Abstract
Background
The cervical vestibular evoked myogenic potential (cVEMP) test measures saccular and inferior vestibular nerve function. The cVEMP can be elicited with different frequency stimuli and interpreted using a variety of metrics. Patients with superior semicircular canal dehiscence (SCD) syndrome generally have lower cVEMP thresholds and larger amplitudes, although there is overlap with healthy subjects. The aim of this study was to evaluate which metric and frequency best differentiate healthy ears from SCD ears using cVEMP.
Methods
Twenty-one patients with SCD and 23 age-matched controls were prospectively included and underwent cVEMP testing at 500, 750, 1,000 and 2,000 Hz. Sound level functions were obtained at all frequencies to acquire threshold and to calculate normalized peak-to-peak amplitude (VEMPn) and VEMP inhibition depth (VEMPid). Third window indicator (TWI) metrics were calculated by subtracting the 250-Hz air-bone gap from the ipsilateral cVEMP threshold at each frequency. Ears of SCD patients were divided into three groups based on CT imaging: dehiscent, thin or unaffected. The ears of healthy age-matched control subjects constituted a fourth group.
Results
Comparing metrics at all frequencies revealed that 2,000-Hz stimuli were most effective in differentiating SCD from normal ears. ROC analysis indicated that for both 2,000-Hz cVEMP threshold and for 2,000-Hz TWI, 100% specificity could be achieved with a sensitivity of 92.0%. With 2,000-Hz VEMPn and VEMPid at the highest sound level, 100% specificity could be achieved with a sensitivity of 96.0%.
Conclusion
The best diagnostic accuracy of cVEMP in SCD patients can be achieved with 2,000-Hz tone burst stimuli, regardless of which metric is used.
Key Words: Cervical vestibular evoked myogenic potential, Superior canal dehiscence, Vestibular nerve function
Introduction
Patients with semicircular canal dehiscence (SCD) syndrome suffer from a variety of auditory and vestibular symptoms caused by a bony defect in the superior semicircular canal that creates a “third window” [Minor et al., 1998]. Acoustic stimulation of an ear with a “third window” causes energy from stapes footplate motion to shunt towards the dehiscence and away from the cochlear partition. As a result, the pressure difference across the basilar membrane decreases, while energy transmission to the vestibular sense organs increases [Ho et al., 2017; Rosowski et al., 2004]. The diagnosis of SCD is generally based on a combination of tests including symptomatology, threshold audiometry and immitance testing, vestibular evoked myogenic potential (VEMP) testing and temporal bone CT imaging [Ho et al., 2017]. These tests can be time consuming and costly.
The cervical vestibular evoked myogenic potential (cVEMP) test measures saccular and inferior vestibular nerve function. When the saccule is acoustically or mechanically stimulated, vestibulocollic projections inhibit the (mostly) ipsilateral sternocleidomastoid muscle (SCM) which can be measured with electromyography [Colebatch et al., 1994]. An SCD third window can lead to low thresholds and large amplitudes on cVEMP testing compared to healthy controls [Benamira et al., 2014; Brantberg, 2009; Brantberg et al., 1999; Colebatch et al., 1994; Hunter et al., 2017; Milojcic et al., 2013; Roditi et al., 2009; Streubel et al., 2001]. Multiple metrics can be used to assess the cVEMP, including the normalized peak-to-peak amplitude (VEMPn), cVEMP threshold, the third window indicator (TWI) [Noij et al., 2018a] and a new metric called VEMP inhibition depth (VEMPid), which estimates the percentage reduction in spike rate of the SCM motoneurons during cVEMP testing and has not previously been used in patients [Prakash et al., 2015].
Previous studies indicated that threshold was more valuable than peak-to-peak amplitude in distinguishing healthy from pathological ears in both SCD and Ménière's disease [Brantberg et al., 1999; Rauch et al., 2004; Taylor et al., 2012]. Thresholds, however, overlap between the SCD and healthy populations, and threshold measurement can be time consuming. Although the cVEMP is not a painful test, longer testing time can result in neck muscle discomfort. A cVEMP-based diagnostic tool for SCD patients that seems to be more useful than cVEMP threshold alone is the TWI [Noij et al., 2018a]. The TWI combines cVEMP threshold with the audiometric low-frequency air-bone gap (ABG) from the ipsilateral ear and improves the differentiation of SCD patients from healthy subjects [Noij et al., 2018a]. The TWI was developed using a retrospective analysis of data and has not been compared to other metrics prospectively.
Although many institutions record cVEMP only with 500-Hz tone bursts, testing at multiple frequencies appears to be a valuable tool in differentiating patients with Ménière's disease [Rauch et al., 2004]. In our institution, clinical cVEMP testing includes stimulation with 500-, 750- and 1,000-Hz tone bursts. In SCD patients, cVEMP amplitude and threshold tuning curves broaden [Taylor et al., 2012]. A similar broadening of tuning has been found in ocular VEMPs (oVEMP) [Manzari et al., 2013]. Manzari et al. used high-frequency stimuli up to 8,000 Hz to evoke oVEMP responses and concluded that 4,000 Hz was the optimal frequency to distinguish dehiscent from healthy ears [Manzari et al., 2013]. These cVEMP and oVEMP findings suggest that stimulation of SCD patients with higher frequencies may be of clinical value. Since cVEMPs above 1,000 Hz were not available in the clinical retrospective data set, TWI has not been evaluated at higher frequencies.
