Abstract
PD-L1 is an immune checkpoint protein that has emerged as a major signaling molecule involved with tumor escape from T cell immune responses. Studies have shown that intra-tumoral expression of PD-L1 can inhibit antitumor immune responses. However, it has recently been shown that expression of PD-L1 on myeloid cells from the tumor is a stronger indicator of prognosis than tumor cell PD-L1 expression. Therefore, it is important to understand the factors that govern the regulation of PD-L1 expression on tumor-infiltrating myeloid cells. We found that immature bone marrow monocytes in tumor-bearing mice had low levels of PD-L1 expression, while higher levels of expression were observed on monocytes in circulation. In contrast, macrophages found in tumor tissues expressed much higher levels of PD-L1 than circulating monocytes, implying upregulation by the tumor microenvironment. We demonstrated that tumor-conditioned media strongly induced increased PD-L1 expression by bone marrow-derived monocytes and TNF-α to be a cytokine that causes an upregulation of PD-L1 expression by the monocytes. Furthermore, we found production of TNF-α by the monocytes themselves to be a TLR2-dependent response to versican secreted by tumor cells. Thus, PD-L1 expression by tumor macrophages appears to be regulated in a different manner than by tumor cells themselves.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-017-1955-5) contains supplementary material, which is available to authorized users.
Keywords: Myeloid, Tumor, Cytokine, Immune checkpoint
Introduction
The tumor microenvironment (TME) plays a critical role in the ability of tumor cells to successfully establish themselves in tissues [1]. Several cell types comprise the TME stroma, including fibroblasts, endothelial cells, macrophages, neutrophils, and B and T cells [2]. Interactions between stromal cells and tumor cells support tumor growth and metastasis [3]. Macrophages are one of the key cellular constituents of the TME, and serve to suppress T cell immune responses, stimulate tumor angiogenesis, promote tumor metastasis, and generate additional genomic instability [4, 5]. The recruitment of monocytes to tumors in response to tumor-derived chemokines, in particular CCL2, is associated with aggressive tumor growth and metastasis [6, 7]. Immature monocytes are also precursors of tumor-associated macrophages (TAM), which have an immune-suppressive phenotype [8].
How TAM suppress tumor immunity is currently an area of intense research interest, and a number of investigations have focused on checkpoint molecules expressed by TAM as a means of negative regulation of T cell function [9, 10]. One key molecule in this T cell suppressive pathway is programmed cell death 1 ligand 1 (PD-L1), which is a transmembrane protein with an IgV and IgC extracellular domain and a short cytoplasmic tail [11]. Ligation of PD-L1 to its receptor programmed cell death 1 (PD-1) on T cells suppresses T cell activation and proliferation, and under normal conditions, functions to maintain peripheral tolerance [12, 13]. Activation of PD-1 by PD-L1 also suppresses B cell production of antibodies and NK cell lytic activity, and binds CD80 to deliver an inhibitory signal to T cells [12–14]. Thus, the primary role of PD-1/PD-L1 signaling is to limit the activity of the immune system as a means of self-regulation [15]. In addition, PD-L1 is also expressed in many tumors where it suppresses ongoing anti-tumor T cell immunity [16–21]. In the presence of high levels of PD-L1 expression in tumor tissues, tumor infiltrating T cells become functionally inactivated and lose the ability to effectively control tumor growth [22].
However, tumor cells are not the only cells in the tumor to express PD-L1. For example, some studies have reported that PD-L1-expressing macrophages were more abundant than PD-L1 expressing tumor cells in tumor biopsy specimens from patients with melanoma, renal cell carcinoma, non-small cell lung, head and neck carcinoma, and colorectal tumors [9, 10]. Despite this fact, little is known about how PD-L1 expression by macrophages is regulated, and specifically how the tumor microenvironment influences PD-L1 expression by TAM.
For tumor cells, IFN-γ is considered the primary cytokine reported to upregulate PD-L1 expression, as evaluated with human, murine, and canine tumor cell lines [19, 23, 24]. However, other cytokines have been found to also upregulate PD-L1 expression. IL-4 and TNF-α were found to synergistically upregulate PD-L1 on certain human cancer cell lines [25]. In another study, monocytes were found to upregulate PD-L1 in response to IL-10 and IL-32γ, and TGF-β induced upregulation of PD-L1 expression on B cells [26]. Thus, other cytokines produced in the tumor microenvironment may exert a significant influence on local PD-L1 expression by TAM.
In the present study, we addressed the questions of how PD-L1 expression changed over time during macrophage differentiation from monocytes to mature macrophages, and which cytokines produced in the local tumor microenvironment were responsible for maintaining high levels of PD-L1 expression by TAM. We found that the level of PD-L1 expression by monocytes and macrophages steadily increased as the cells matured from monocytes in the bone marrow and bloodstream to mature macrophages in tissues. Surprisingly, endogenous IFN-γ production was found not to be required for maintenance or upregulation of PD-L1 expression on macrophages in tumor tissues. Instead, we found that endogenous production of TNF-α by tumor-infiltrating macrophages was the more important driver of PD-L1 expression by TAM. Moreover, the source of TNF-α production was macrophages themselves, in response to versican produced by tumor cells. These tumor-derived factors led to upregulated PD-L1 expression in a TLR2-dependent fashion. Taken together, these findings suggest that PD-L1 expression by TAM is regulated both by developmental age of the cells and by locally produced inflammatory cytokines, especially TNF-α. The relevance of these findings to current PD-1 and PD-L1 targeted immunotherapies for cancer is also discussed.
Materials and methods
Mice and tumor models
Wild-type (WT), IFN-γ−/− mice (strain B6.129S7-Ifng tm1Ts/J), TLR2−/− mice (strain B6.129-Tlr2tm1Kir/J), and TNFR−/− mice (strainB6.129 S-Tnfrsf1atm1Imx Tnfrsf1btm1Imx/J) bred on the C57Bl/6 background were acquired from Jackson Laboratories (Sacramento, CA), as were BALB/c mice. C57Bl/6 were inoculated s.c in the flank with 1 × 106 B16.F10 melanoma cells in 100 ul PBS (Gibco, Grand Island, NY). BALB/c mice were inoculated similarly with 4T1 mammary carcinoma cells. According to the study IACUC protocols, the mice were humanely euthanized when the tumor diameters reached 10 mm or less. Tumor area was determined the day the mice were euthanized, with tumor area calculated as length x width in mm.
Murine cell lines
B16.F10 melanoma cells and 4T1 mammary carcinoma cells were obtained from ATCC (Manassas, VA). These cell lines were screened by PCR to ascertain that they were of murine origin [27].
