Abstract
Purpose
Umbelliferone (Umb), a member of coumarin family, is found in many plants and is a promising molecule with potential anti-inflammatory, anti-oxidative, and anti-tumor activities. However, the effect of Umb on arthritis remains unclear.
Methods
A rat model with Freund’s complete adjuvant (FCA)-induced arthritis was developed and used to test the efficacy of Umb on arthritis rats. Rats were given an intragastric injection of Umb (20 and 40 mg/kg) once daily from days 21 to 28 after the administration of FCA. Hind paw volume was assessed using a volume meter. The pro-inflammatory cytokine levels and prostaglandin E2 (PEG2) level in serum and synovial fluid were detected by ELISA. HE staining was used to determine representative histological changes in joint tissues, and Western blot analysis was employed to study the effects of Umb on MAPK/NF-κB signaling pathway.
Results
Our results showed that Umb suppressed the release of IL-6, IL-1β, tumor necrosis factor-alpha, and PEG2. In addition, Umb could also dramatically ameliorate the pathological changes observed in rat joints. Based on the results of Western blot, we also observed that Umb could strikingly suppress the expression of MAPK/NF-κB pathway molecules.
Conclusion
These results proved that treatment with Umb is very effective for arthritis and inhibiting the MAPK/NF-κB signaling pathway may be a potential therapeutic target for treatment of arthritis.
Keywords: umbelliferone, arthritis, inflammation, MAPK/NF-κB pathway
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease with the main clinical manifestation of systemic complications, including synovial inflammation, joint lesions, and bone damage.1 It is a clinical syndrome that includes several disease subsets and usually involves several inflammatory cascades, finally leading to persistent synovial inflammation and associated damage to articular cartilage and underlying bone.2 Currently, 0.5%–1% of people around the world suffer from arthritis.3 However, the pathological mechanism of RA and its etiological factors remain largely unknown. Therefore, it is urgent to explore its potential mechanism and seek a safer alternative drug to ameliorate the inflammatory pathological progress in the patients with RA.
Inflammatory mediators are known to play a central role in RA.4 It is widely believed that persistent excess inflammatory cytokines, such as IL-6, IL-1β, and tumor necrosis factor-alpha (TNF-α), contribute to the development of chronic inflammation and RA.5 Meanwhile, MAPK/NF-κB pathway has been proven to play an important role in regulating the release of inflammatory mediators.6
Umbelliferone (Umb) or 7-hydroxycoumarin, widely found in many plants such as carrot, coriander, and garden angelica, is a derivative of coumarin. Recently, many studies reported on the various pharmacological properties of Umb, such as anti-oxidant, anti-inflammation, anti-diabetic, and anti-tumor activities.7–9 In addition, there are also reports that focus on its renoprotective effects by regulation of Nrf-2/Keap-1 and P38MAPK/NF-κB pathways.10 These studies suggested that Umb might be a potential compound that can be used for the treatment of RA. Therefore, we examined the effects of Umb on RA and its underlying mechanism in the present study.
Materials and methods
Reagents
Umb (purity ≥98%) was purchased from National Institutes for Food and Drug Control (Beijing, China). Freund’s complete adjuvant (FCA) was obtained from Sigma-Aldrich Co. (St Louis, MO, USA). TNF-α, IL-6, IL-1β, and prostaglandin E2 (PEG2) ELISA kits were purchased from Nanjing KeyGen BiotechCo., Ltd. (Nanjing, China). All antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA).
Animal and experimental protocol
Sprague Dawley rats (180–220 g) were purchased from the Animal Center of Tongji Medical College (Wuhan, China). Rats were maintained in a specific pathogen-free laboratory in the Animal Center of Tongji Medical College under controlled temperature (22°C–24°C) and a 12-hour light/12-hour dark cycle environment. Standard food and water were given ad libitum according to the Animal Studies Committee of Tongji Medical College. All experimental steps complied with the Tongji Medical College guidelines for the care and use of animals and were approved by the Medical Ethics Committee of Tongji Medical College.
