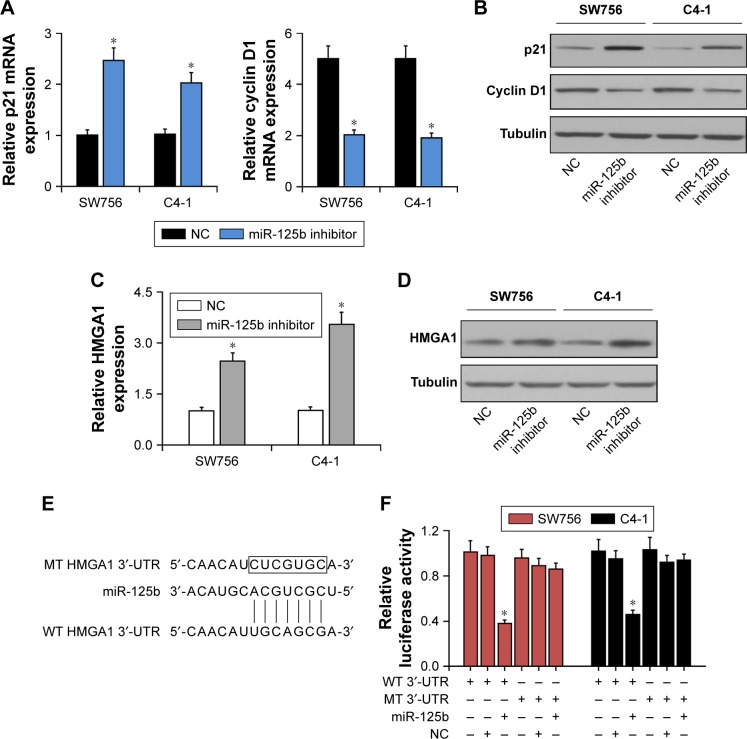
Inhibition of miR-125b upregulated p21Cip1 expression and downregulated cyclin D1 expression
Since overexpression of miR-125b inhibitors appeared to be closely associated with the CC cell proliferation, we further explored the impact of miR-125b on the CDK inhibitor p21Cip1 or the CDK modulator cyclin D1. RT-qPCR and Western blot analysis showed that relative to those in the cells transfected with the control, the levels of p21Cip1 expression were upregulated while the levels of cyclin D1 expression were decreased in the CC cells transfected with miR-125b inhibitors (Figure 4A and B). Taken together, our hypothesis that miR-125b plays a crucial role in the development and progression of the CC cells was verified by the results.
Figure 4.
HMGA1 was a direct target gene for miR-125b. (A) RT-qPCR was used to detect the expression levels of p21 and cyclin D1 mRNA in the SW756 and C4-1 cells after transfection with miR-125b or NC. (B) Western blot analysis was used to detect the expression levels of p21 and cyclin D1 proteins in SW756 and C4-1 cells after transfection with miR-125b or NC. (C) RT-qPCR was used to detect the expression levels of HMGA1 mRNA in SW756 and C4-1 cells after transfection with miR-125b or NC. (D) Western blot analysis was used to detect the expression levels of HMGA1 protein in SW756 and C4-1 cells after transfection with miR-125b or NC. (E) The miR-125b and its putative binding sequences in HMGA1 3′-UTR. (F) SW756 and C4-1 cells were transfected with plasmids containing WT or MT-type HMGA1 3′-UTR and miR-125b inhibitor oligonucleotides for luciferase reporter gene experiments; *P<0.05.