Tissue preparation for flow cytometry
Mice were euthanized before spleens and tumor tissues were harvested. Tumor tissues were minced with fine scissors, then digested with collagenase (Sigma Aldrich, St. Louis, MO) for 30 min at 37 °C and filtered to obtain a single cell suspension. Spleen cells were collected by forcing cells through 70 micron filters with cell culture medium. Tissue and blood samples were lysed with ammonium-chloride-potassium (ACK) lysis buffer (0.5% Phenol Red solution, 150mM ammonium chloride, 10 mM potassium bicarbonate, 0.1 mM disodium salt dihydrate) and the remaining cells were washed with cell culture medium in preparation for flow cytometric staining.
Bone marrow collection and monocyte enrichment
Bone marrow was collected from tibias and femurs by forcing cells out of the marrow cavity, using tissue culture medium and a 25# needle. The bone marrow cells were then lysed with ACK lysis buffer and washed with medium before plating in 24-well polystyrene cell culture plates (Falcon, Durham, NC) for the selection of adherent monocytes. Adherent bone marrow-derived monocytes were then harvested by pipetting with ice-cold 2 mM EDTA in PBS (Gibco).
Generation of conditioned medium
Tumor cells were cultured in the same cell culture medium described previously. To generate tumor-conditioned medium, tumor cells were seeded into 24-well plates at 75,000 cells/ml and conditioned medium was collected 24 h later. The CM was then centrifuged to remove any remaining cells.
Co-culture studies and conditioned medium effects on monocyte PD-L1 expression
Bone marrow-derived monocytes were selected by adherence as described above and washed with PBS. Tumor cells at 50,000 cells/ml or tumor CM at 50% were added to cultures of bone marrow-derived monocytes. After 18 h of culture, monocytes were harvested as described above and processed for flow cytometry. These culture conditions routinely yielded monocyte cultures that were at least 80–90% pure.
Flow cytometry
Cells prepared from tumor and liver tissues, as well as bone marrow-derived monocytes, blood monocytes, and TAM were obtained as described above and placed in FACS buffer for immunostaining. Nonspecific antibody binding was blocked by addition of normal mouse serum and 0.01% Fc-specific antiserum (CD16/32, clone 93, eBioscience, San Diego, CA).
Cells were immunostained with the following antibodies: CD45 Pacific Orange (Clone 30-F11) from Invitrogen (Grand Island, NY) to identify hematopoietic cells, and with PD-L1 PE (clone MIH5), CD11b Pacific blue (clone M1/70), F4-80 APC (clone Cl:A3-1), Ly6C biotin (AL-21), Ly6G Alexa 488 (clone 1A8), CCR2 APC (clone 475,301), and CD11c FITC (clone N418). Cells were also stained with appropriately matched isotype antibodies to assure specificity of immunostaining. All antibodies were purchased from eBioscience unless otherwise noted.
Prior to analysis, 7-AAD viability dye (eBioscience) was added to flow samples to exclude dead cells. Cells were analyzed using a Beckman Coulter Gallios flow cytometer (Brea, CA) and FlowJo Software (Ashland, OR).
In other experiments, intracellular cytokine expression was quantitated. To measure intracellular expression of TNF-α, the cells were initially stained for surface markers before fixation with paraformaldehyde (PFA), and permeabilization with 0.25% saponin. Cells were then washed and immunostained with an anti-TNF-α PE-conjugated antibody (clone MP6-XT22, eBioscience).
Cytokines for PD-L1 upregulation by monocytes and macrophages
The following recombinant murine cytokines were purchased from Peprotech (Rocky Hill, NJ): TNF-α, TGF-β, IL-10, MCP-1, IFN-γ, GM-CSF, and IL-3. Each cytokine was used at 10 ng/ml, according to the manufacturer’s suggested working concentrations and based on titration studies done in our laboratory (data not shown). Bone marrow-derived monocytes were stimulated with these cytokines for 24 h prior to analysis of PD-L1 expression by flow cytometry.
Neutralizing antibodies
A TNF-α neutralizing antibody (clone NF-7, Abcam, Cambridge, UK) was used at 5 ug/ml in tumor CM prior to treatment of monocytes with tumor CM. A rabbit polyclonal versican antibody (Santa Cruz, Santa Cruz, CA) was used at 200 ug/ml in tumor CM for 4 h at 4 C. This was followed by incubation of CM and anti-versican antibodies with protein A Sepharose beads (Abcam) overnight according to manufacturer’s protocol for immunoprecipitation of antibodies. The beads were removed from the CM by centrifugation prior to CM being added to monocyte cultures.
ELISA
Cell culture supernatants were collected and centrifuged for the removal of cells. Cytokines were measured using specific ELISA kits for murine IFN-γ and TNF-α (R&D Systems, Minneapolis, MN), and assays were performed according to manufacturer directions.
Tissue immunofluorescence staining and imaging
Tumor tissues were harvested and fixed in periodate-lysine-paraformaldehyde (PLP) for 24 h before transferring to a 30% glucose solution for another 24 h, all at 4 °C. Afterwards, tissues were embedded in OCT (Optimal Cutting Temperature compound), frozen at −80 °C, and cryosectioned to a thickness of 5 microns. The tissues were mounted on glass slides and blocked with 5% donkey serum for 30 min prior to staining.
For analysis of PD-L1 expression, we used an unlabeled antibody (clone 10F.9G2, BioXcell, West Lebanon, NH). For analysis of intracellular TNF-α expression, a directly conjugated antibody (clone MP6-XT22) and a matched irrelevant isotype control antibody from eBioscience were used. For detection of macrophages, an unconjugated F4-80 antibody (clone BM8) was used.
Antibodies for PD-L1 and F4-80 and their matched isotype controls were used overnight at 4 °C, followed by a donkey anti-rat antibody (Jackson Immunoresearch, West Grove, PA) for 30 min to detect primary antibody binding. For dual staining, antibodies for intracellular cytokines were diluted in 0.25% Saponin diluted in PBST overnight at 4 °C. Finally, the tissues were stained with DAPI to identify nucleated cells and coverslipped with Prolong Diamond mounting media (LifeTech, Carlsbad, CA) before imaging. Controls included immunostaining with appropriate concentrations of irrelevant isotype-matched antibodies. Figures were then compiled using Adobe Photoshop.