All rats were randomly divided into five groups with ten rats per group: control group (control), adjuvant arthritis (AA) group, AA + diclofenac sodium (DS, 5 mg/kg) group, AA + Umb (20 mg/kg) group, and AA + Umb (40 mg/kg) group. AA animal model was induced as described in a previous study.11 FCA was administrated subcutaneously in the palmar surface of left hind paw on day 0 to establish the arthritis rat model. Rats in AA + Umb groups were stimulated by arthritis and then orally administrated with Umb (20 and 40 mg/kg) suspended in 200 μL of a 0.5% carboxymethyl cellulose sodium (CMC–Na) mixture once a day from day 21 to 28. The rats in AA + DS groups were stimulated by AA and then orally administrated with DS (5 mg/kg) once a day from day 21 to 28. Arthritis was induced in rats of control and AA groups and then equal amount of 0.5% CMC–Na was given orally in a similar fashion.
Hind paw volume (HPV) analysis
The change in the HPV was monitored to study the severity of arthritis disease.12 The size of left hind paw was assessed every seventh day from day 1 to 28 using YLS-7A volume meter (Shandong Academy of Medical Sciences Equipment Station, Shandong, China).
Detections of cytokines
At the end of the animal experiments, xylazine (12 mg/kg) and ketamine (80 mg/kg) were injected intraperitoneally to anesthetize all the animals, which were subsequently exsanguinated for sacrifice. Blood and synovial fluid were collected and centrifuged at 3,500 rpm for 15 minutes and the supernatant was separated and used for the detection of cytokines. The levels of cytokines, including IL-6, IL-1β, and TNF-α, and PEG2 were detected by using ELISA kits in accordance with the manufacturer’s directions.
Histological study
Joint tissue specimens were harvested from left hind paws of each rat and fixed in 10% (V/V) neutral buffered formalin and subjected to decalcification. After that, samples were embedded in paraffin and sliced into 4 μm thick slices. HE staining was then processed using standard methods.
Western blot analysis
After the rats were euthanized, the synovium of knee joints was isolated and cut into pieces. The samples were snap frozen in liquid nitrogen, homogenized, and lysed in ice-cold RIPA buffer (0.1% phenylmethylsulfonyl fluoride) and then centrifuged at 12,000× g for 20 minutes. The protein concentration was determined by BCA kit (Beyotime Biotechnology, Nanjing, China) according to the manufacturer’s instructions. The protein extracts were separated by 8% SDS-PAGE and then transferred onto polyvinylidene fluoride (PVDF) membranes. The PVDF membranes were blocked with 5% fat-free milk for 2 hours and incubated with respective primary rabbit antibodies (all antibodies were obtained from Cell Signaling Technology: P-Jnk, #4688; Jnk, #9252; P-ERK, #4370; ERK, #9102; P-P38, #4511; P38, #9212; P-P65, #3033; P65, #8242; P-IκBα, #5209; IκBα, #4812; GAPDH, #51748) in 5% BSA (1:1,000) at 4°C overnight. Blots were washed with Tris-buffered saline/Tween 20 (TBST) and incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:10,000) for 1 hour at room temperature. Immunoreactive bands were visualized using enhanced chemiluminescence (Bio-Rad Laboratories Inc., Hercules, CA, USA), and the results were observed by using a Bio-Rad ChemiDoc XRS system and intensities were determined using image analysis software (Bio-Rad Laboratories).
Statistical analysis
All results were expressed as mean ± SD. Statistical analysis was performed by using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA). The values were calculated from the mean of three independent experiments and analyzed by one-way ANOVA with Tukey’s multiple comparison test. The results were considered significant when P<0.05 and P<0.01.
Results
Effect of Umb on rat HPV
The alterations in HPV at days 0, 7, 14, 21, and 28 were investigated to evaluate the severity of swelling and polyarthritis caused by FCA-stimulated adjuvant arthritis. Interestingly, the hind paw swelling significantly reversed after administration of Umb (20 and 40 mg/kg) or DS (5 mg/kg) in contrast with that of the AA rats (Figure 1).
Figure 1.
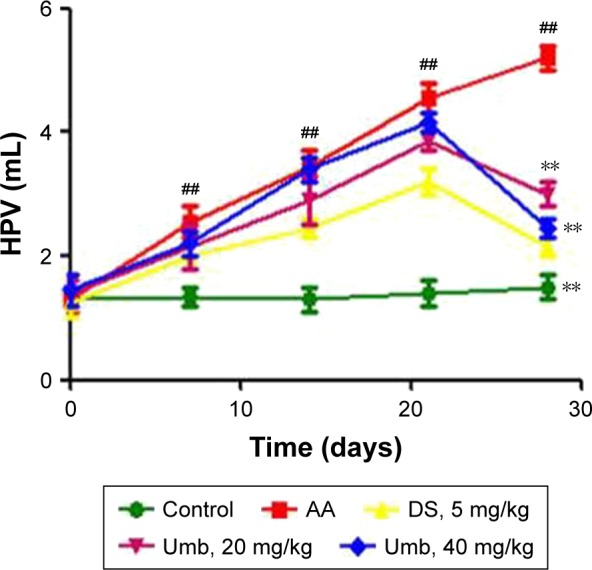
Effect of Umb on rat HPV.
