Abstract
Background:
Accurate and reliable vascular features extracted from 3D time-of-flight (TOF) magnetic resonance angiography (MRA) can help evaluate cerebral vascular diseases and conditions. The goal of this study was to evaluate the reproducibility of an intracranial artery feature extraction (iCafe) algorithm for quantitative analysis of intracranial arteries from TOF MRA.
Methods:
Twenty-four patients with known intracranial artery stenosis were recruited and underwent two separate MRA scans within 2 weeks of each other. Each dataset was blinded to associated imaging and clinical data and then processed independently using iCafe. Inter-scan reproducibility analysis was performed on the 24 pairs of scans while intra- /inter-operator reproducibility and stenosis detection were assessed on 8 individual MRA scans. After tracing the vessels visualized on TOF MRA, iCafe was used to automatically extract the locations with stenosis and eight other vascular features. The vascular features included the following six morphometry and two signal intensity features: artery length (total, distal, and proximal), volume, number of branches, average radius of the M1 segment of the middle cerebral artery, and average normalized intensity of all arteries and large vertical arteries. A neuroradiologist independently reviewed the images to identify locations of stenosis for the reference standard. Reproducibility of stenosis detection and vascular features was assessed using Cohen’s kappa, the intra-class correlation coefficient (ICC), and within-subject coefficient of variation (CV).
Results:
The segment-based sensitivity of iCafe for stenosis detection ranged from 83.3-91.7% while specificity was 97.4%. Kappa values for inter-scan and intra-operator reproducibility were 0.73 and 0.77, respectively. All vascular features demonstrated excellent inter-scan and intra-operator reproducibility (ICC = 0.91-1.00, and CV = 1.21-8.78% for all markers), and good to excellent inter-operator reproducibility (ICC = 0.76-0.99, and CV = 3.27-15.79% for all markers).
Conclusion:
Intracranial artery features can be reliably quantified from TOF MRA using iCafe to provide both clinical diagnostic assistance and facilitate future investigative quantitative analyses.
Keywords: reproducibility, Magnetic Resonance Angiography (MRA), stenosis detection, feature measurement, intracranial artery
Background
Atherosclerosis is a major cause of cardio- and cerebrovascular disease mortality and morbidity globally [1–3]. Plaque progression leads to arterial stenosis and/or occlusion, which may cause downstream ischemic events due to atherothrombosis or hypoperfusion [4]. As a subset of atherosclerosis, intracranial atherosclerosis accounts for about 30-50% and 10% of cerebrovascular ischemic events in Asian and Caucasian populations, respectively [5].
In current clinical practice, luminal stenosis measured via angiographic techniques (such as 3D time-of-flight [TOF] MR angiography [MRA]) is the primary criterion for assessing disease severity in intracranial atherosclerosis [6]. However, these images also contain important information about the overall status of the vascular networks and blood flow that is not routinely considered by reviewing radiologists. For example, the condition of the circle of Willis (CoW) and the degree of collateralization is an important determinant in the risk for ischemic events, for monitoring the effectiveness of medical and interventional treatment over time, and understanding the interplay of blood supply to sub-regions of the brain [7–9]. In addition, 3D TOF signal intensity is also associated with blood flow velocity, and flow rate quantification for intracranial arteries is an indicator for hypothermia and dementia [10,11]. Thus, digital reconstruction of the whole intracranial vascular map from intracranial TOF MRA datasets could provide a method to analyze structure and intensity features of each artery or artery group so that cerebral vascular disease and conditions can be evaluated from the perspective of blood supply and variations in vascular anatomy. As vascular structure and function are important factors for maintaining brain health, an accurate and comprehensive quantification of the intracranial vascular map may provide novel biomarkers for assessing brain conditions, such as cognitive function and the effects of aging.
Intracranial artery feature extraction (iCafe) [12] is a novel arterial feature extraction tool that was recently developed to trace intracranial arteries from 3D TOF MRA scans and comprehensively quantify morphometry and signal intensity features of the intracranial arterial tree globally or for individual arteries or vascular groups. Intracranial stenosis can also be detected and quantified using iCafe, with a reported sensitivity and positive predictive value of 85% [12]. However, to date, reproducibility of iCafe measurements using TOF MRA has not been established. The variations come from both imaging-dependent steps and operator-dependent steps. From the perspective of imaging, it is well known that turbulent, slow, or in-plane vessel flow will cause loss of intraluminal signal [13] and may lead to apparent differences between scans. Additionally, the operator-dependent steps in iCafe may also lead to different results even for same image, given the complexity and substantial inter-individual variation of the cerebral arterial network [14]. Assessing the influence of these operator-dependent steps in iCafe is required to understand the performance of the technique.