Abbreviations: RT-qPCR, real-time quantitative PCR; NC, negative control; WT, wild type; MT, mutant.
miR-125b regulated PI3K/Akt pathway through suppression of HMGA1 in the CC cells
Western blot and RT-qPCR were performed to detect the levels of HMGA1 expression in the SW756 and C4-1 cells. The expression of HMGA1 was significantly upregulated in the CC cells which were transfected with miR-125b inhibitors (Figure 4C and D). The study showed that HMGA1 is a potential target gene for miR-125b in the clinical tissue specimens and CC cell lines. Luciferase reporter gene analysis was performed to further verify whether miR-125b targeted the 3′-UTR of HMGA1 directly in the CC cells. The target sequences of WT HMGA1 3′-UTR (WT 3′-UTR) or MT HMGA1 3′-UTR (MT 3′-UTR) were cloned into the luciferase reporter vector (Figure 4E). Transfection with miR-125b inhibited the luciferase activity of the WT HMGA1 3′-UTR luciferase reporter plasmids in the SW756 and C4-1 cells, but MT HMGA1 3′-UTR attenuated the inhibitory effect of miR-125b (Figure 4F). It can be concluded here that HMGA1 is the target gene for miR-125b. Moreover, Western blot results indicated that miR-125b knockdown can be reversed by the downregulation of HMGA1, showing that PI3K/Akt pathway was regulated by miR-125b through HMGA1 suppression (Figure 5).
Figure 5.
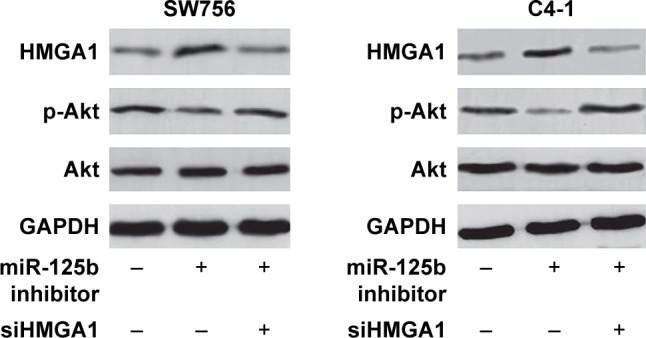
miR-125b regulated PI3K/Akt pathway through suppression of HMGA1. The primary Akt protein level was detected after co-transfection with miRNA inhibitor and siHMGA1 in SW756 and C4-1 cells.
Discussion
It had been indicated in previous studies that miRNAs were dysregulated in most of the human malignancies, and their dysregulation was associated with tumor development and progression.13 It has been reported that with the malignant progression and development of CC, many miRNAs were significantly dysregulated and acted as tumor suppressors or oncogenes, such as miR-497, miR-195, and miR-187.14–16 In the present study, the levels of miR-125b expression in CC cell lines and tissues of patients were upregulated remarkably. miR-125b overexpression was also significantly related to the downregulation of HMGA1, poor clinicopathological features, poor OS and PFS, and poor prognosis. Moreover, inhibition of the miR-125b expression can induce cell cycle arrest at G1 phase and prevent the growth and proliferation of CC cell lines. It was shown in in vitro studies that HMGA1 could be a direct target gene for miR-125b in the CC cells. It was shown in this study that miR-125b could play a crucial role in the carcinogenesis and development of CC.
Accumulating evidence verified that the impact of miR-125b on the development of cancers was extremely complex. miR-125b functions as both oncogene and tumor suppressor, in gastric cancer cells, bladder carcinoma, glioma hepatocellular carcinoma, and colorectal cancer.7,8,17–19 Since the function of miR-125b in the CC has not been clarified, the purpose of this study was to explore the potential biological functions of miR-125b in the CC. The study showed that the proliferation and growth of HeLa and C4-1 cells were significantly inhibited by the inhibition of miR-125b expression through inducing cell cycle arrest. Moreover, miR-125b was remarkably related to poor clinicopathological features, and poor survival and prognosis in patients with CC, suggesting that miR-125b may have a crucial function as carcinogenic miRNA in the progression and development of CC. It is worth noting that this study further verified that the differential impact of miRNAs, such as miR-125b, in various kinds of cancers relied on the type of cancer as well as on the environment of the cell.