Notes: Rats were given an intragastric injection of Umb (20 and 40 mg/kg) once daily from day 21 to 28 after the administration of FCA. The size of left hind paw was assessed every seventh day from day 1 to 28. Values represent the mean ± SD and are representative of two independent experiments. ##P<0.01, indicates significant difference from the values in the control group; **P<0.01 indicates significant difference from the values in the AA group.
Abbreviations: Umb, umbelliferone; HPV, hind paw volume; FCA, Freund’s complete adjuvant; AA, adjuvant arthritis; DS, diclofenac sodium.
Effect of Umb on the expression of cytokines in serum
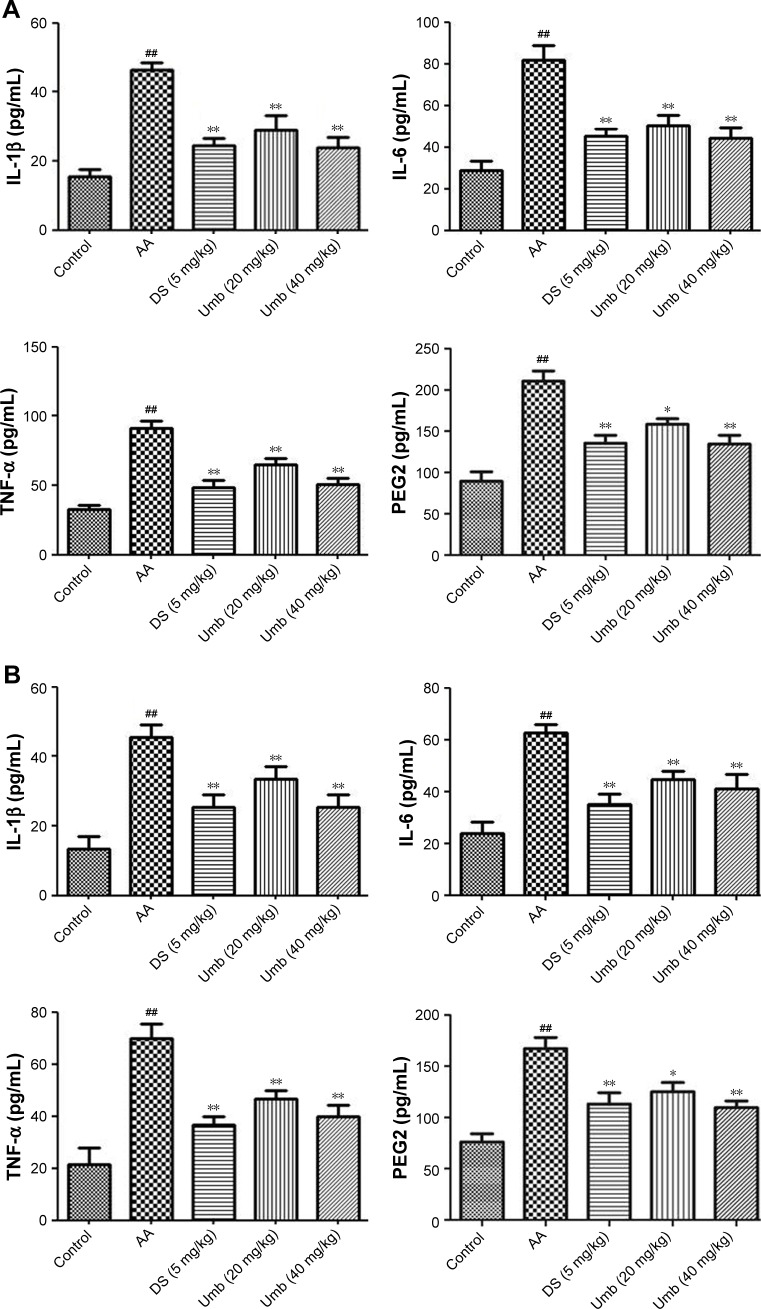
As shown in Figure 2, the levels of TNF-α, IL-6, IL-1β, and PEG2 in serum and synovial fluid were obviously increased in AA group compared with those in the control group using the ELISA kits. However, the Umb or DS treatment groups exhibited decreased levels of IL-1β, IL-6, TNF-α, and PEG2 in serum and synovial fluid, especially in the high dosage group.