Digital reconstruction tools to extract vascular features, such as length and tortuosity, from TOF MRA have previously been developed for quantitative analysis of intracranial arteries. Artery length, fractal dimension, and tortuosity were extracted by Neuron_Morpho in [19] to determine their relation with age in a group of 61 healthy subjects. Vessel number, average radius, and tortuosity were acquired using in-house C++ software for 14 subjects in [22] to determine the effects of exercise on cerebral vasculature health. Nowinski, et. al [17] developed a semi-automated intracranial artery reconstruction tool able to model a complete arterial map for a healthy subject with comprehensive artery labels, but the tool requires immense human labor. Compared with iCafe, the previous methods: 1) have extracted vascular features that are very limited, and signal intensity features are not available, 2) most artery labeling schemes are limited to left or right side of PCA, ACA, and MCA, and lack information on other arteries or artery groups (such as proximal/distal), 3) none of these artery feature extraction tools have been assessed for reproducibility before application in population studies, 4) excessive processing time and/or human supervision make some of the tools inapplicable to large population studies, and 5) most of the tools have not been tested on patients with stenosis.
In the current study, we assess the reproducibility of iCafe measurements in a cohort which underwent two separate MRA scans during separate sessions within two weeks of each other. We evaluated inter-scan, intra-operator, and inter-operator reproducibility of representative morphometry and intensity features of the intracranial arteries, as well as inter-scan and intra-operator agreement of stenosis point detection by iCafe, as one of the potential clinical applications, although iCafe is mainly used as a research tool currently.
Materials and Methods
Patient studies
After institutional review board review and approval, 24 subjects with documented intracranial arterial stenosis were enrolled in the study after obtaining informed consent. Subjects were scanned during two different scan sessions on a 3T Philips Ingenia Scanner (Philips Healthcare, Best, the Netherlands) using a standard head coil. Imaging parameters for TOF MRA were as follows: TR/TE = 14.7/3.5 ms, flip angle = 18°, acquired in-plane resolution = 0.6 mm×0.6 mm, interpolated in-plane resolution = 0.3 mm×0.3 mm, slice thickness = 1.4 mm, FOV = 190mm*190mm, matrix = 360*228, acquisition time = 118.9±20.2s. The mean time interval between the two scans was 10.2±3.0 days.
Feature extraction
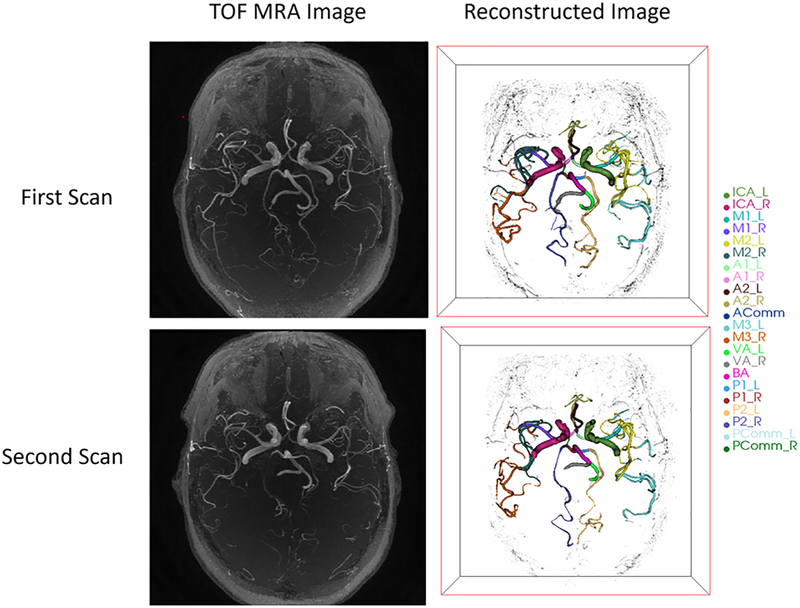
Trained iCafe operators analyzed all images. MRA images were resampled using bi-cubic interpolation for isotropic resolution in 3D space. The fields of view were aligned for each pair of images. As the intensity values in magnitude MRI images do not have a fixed meaning, a fast and accurate intensity normalization method by Nyul [15] was used to adjust intensities among cases in the database. Artery regions were then traced using an improved open-curve active contour model and anatomical names of each artery were labeled using a probability model in iCafe as described in [12]. Traces and labels were able to be corrected by the operator when errors occurred. Artery traces from two scans of the same subject are shown in Figure 1.
Fig 1.