In order to determine the underlying molecular mechanism involved in miR-125b-mediated changes in biological properties, HMGA1, a structural transcription factor, can be attached to AT-rich regions within the small grooves in DNA and can be made to participate in many basic physiological processes of cells, including cell cycle progression, embryonic development, tumor transformation, differentiation, apoptosis, cellular metabolism, and DNA repair. It has been indicated in recent studies that HMGA1 can produce an impact on the proliferation of cells via regulating the cyclin D and cyclin E expression by interacting with retinoblastoma protein in human T leukemia cells.20 Nevertheless, the molecular mechanism and role of HMGA1 in the progression of CC remain unclear. The PI3K/Akt signaling pathway is a classically crucial signaling pathway that is associated with many cellular roles, such as cell proliferation, adhesion, migration, metabolism, as well as cell survival.21 In addition, the PI3K/Akt signaling pathway can control the drug resistance, transformation, proliferation, apoptosis, growth, and many other biological functions of several kinds of cancer cells.22 Recent studies have indicated that the PI3K/Akt signaling pathway is closely related to the carcinogenesis and progression of the CC cells, which has been a potential target to prevent and treat CC.23
The results of this study verified the hypothesis that miR-125b is involved in the regulation of HMGA1 expression. Luciferase reporter gene analysis revealed that HMGA1 could be a direct target gene for miR-125b within the CC cells. miR-125b was capable of directly regulating the expression levels of HMGA1 via targeting the mRNA 3′-UTR. It was further indicated in the study that miR-125b knockdown could be reversed by downregulation of HMGA1, indicating that miR-125b regulated PI3K/Akt pathway through suppression of HMGA1.
Conclusion
This study demonstrated that miR-125b can mediate the proliferation and progression of CC cells by downregulating the HMGA1 expression. miR-125b played a key role in cell cycle regulation and cell proliferation in CC. Understanding of the exact mechanism by which miR-125b exerts an impact on the proliferation of tumor cells can deepen our biological knowledge on CC. Inhibition of miR-125b expression is expected to become a new strategy to treat CC. Therefore, we expect that the findings of this study, that is, targeting of the HMGA1-related signaling pathway could be a clinical treatment strategy for CC, would be further explored in future research works.
Ethical approval and patient consent
This study was approved by the Ethics Committee of Linyi Central Hospital. All patients signed the written informed consent for this study.
Footnotes
Author contributions
Conception and intellectual input: JX; designed and performed the experiments: BS, LZ, LY, and FL; manuscript drafting: YZ; data interpretation and statistical analyses: CL. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cervical cancer analysis reveals new mutations Cancer Discov. 2017;7(4):344. doi: 10.1158/2159-8290.CD-NB2017-020. [DOI] [PubMed] [Google Scholar]
- 2.Alldredge JK, Tewari KS. Clinical trials of antiangiogenesis therapy in recurrent/persistent and metastatic cervical cancer. Oncologist. 2016;21(5):576–585. doi: 10.1634/theoncologist.2015-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu DM, Shi J, Liu T, et al. Integrated analysis reveals down-regulation of SPARCL1 is correlated with cervical cancer development and progression. Cancer Biomark. 2018;21(2):355–365. doi: 10.3233/CBM-170501. [DOI] [PubMed] [Google Scholar]
- 4.Dizon DS, Mackay HJ, Thomas GM, et al. State of the science in cervical cancer: where we are today and where we need to go. Cancer. 2014;120(15):2282–2288. doi: 10.1002/cncr.28722. [DOI] [PubMed] [Google Scholar]