Figure 2.
Effect of Umb on pro-inflammatory cytokine levels and PEG2 level.
Notes: Rats were given an intragastric injection of Umb (20 and 40 mg/kg) once a day from day 21 to 28 after the administration of FCA. The concentrations of IL-1β, IL-6, TNF-α, and PEG2 were determined in serum (A) and synovial fluid (B). Values represent the mean ± SD and are representative of two independent experiments. ##P<0.01 indicates significant difference from the values in the control group; *P<0.05, **P<0.01, indicate significant difference from the values in the AA group.
Abbreviations: Umb, umbelliferone; PEG2, prostaglandin E2; FCA, Freund’s complete adjuvant; TNF-α, tumor necrosis factor-alpha; AA, adjuvant arthritis; DS, diclofenac sodium.
Effect of Umb on histological assessment
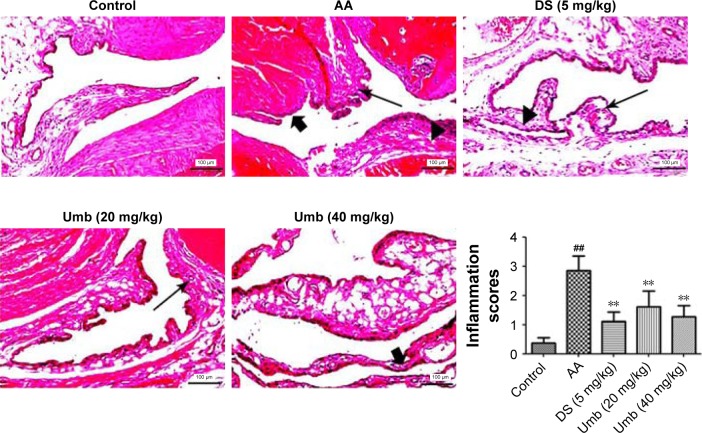
As shown in Figure 3, joints in the control group showed normal tissue architecture and cell structure. However, obvious joint destruction with the characteristics of articular cartilage lesion, cellular infiltration, epithelial cell degeneration, synovial tissue vasodilation, and congestion was observed in AA rats. On the contrary, joints in the Umb (20 and 40 mg/kg) or DS (5 mg/kg) group were effectively protected from synovial tissue edema, granulocyte/mononuclear cell infiltration, epithelial cell degeneration, and articular cartilage erosion, suggesting Umb may display a protective effect on arthritis.
Figure 3.
Histological assessment (×200).
Notes: Rats were given an intragastric injection of Umb (20 and 40 mg/kg) once a day from day 21 to 28 after the administration of FCA. HE staining was done to study cellular infiltration (►), synovial tissue vasodilation and congestion (<img>), and articular cartilage lesion (→). Representative histological changes in joint tissues were obtained from mice of different groups. Values represent the mean ± SD and are representative of two independent experiments. ##P<0.01 indicates significant difference from the values in the control group; **P<0.01 indicates significant difference from the values in the AA group.
Abbreviations: Umb, umbelliferone; FCA, Freund’s complete adjuvant; AA, adjuvant arthritis; DS, diclofenac sodium.