Statistical analysis
Statistical comparisons between those data sets with two treatment groups were done using non-parametric t-tests (Mann–Whitney test). Comparisons between three or more groups were done using ANOVA, followed by Dunnet’s or Tukey multiple means post-test. Analyses were done using Prism6 software (GraphPad, La Jolla, CA). For all analyses, statistical significance was determined for p < 0.05.
Results
Role of endogenous IFN-γ regulation of PD-L1 expression by monocytes and tumor macrophages
Our studies and those of others have found that IFN-γ can significantly upregulate PD-L1 expression by both tumor cells and macrophages [23, 28]. Using bone marrow-derived macrophages and monocytes, we confirmed that exposure to IFN-γ resulted in significant upregulation of PD-L1 expression by monocytes, as well as by tumor cells (data not shown). Moreover, a previous investigation evaluated the role of endogenous cytokines in regulating PD-L1 expression by tumor cells and myeloid cells in vivo, and concluded that IFN-γ produced by inflammatory cells stimulated tumor cells to increase their PD-L1 expression [29]. However, this previous study did not conclusively assess the role of IFN-γ in regulating PD-L1 expression by tumor-associated macrophages.
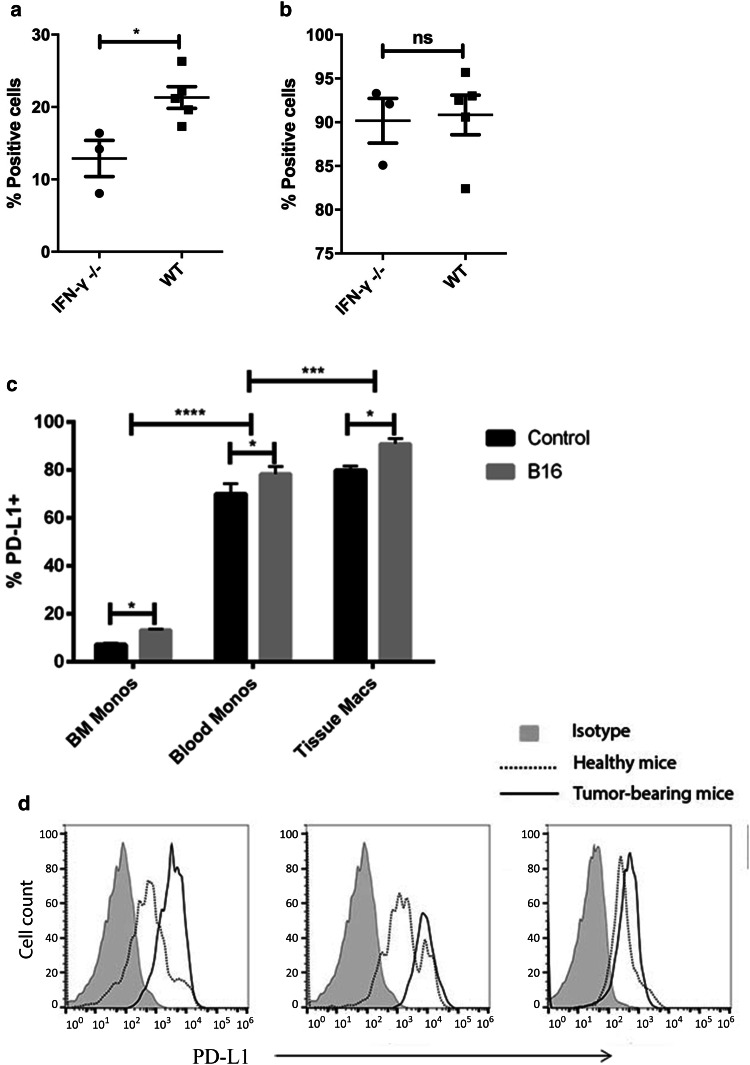
Therefore, we used mice lacking expression of IFN-γ to address more fully the role of endogenous IFN-γ in regulating both tumor and TAM PD-L1 expression in vivo. B16.F10 tumors were grown in both WT and IFN-γ−/− mice (n = 5 per group), and tumor tissues were processed for flow cytometry for assessment of PD-L1 expression by tumor cells and TAM. We found that CD45− tumor cells in IFN-γ−/− mice expressed significantly less PD-L1 than tumor cells obtained from WT animals (Fig. 1a). However, macrophages in tumor tissues from WT and IFN-γ−/− mice expressed similar levels of PD-L1, based on both MFI and % positive analysis (Fig. 1b). While these data confirm previous studies with respect to the essential role for IFN-γ in regulating tumor cell PD-L1 expression, the new findings suggested that PD-L1 expression by TAM was regulated in an IFN-γ-independent fashion.
Fig. 1.
PD-L1 expression by tumor cells, monocytes, and macrophages in vivo. B16 tumors cells were established s.c. in WT and IFN-γ−/− mice (n = 3–5 animals per group), as noted in Methods. Single cell suspensions were prepared from excised tumor tissues and flow cytometry was used to compare PD-L1 expression by CD45− tumor cells in (a) and by tumor-associated macrophages (CD45+/CD11b+/F4-80+) in (b) obtained from the two groups of mice. The mean percentage of PD-L1+ cells present in tumor tissues from WT and IFN-γ−/− are depicted and the mean percentages were compared statistically using a non-parametric t-test. In (c), bone marrow monocytes (CD11b+/Ly6C+/Ly6G-), circulating monocytes (CD11b+/Ly6C+/Ly6G−), and tissue macrophages (CD45+/ CD11b+) were harvested from the spleens of healthy mice and from tumors of mice with established s.c. B16 tumors (n = 4–5 mice per group) and PD-L1 expression was quantitated by flow cytometry. The level of expression of PD-L1 on the cells is shown as histograms of geometric MFI in (d) where gray filled = isotype stain, dotted line = cells from healthy mice, and solid line = cells from tumor-bearing mice from bone marrow, blood, and tissues. The mean percentages of PD-L1+ cells in healthy mice and mice with tumors were compared statistically using a non-parametric t-test, and mean percentages of PD-L1+ cells in different tissues from healthy and tumor-bearing mice were compared using two-tailed ANOVA, with Tukey post-test. Groups means with statistically significant differences were denoted as * = p < 0.05, *** = p < 0.0005, and **** = p < 0.0001. Similar results were obtained in two additional, independent experiments
Effect of monocyte maturation into macrophages on PD-L1 expression
We next sought to determine the role of monocyte differentiation into tissue macrophages on regulation of PD-L1 expression. For example, it is possible that macrophages in tumor tissues express higher levels of PD-L1 simply as a result of maturation-related changes. Therefore, we used flow cytometry to examine the level of PD-L1 expression by immature monocytes in bone marrow, circulating monocytes, and macrophages in normal and tumor tissues.