The current study was designed to prospectively test SCD patients and systematically evaluate the same sound levels, frequencies and metrics in every subject, something that could not be done in our retrospective study [Noij et al., 2018a]. 2,000 Hz was included to evaluate cVEMP metrics at a higher frequency. Although Manzari et al. [2013] concluded that 4,000 Hz was optimum for oVEMP measurements, we decided not to add another frequency to our protocol because this would increase testing time and because intense 4,000-Hz tones can be very uncomfortable. The aim of this prospective study was to determine which cVEMP metric and which stimulus frequency best identify SCD patients to optimize the applicability of cVEMP as a diagnostic tool in SCD patients.
Methods
Subjects
Between April 2016 and November 2017, twenty-one patients with symptomatic and radiographically confirmed SCD and 23 age-matched healthy controls were prospectively included in this study. Since middle ear pathology can influence cVEMP outcomes (decreased amplitude and increased thresholds), patients and control subjects with middle ear pathology were excluded. Middle ear pathology was identified by a combination of exam findings, including threshold and immitance audiometry. Those with an ABG > 10 dB at any tested frequency were further evaluated with immittance audiometry. Ears with an ABG > 10 dB in combination with abnormal tympanograms and/or absent reflexes were excluded from this study. For the healthy controls, additional exclusion criteria were a history of hearing loss, vertigo, balance problems, neurological disorders and musculoskeletal disease. This study was approved by the Human Studies Committee of the Massachusetts Eye and Ear Infirmary (protocol No. 13-097H, principal investigator: S.D. Rauch).
Radiology
Eleven patients underwent CT imaging at our institution, and 10 patients had CT imaging from an outside hospital. For 7 patients, a cone beam CT scanner (3D Accuitomo 170; J. Morita, Irvine, CA, USA) was used generating images with a slice thickness of 0.5 mm and an axial pixel dimension of 0.125 mm. Multidetector row CT scanners were used for all other patients, generating images with an average slice thickness of 0.70 mm (0.5–1.25 mm). All superior semicircular canals were evaluated by a neuroradiologist at our institution using reformatted images in Stenvers and Pöschl planes created with multiplanar reconstruction. Stenvers plane images were perpendicular, and Pöschl images were parallel to the plane of the superior semicircular canal. The neuroradiologist evaluating these images subjectively determined whether the semicircular canals were dehiscent, had a thin layer of bone covering the canal or were normal in appearance. Based on the radiologist's interpretation, the results from each side of each subject were divided into three groups: dehiscent, thin bone or unaffected (“unaffected” denotes a normal bone-covered superior canal contralateral to an SCD ear).
Audiometry
Air and bone conduction tonal thresholds were measured for all subjects at octave frequencies from 250 to 4,000 Hz. If the difference between air and unmasked bone conduction thresholds was larger than 10 dB HL, bone conduction thresholds were masked. The ABG was calculated at each tested frequency by subtracting the bone conduction threshold from the air conduction threshold. Ears with either an ABG of ≤10 dB at any frequency or an ABG > 10 dB in combination with normal tympanograms and present acoustic reflexes were considered to have normal middle ear function and were included in this study.
Cervical Vestibular Evoked Myogenic Potential
cVEMPs were obtained with subjects sitting up straight and the head turned away from the stimulated ear to elicit contraction of the ipsilateral SCM. Electromyographic (EMG) activity was recorded from 4 surface electrodes: a positive electrode on the middle belly of each SCM, a reference electrode at the midpoint between SCM attachments to the sternum, and a ground electrode on the midline forehead. Ipsilateral SCM EMG amplitude was monitored, and subjects contracted their SCM to produce > 45 μV root mean square. All subjects were able to maintain SCM contractions above this limit, and the few waveforms < 45 μV root mean square were not included. EMG activity was amplified, bandpass-filtered and sampled at 50 kHz with a 16-bit analog-to-digital converter (National Instruments).
cVEMPs were obtained using 500-, 750-, 1,000- and 2,000-Hz tone bursts generated by custom-programmed evoked potential software (National Instruments 16-bit digital I/O board) using a Blackman gating function with 2 cycle (4.0 ms at 500 Hz, 2.5 ms at 750 Hz, 2 ms at 1,000 Hz, 1 ms at 2,000 Hz) rise and fall times and no plateau. Tone bursts were presented monaurally via circumaural headphones (Telephonics TDH-49) at a repetition rate of 13 bursts/s; cVEMP responses averaged between 200 and 300 single sweeps. In the SCD group, tone bursts were presented at 83, 93, 103 and 123 dB peak sound pressure level (peSPL), while in the healthy control group, tone bursts were presented at 93, 103, 113 and 123 dB peSPL (123 dB peSPL is equivalent to 90 dB nHL). SCD patients generally have lower thresholds and the protocol with a large sound-level step was chosen to reduce testing time. Presentation orders for sound level, frequency and side were randomized.
VEMP Metrics
The collection of sound level functions at all frequencies allowed for the calculation of VEMPn and VEMPid at every sound level and for determining threshold. The cVEMP waveform was normalized using trace-by-trace normalization [van Tilburg et al., 2014]. Trace-by-trace normalization divides each raw EMG trace by the root mean square value of that trace's electromyogram (the trace electromyogram includes all 77 ms of the time from one stimulus to the next). VEMPn was obtained by measuring the amplitude difference between P1 and N1 of the average normalized waveform.