3D visualization of traced arteries in iCafe from two scans of the same subject
Locations of stenosis ≥10% were automatically identified by iCafe from the vessel traces using the same method described in [12]. Briefly, the stenosis detection algorithm was only applied to 11 large proximal arteries per subject due to image resolution and quality limitations. These arteries included the basilar artery (BA) and bilateral internal carotid arteries (ICA) (carotid siphon to the bifurcation into M1 and A1), M1 segments of middle cerebral artery (MCA), A1 segments of anterior cerebral artery (ACA), P1 segments of posterior cerebral artery (PCA), and intracranial vertebral artery (VA) segments. Furthermore, 8 representative morphometry and intensity features were automatically extracted from the vessel traces by iCafe (Table 1). The morphometry features selected in this study included length, volume, and number of branches for all intracranial artery segments with complete coverage on MRA (including the segments of the ACA, MCA, and PCA but not including the ICA, BA, and VA), and average radius of the M1. We further divided length measurements into proximal and distal artery groups to see whether the feature extraction reproducibility differed between relative proximity with the CoW and with artery sizes. The M1, A1, and P1 segments were included in the proximal artery group. The distal artery group included the M2 and M3+ segments of the MCA, the A2+ segments of the ACA and the P2+ segments of the PCA. The ICA, BA and VA were excluded from length calculations as these arteries may not be fully covered by the scan. The posterior communicating artery (Pcomm), the anterior communicating artery (Acomm), and the ophthalmic artery (OA) were excluded from this sub-analysis considering their proximity to the CoW and small radius. Only average radius of the M1 segment of the MCA was calculated because approximately 40% of symptomatic intracranial atheromas are located in the MCA [1] and an average of all segments would be difficult to interpret, as the average radius varies substantially from segment to segment. For signal intensity features, we selected average normalized intensity for all the arteries, and the subgroup of large vertically oriented arterial segments where there would be minor impact from in-plane vessel flow. Large vertical arteries include the ICA, BA and VA.
Table 1:
Features for reproducibility test
| FEATURE NAME | FEATURE DEFINITION |
|---|---|
| Total Length | Combined length of all valid (ICA, BA, VA are not included due to their incomplete coverage shown from images) intracranial artery segments visualized in the 3D MRA acquisition. |
| Total Volume | Volume of all valid intracranial arteries visualized in the 3D acquisition. This calculation is based on the cylinder model with varying radius along the centerline. |
| Proximal Length | Length of major arteries near the Circle of Willis, including M1 middle cerebral artery (MCA), A1 anterior cerebral artery (ACA), P1 posterior cerebral artery (PCA). |
| Distal Length | Length of the M2/3+ MCA, A2+ ACA and P2+ PCA. |
| Total Branches | Number of branches (bifurcation to bifurcation or branch end) of arteries. |
| Average M1 Radius | Average radius of M1 MCA segments. |
| Average Normalized Intensity | Average signal intensity of each centerline point along all arteries after image normalization. |
| Average Normalized Intensity for large arteries | Average normalized intensity of arteries with relatively large radius and vertical orientation including: ICA, Vertebral Artery (VA), Basilar Artery (BA). |
Feature reproducibility
Intra- and inter-operator reproducibility of the 8 vascular features from iCafe and inter-scan reproducibility of iCafe stenosis detection were performed as an initial assessment using 8 randomly selected subjects. Inter-scan reproducibility of the 8 vascular features from iCafe was assessed using all 24 subjects.
To provide a reference standard for the stenosis detection analysis, an experienced neuroradiologist independently reviewed the 2D slices of the MRA images and recorded all segments with ≥10% stenosis based on visual assessment. For intra-operator reproducibility, the same set of images were analyzed two times, separated by at least one month between reviews. For inter-operator reproducibility, the same set of images was analyzed by another reviewer, independent of the first review, and without reaching consensus. For inter-scan reproducibility, all 48 scans (2 × 24 subjects) were randomized and analyzed at a rate of one per day.
The iCafe stenosis detection results were analyzed on a per-segment basis and the reproducibility of the 8 vascular features was analyzed on a per-subject basis. The reproducibility of iCafe stenosis detection was summarized using Cohen’s kappa and diagnostic performance was summarized using sensitivity and specificity based on the blinded neuroradiologist review. Intra-/inter operator and inter-scan reproducibility of the 8 vascular features were summarized using the intra-class correlation coefficient (ICC) and the within-subject coefficient of variation (CV). Bland-Altman plots were used to visualize the differences. R studio (version 3.4.2) was used for statistical analysis. ICC values of less than 0.5 are indicative of poor reproducibility, values between 0.5 and 0.75 indicate moderate reproducibility, values between 0.75 and 0.9 indicate good reproducibility, and values greater than 0.90 indicate excellent reproducibility [16].
Results
Sixteen males and eight females were recruited for this study. Subjects included in this study had an age range from 45- 81, and a mean age of 61.0±9.6.
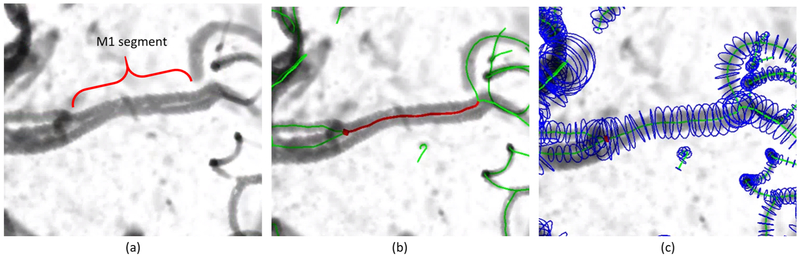
Intracranial artery structures of each subject were generated using iCafe with manual corrections applied after automated vessel segment, from which the vascular features were extracted. The processing time needed for each case was between 20 minutes to 1 hour. An example of generated artery tracing and extracted features is shown in Figure 2.
Fig 2.