Effects of Umb on the expression of MAPK/NF-κB signaling molecules in synovial tissue
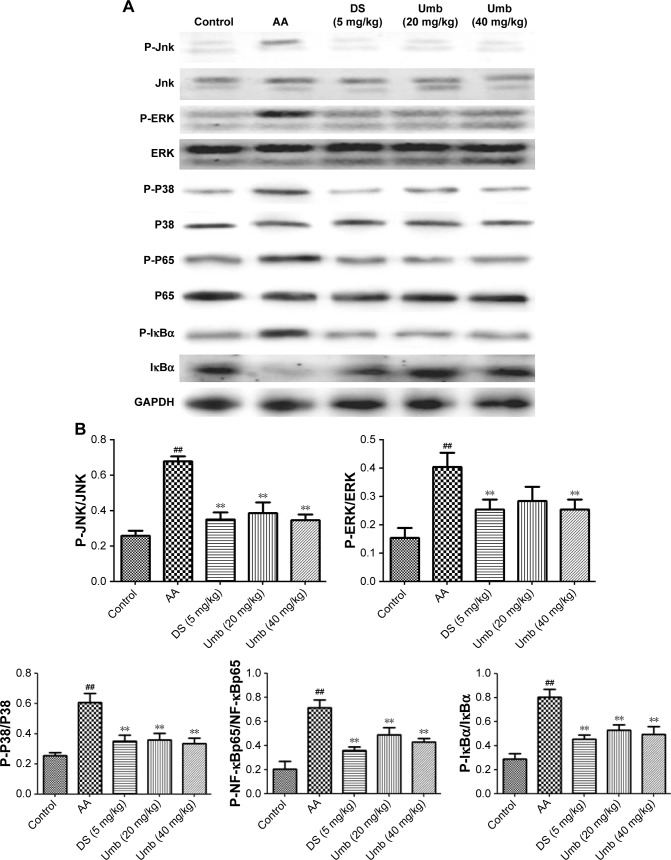
To further investigate the anti-inflammatory mechanism of Umb on FCA-induced adjuvant arthritis in rats, the inflammation-related signaling pathway of MAPK/NF-κB was studied. Evidences have reported that upregulation of MAPK pathway would activate NF-κB which is followed by the activation of downstream signaling pathways.13,14 As shown in Figure 4, the expression levels of p-JNK, p-ERK, p-P38, p-NF-κBp65, and p-IκBα were upregulated in AA rats after exposure to FCA, while treatments with Umb (20 and 40 mg/kg) or DS (5 mg/kg) effectively downregulated the above mentioned protein levels in synovial tissue, suggesting the involvement of MAPK/NF-κB signaling in the pathogenesis of arthritis induced by FCA.
Figure 4.
Effects of Umb on the expression of MAPK/NF-κB signaling in synovial tissue.
Notes: (A) The bands of Western blot. (B) The densitometric analysis of bands. Rats were given an intragastric injection of Umb (20 and 40 mg/kg) once a day from day 21 to 28 after the administration of FCA. Protein samples were analyzed by Western blot with specific antibodies. Values represent the mean ± SD and are representative of two independent experiments. ##P<0.01, indicates significant difference from the values in the control group; **P<0.01 indicates significant difference from the values in the AA group.
Abbreviations: Umb, umbelliferone; FCA, Freund’s complete adjuvant; AA, adjuvant arthritis; DS, diclofenac sodium.
Discussion
Humans produce a large number of adverse substances in response to a harmful stimulus, which may further induce RA. The release of toxic substances leads to synovitis in cartilage. Inflammatory response is an important pathophysiological mechanism that is initiated with the injection of the FCA. FCA-induced rat arthritis is a widely used animal model for demonstrating autoimmune inflammation, as it presents characteristics similar to those observed in human RA damage.15 After the injection of FCA, this model successfully exhibited obvious HPV changes, which is one of the major factors in assessing the degree of inflammation and curative efficacy of drugs. As a non-steroidal anti-inflammatory analgesic, DS is also a commonly used drug for the treatment of RA, which mainly acts by inhibiting the synthesis of prostaglandins to produce anti-inflammatory and analgesic effects. DS is often used as a positive drug for arthritis animal experiments.16 Consistent with this finding, our results showed that treatment with Umb or DS exhibited inhibitory effect on hind paw swelling.
Moreover, there are increasing evidences to point out that pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, are involved in the pathogenesis of RA. The release of these cytokines further promotes the process of inflammation and pain, as well as cartilage and bone destruction.17 Emerging evidence has indicated that TNF-α and IL-6 participated in synovial proliferation and induced cartilage destruction, while IL-1β played dominant roles in the process of RA.18 IL-6 may result in joint damage because of its high expression in the rheumatoid synovium.19,20 From our results, we found that Umb could reduce the concentrations of inflammatory cytokines in FCA-induced arthritis.