We found that indeed, the level of PD-L1 expression by monocytes increased as the cells age, with the lowest levels of expression in the bone marrow and the highest levels of expression observed in spleen and tumor tissues (Fig. 1c, d). PD-L1 expression was measured on immature bone marrow monocytes (CD11b+/Ly6C+/Ly6G−), circulating monocytes (CD11b+/Ly6C+/Ly6G−), and either tissue macrophages from the spleen or TAM from melanoma-bearing mice (CD11b+/F4-80+). Importantly, in tumor tissues the more recently emigrated inflammatory monocytes (as assessed by higher levels of expression of CCR2 [6, 7]) expressed higher levels of PD-L1 than resident macrophages, suggesting that the newly arrived monocytes further upregulated their PD-L1 expression once they reached tumor tissues.
In vitro regulation of monocyte PD-L1 expression by tumor-conditioned medium
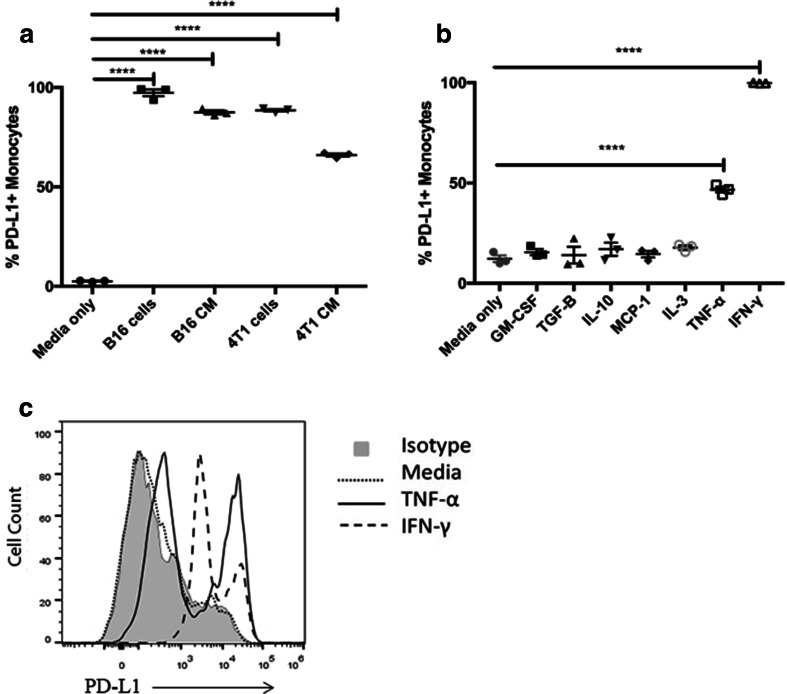
These findings suggested that factors produced locally in tumor tissues were responsible for upregulation of PD-L1 expression. To address this question, we developed a co-culture system, in which immature bone marrow-derived monocytes were co-cultured with tumor cells, using transfer of conditioned medium (CM). We found that culture of monocytes with tumor CM resulted in rapid and significant upregulation of PD-L1 expression (Fig. 2a). For example, exposure to as little as 25% CM triggered significant PD-L1 upregulation, and that the effect occurred within 18 h (data not shown). It was also observed that CM from certain tumor cell lines led to greater upregulation of PD-L1 than others (Fig. 2a).
Fig. 2.
Effects of tumor cells, tumor CM, and cytokines on monocyte PD-L1 expression. a B16 and 4T1 tumor cells were grown as monolayers, then co-cultured with bone marrow-derived monocytes for 24 h, as described in Methods. In other studies, bone marrow-derived monocytes were cultured with CM from tumor cells. The effects of co-culture with live tumor cells or with tumor CM on monocyte PD-L1 expression was determined by flow cytometry. b Bone marrow-derived monocytes were prepared as described in Methods and incubated with the following murine recombinant cytokines (TNF-α, TGF-β, IL-10, MCP-1, IFN-γ, GM-CSF, and IL-3) at a concentration of 10 ng/ml. After overnight culture, the monocytes were collected and immunostained for flow cytometric analysis of PD-L1 expression. c Histograms of monocyte PD-L1 expression following exposure to TNF-α or IFN-γ. The percentage of PD-L1+ cells was compared between monocytes cultured in medium only and monocytes exposed to tumor cells, tumor CM, or cytokines using one-tailed ANOVA, followed by Dunnet’s multiple means comparison. Statistically significant differences were denoted as ** = p < 0.005, *** = p < 0.0005, and **** = p < 0.0001. These data are representative of 4 repeated experiments with similar results
Cytokine production in tumor and monocyte co-cultures
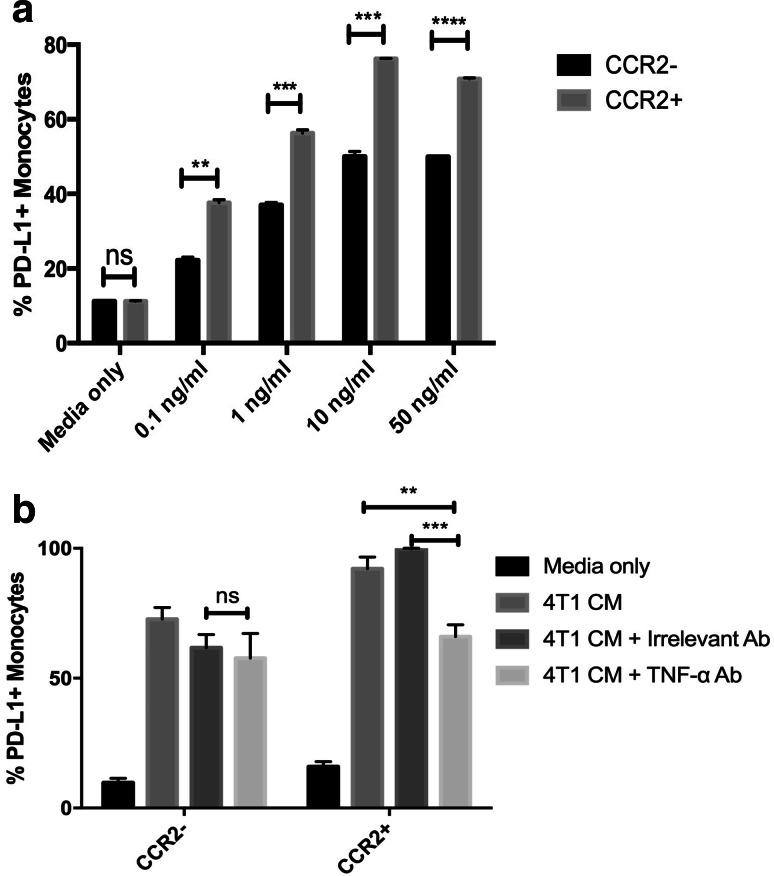
Several secreted cytokines or growth factors were considered candidates for the secreted PD-L1 upregulation, based on previous work [25, 26, 30–32], including GM-CSF, MCP-1, IL-10, IL-3, TGF-β, or TNF-α. To help identify the relevant factors, we first screened recombinant cytokines to identify those that most strongly upregulated PD-L1 expression in the bone marrow monocyte system (Fig. 2b). Using this screen, we identified IFN-γ and TNF-α as potential candidate cytokines (Fig. 2c). TNF-α is a strong regulator of monocyte PD-L1 expression [33], and CCR2+ inflammatory monocytes are especially responsive to this cytokine (Fig. 3a) [34, 35].