VEMPid is a template correlation method that estimates the percentage reduction in spike rate of the SCM motoneurons that is elicited by acoustic stimulation of the saccule. In this study, VEMPid was calculated using a generic template created from cVEMP responses of healthy subjects [Noij et al., 2018b; Prakash et al., 2015]. Template correlation values were calculated using the point-by-point correlation of each individual trace in a cVEMP response with the generic template. VEMPid was calculated by dividing the mean of all 200–300 template correlation values by the standard deviation of the template correlation values and multiplying by 0.2. Although Prakash et al. originally used a subject-specific template, a generic template can be used as long as the generic template latency is adjusted to the patient's response [Noij et al., 2018b; Prakash et al., 2015].
cVEMP threshold was determined after responses had been measured for the 4 preset sound levels. Starting at the highest sound level for which no cVEMP response was observed, recordings were made by increasing the sound level in 5-dB steps until a response was observed. Threshold was defined as the lowest sound level at which a cVEMP was present, as subjectively determined by the tester assessing the shape, size and latency of the response. If no response was present at our equipment limit (133 dB peSPL), the threshold was considered to be 10 dB above this limit.
TWIs were calculated by subtracting the 250-Hz ABG from the ipsilateral cVEMP threshold at each frequency. For example: a subject with a cVEMP threshold of 103 dB peSPL at a given frequency and a 250-Hz ABG of 15 dB has a TWI of 88 dB at this frequency [Noij et al., 2018a].
Data Analysis
An independent t test was performed to compare age of the SCD and healthy control groups. Full factorial analyses of variance (ANOVAs) were performed to examine differences in ABG and cVEMP metrics (nVEMP, VEMPid, cVEMP threshold and TWI) among the four groups (dehiscent, thin, unaffected and healthy control) and to evaluate the effect of stimulus frequency on the different cVEMP metrics. Group, frequency and sound level were considered fixed factors, while subject was considered a random factor. Post hoc pairwise comparisons using a Bonferroni adjustment for multiple comparisons were performed to compare groups and frequencies. Receiver-operating characteristic (ROC) curves were used to compare the different metrics and frequencies in their ability to distinguish dehiscent from healthy-control superior semicircular canals.
Results
Patient Characteristics
Twenty-one patients with a radiographically confirmed SCD on at least one side were included (11 female, mean age: 50.1 years; range: 35–67 years). Based on CT imaging, 25/42 ears were categorized as dehiscent, 9/42 as thin and 8/42 as unaffected. One of the “unaffected” ears was excluded based on audiometry indicating a potential middle ear problem, leaving 7 unaffected ears for analysis. We included 23 age-matched controls (14 female, mean age: 51.8 years, range: 26–68 years). There was no significant difference in age between the SCD and control group (p = 0.619).
Air-Bone Gap
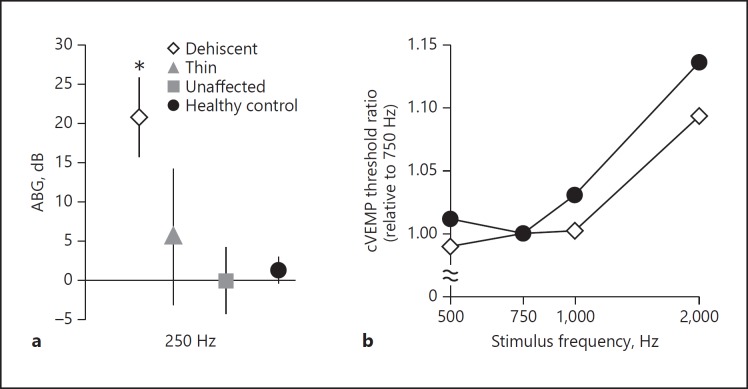
ABGs were calculated at 250 Hz for every group (Fig. 1a). As expected, the dehiscent ears had significantly larger ABGs compared to thin (p = 0.001), unaffected (p < 0.001) or healthy-control ears (p < 0.001). No significant differences were found for any of the other combinations.
Fig. 1.
a Average air-bone gap (ABG) at 250 Hz for each group. Error bars represent 95% confidence intervals. The ABG was significantly higher for the dehiscent group (asterisk) compared to thin, unaffected and healthy-control groups. There were no significant differences among unaffected, thin and healthy-control ears. b Average cVEMP threshold tuning relative to the 750-Hz threshold. As can be appreciated visually, the dehiscent-ear tuning curve is flatter than the healthy-ear tuning curve.
Cervical Vestibular Evoked Myogenic Potentials
cVEMP thresholds and TWIs were significantly lower for the dehiscent group compared to thin (p < 0.001), unaffected (p < 0.001) and healthy-control ears (p < 0.001), regardless of frequency (Fig. 2a, b). No significant differences were found for any other combination. Regarding frequency, thresholds and TWIs were significantly higher in all four subject groups at 2,000 Hz compared to 500 (p < 0.001), 750 (p < 0.001) and 1,000 Hz (p < 0.001). No significant differences were found for any other frequency combination. At 2,000 Hz, a response could not be elicited in 19/46 of the healthy control ears, while a response could always be elicited in the dehiscent ears.
Fig. 2.
Average cVEMP threshold (a) and third window indicator (TWI) (b) for 500, 750, 1,000 and 2,000 Hz. Error bars represent the 95% confidence intervals. Both cVEMP thresholds and the TWI were significantly lower in the dehiscent group compared to the thin, unaffected and healthy-control groups.