(a) M1 segment (indicated by the red bracket) of the Middle Cerebral Artery (MCA) from one of the subjects in this study (b) Traced artery centerlines in green and red lines. The red line indicates the selected M1 segment. Length of M1 segment is calculated as 17.16mm (c) Artery centerlines in green lines and radius estimation for each point along the centerline in blue circles. Volume of M1 segment is calculated as 174.49mm3, average radius is calculated as 1.69mm
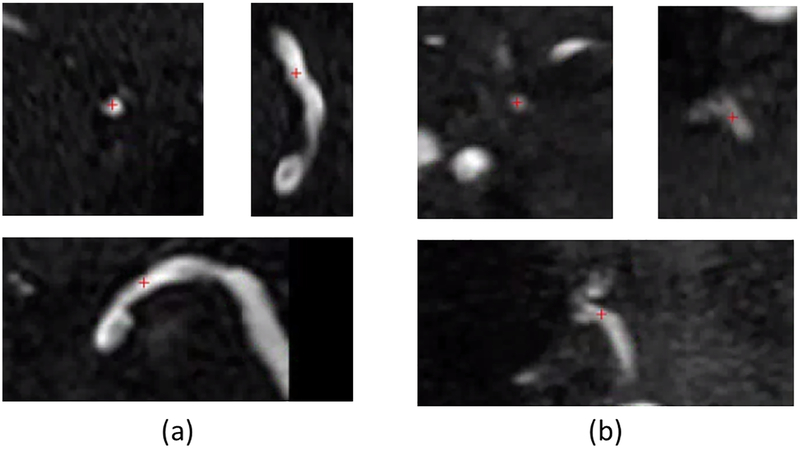
In the eight subjects included in the stenosis detection analysis, stenoses were identified by the neuroradiologist in 12 arteries from 5 subjects. As shown in Table 2, the segment-based sensitivity of iCafe stenosis detection ranged from 83.3-91.7% and the specificity was 97.4% across all reading sessions. The estimated kappa values for intra-operator and inter-scan reproducibility of stenosis detection were 0.73 and 0.77, respectively. Across the three reading sessions, iCafe missed a stenosis in three locations (one each in the BA, A1, and M1). In each case, iCafe detected some narrowing (6.2%-7.8%) but it was not large enough to be considered stenosis (below the pre-specified threshold of 10%). For stenoses identified by iCafe but not by the human reviewer, and after confirmation with the human reviewer, these disagreements were considered to be due to partial volume effects or flow dephasing. One example of iCafe missed stenosis position and one example of iCafe falsely detected stenosis are shown in Figure 3 (a) and (b), respectively.
Table 2:
Segment-based diagnostic performance of iCafe stenosis detection across three evaluations
| Evaluation | Sensitivity | Specificity |
|---|---|---|
| First scan | 11/12 (91.7%) | 74/76 (97.4%) |
| Second scan | 10/12 (83.3%) | 74/76 (97.4%) |
| Repeated analysis of first scan | 11/12 (91.7%) | 74/76 (97.4%) |
| Average | 88.9% | 97.4% |
Fig 3.
Cross-sectional plane (top left) and two perpendicular planes of the cross-sectional plane (top right and bottom) of one of the positions detected by the radiologist but not iCafe (a) and one of the positions detected by iCafe bit not the Radiologist (b). Red cross shows the location of the stenosis position. Stenosis percent of location in (a) is 6.2% calculated from iCafe, below the threshold. Stenosis percent of location in (b) is 12.1% calculated from iCafe, above the threshold.
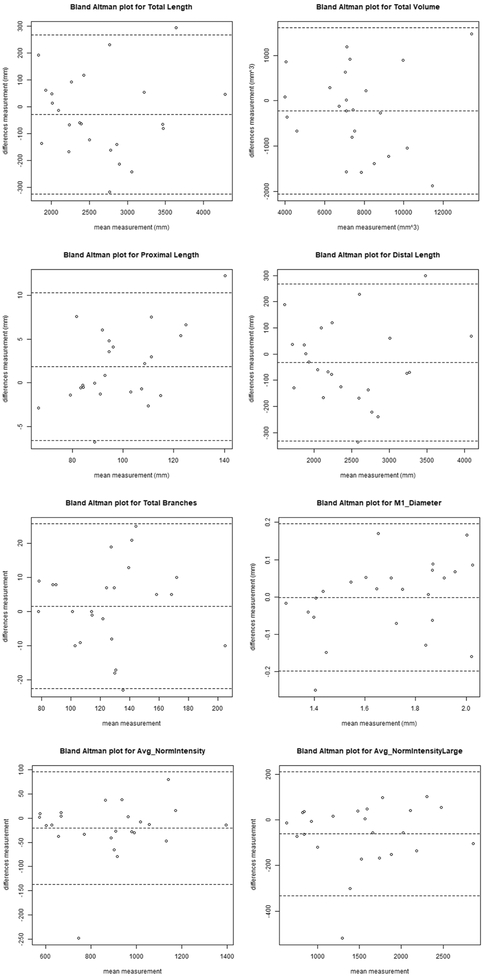
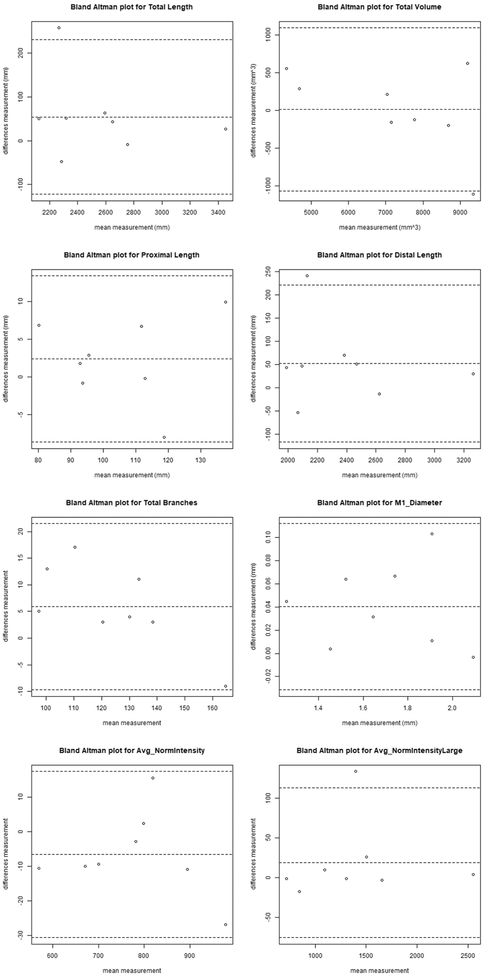