Fig. 3.
Neutralization of TNF-α significantly blocks upregulation of monocyte PD-L1 expression by tumors. a Bone marrow-derived monocytes were treated with increasing concentrations of recombinant TNF-α (0.1–50 ng/ml), and PD-L1 expression was compared between inflammatory monocytes (CD11b+/Ly6C+/Ly6G−/CCR2+) and non-inflammatory monocytes (CD11b+/Ly6C+/Ly6G−/CCR2−). b Conditioned medium from 4T1 cells was treated with a TNF-α neutralizing antibody (or isotype control antibody), then incubated with triplicate wells of bone marrow-derived monocytes for 24 h. Effects of tumor CM on monocyte PD-L1 expression was assessed on both inflammatory monocytes (CD11b+/Ly6C+/Ly6G−/CCR2+) and non-inflammatory monocytes (CD11b+/Ly6C+/Ly6G−/CCR2−). Similar results were obtained in three additional independent experiments. Statistical comparison of TNF-α concentrations was done by two-tailed ANOVA, followed by Tukey’s multiple means comparison. Statistically significant differences were denoted as * = p < 0.05 and ** = p < 0.005
To directly address the role of each cytokine, neutralization studies were done. Here we found that neutralization of GM-CSF and IL-10 did not result in significant reduction in PD-L1 upregulation in response to tumor CM (data not shown). However, when tumor CM was treated with TNF-α neutralizing antibody, the upregulation of PD-L1 was significantly abrogated (Fig. 3b). Furthermore, CCR2+ monocytes showed significantly lower expression of PD-L1 in comparison to CCR2− monocytes, supporting the previous experiment using exogenous recombinant TNF-α in that CCR2+ inflammatory monocytes were more sensitive to regulation by TNF-α (Fig. 3b). These findings suggested therefore that TNF-α might be the more important cytokine in the tumor environment for upregulation of PD-L1 expression.
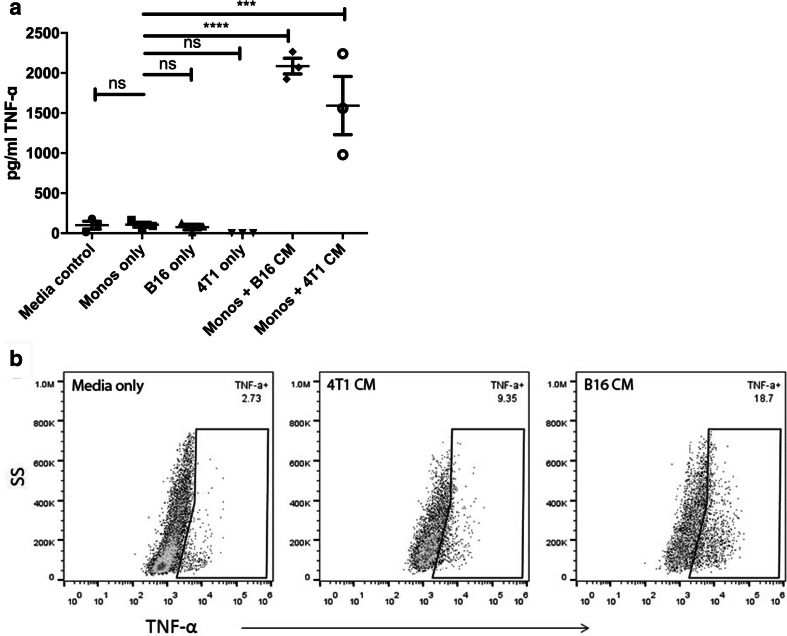
The concentrations of TNF-α in tumor and monocyte CM were then measured by ELISA, and these concentrations correlated with the degree of observed PD-L1 upregulation by co-cultured monocytes (Fig. 4a). First, we found that CM from any of the cells alone contained undetectable concentrations of TNF-α, which indicates that tumor cells themselves were not the source of TNF-α production. Therefore, we examined TNF-α concentrations in the CM of monocytes that had been co-cultured with tumor CM. We observed high concentrations of TNF-α in the monocyte CM, suggesting production by the monocytes themselves. We also used intracellular staining and found TNF-α production by monocytes cultured with tumor CM (Fig. 4b). In contrast, IFN-γ concentrations in the same CM were undetectable (data not shown). Thus, it appeared that tumor cells in culture spontaneously secreted a factor that stimulated monocytes to produce TNF-α, which in turn led to greater upregulation of PD-L1 expression.
Fig. 4.