On average, 750 Hz yielded the best response in the healthy control ears (Fig. 1b). To evaluate whether there were differences in frequency tuning between dehiscence patients and healthy controls, tuning curves were calculated using the 750-Hz threshold as the reference. As can be appreciated visually, the tuning curve for dehiscent ears is slightly flatter than the healthy-control tuning curve (Fig. 1b).
For both VEMPn and VEMPid, there was a significant interaction between frequency and stimulus level (F = 6.106, p < 0.001 and F = 8.771, p < 0.001), with a larger effect of frequency at the highest sound levels (Fig. 3). There was also a significant interaction between dehiscence groups and stimulus level for both VEMPn and VEMPid (F = 65.688, p < 0.001 and F = 78.942, p < 0.001), with a larger effect of stimulus level in the dehiscent group compared to the other groups. As seen previously, VEMPid decreased to zero at the lower stimulus levels (i.e. when no response was present), while the VEMPn plateaued above zero (Fig. 3) [Noij et al., 2017].
Fig. 3.
Top: average normalized peak-to-peak amplitude (VEMPn). Bottom: average VEMP inhibition depth (VEMPid). Data from 500 (a, e), 750 (b, f), 1,000 (c, g) and 2,000 Hz (d, h) for multiple sound levels. Error bars represent 95% confidence intervals. The effect of frequency is significantly larger at the higher sound levels, and the effect of stimulus level is significantly larger in the dehiscent group compared to the other groups.
Because cVEMP outcomes are known to be affected by age, we used Pearson correlation coefficients to evaluate whether age influenced our cVEMP outcomes. Similar to findings by others, VEMPn significantly decreased with age for all frequencies in the healthy control group, while threshold significantly increased (data not shown) [Rosengren et al., 2011; Welgampola and Colebatch, 2001]. In the dehiscent group there was no significant effect of age, indicating that the presence of a dehiscence reduces the age effect (data not shown).
ROC Curves
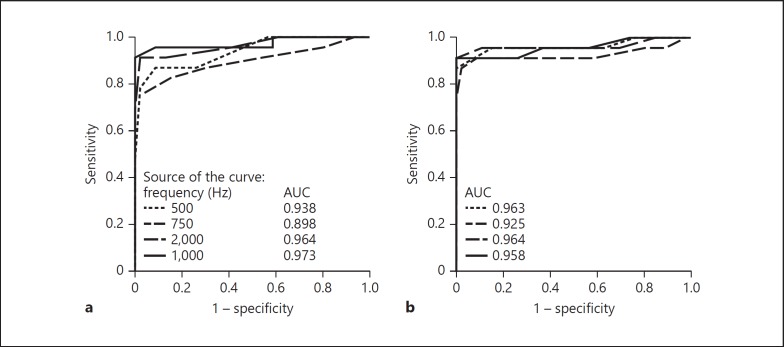
ROC curves were created to compare the ability of the four metrics (cVEMP threshold, TWI, VEMPn and VEMPid) and the four frequencies (500, 750, 1,000 and 2,000 Hz) to distinguish dehiscent from the healthy ears. Based on the area under the curve (AUC), 2,000 Hz was the best frequency for VEMP metrics to distinguish healthy from dehiscent ears (Fig. 4, 5). However, for the TWI, AUCs were slightly better at 500 and 1,000 Hz (Fig. 4).
Fig. 4.
Receiver-operating characteristic curves displaying sensitivity of detecting a dehiscence versus false positive rate (1 – specificity) for cVEMP thresholds (a) and third window indicators (TWIs) (b) for 500, 750, 1,000 and 2,000 Hz. Insets show the frequency key and the corresponding area under the curve (AUC). cVEMP threshold and TWI data have 5-dB steps so that normal and affected subjects can have the same threshold. Thus, a change in cutoff value can produce a change in both the sensitivity and specificity at once, which causes points to be connected by diagonal lines.
Fig. 5.
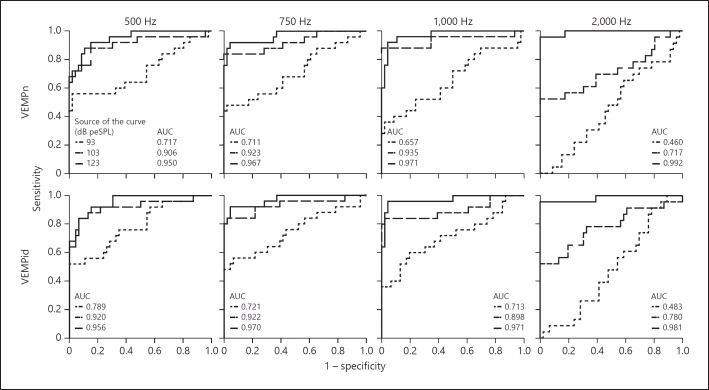
Receiver-operating characteristic (ROC) curves displaying sensitivity of detecting a dehiscence versus false positive rate (1 – specificity) for normalized peak-to-peak amplitude (VEMPn) (top row) and VEMP inhibition depth (VEMPid) (bottom row) for 500, 750, 1,000 and 2,000 Hz at multiple sound levels. Insets show the frequency key and the corresponding area under the curve (AUC).