- 5.Lee YS, Dutta A. MicroRNAs in cancer. Annu Rev Pathol. 2009;4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin VY, Chu KM. MiRNA as potential biomarkers and therapeutic targets for gastric cancer. World J Gastroenterol. 2014;20(30):10432–10439. doi: 10.3748/wjg.v20.i30.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie H, Liao X, Chen Z, et al. LncRNA MALAT1 inhibits apoptosis and promotes invasion by antagonizing miR-125b in bladder cancer cells. J Cancer. 2017;8(18):3803–3811. doi: 10.7150/jca.21228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka H, Hazama S, Iida M, et al. miR-125b-1 and miR-378a are predictive biomarkers for the efficacy of vaccine treatment against colorectal cancer. Cancer Sci. 2017;108(11):2229–2238. doi: 10.1111/cas.13390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, Zhang X, Ma Y, et al. Ascites promotes cell migration through the repression of miR-125b in ovarian cancer. Oncotarget. 2017;8(31):51008–51015. doi: 10.18632/oncotarget.16846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bu Q, You F, Pan G, et al. MiR-125b inhibits anaplastic thyroid cancer cell migration and invasion by targeting PIK3CD. Biomed Pharmacother. 2017;88:443–448. doi: 10.1016/j.biopha.2016.11.090. [DOI] [PubMed] [Google Scholar]
- 11.He Y, Lin J, Ding Y, et al. A systematic study on dysregulated microRNAs in cervical cancer development. Int J Cancer. 2016;138(6):1312–1327. doi: 10.1002/ijc.29618. [DOI] [PubMed] [Google Scholar]
- 12.Granados-Lopez AJ, Ruiz-Carrillo JL, Servin-Gonzalez LS, et al. Use of mature miRNA strand selection in miRNAs families in cervical cancer development. Int J Mol Sci. 2017;18:2. doi: 10.3390/ijms18020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang WT, Chen YQ. Circulating miRNAs in cancer: from detection to therapy. J Hematol Oncol. 2014;7:86. doi: 10.1186/s13045-014-0086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhang D, Wang F, Xu D, Guo Y, Cui W. Serum miRNAs panel (miR-16-2*, miR-195, miR-2861, miR-497) as novel non-invasive biomarkers for detection of cervical cancer. Sci Rep. 2015;5:17942. doi: 10.1038/srep17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q, Han LR, Zhou YX, Li Y. MiR-195 suppresses cervical cancer migration and invasion through targeting Smad3. Int J Gynecol Cancer. 2016;26(5):817–824. doi: 10.1097/IGC.0000000000000686. [DOI] [PubMed] [Google Scholar]
- 16.Liang H, Luo R, Chen X, Zhao Y, Tan A. miR-187 inhibits the growth of cervical cancer cells by targeting FGF9. Oncol Rep. 2017;38(4):1977–1984. doi: 10.3892/or.2017.5851. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Wu JG, Wang JJ, Jiang X, et al. MiR-125b promotes cell migration and invasion by targeting PPP1CA-Rb signal pathways in gastric cancer, resulting in a poor prognosis. Gastric Cancer. 2015;18(4):729–739. doi: 10.1007/s10120-014-0421-8. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Zheng J, Chen L, Diao H, Liu Y. Predictive and prognostic roles of abnormal expression of tissue miR-125b, miR-221, and miR-222 in glioma. Mol Neurobiol. 2016;53(1):577–583. doi: 10.1007/s12035-014-9017-x. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Hu J, Zhou K, et al. Serum exosomal miR-125b is a novel prognostic marker for hepatocellular carcinoma. Onco Targets Ther. 2017;10:3843–3851. doi: 10.2147/OTT.S140062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xi Y, Li YS, Tang HB. High mobility group A1 protein acts as a new target of Notch1 signaling and regulates cell proliferation in T leukemia cells. Mol Cell Biochem. 2013;374(1–2):173–180. doi: 10.1007/s11010-012-1517-2. [DOI] [PubMed] [Google Scholar]
- 21.Cheng HW, Chen YF, Wong JM, et al. Cancer cells increase endothelial cell tube formation and survival by activating the PI3K/Akt signalling pathway. J Exp Clin Cancer Res. 2017;36(1):27. doi: 10.1186/s13046-017-0495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang C, Wang MH, Zhou JD, Chi Q. Upregulation of miR-542-3p inhibits the growth and invasion of human colon cancer cells through PI3K/AKT/survivin signaling. Oncol Rep. 2017;38(6):3545–3553. doi: 10.3892/or.2017.5851. [DOI] [PubMed] [Google Scholar]
- 23.Bahrami A, Hasanzadeh M, Hassanian SM, et al. The potential value of the PI3K/Akt/mTOR Signaling pathway for assessing prognosis in cervical cancer and as a target for therapy. J Cell Biochem. 2017;118(12):4163–4169. doi: 10.1002/jcb.26118. [DOI] [PubMed] [Google Scholar]