Tumor cells and tumor-secreted factors stimulate TNF-α production by monocytes. a Bone marrow monocytes were cultured overnight with B16 cells or 4T1 cells, or with tumor CM derived from these cells. Medium from monocyte cultures was collected 24 h after co-culture with live tumor cells or with tumor CM, and IFN-γ and TNF-α concentrations were determined by ELISA. b Bone marrow-derived monocytes were cultured overnight with tumor CM then incubated with Brefeldin A for 4 h and immunostained for detection of intracellular TNF-α expression by flow cytometry. Appropriate isotype control antibodies were used to assess the specificity of TNF-α staining. Similar results were obtained in three additional independent experiments. Statistical comparison of TNF-α concentrations was done by one-tailed ANOVA, with Dunnet’s post-test. Statistically significant differences were denoted as *** = p < 0.0005 and **** = p < 0.0001
Regulation of monocyte TNF-α production by tumor-secreted factors
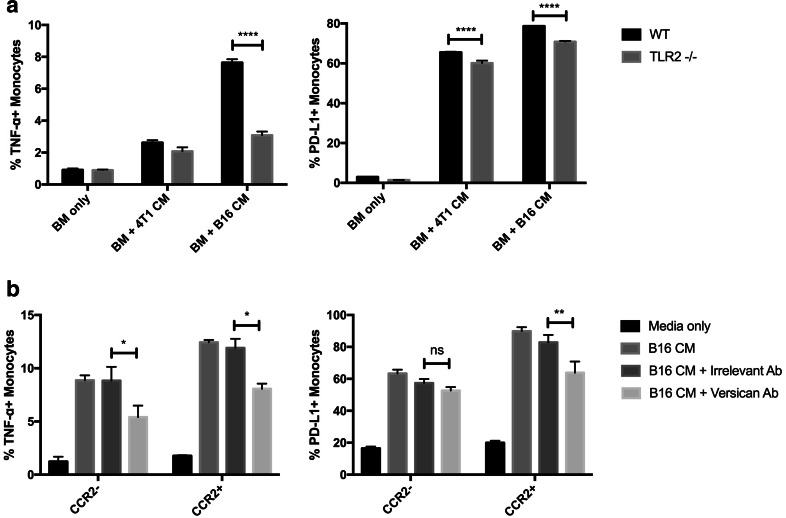
Previous studies have described factors spontaneously secreted by tumor cells that led to TNF-α production by TAM [36, 37]. These factors were also reported to stimulate macrophages to produce TNF-α in a TLR2-dependent manner. Therefore, we first used TLR2−/− bone marrow monocytes to elucidate the role of TLR2-dependent signaling in monocyte upregulation of TNF-α production, and ultimately PD-L1 upregulation. We found that monocytes unable to signal via the TLR2 pathway failed to secrete TNF-α and to upregulate PD-L1 (Fig. 5a). Thus, this finding was consistent with a signaling pathway similar to that described previously for tumor-induced macrophage TNF-α production, leading to upregulated PD-L1 expression [36]. In addition, removal of versican from CM led to an abrogated upregulation of TNF-α production and PD-L1 expression compared to monocytes cultured with tumor CM alone (Fig. 5b). These data suggest that versican is likely a primary mediator of TNF-α production and PD-L1 expression upregulation by monocytes.
Fig. 5.
Versican secreted by tumor cells induce monocyte TNF-α production via TLR2 signaling pathway. a Bone marrow monocytes were cultured overnight with B16 cells or 4T1 cells, or with tumor CM derived from these cells. Medium from monocyte cultures was collected 24 h after co-culture with live tumor cells or with tumor CM, and IFN-γ and TNF-α concentrations were determined by ELISA. b Bone marrow-derived monocytes were cultured overnight with tumor CM then incubated with Brefeldin A for 4 h and immunostained for detection of intracellular TNF-α expression by flow cytometry. Appropriate isotype control antibodies were used to assess the specificity of TNF-α staining. Similar results were obtained in three additional independent experiments. Statistical comparison of TNF-α concentrations was done by one-tailed ANOVA, with Dunnet’s post-test. Statistically significant differences were denoted as *** = p < 0.0005 and **** = p < 0.0001
Cellular source of TNF-α production in tumor tissues and role of TNF-α in vivo
Finally, experiments were done to determine the in vivo source of TNF-α production within tumor tissues. Using tissues from subcutaneous B16 tumors, we found strong, constitutive expression of both TNF-α and PD-L1 throughout tumor tissues (Fig. 6a). Co-localization studies revealed the source of TNF-α production was primarily F4/80+ macrophages, thus confirming in vivo the feedback loop described by our in vitro studies (Fig. 6b). We also conducted studies using TNFR−/− mice to assess the role of TNF-α in regulating tumor grown in the B16 model. We found B16 tumors grown in TNFR−/− mice were significantly smaller than those grown in WT mice (Supplemental Figure). Furthermore, there were significantly decreased numbers of PD-L1+ tumor-associated macrophages and dendritic cells but not tumor cells from TNFR−/− mice (Fig. 6c), supporting the results observed with in vitro studies.
Fig. 6.
In vivo TNF-α production within tumor tissues and effects on tumor-associated macrophage PD-L1 expression. a B16 tumors were collected from the s.c. tissues of mice, cryosectioned, and immunostained for detection of F4/80 (green) and PD-L1 expression (red and counterstained with DAPI (blue). b Tumor tissues were immunostained for co-localization of F4/80 (green) and TNF-α (red), and counterstained with DAPI (blue) for nuclear detection as noted in Methods. Depicted are representative images obtained from 6 separate tumors processed and immunostained for PD-L1, TNF-α, and F4/80. c Tumor tissues from WT and TNFR−/− mice were processed into single cell suspension for flow cytometric analysis of PD-L1 expression by macrophages (CD45+/CD11b+/Ly6G−/F4-80+) and dendritic cells (CD11b+/CD11c+). Mean percentages of PD-L1+ cells were compared using non-parametric t-tests, with * = p < 0.05
Discussion
Factors that regulate PD-L1 expression on TAM and monocytes have not been previously identified, which provided the impetus for the current study. In our investigations, we found that monocytes progressively upregulated PD-L1 expression as they matured and entered tumor tissues. In addition, we also identified a feed-forward loop regulating PD-L1 expression on TAM, wherein tumor-secreted versican triggered TNF-α secretion by macrophages. This in turn stimulated local upregulation of PD-L1 expression by the same macrophages. These findings suggest that PD-L1 expression by macrophages and monocytes in tumor tissues is regulated primarily by maturation and by locally produced TNF-α. Macrophage PD-L1 expression was largely independent of local IFN-γ production, in contrast to tumor cells, which were found to be much more dependent on IFN-γ for upregulated PD-L1 expression. These results provide therefore a clearer understanding of the regulation of PD-L1 in the TME.
Consistent with previous reports, we found that tumor cell PD-L1 expression was primarily regulated by endogenous production of IFN-γ [19, 23], in as much as PD-L1 expression was significantly reduced in IFN-γ−/− mice (Fig. 1a). However, expression of PD-L1 by TAMs in IFN-γ−/− mice was unchanged compared to WT animals. These findings suggested alternative, IFN-γ-independent mechanisms for regulation of PD-L1 expression on TAM and monocytes.
One mechanism regulating PD-L1 expression by macrophages identified in this study was cellular maturation. For example, we observed that the level of PD-L1 expression (both in terms of the total percentage of PD-L1+ cells and the overall level of PD-L1 expressed by each cell) underwent significant upregulation as cells matured from bone marrow monocytes, to circulating monocytes, to macrophages in tumor and spleen tissues (Fig. 1b). Thus, monocytes likely become more effective at downregulating T cell responses as they mature and enter tissues, which is consistent with the normal immune homeostatic role of the PD-1/PD-L1 axis [12, 13].