We think that a more useful ROC measure is the sensitivity when there is 100% specificity. Using sensitivity of the 123 dB peSPL data, 2,000 Hz was the best frequency for every metric to distinguish healthy from dehiscent ears (Fig. 4, 5). For cVEMP threshold and TWI at 2,000 Hz, a specificity of 100% could be reached with a sensitivity of 92% (Table 1; Fig. 4) for cutoffs of 118 and 108 dB peSPL, respectively. These values correspond to a positive predictive value of 100% and a negative predictive value of 95.8%, meaning that all patients with a positive test (cVEMP threshold < 118 dB peSPL or TWI < 108 dB) have a dehiscent SCC on CT (Table 1; Fig. 4). A higher sensitivity of 96% for 100% specificity can be reached for both VEMPn and VEMPid at 2,000 Hz and 123 dB peSPL (Table 1; Fig. 5) for cutoff values of 0.67 and 9.2%, respectively. That is, all patients with a positive test (VEMPn > 0.67 and VEMPid > 9.2%) have a dehiscent SCC on CT (Table 1; Fig. 5). An exact McNemar's test evaluated the difference in sensitivity (92 vs. 96%) for the different metrics and revealed no statistically significant difference (p = 1.000).
Table 1.
Sample criterion values and the corresponding sensitivities and specificities for the ROC curves in Figures 4 and 5
| Threshold |
TWI |
VEMPn (123 dB peSPL) |
VEMPid (123 dB peSPL) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| criterion value, dB peSPL | sensitivity, % | specificity, % | criterion value, dB | sensitivity, % | specificity, % | criterion value (dimensionless) | sensitivity, % | specificity, % | criterion value % | sensitivity, % | specificity, % | |
| 500 Hz | 98 | 52.0 | 100.0 | 103 | 88.0 | 100.0 | 1.44 | 68.0 | 100.0 | 20.9 | 68.0 | 100.0 |
| 103 | 80.0 | 97.8 | 108 | 96.0 | 84.8 | 1.00 | 84.0 | 91.3 | 15.0 | 84.0 | 93.5 | |
| 108 | 88.0 | 93.5 | 113 | 96.0 | 60.9 | 0.75 | 92.0 | 78.3 | 10.0 | 92.0 | 82.6 | |
| 118 | 100.0 | 43.5 | 123 | 100.0 | 21.7 | 0.49 | 100.0 | 56.5 | 7.2 | 100.0 | 69.6 | |
| 750 Hz | 103 | 76.0 | 100.0 | 93 | 76.0 | 100.0 | 1.31 | 76.0 | 100.0 | 19.0 | 80.0 | 100.0 |
| 113 | 88.0 | 69.6 | 103 | 92.0 | 91.3 | 1.00 | 92.0 | 93.5 | 15.0 | 84.0 | 95.7 | |
| 123 | 96.0 | 19.6 | 113 | 92.0 | 58.7 | 0.75 | 92.0 | 78.3 | 10.0 | 92.0 | 84.8 | |
| 128 | 100.0 | 6.5 | 133 | 100.0 | 2.2 | 0.51 | 100.0 | 63.0 | 6.8 | 100.0 | 63.0 | |
| 1,000 Hz | 103 | 68.0 | 100.0 | 103 | 92.0 | 100.0 | 1.73 | 60.0 | 100.0 | 27.5 | 64.0 | 100.0 |
| 108 | 92.0 | 97.8 | 113 | 96.0 | 76.1 | 1.00 | 92.0 | 95.7 | 20.0 | 68.0 | 97.8 | |
| 118 | 96.0 | 58.7 | 123 | 96.0 | 71.7 | 0.75 | 96.0 | 87.0 | 15.0 | 84.0 | 97.8 | |
| 123 | 100.0 | 39.1 | 133 | 100.0 | 4.3 | 0.54 | 100.0 | 65.2 | 4.4 | 100.0 | 50.0 | |
| 2,000 Hz | 118 | 92.0 | 100.0 | 108 | 92.0 | 100.0 | 0.67 | 96.0 | 100.0 | 9.2 | 96.0 | 100.0 |
| 123 | 96.0 | 91.3 | 118 | 92.0 | 97.8 | 0.50 | 96.0 | 95.7 | 7.0 | 96.0 | 97.8 | |
| 128 | 96.0 | 67.4 | 128 | 96.0 | 63.0 | 0.40 | 96.0 | 82.6 | 5.0 | 96.0 | 93.5 | |
| 138 | 100.0 | 41.3 | 138 | 100.0 | 26.1 | 0.28 | 100.0 | 82.6 | 1.9 | 100.0 | 60.9 | |
If all cases with a threshold or third window indicator (TWI) below the criterion value or a VEMPn or VEMPid above the criterion value are assumed to indicate the presence of a dehiscence, then the data show that this judgment will have the sensitivity and specific- ity listed. For example, for cVEMP threshold at 2,000 Hz (lower left in the Table), a 118-dB criterion cutoff value corresponds to a 92% sensitivity and a 100% specificity (italicized in the Table). This means that none of the healthy ears had a 2,000-Hz threshold below 118 dB peSPL (specificity = 100%), while 23 out of 25 ears with SCD met this criterion (sensitivity = 92%).
