Abstract
Current genetic detection methods require gene isolation, gene amplification and detection with a fluorescent-tagged probe. They typically require sophisticated equipment and expensive fluorescent probes, rendering them not widely available for rapid acute infection diagnoses at the point of care to ensure timely treatment of the diseases. Here we report a rapid genetic detection method that can detect the bacterial gene directly from patient stools using a piezoelectric plate sensor (PEPS) in conjunction with a continuous flow system with two temperature zones. With stools spiked with sodium dodecyl sulfate (SDS) in situ bacteria lysing and DNA denaturation occurred in the high-temperature zone whereas in situ specific detection of the denatured DNA by the PEPS occurred in the lower-temperature zone. The outcome was a rapid genetic detection method that directly detected bacterial genes from stool in <40 min without the need of gene isolation, gene amplification, or expensive fluorescent tag but with polymerase chain reaction (PCR) sensitivity. In 40 blinded patient stools, it detected the toxin B gene of Clostridium difficile with 95% sensitivity and 95% specificity. The all-electrical, label-free nature of the detection further supports its potential as a low-cost genetic test that can be used at the point of care.
Keywords: piezoelectric plate sensor, in situ, isolation-free, amplification-free, label-free, stool bacteria genetic detection
1. INTRODUCTION
In underdeveloped countries where resources are limited, diarrheal infections (DIs) are a leading cause of illness and a major healthcare challenge, with 4.6 million episodes and 3 million deaths each year worldwide (Guerrant et al. 2002). Conventional methods for detecting enteropathogens involve days of separate culture steps, microscopy, followed by biochemical identification, and serotyping – all of which is too slow for timely identification of an outbreak or bioterrorism attack response. The recent advent of quantitative real-time polymerase chain reaction (qPCR) methods have overcome the need for prior enrichment and cultivation of the pathogens and permit rapid detection of pathogenic bacteria in food, and in stool (Chapin et al. 2011; Culbreath et al. 2012; Grys et al. 2009) with high sensitivity and specificity. Unfortunately, qPCR requires DNA isolation, amplification, and expensive fluorescent labeling which is too costly for widespread use. Detecting ribosomal RNA (rRNA) for bacterial infection requires an additional step of reverse transcription to convert RNA to DNA before amplification and detection. In developed countries, DIs such as that caused by Clostridium difficile (CD) (Cagle et al. 2013; Gerding et al. 1995) are a serious healthcare-associated infection. In the US alone in 2011, CD caused more than 500,000 infections and 29,000 deaths within 30 days of the initial diagnosis (CDC 2016). The cost to treat CD infection (CDI) was over $3B a year in the US alone (Ghantoji et al. 2010; Moody et al. 2012a, b; O'Brien et al. 2007). Traditional CDI diagnosis relies on stool culture (SC), cytotoxin neutralization assay (CTA), or toxigenic culture (TC) (Cohen et al. 2010). These tests take days and are clinically impractical. Presently, TC is only used as the gold standard for evaluating the sensitivity and specificity of a new test (Cohen et al. 2010). Current CDI diagnosis relies on CD toxins enzyme immunoassay (EIA) together with glutamate dehydrogenase (GDH) antigen EIA (Carrico 2008; Cohen et al. 2010). Although stool toxin EIA is specific and relatively fast (hours to 1 day), the sensitivity of testing for EIA is only 60% (Bartlett and Gerding 2008; Carrico 2008; Chapin et al. 2011; Cohen et al. 2010; Delmee et al. 2005). Nucleic acid amplification test (NAAT) using quantitative real-time polymerase chain reaction (qPCR)(Chapin et al. 2011; Culbreath et al. 2012) to detect tcdB gene and NAAT using loop-mediated isothermal amplification (LAMP) to detect tcdA (Pancholi et al. 2012) are sensitive and specific. However, both qPCR and LAMP require an expensive fluorescent probe and are not widely available. Therefore, there is a need to develop a low-cost method that can rapidly detect genetic markers of bacteria in stool to help rapidly diagnose DIs.