Our investigations also revealed a previously unrecognized role for TNF-α in regulating PD-L1 expression by TAM. We found that TNF-α produced by monocytes themselves, in response to tumor-secreted versican, was the key cytokine responsible for upregulated PD-L1 expression on monocytes (Fig. 4). The tumor-secreted versican triggered TNF-α production in a TLR2-dependent manner, consistent with the previously reported pro-inflammatory effects of tumor extracellular matrix proteins [36]. We also determined that less mature monocytes (pro-inflammatory CCR2+ monocytes) were more responsive to TNF-α than non-inflammatory, CCR2− monocytes (Fig. 3). This finding suggests that within the TME, newly recruited inflammatory monocytes are likely to be a major source of high level PD-L1 myeloid cells, which would have the greatest negative impact on tumor-infiltrating T cells. These pro-inflammatory, PD-L1hi monocytes are also the most likely to be recruited in response to T cell-generated inflammation and CCL2 production, serving to counter-balance T cell inflammatory stimuli with anti-inflammatory immune regulatory responses [6, 7].
Previous studies have reported that monocyte-derived IL-10 was an important stimulus for PD-L1 expression upregulation by human monocytes, and that TNF-α exerted only a marginal effect on PD-L1 expression [38]. In contrast, we found that IL-10 had little effect on monocyte PD-L1 expression, and that TNF-α was a very potent signal for PD-L1 upregulation. These differing findings may be explained by use of different monocyte populations (ie bone marrow vs blood) obtained using different culture techniques. In addition, it is also possible that the murine and human monocytes respond differently to IL-10 and TNF-α.
It was also reported previously that bone marrow-derived macrophages could trigger upregulated PD-L1 expression by tumor cells upon physical contact, in a mechanism that that was reported to be dependent on contact between macrophage CD11b and tumor cells [39]. However, the results of this prior study could also be explained by tumor-induced secretion of macrophage TNF-α, since their studies did not examine the effects of CM from the co-cultures on tumor PD-L1 expression.
In our studies, we found that tumor cells regulated monocyte PD-L1 expression via a contact-independent mechanism. TNF-α was produced by monocytes when cultured with tumor CM (Fig. 2), and neutralization assays revealed that TNF-α was the principle cytokine responsible for this effect (Fig. 3). We did not use mature macrophages for these assays because they have such high levels of PD-L1 expression that a treatment effect cannot be discerned (data not shown). Furthermore, monocytes derived from TLR2−/− mice showed decreased sensitivity to B16 CM, suggesting a TLR2-dependent signaling mechanism for regulating TNF-α secretion (and ultimately PD-L1 upregulation) by TAM. We found that the removal of versican from B16 CM significantly suppressed upregulation of TNF-α production and PD-L1 expression by monocytes cultured with B16 CM, indicating that versican produced by tumor cells plays a central role in regulating monocytes (Fig. 5).
Interestingly, monocytes from TLR2−/− mice exhibited no decrease in TNF-α secretion compared to WT monocytes following exposure to 4T1 CM. This finding suggests an alternative mechanism by which CM from 4T1 cells induced TNF-α secretion. One possibility is production of MMP-3 by 4T1 cells, as they have been reported previously to produce MMP-3 in culture, which in turn was shown to induce macrophages to produce TNF-α [40, 41].
Furthermore, we observed large numbers of macrophages expressing TNF-α and PD-L1 within tumor tissues (Fig. 6), thus providing in vivo validation of the in vitro observations. We also observed that growth of B16 tumors was significantly suppressed in TNFR−/− mice with decreased expression of PD-L1 by TAM and dendritic cells but not on tumor cells, indicating the overall importance of PD-L1 expression by TAM in the regulation of tumor growth (Supplemental Figure and Fig. 6c). The reduction in PD-L1 expression by TAM from tumors in TNFR−/− mice would also be expected to enhance anti-tumor immunity, which could account for the reduced tumor growth. These data provide additional in vivo support for the existence of a TNF-α—PD-L1 immune regulatory pathway in tumors.
In summary, our studies provide evidence for a new immune pathway for regulation of PD-L1 expression by monocytes and macrophages in tumor tissues, and potentially in other organs undergoing inflammatory insults. This pathway requires the release of pro-inflammatory mediators (such as versican) from tumors, plus the presence of inflammatory monocytes and macrophages that respond to the inflammation-associated signals by releasing TNF-α in a TLR2-dependent mechanism. The local release of TNF-α in turn leads to upregulation of PD-L1 expression, thereby triggering local immune suppression. Interruption of this pathway could have important therapeutic implications as a means of enhancing anti-tumor immunity. Thus, improved understanding of this and other pathways for regulation of PD-L1 expression by inflammatory monocytes TAM in tumor tissues will be important to the design of newer approaches to tumor immunotherapy [42–44].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
These studies were supported by a grant from the Shipley Foundation.
Author contributions
Conception of this study is credited to Genevieve Hartley and Steven Dow while experimental and analytical work was performed by Genevieve Hartley, Daniel Regan, and Amanda Guth. The manuscript was then prepared by Genevieve Hartley and Steven Dow.
Abbreviations
- CM
Conditioned medium
- PD-1
Programmed cell death 1
- PD-L1
Programmed cell death 1 ligand 1
- TAM
Tumor-associated macrophage
- TME
Tumor microenvironment
- WT
Wild-type
Ethical approval
The authors declare no conflict of interest.
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of Colorado State University.