Discussion
This study investigated the cVEMP metric and frequency that were best at distinguishing healthy from dehiscent semicircular canals. At the highest sound level used for direct comparisons (123 dB peSPL) and for every metric used, 2,000 Hz produced the highest sensitivity in detecting a dehiscence with 100% specificity. Sensitivities of ≥92% could be reached for every metric, and VEMPn and VEMPid reached the highest sensitivity (96.0%). Although 2,000 Hz is not the frequency with the largest amplitudes or lowest thresholds, the 2,000-Hz, 123 dB peSPL stimuli provided the best separation between healthy and dehiscent ears. This was partly because healthy ears did often not have robust cVEMP responses at 2,000 Hz, while dehiscent ears did. At 2,000 Hz, VEMPn and VEMPid grow more with sound level in dehiscent ears than in patients with normal or thin bony covering of the superior semicircular canal (Fig. 3).
At 103 dB peSPL, VEMPn and VEMPid at 500, 750 and 1,000 Hz were better than 2,000 Hz at separating dehiscent and healthy semicircular canals (Fig. 5). The 2,000-Hz data may do more poorly at levels below 123 dB peSPL because of differences in threshold across frequencies. Thresholds at 2,000 Hz were higher for all subject groups (Fig. 2), and 103 dB peSPL was not always sufficient to elicit a response at 2,000 Hz. At 103 dB peSPL, 750- and 1,000-Hz VEMPn and VEMPid provided good sensitivities (80 and 88%) with 100% specificity (Fig. 5), and comparable sensitivities were obtained with the TWI. Therefore, it could be argued that to reduce noise exposure the use of lower frequencies and lower sound levels is a reasonable alternative to a 2,000-Hz, 123 dB peSPL stimulus.
Compared to the standard multifrequency testing in our clinic, a single measurement using a 123 dB peSPL 2,000-Hz tone burst would reduce the total sound exposure. The 2,000-Hz tone burst has 2 cycle (1 ms) rise and fall times without a plateau so that the 123 dB peSPL is reached only momentarily at the sound peak. The brevity of this stimulus reduces the risk for acoustic trauma.
Omitting a cVEMP stimulus level between 103 and 123 dB peSPL is a limitation of this study. Because we wanted to compare 4 frequencies, obtain threshold at every frequency and keep testing time within 1.5 h, it was necessary to take a large step (123 to 103 dB peSPL) in the SCD group. It should be investigated whether the VEMPn and VEMPid separation of SCD and healthy ears at 2,000 Hz is equally good at stimulus levels between 103 and 123 dB peSPL.
The use of VEMPn and VEMPid is more favorable than cVEMP threshold because they require only one recording at each frequency and are less time-consuming. We favor the use of VEMPid because it has a “meaningful zero” value, i.e. VEMPid averages zero when no response is present, whereas VEMPn has a floor value caused by the EMG noise (Fig. 3). The “meaningful zero” makes VEMPid easier to interpret [Noij et al., 2017]. This is the first reported clinical application of VEMPid. At present there is no commercial device that calculates VEMPid from cVEMP recordings. However, the required calculations are described in Prakash et al. [2015] and Noij et al. [2018b], and no difference in the cVEMP measurement setup is required.
Comparisons with Previous Studies
Multiple studies have looked at the usefulness of cVEMP amplitude or threshold in SCD patients [Brantberg and Verrecchia, 2009; Janky et al., 2013; Zhou et al., 2007; Zuniga et al., 2013], and some have looked at both [Govender et al., 2016; Taylor et al., 2012; Welgampola et al., 2008]. Fife et al. [2017] summarized the outcomes of these studies and concluded that a sensitivity of 100% in combination with a specificity of 93% could be reached using normalized cVEMP amplitude. Using cVEMP threshold gives sensitivities ranging from 86 to 91%, with specificities ranging from 90 to 96% [Fife et al., 2017]. There are some limitations in comparing these studies, including the differences in methods used to obtain the cVEMP (e.g. click vs. tone burst and differences in stimulus level) and the reporting of outcomes (sensitivity and specificity are not always reported). Our study systematically compares the use of 4 different metrics at 4 different frequencies at multiple sound levels using the same methods in every subject, allowing for a reliable comparison. We favor the use of a cutoff value with a 100% specificity to avoid any false diagnosis of SCD in patients without this disorder. As can be appreciated in Table 1 (see threshold at 500 Hz), a large drop in sensitivity could be observed if a specificity of 100% is given priority. This highlights the importance of using a 2,000-Hz tone burst, because this frequency gives a high sensitivity (≥92%) in combination with a 100% specificity.
We found a slightly flatter tuning curve for dehiscent ears than for healthy-control ears (Fig. 1b), which is similar to findings of Taylor et al. [2012]. Taylor et al. investigated cVEMP frequency tuning in SCD patients and found that cVEMPs in SCD ears tended to tune to lower frequencies and that cVEMP thresholds at 500 and 2,000 Hz were equally good at separating healthy from dehiscent ears [Taylor et al., 2012]. This last finding is in contrast with our findings. Although our AUCs from 500 and 2,000 Hz do not show large differences for each metric (Fig. 4, 5), the point in the ROC curve that corresponds to the highest sensitivity with 100% specificity does differ and is higher at 2,000 Hz for all metrics (Table 1; Fig. 4, 5). For the clinical value of the test, the sensitivity and specificity provide more useful information than the AUC. For example, AUCs for cVEMP thresholds at 500 and 2,000 Hz were 0.938 and 0.973, respectively (Fig. 4), while the sensitivity with 100% specificity was 52% for 500 Hz and 92% for 2,000 Hz, which is a clinically relevant difference (Table 1).