For the other eight vascular features, inter-scan reproducibility was excellent, with ICCs ranging from 0.91-0.97 and CVs from 3.29-8.78% (Table 3). The intra-operator reproducibility was similar to inter-scan reproducibility, with ICCs ranging from 0.91-1.00 and CVs from 1.21-5.39%. In particular, the ICC estimates ranged from 0.95-0.97 for proximal and distal length, and between 0.99-1 for average normalized intensity across all arteries and large arteries only. Bland-Altman plots of the 8 features for the inter-scan evaluation are shown in Figure 4, and for the intra-operator evaluation in Figure 5.
Table 3:
Reproducibility of eight iCafe vascular features
| Features | Inter-scan | Intra-operator | Inter-operator | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean+-SD | ICC | 95% CI | CV | Mean+-SD | ICC | 95% CI | CV | Mean+-SD | ICC | 95% CI | CV | |
| Total Length (mm) | 2639.7+-636.2 | 0.97 | 0.94-0.99 | 4.03% | 2555.7+-423.1 | 0.97 | 0.86-0.99 | 2.78% | 2659+-439.3 | 0.85 | 0.38-0.97 | 6.85% |
| Total Volume (mm3) | 7597.2+-2259.6 | 0.92 | 0.82-0.96 | 8.78% | 7272.3+-1914.4 | 0.96 | 0.83-0.99 | 5.01% | 8010+-2315.8 | 0.76 | −0.05-0.95 | 15.79% |
| Proximal Length (mm) | 98.8+-16.9 | 0.96 | 0.91-0.98 | 3.29% | 105.4+-18.3 | 0.95 | 0.8-0.99 | 3.86% | 104.8+-19.7 | 0.85 | 0.29-0.97 | 7.78% |
| Distal Length (mm) | 2469.5+-625.5 | 0.97 | 0.93-0.99 | 4.39% | 2376.9+-421.2 | 0.97 | 0.87-0.99 | 2.85% | 2471.5+-442.2 | 0.85 | 0.44-0.97 | 7.21% |
| Total Branches | 126.3+-30.4 | 0.92 | 0.83-0.97 | 6.81% | 124.4+-22.1 | 0.91 | 0.56-0.98 | 5.39% | 127.2+-18.9 | 0.78 | 0.19-0.95 | 7.40% |
| Average M1 Radius | 1.7+-0.2 | 0.91 | 0.81-0.96 | 4.12% | 1.7+-0.3 | 0.98 | 0.75-1.00 | 2.21% | 1.7+-0.2 | 0.77 | 0.25-0.95 | 7.28% |
| Average Normalized Intensity | 880.8+-215.2 | 0.96 | 0.91-0.98 | 4.96% | 776.7+-129 | 0.99 | 0.97-1.00 | 1.21% | 758.2+-123 | 0.96 | 0.22-0.99 | 3.27% |
| Average Normalized Intensity for large arteries | 1535.2+-598 | 0.97 | 0.92-0.99 | 6.85% | 1383.5+-569.7 | 1.00 | 0.98-1.00 | 2.48% | 1373.8+-555.5 | 0.99 | 0.96-1.00 | 3.63% |
Fig 4.
Bland-Altman plot of the vascular features inter-scan reproducibility. The central dashed line indicates the mean difference and the outer two dashed lines indicate the upper and lower limits of agreement (mean plus 2 times the standard deviation of differences).
Fig 5.
Bland-Altman plot of the vascular features intra-operator reproducibility. The central dashed line indicates the mean difference and the two outer dashed lines indicate the upper and lower limits of agreement (mean plus 2 times the standard deviation of differences).