References
- 1.Tarin D, Price JE. Influence of microenvironment and vascular anatomy on “metastatic” colonization potential of mammary tumors. Cancer Res. 1981;41(9 Pt 1):3604–3609. [PubMed] [Google Scholar]
- 2.Schiavoni G, Gabriele L, Mattei F. The tumor microenvironment: a pitch for multiple players. Front oncol. 2013;3:90. doi: 10.3389/fonc.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keskinov AA, Shurin MR. Myeloid regulatory cells in tumor spreading and metastasis. Immunobiology. 2015;220(2):236–242. doi: 10.1016/j.imbio.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res. 2012;4(4):376–389. [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012;22(4):275–281. doi: 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao LL, Shi SJ, Chen LB, Huang GC. Expression of monocyte chemotactic protein-1/CCL2 in gastric cancer and its relationship with tumor hypoxia. World J Gastroenterol. 2014;20(15):4421–4427. doi: 10.3748/wjg.v20.i15.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70(5):325–330. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Trans Med. 2012;4(127):127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S, Liu Y, Strome SE, Chen L, Tamada K. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116(8):1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanoni D, Tavecchio S, Recalcati S, Balice Y, Venegoni L, Fiorani R, Crosti C, Berti E. New monoclonal antibodies against B-cell antigens: possible new strategies for diagnosis of primary cutaneous B-cell lymphomas. Immunol Lett. 2011;134(2):157–160. doi: 10.1016/j.imlet.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Qian J, Lu Y, Li H, Bao H, He D, Liu Z, Zheng Y, He J, Li Y, Neelapu S, Yang J, Kwak LW, Yi Q, Cai Z. Immune evasion of mantle cell lymphoma: expression of B7-H1 leads to inhibited T-cell response to and killing of tumor cells. Haematologica. 2013;98(9):1458–1466. doi: 10.3324/haematol.2012.071340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 19.Soliman H, Khalil F, Antonia S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS One. 2014;9(2):e88557. doi: 10.1371/journal.pone.0088557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 21.Wintterle S, Schreiner B, Mitsdoerffer M, Schneider D, Chen L, Meyermann R, Weller M, Wiendl H. Expression of the B7-related molecule B7-H1 by glioma cells: a potential mechanism of immune paralysis. Cancer Res. 2003;63(21):7462–7467. [PubMed] [Google Scholar]
- 22.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 23.Abiko K, Matsumura N, Hamanishi J, Horikawa N, Murakami R, Yamaguchi K, Yoshioka Y, Baba T, Konishi I, Mandai M. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br J Cancer. 2015;112(9):1501–1509. doi: 10.1038/bjc.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartley G, Faulhaber E, Caldwell A, Coy J, Kurihara J, Guth A, Regan D, Dow S. Immune regulation of canine tumour and macrophage PD-L1 expression. Vet Comp Oncol. 2016 doi: 10.1111/vco.12197. [DOI] [PubMed] [Google Scholar]
- 25.Quandt D, Jasinski-Bergner S, Muller U, Schulze B, Seliger B. Synergistic effects of IL-4 and TNFalpha on the induction of B7-H1 in renal cell carcinoma cells inhibiting allogeneic T cell proliferation. J Trans Med. 2014;12:151. doi: 10.1186/1479-5876-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taube JM, Young GD, McMiller TL, Chen S, Salas JT, Pritchard TS, Xu H, Meeker AK, Fan J, Cheadle C, Berger AE, Pardoll DM, Topalian SL. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin Cancer Res. 2015;21(17):3969–3976. doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Donoghue LE, Rivest JP, Duval DL. Polymerase chain reaction-based species verification and microsatellite analysis for canine cell line validation. J Vet Diagnos Inves. 2011;23(4):780–785. doi: 10.1177/1040638711408064. [DOI] [PubMed] [Google Scholar]
- 28.Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Dual Faces of IFNgamma in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro- and Antitumor Immunity. Clin Cancer Res. 2016;22(10):2329–2334. doi: 10.1158/1078-0432.CCR-16-0224. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Feng Y, Lu L, Wang H, Dai L, Li Y, Zhang P. Interferon-gamma-induced PD-L1 surface expression on human oral squamous carcinoma via PKD2 signal pathway. Immunobiology. 2012;217(4):385–393. doi: 10.1016/j.imbio.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Soares KC, Rucki AA, Wu AA, Olino K, Xiao Q, Chai Y, Wamwea A, Bigelow E, Lutz E, Liu L, Yao S, Anders RA, Laheru D, Wolfgang CL, Edil BH, Schulick RD, Jaffee EM, Zheng L. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother. 2015;38(1):1–11. doi: 10.1097/CJI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Garcia M, Porichis F, de Jong OG, Levi K, Diefenbach TJ, Lifson JD, Freeman GJ, Walker BD, Kaufmann DE, Kavanagh DG. Expression of PD-L1 and PD-L2 on human macrophages is up-regulated by HIV-1 and differentially modulated by IL-10. J Leukoc Biol. 2011;89(4):507–515. doi: 10.1189/jlb.0610327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee I, Wang L, Wells AD, Ye Q, Han R, Dorf ME, Kuziel WA, Rollins BJ, Chen L, Hancock WW. J Immunol. 2003;171(12):6929–6935. doi: 10.4049/jimmunol.171.12.6929. [DOI] [PubMed] [Google Scholar]
- 33.Ou JN, Wiedeman AE, Stevens AM. TNF-alpha and TGF-beta counter-regulate PD-L1 expression on monocytes in systemic lupus erythematosus. Sci Rep. 2012;2:295. doi: 10.1038/srep00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia L, Lu J, Xiao W. Blockage of TNF-alpha by infliximab reduces CCL2 and CCR2 levels in patients with rheumatoid arthritis. J Investig Med. 2011;59(6):961–963. doi: 10.2310/JIM.0b013e31821c0242. [DOI] [PubMed] [Google Scholar]
- 35.Weber C, Draude G, Weber KS, Wubert J, Lorenz RL, Weber PC. Downregulation by tumor necrosis factor-alpha of monocyte CCR2 expression and monocyte chemotactic protein-1-induced transendothelial migration is antagonized by oxidized low-density lipoprotein: a potential mechanism of monocyte retention in atherosclerotic lesions. Atherosclerosis. 1999;145(1):115–123. doi: 10.1016/S0021-9150(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 36.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thorn M, Guha P, Cunetta M, Espat NJ, Miller G, Junghans RP, Katz SC. Tumor-associated GM-CSF overexpression induces immunoinhibitory molecules via STAT3 in myeloid-suppressor cells infiltrating liver metastases. Cancer Gene Ther. 2016;23(6):188–198. doi: 10.1038/cgt.2016.19. [DOI] [PubMed] [Google Scholar]
- 38.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206(6):1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noh H, Hu J, Wang X, Xia X, Satelli A, Li S. Immune checkpoint regulator PD-L1 expression on tumor cells by contacting CD11b positive bone marrow derived stromal cells. Cell Commun Signal. 2015;13:14. doi: 10.1186/s12964-015-0093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steenport M, Khan KM, Du B, Barnhard SE, Dannenberg AJ, Falcone DJ. J immunol. 2009;183(12):8119–8127. doi: 10.4049/jimmunol.0901925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kano A. Tumor cell secretion of soluble factor(s) for specific immunosuppression. Sci Rep. 2015;5:8913. doi: 10.1038/srep08913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.