Current genetic detection technologies under development rely on fluorescence (Hammond et al. 2007), quartz crystal microbalance (QCM) (Feng et al. 2007; Passamano and Pighini 2006), electrochemical (Gasparac et al. 2004) binding of nano-metal particles (Park et al. 2002), surface plasmon resonance (SPR) (He et al. 2000), silicon-based microcantilever sensor as well as piezoelectric microcantilever sensor. For DNA detection, nanoparticle amplified QCM exhibited a concentration sensitivity of 1 pM (Mao et al. 2006). Nanoparticle enhanced SPR exhibits concentration sensitivity of 10-100 aM (Gifford et al. 2010). The electrochemical methods involving nanofibers and nanotubes also exhibited concentration sensitivity of about 30 fM (Yang et al. 2009). Nanowires (Andreu et al. 2006; Gao et al. 2007; Hahm and Lieber 2003; Zhang et al. 2010; Zheng et al. 2005) and nanotubes (Chang et al. 2007; Wang et al. 2003b) exhibited concentration sensitivity ranging from 100 fM to 1 fM. Microcantilevers coupled with nano-metal particles exhibited 0.01 nM concentration sensitivity (Su et al. 2003). Although methods such as QCM, SPR, silicon-based microcantilever sensor as well as lead zirconate titanate (PZT) piezoelectric microcantilever sensor (PEMS) (Rijal and Mutharasan 2007; Zheng et al. 2011) are label-free, the sensitivity is still many orders of magnitude away from the attomolar (10−18M) requirement (Caruso et al. 1997). Similarly, the 10−16 M sensitivity achieved by magnetic beads isolation coupled with electrochemical enhancement is still not sufficient (Wang et al. 2003a). Nano-scale mechanical imaging by atomic force microscopy (AFM) can differentiate unhybridized single-stranded DNAs (ssDNAs) from hybridized double-stranded DNAs (dsDNAs) at attomolar sensitivity but it requires sophisticated instrumentation such as AFM (Husale et al. 2009). Although a GaN nanowire extended-gate field-effect-transistor could detect attomolar concentrations of target DNA (tDNA) in situ (Chen et al. 2011), the detection signal (0.2V) at 10−18 M is not very different from that (0.3V) at 10−6M - making it not suitable for DNA quantification. Streptavidin horseradish peroxidase functionalized carbon nanotubes can detect DNA at 10−18 M, however it requires labeling and not in situ (Gao et al. 2011). Label-free carbon nanotube impedance biosensors can only detect DNA at 100 aM, but this is not sufficient for clinical applications (Kurkina et al. 2011). Although electrochemical biosensors have been shown to reach attomolar sensitivity, they require electrocatalysis (Soleymani et al. 2009) or magnetic beads amplification (Loaiza et al. 2008) and are neither label-free nor realtime.
Lead magnesium niobate-lead titanate (Pb(Mg1/3Nb2/3)O3)0.65-(PbTiO3)0.35 (PMN-PT) piezoelectric plate sensor (PEPS) consists of sole a PMN-PT freestanding film 8 μm in thickness (Shih et al. 2006) coated with gold electrodes and electrically insulated by a 3- mercaptopropyltrimethoxysilane (MPS) coating. Receptor specific to a biomarker is immobilized on the surface of the electrical insulation layer. Binding of the tDNA to the probe DNA (pDNA) on the PEPS surface shifts the PEPS length-extension-mode (LEM) or width-extension mode (WEM) resonance peak frequency (f). tDNA detection is achieved by directly immersing a probe-coated PEPS in the biological fluid of interest and monitoring the LEM or WEM resonance frequency shift (Δf) in real time. What is remarkable about the PMN-PT PEPS—(PEPS hereafter) is its sensitivity—it has demonstrated PCR-like 60 copies/ml concentration sensitivity in detecting single-stranded tDNA in urine without DNA amplification (Kirimli et al. 2014, 2015). Such sensitivity is a result of the PEPS’s ability to self-enhance its detection Δf 1000-fold via binding stress-induced crystalline orientation switching that is unique of the thin PMN-PT layer (Wu et al. 2016). Given its sensitivity and its label-free nature, the goal of this study is to investigate if PEPS could directly detect genetic signatures of enteropathogens from stool without DNA isolation or amplification for potential low-cost, rapid diarrheal infection detection.