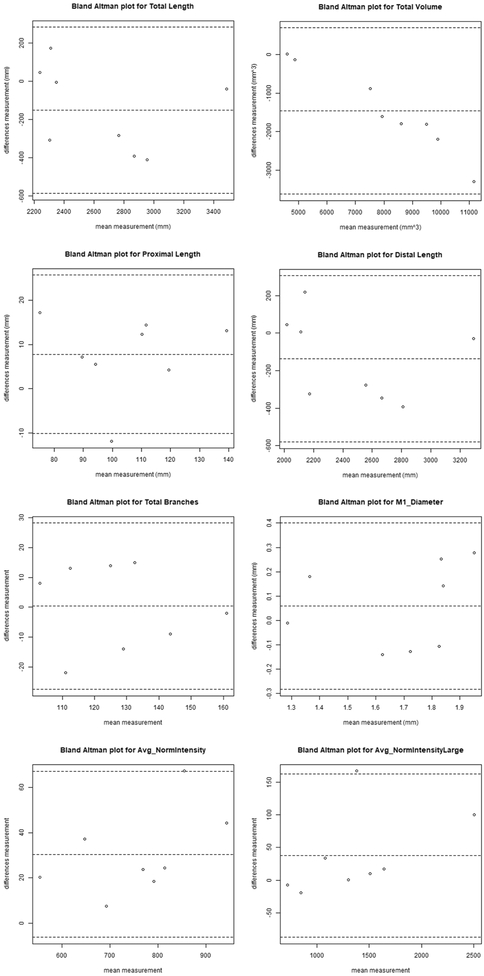
The inter-operator reproducibility fell between good to excellent, with ICCs ranging from 0.76-0.99 and CVs from 3.27-15.79%. Bland-Altman plots of the 8 features for the intra-operator evaluation are shown in Figure 6.
Fig 6.
Bland-Altman plot of the vascular features inter-operator reproducibility. The central dashed line indicates the mean difference and the two outer dashed lines indicate the upper and lower limits of agreement (mean plus 2 times the standard deviation of differences).
Discussion
In this study assessing a novel, semi-automated method (iCafe) that provides quantitative measurements of intracranial vasculature on TOF-MRA, we found: 1) both stenosis detection and quantification of artery features agree well for inter-scan, intra-operator and inter-operator reproducibility analyses; 2) reproducibility for the lengths of proximal and distal arteries was similar; and 3) reproducibility of the average normalized intensity for the large artery subgroup was similar to the reproducibility for the whole group.
Significance
Accurate and repeatable digital reconstruction of intracranial arterial topography from TOF MRA images has the potential to augment traditional qualitative analysis of intracranial artery disease and vascular health.
As the geometry of the human vasculature is complex, and the majority of the arteries (72.39%) have a radius smaller than 1 mm [17], reconstructing an accurate and complete intracranial arterial map is challenging. To achieve this, iCafe differs from other automated intracranial artery segmentation and quantification techniques [18–20] in four major ways: 1) iCafe utilizes a comprehensive intracranial artery labeling scheme (24 artery types) to aggregate comprehensive vascular features (12 morphometry and 16 intensity features for each artery) according to their anatomical groups, so that a complete list of vascular features can be acquired from the brain vascular map both globally and regionally; 2) accurate reconstruction of arterial maps is critical for vascular feature extraction. iCafe is a semi-automated technique which allows users to supervise automated artery tracing and labeling. A series of manual operations can be applied in iCafe to correct mistakes following automated steps to allow high accuracy of vascular map reconstruction; 3) location of bifurcation and stenosis, as well as artery length measurements have been previously validated by comparison with an expert neuroradiologist’s interpretation as the gold standard. iCafe achieved 94% sensitivity and 85% positive predictive values (PPV) for localization of bifurcations, and 85% sensitivity and PPV for detection of stenosis in our previous work [12]; 4) a deep convolutional neural network [21] was recently incorporated into iCafe for better artery region segmentation, and it is expected to continue to improve accuracy through accumulation of processed data.
Potential clinical uses
Detection of stenosis of the intracranial arteries is one of the potential clinical uses for iCafe, and it requires the reconstructed vascular map to have accurate radius estimation for each point in the map. A group of subjects with intracranial arterial stenosis was enrolled in this study. Unlike young healthy subjects, whose intracranial arterial trees are likely to be regular and arteries may have relatively higher signal intensity in TOF MRA, subjects in this dataset are closer to those in clinical applications so that this reproducibility assessment may have potential clinical value.
For iCafe, after reproducibility assessment, quantitative and comprehensive features are available to provide reliable evaluation of intracranial vascular structure and blood flow, which have potential clinical applications. For example, identification of a complete CoW with iCafe provides an indicator of the connectivity between different intracranial vascular territories, and the regional topographical features provided by iCafe provides quantitative measures of the degree of vascular arborization and collateralization. As TOF MRA is a frequently used sequence in clinical and research applications, generating intracranial arterial maps in large populations is feasible.