Multiple signaling pathways are responsible for the generation and secretion of inflammatory cytokines and play important roles in the pathogenesis of RA. Recently, many studies have proved that MAPK pathway plays an important role in arthritis. Previous literature have also shown the MAPK signaling which may mediate NF-κB pathway.21 The transcriptional factor NF-κB, involved in many inflammation diseases, is an essential molecule that controls the production of various mediators (including IL-1β, IL-6, and TNF-α).22 In normal cells, inactivated NF-κB is absorbed by the IκB protein in the cytoplasm. The two subunits of the IKK complex, IKKα and IKKβ, are the main regulator factors that control the IκB production.23 After the phosphorylation and degradation of IκB, NF-κB is immediately transferred to the nucleus which in turn drives the expression of cytokines and inflammatory mediators.24 To further identify the molecular mechanism related to AA, Western blot evaluation was performed. Our present study indicated that the inhibition effect of Umb on MAPK signaling was proved to be activated and successful. Furthermore, the suppression of MAPK cascade could effectively block the phosphorylations of IκB and NF-κB. An analog of Umb, Umb β-D-galactopyranoside, has also been reported to exhibit anti-arthritic properties, but the key molecular targets of this study were COX-1 and COX-2, which are bifunctional enzymes.25 These enzymes show both cyclooxygenase and catalase activities, and are key molecules that catalyze the conversion of arachidonic acid to prostaglandins. But in our study, we focused on the MAPK/NF-κB pathway, a very classic inflammatory management pathway. Both Umb and Umb β-D-galactopyranoside were confirmed to exert protective effect on arthritic rats, which has important guiding significance for their later development.
Conclusion
Our study confirmed the hypothesis that Umb exhibited protective effects on FCA-induced arthritis, as evidenced by suppressing the increase in HPV and ameliorating histological changes observed in joint tissues. The mainly involved mechanism was the anti-inflammatory role of Umb through MAPK/NF-κB pathways. Our study provided a potential candidate compound for the treatment of arthritis.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No 81141077) and National Key Research and Development Program (2017YFC1103804) and Hubei Provincial Natural Science Foundation (2018CFB423).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wang J, Wei T, Gao J, et al. Effects of Naringenin on inflammation in complete freund’s adjuvant-induced arthritis by regulating Bax/Bcl-2 balance. Inflammation. 2015;38(1):245–251. doi: 10.1007/s10753-014-0027-7. [DOI] [PubMed] [Google Scholar]
- 2.Valbona D, Argjent T, Teuta B. Epidemiology of rheumatoid arthritis in Tirana, Albania. Mater Sociomed. 2013;25(2):96–97. doi: 10.5455/msm.2013.25.96-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta M, Sasmal S, Mukherjee A. Therapeutic effects of acetone extract of saraca asoca seeds on rats with adjuvant-induced arthritis via attenuating inflammatory responses. ISRN Rheumatol. 2014;2014:959687. doi: 10.1155/2014/959687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smolen JS, Steiner G. Therapeutic strategies for rheumatoid arthritis. Nat Rev Drug Discov. 2003;2(6):473–488. doi: 10.1038/nrd1109. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann M. Many cytokines are very useful therapeutic targets in disease. J Clin Invest. 2008;118(11):3533–3536. doi: 10.1172/JCI37346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng Y, Tang K, Chen R, et al. Effects of Shugan-Jianpi recipe on the expression of the p38 MAPK/NF-kappaB signaling pathway in the hepatocytes of NAFLD rats. Medicines (Basel) 2018;5(3):106. doi: 10.3390/medicines5030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijayalakshmi A, Sindhu G. Dose responsive efficacy of umbelliferone on lipid peroxidation, anti-oxidant, and xenobiotic metabolism in DMBA-induced oral carcinogenesis. Biomed Pharmacother. 2017;88:852–862. doi: 10.1016/j.biopha.2017.01.064. [DOI] [PubMed] [Google Scholar]