Manzari et al. [2013] tested high-frequency stimuli for oVEMP measurements and found that 4,000 Hz was the best frequency to separate healthy from dehiscent ears with 100% specificity and sensitivity. While a slightly higher sensitivity at 4,000 Hz with cVEMP is a possibility, our decision to use 2,000-Hz stimuli but not 4,000-Hz ones was made based on the increased patient discomfort that would be caused by both the discomfort of the high-frequency sound itself and the increased testing time by adding another frequency.
Although the present study included only 21 SCD patients, the sensitivity (with 100% specificity) of our 500-Hz TWI is comparable to the TWI sensitivity from our larger retrospective study of 140 SCD patients (88 vs. 82%; both with cutoff values of 103 dB) [Noij et al., 2018a]. This indicates that our cohort is representative of this patient population. In the current study, thin-group TWIs were slightly smaller than healthy-group TWIs (Fig. 2), but the differences were not statistically significant. Such differences were not seen in the larger retrospective TWI study [Noij et al., 2018a], which indicates that the differences in the present study probably originate from the smaller number of subjects. Based on results of this study, we recommend measuring cVEMP using a 2,000-Hz tone burst stimulus in suspected SCD patients. The use of VEMPn or VEMPid at a high stimulus level allows for a short test since only one recording is necessary on each side, significantly decreasing testing time, patient discomfort and noise exposure.
Conclusion
For all cVEMP metrics used, the best diagnostic accuracy of cVEMP in SCD patients is achieved with 2,000-Hz tone burst stimuli. We recommend the use of 2,000 Hz in clinical cVEMP testing of patients in whom SCD is suspected.
Disclosure Statement
The authors declare that they have no conflict of interest.
Acknowledgements
This work was conducted with support from Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic health care centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
The authors would like to thank Miranda Janssen, Raymond van de Berg and Herman Kingma from the Department of Otolaryngology and Head and Neck Surgery of Maastricht University Medical Center for their contributions in the statistical analyses and for reviewing a prepublished version of this paper and suggesting edits that improved its precision and comprehensibility.
References
- 1.Benamira LZ, Alzahrani M, Saliba I. Superior canal dehiscence: can we predict the diagnosis? Otol Neurotol. 2014 Feb;35((2)):338–43. doi: 10.1097/MAO.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 2.Brantberg K. Vestibular evoked myogenic potentials (VEMPs): usefulness in clinical neurotology. Semin Neurol. 2009 Nov;29((5)):541–7. doi: 10.1055/s-0029-1241042. [DOI] [PubMed] [Google Scholar]
- 3.Brantberg K, Bergenius J, Tribukait A, Krister Brantberg, Johan Bergenius. Vestibular-evoked myogenic potentials in patients with dehiscence of the superior semicircular canal. Acta Otolaryngol. 1999;119((6)):633–40. doi: 10.1080/00016489950180559. [DOI] [PubMed] [Google Scholar]
- 4.Brantberg K, Verrecchia L. Testing vestibular-evoked myogenic potentials with 90-dB clicks is effective in the diagnosis of superior canal dehiscence syndrome. Audiol Neurotol. 2009;14((1)):54–8. doi: 10.1159/000153435. [DOI] [PubMed] [Google Scholar]
- 5.Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994 Feb;57((2)):190–7. doi: 10.1136/jnnp.57.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fife TD, Colebatch JG, Kerber KA, Brantberg K, Strupp M, Lee H, et al. Practice guideline: Cervical and ocular vestibular evoked myogenic potential testing: Report of the Guideline DevelopmentDissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2017 Nov;89((22)):2288–96. doi: 10.1212/WNL.0000000000004690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Govender S, Fernando T, Dennis DL, Welgampola MS, Colebatch JG. Properties of 500Hz air- and bone-conducted vestibular evoked myogenic potentials (VEMPs) in superior canal dehiscence. Clin Neurophysiol. 2016 Jun;127((6)):2522–31. doi: 10.1016/j.clinph.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Ho ML, Moonis G, Halpin CF, Curtin HD. Spectrum of Third Window Abnormalities: Semicircular Canal Dehiscence and Beyond. AJNR Am J Neuroradiol. 2017 Jan;38((1)):2–9. doi: 10.3174/ajnr.A4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter JB, Patel NS, O'Connell BP, Carlson ML, Shepard NT, McCaslin DL, et al. Cervical and Ocular VEMP Testing in Diagnosing Superior Semicircular Canal Dehiscence. Otolaryngol Head Neck Surg. 2017 May;156((5)):917–23. doi: 10.1177/0194599817690720. [DOI] [PubMed] [Google Scholar]