With further development, iCafe may provide information regarding disease course. Future applications of iCafe include potentially predicting the success of endovascular interventions for acute ischemic stroke from CTA data. In addition, combined with vessel wall imaging for clinical diagnosis, quantitative measures extracted from TOF MRA using iCafe may help show the extent of improvement in response to medical therapies for vasculitis, reversible cerebral vasoconstriction syndrome or other intracranial vasculopathies.
Reliable quantitative arterial measurements
The reproducibility analysis of all the other vascular features showed excellent agreement for both inter-scan and intra-operator evaluations, and good agreement for inter-operator evaluations, indicating the quantitative measurements of intracranial arteries are highly reliable. The robust measurement of both proximal and distal artery lengths indicates the detailed structural information of distal branches can be consistently reflected in TOF MRA as well as proximal arteries.
The similarly excellent average normalized intensity reproducibility of the large artery subgroup and the whole group (large and smaller distal arterial segments) showed that loss of signal for in-plane vessel flow was not significant. Further, since TOF signal intensity is partially related to blood flow velocity, the reproducibility of signal intensity features indicates consistent blood flow during two MRA scans acquired within a short time interval.
The signal intensity features of intracranial arteries have generally been ignored in previous studies. But with signal intensity reproducibility validated, we may start to discover the potential applications of these intensity features, especially in quantification of the velocity of brain artery flow, which could be very informative. An example is assessing the reduced artery flow velocity that results in the post-stenotic artery. Quantitative information on reduced flow might provide additional value for treatment decisions. This feature, based on quantitative flow evaluation, might also help differentiate between subjects with stenosis who will be likely to suffer a stroke. iCafe can extract 1,345 features from 12 morphometric categories and 16 intensity categories for each artery, grouped by 24 artery types or 34 vascular groups [12]. We believe the selection of a subset of 8 features in our reproducibility test represents most of the important and independent features. Reproducibility for all features was not attempted since most of the morphometric features are inter-dependent and inter-related. For example, artery surface area is a feature category related to radius and length, so its reproducibility is assumed to be excellent when both radius and length show good performance. The other intensity categories, such as ten percentiles of each artery, were not included in this study, as their importance is not as high as mean intensity of arteries.
Limitations
As the brain vasculature is a complex network with substantial inter-individual variation, the processing and correction time to fully model one intracranial vascular map can be as long as 8 weeks [17] using traditional methods [23]. For iCafe, with improved artery tracing, labeling methods and multiple practical 3D artery editing tools, the processing time needed for each case is between 20 minutes to 1 hour, most of which is spent checking or correcting each of the more than one hundred arterial traces (starting/ending position, direction, tortuosity and connection). All the subjects used in this study need human corrections, and most of the labor is focused on small arteries in distal branches, where the signal intensity is relatively weak and the artery structure is not clearly identifiable. For stenosis detection performed on the 11 larger intracranial arterial segments, automated artery tracing with little human intervention is robust. Considering the processing time to generate whole vascular maps with high accuracy, only eight cases were selected for the intra-operator and stenosis detection assessments. With continued improvement of the iCafe algorithm, such as applying artificial intelligence techniques to improve and automate image processing, we believe the processing time can be decreased.
We tested reproducibility on a relatively small dataset. Recruitment of larger populations of subjects with a broader range of diseases will permit further refinement of the algorithm and more robust assessment of reproducibility. The MRA data was collected from a single scanner with the same MRA protocol. The variability caused by using different MR scanners or different MRA parameters was not evaluated.
Conclusion
iCafe is a highly reproducible image analysis tool that can reliably detect locations of stenosis and extract intracranial arterial morphometry and intensity features from TOF MRA to provide both clinical diagnostic assistance and facilitate future investigative quantitative analyses.
Acknowledgements
We are grateful to the support of NVIDIA Corporation with donation of the Titan Xp GPU.
Funding
This research was supported by grants from the National Institutes of Health (R01-NS083503-03, R01-NS092207-01A1, R01-HL103609-01A1 and 1R56NS092207) and Philips Healthcare.
List of abbreviations
- TOF
time-of-flight
- CoW
circle of Willis
- MRA
magnetic resonance angiography
- iCafe
intracranial artery feature extraction
- ICC
intra-class correlation coefficient
- CV
coefficient of variation
- MCA
middle cerebral artery
- ACA
anterior cerebral artery
- PCA
posterior cerebral artery
- ICA
internal carotid artery
- BA
basilar artery
- VA
vertebral artery
- Pcomm
posterior communicating artery
- Acomm
anterior communicating artery
- OA
ophthalmic artery
- PPV
positive predictive values
Footnotes
Ethics approval and consent to participate
This study was approved by institutional review board, and all subjects gave written informed consent prior to enrollment in the study.
Consent for publication
Written informed consent was obtained from all participants for inclusion of their data in publications.
Availability of data and material
The datasets generated and analyzed during the current study are not publicly available due institutional review board restrictions but are available from the corresponding author on reasonable request.
iCafe is available for other groups for non-commercial research under an academic license. Please contact Dr. Chun Yuan (cyuan@uw.edu) to request an executable file.