- 8.Kumar V, Ahmed D, Verma A, Anwar F, Ali M, Mujeeb M. Umbel-liferone β-D-galactopyranoside from Aegle marmelos (L.) corr. an ethnomedicinal plant with antidiabetic, antihyperlipidemic and anti-oxidative activity. BMC Complement Altern Med. 2013;13(1):273. doi: 10.1186/1472-6882-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Islam MN, Choi RJ, Jin SE, et al. Mechanism of anti-inflammatory activity of umbelliferone 6-carboxylic acid isolated from Angelica decursiva. J Ethnopharmacol. 2012;144(1):175–181. doi: 10.1016/j.jep.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 10.Hassanein EHM, Mohamed WR, Shalkami AS, Khalaf MM, Hemeida RAM. Renoprotective effects of umbelliferone on methotrexate-induced renal injury through regulation of Nrf-2/Keap-1, P38MAPK/NF-kappaB, and apoptosis signaling pathways. Food Chem Toxicol. 2018;116(Pt B):152–160. doi: 10.1016/j.fct.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 11.Chang X, He H, Zhu L, et al. Protective effect of apigenin on Freund’s complete adjuvant-induced arthritis in rats via inhibiting P2X7/NF-κB pathway. Chem Biol Interact. 2015;236:41–46. doi: 10.1016/j.cbi.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Coelho M, Reis P, Gava V, et al. Anti-arthritic effect and subacute toxicological evaluation of Baccharis genistelloides aqueous extract. Toxicol Lett. 2004;154(1–2):69–80. doi: 10.1016/j.toxlet.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Xueyang D, Zhanqiang M, Chunhua M, Kun H. Fasudil, an inhibitor of Rho-associated coiled-coil kinase, improves cognitive impairments induced by smoke exposure. Oncotarget. 2016;7(48):78764–78772. doi: 10.18632/oncotarget.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen T, Guo Q, Wang H, et al. Effects of esculetin on lipopolysaccharide (LPS)-induced acute lung injury via regulation of RhoA/Rho Kinase/NF-κB pathways in vivo and in vitro. Free Radic Res. 2015;49(12):1459–1468. doi: 10.3109/10715762.2015.1087643. [DOI] [PubMed] [Google Scholar]
- 15.Lan Z, Wei M, Chen L, Xie G, Liu X, Zhang X. Role of sinomenine on complete Freund’s adjuvant-induced arthritis in rats. IUBMB Life. 2016;68(6):429–435. doi: 10.1002/iub.1499. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann P, Butt E, Feher A, et al. Electroporation-enhanced transdermal diclofenac sodium delivery into the knee joint in a rat model of acute arthritis. Drug Des Devel Ther. 2018;12:1917–1930. doi: 10.2147/DDDT.S161703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T, Mou Y, Tan J, et al. The protective effect of CDDO-Me on lipopolysaccharide-induced acute lung injury in mice. Int Immunopharmacol. 2015;25(1):55–64. doi: 10.1016/j.intimp.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Newton RC, Decicco CP. Therapeutic potential and strategies for inhibiting tumor necrosis factor-α. J Med Chem. 1999;42(13):2295–2314. doi: 10.1021/jm980541n. [DOI] [PubMed] [Google Scholar]
- 19.Jiang W, Luo F, Lu Q, et al. The protective effect of Trillin LPS-induced acute lung injury by the regulations of inflammation and oxidative state. Chem Biol Interact. 2016;243:127–134. doi: 10.1016/j.cbi.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Jazayeri JA, Carroll GJ, Vernallis AB. Interleukin-6 subfamily cytokines and rheumatoid arthritis: role of antagonists. Int Immunopharmacol. 2010;10(1):1–8. doi: 10.1016/j.intimp.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Zhu L, Chen T, Chang X, et al. Salidroside ameliorates arthritis-induced brain cognition deficits by regulating Rho/ROCK/NF-κB pathway. Neuropharmacology. 2016;103:134–142. doi: 10.1016/j.neuropharm.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Zhou R, Luo F, Lei H, et al. Liujunzi Tang, a famous traditional Chinese medicine, ameliorates cigarette smoke-induced mouse model of COPD. J Ethnopharmacol. 2016;193:643–651. doi: 10.1016/j.jep.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Q, Yi M, Guo Q, et al. Protective effects of polydatin on lipopolysaccharide-induced acute lung injury through TLR4-MyD88-NF-κB pathway. Int Immunopharmacol. 2015;29(2):370–376. doi: 10.1016/j.intimp.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Chen T, Jiang W, Zhang H, et al. Protective effect of trillin against ethanol-induced acute gastric lesions in an animal model. RSC Adv. 2016;6(24):20081–20088. doi: 10.1039/C5RA21158A. [DOI] [Google Scholar]
- 25.Kumar V, Al-Abbasi FA, Verma A, Mujeeb M, Anwar F. Umbelliferone β-D-galactopyranoside exerts anti-inflammatory effect by attenuating COX-1 and COX-2. Toxicol Res (Camb) 2015;4(5):1427. doi: 10.1039/C5TX90017D. [DOI] [Google Scholar]