- 10.Janky KL, Nguyen KD, Welgampola M, Zuniga MG, Carey JP. Air-conducted oVEMPs provide the best separation between intact and superior canal dehiscent labyrinths. Otol Neurotol. 2013 Jan;34((1)):127–34. doi: 10.1097/MAO.0b013e318271c32a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manzari L, Burgess AM, McGarvie LA, Curthoys IS. An indicator of probable semicircular canal dehiscence: ocular vestibular evoked myogenic potentials to high frequencies. Otolaryngol Head Neck Surg. 2013 Jul;149((1)):142–5. doi: 10.1177/0194599813489494. [DOI] [PubMed] [Google Scholar]
- 12.Milojcic R, Guinan JJ, Jr, Rauch SD, Herrmann BS. Vestibular evoked myogenic potentials in patients with superior semicircular canal dehiscence. Otol Neurotol. 2013 Feb;34((2)):360–7. doi: 10.1097/mao.0b013e31827b4fb5. [DOI] [PubMed] [Google Scholar]
- 13.Minor LB, Solomon D, Zinreich JS, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998 Mar;124((3)):249–58. doi: 10.1001/archotol.124.3.249. [DOI] [PubMed] [Google Scholar]
- 14.Noij KS, Duarte MJ, Wong K, Cheng YS, Masud S, Herrmann BS, et al. Toward Optimizing Cervical Vestibular Evoked Myogenic Potentials (cVEMP): Combining Air-Bone Gap and cVEMP Thresholds to Improve Diagnosis of Superior Canal Dehiscence. Otol Neurotol. 2018 Feb;39((2)):212–20. doi: 10.1097/MAO.0000000000001655. [DOI] [PubMed] [Google Scholar]
- 15.Noij KS, Herrmann BS, Rauch SD, Guinan JJ., Jr Toward optimizing VEMP: Normalization Reduces the Need for Strong Neck Muscle Contraction. Audiol Neurotol. 2017;22:282–91. doi: 10.1159/000485022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noij KS, van Tilburg MJ, Herrmann BS, Marciniak P, Rauch SD, Guinan JJ., Jr Toward Optimizing VEMP: Calculating VEMP Inhibition Depth With a Generic Template. Ear Hear. 2018 Apr;39((6)):1199–206. doi: 10.1097/AUD.0000000000000579. [DOI] [PubMed] [Google Scholar]
- 17.Prakash SR, Herrmann BS, Milojcic R, Rauch SD, Guinan JJ., Jr Evaluating Inhibition of Motoneuron Firing From Electromyogram Data to Assess Vestibular Output Using Vestibular Evoked Myogenic Potentials. Ear Hear. 2015 Sep-Oct;36((5)):591–604. doi: 10.1097/AUD.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 18.Rauch SD, Zhou G, Kujawa SG, Guinan JJ, Herrmann BS. Vestibular evoked myogenic potentials show altered tuning in patients with Ménière's disease. Otol Neurotol. 2004 May;25((3)):333–8. doi: 10.1097/00129492-200405000-00022. [DOI] [PubMed] [Google Scholar]
- 19.Roditi RE, Eppsteiner RW, Sauter TB, Lee DJ. Cervical vestibular evoked myogenic potentials (cVEMPs) in patients with superior canal dehiscence syndrome (SCDS) Otolaryngol Head Neck Surg. 2009 Jul;141((1)):24–8. doi: 10.1016/j.otohns.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Rosengren SM, Govender S, Colebatch JG. Ocular and cervical vestibular evoked myogenic potentials produced by air- and bone-conducted stimuli: comparative properties and effects of age. Clin Neurophysiol. 2011 Nov;122((11)):2282–9. doi: 10.1016/j.clinph.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Rosowski JJ, Songer JE, Nakajima HH, Brinsko KM, Merchant SN. Clinical, experimentaland theoretical investigations of the effect of superior semicircular canal dehiscence on hearing mechanisms. Otol Neurotol. 2004 May;25((3)):323–32. doi: 10.1097/00129492-200405000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Streubel SO, Cremer PD, Carey JP, Weg N, Minor LB. Vestibular-evoked myogenic potentials in the diagnosis of superior canal dehiscence syndrome. Acta Otolaryngol Suppl. 2001;545:41–9. doi: 10.1080/000164801750388090. [DOI] [PubMed] [Google Scholar]
- 23.Taylor RL, Bradshaw AP, Halmagyi GM, Welgampola MS. Tuning characteristics of ocular and cervical vestibular evoked myogenic potentials in intact and dehiscent ears. Audiol Neurotol. 2012;17((4)):207–18. doi: 10.1159/000336959. [DOI] [PubMed] [Google Scholar]
- 24.van Tilburg MJ, Herrmann BS, Guinan JJ, Jr, Rauch SD. Normalization reduces intersubject variability in cervical vestibular evoked myogenic potentials. Otol Neurotol. 2014 Sep;35((8)):e222–7. doi: 10.1097/MAO.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 25.Welgampola MS, Colebatch JG. Vestibulocollic reflexes: normal values and the effect of age. Clin Neurophysiol. 2001 Nov;112((11)):1971–9. doi: 10.1016/s1388-2457(01)00645-9. [DOI] [PubMed] [Google Scholar]
- 26.Welgampola MS, Myrie OA, Minor LB, Carey JP. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology. 2008 Feb;70((6)):464–72. doi: 10.1212/01.wnl.0000299084.76250.4a. [DOI] [PubMed] [Google Scholar]
- 27.Zhou G, Gopen Q, Poe DS. Clinical and diagnostic characterization of canal dehiscence syndrome: a great otologic mimicker. Otol Neurotol. 2007;28((7)):920–6. [PubMed] [Google Scholar]
- 28.Zuniga MG, Janky KL, Nguyen KD, Welgampola MS, Carey JP. Ocular versus cervical VEMPs in the diagnosis of superior semicircular canal dehiscence syndrome. Otol Neurotol. 2013 Jan;34((1)):121–6. doi: 10.1097/MAO.0b013e31827136b0. [DOI] [PMC free article] [PubMed] [Google Scholar]