Competing interests
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke. 1986;17:648–55. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJL, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Zhou Y, Zhang S, Ding W, Lou M. Cerebral blood flow evaluation of intensive rosuvastatin therapy in stroke/transient ischemic attack patients with intracranial arterial atherosclerotic stenosis study: Rationale and design. Brain Behav. 2017;7:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qureshi AI, Feldmann E, Gomez CR, Johnston SC, Kasner SE, Quick DC, et al. Intracranial atherosclerotic disease: An update. Ann Neurol. 2009;66:730–8. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Y, Peng W, Teng Z, Gillard JH, Hong B, Liu Q, et al. Local blood pressure associates with the degree of luminal stenosis in patients with atherosclerotic disease in the middle cerebral artery. Biomed Eng Online. BioMed Central; 2016;15:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang F, Fukasaku K, Liu H, Takagi S. A computational model study of the influence of the anatomy of the circle of willis on cerebral hyperperfusion following carotid artery surgery. Biomed Eng Online. BioMed Central; 2011;10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alastruey J, Parker KH, Peiró J, Byrd SM, Sherwin SJ. Modelling the circle of Willis to assess the effects of anatomical variations and occlusions on cerebral flows J Biomech. Elsevier; 2007;40:1794–805. [DOI] [PubMed] [Google Scholar]
- 9.Long Q, Luppi L, König CS, Rinaldo V, Das SK. Study of the collateral capacity of the circle of Willis of patients with severe carotid artery stenosis by 3D computational modeling J Biomech. Elsevier; 2008;41:2735–42. [DOI] [PubMed] [Google Scholar]
- 10.Rosomoff HL, Holaday DA. Cerebral blood flow and cerebral oxygen consumption during hypothermia. Am J Physiol. 1954;179:85–8. [DOI] [PubMed] [Google Scholar]
- 11.Hachinski VC, Iliff LD, Zilhka E, Du Boulay GH, McAllister VL, Marshall J, et al. Cerebral Blood Flow in Dementia Arch Neurol. American Medical Association; 1975;32:632–7. [DOI] [PubMed] [Google Scholar]
- 12.Chen L, Mossa-Basha M, Balu N, Canton G, Sun J, Pimentel K, et al. Development of a quantitative intracranial vascular features extraction tool on 3D MRA using semiautomated open-curve active contour vessel tracing. Magn Reson Med. 2018;79:3229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wehrli FW, Shimakawa A, Gullberg GT, MacFall JR. Time-of-flight MR flow imaging: selective saturation recovery with gradient refocusing. Radiology. 1986;160:781–5. [DOI] [PubMed] [Google Scholar]
- 14.Alpers BJ, Berry RG, Paddison RM. Anatomical Studies of the Circle of Willis in Normal Brain Arch Neurol Psychiatry. American Medical Association; 1959;81:409–18. [DOI] [PubMed] [Google Scholar]
- 15.Nyul LG, Udupa JK, Zhang X. New variants of a method of MRI scale standardization. IEEE Trans Med Imaging. 2000;19:143–50. [DOI] [PubMed] [Google Scholar]
- 16.Koo TK, Li MY. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research J Chiropr Med. Elsevier B.V.; 2016;15:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowinski WL, Volkau I, Marchenko Y, Thirunavuukarasuu A, Ng TT, Runge VM. A 3D model of human cerebrovasculature derived from 3T magnetic resonance angiography. Neuroinformatics. 2009;7:23–36. [DOI] [PubMed] [Google Scholar]
- 18.Aylward SR, Bullitt E. Initialization, noise, singularities, and scale in height ridge traversal for tubular object centerline extraction. IEEE Trans Med Imaging. 2002;21:61–75. [DOI] [PubMed] [Google Scholar]
- 19.Wright SN, Kochunov P, Mut F, Bergamino M, Brown KM, Mazziotta JC, et al. Digital reconstruction and morphometric analysis of human brain arterial vasculature from magnetic resonance angiography Neuroimage. Elsevier Inc.; 2013;82:170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng H, Ruan Z, Long F, Simpson JH, Myers EW. V3D enables real-time 3D visualization and quantitative analysis of large-scale biological image data sets Nat Biotechnol. Nature Publishing Group; 2010;28:348–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Xie Y, Sun J, Balu N, Mossa-Basha M, Pimentel K, et al. 3D intracranial artery segmentation using a convolutional autoencoder. Proc - 2017 IEEE Int Conf Bioinforma Biomed BIBM 2017 IEEE; 2017. p. 714–7. [Google Scholar]
- 22.Bullitt E, Rahman FN, Smith JK, Kim E, Zeng D, Katz LM, et al. The effect of exercise on the cerebral vasculature of healthy aged subjects as visualized by MR angiography. Am J Neuroradiol. 2009;30:1857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson DL, Noble JA. An adaptive segmentation algorithm for time-of-flight MRA data. IEEE Trans Med Imaging. 1999;18:938–945. [DOI] [PubMed] [Google Scholar]