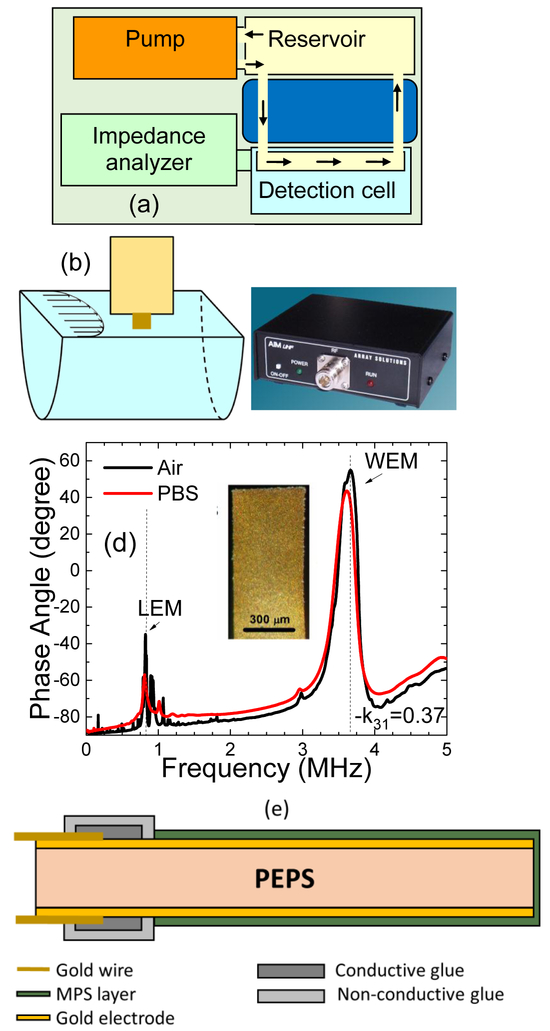
To achieve direct bacterial genetic detection from stool without DNA isolation, a unique continuous flow system was used consisting of two temperature zones—a higher-temperature reservoir kept at 95°C where bacteria were loaded and a low-temperature detection cell at the detection temperature where detection of the tDNA took place. Figure 1(a) illustrates the total detection system complete with the above-mentioned flow system, a pump that pushed the flow, and an electrical impedance analyzer that measured the PEPS resonance frequency shift, Δf, with time. In each test, the stool sample was first loaded in the high-temperature reservoir for 10 min to completely lyse the bacteria and de-hybridize the DNA, followed by flowing the stool in thin tubing through a cooling medium (room-temperature water). The narrow tubing allowed the DNA molecules to stay de-hybridized when they reached the detection cell. A PEPS coated with the probe complementary to the tDNA was located at the center of the detection cell (see Fig. 1(b)) to detect the tDNA for 30 min. A low-cost, portable electrical impedance analyzer (Fig. 1(c)) was used to measure the Δf of the WEM resonance at the peak of around 3.6 MHz (see Fig. 1(d)) due to the binding of the tDNA to the probe on the PEPS surface in real time. All detections in this study were carried out by monitoring the WEM resonance peak frequency shift. The optical microphotograph of a PEPS is shown as an insert in (d) and a schematic of the cross-section of a PEPS is shown in Fig. 1(e).
Fig. 1:
(a) A schematic of the PEPS detection system consisting a reservoir at 95°C where the stool is loaded, bacteria lysed, DNA released and de-hybridized, a cooling module to fast cool the stool for the DNA to remain de-hybridized, a detection cell at the detection temperature where specific detection of target DNA (tDNA) occurs, a pump to circulate the stool, and a portable electrical impedance analyzer to measure PEPS resonance frequency shift, Δf, (b) the blow-up of the detection cell where a PEPS vertically situated at the center of the flow with its major faces parallel to the direction of the flow, (c) a photograph of the portable AIM 4170C impedance analyzer that read the resonance spectra of the PEPS, (d) typical PEPS phase angle-versus -frequency resonance spectra in air (black) and in PBS (red) where the dashed lines denote the first length extension-mode (LEM) resonance peak and the first width-extension-mode (WEM) resonance peak, and (e) a schematic of the cross-section of the PEPS. The insert in (d) shows an optical micrograph of a PEPS viewed from the top gold electrode. WEM resonance peak frequency, f, was monitored for all detections in this study.
2. MATERIAL AND METHODS
2.1. Escherichia coli O157:H5 (EC) culture
Shinga-like toxins-producing Escherichia coli (E. coli) O157:H7 (ATCC 43895) and negative control E. coli (ATCC 25922) were grown into 5 ml Trypticase soy broth (TSB) (Difco, USA) overnight at 37° C with shaking. For bacterial DNA (baDNA) extraction, the culture was harvested, and genomic DNA was extracted with DNeasy Blood & Tissue kit (Qiagen, USA) and the concentration was adjusted to 100 ng/μL before dilution and spiking. For EC spiking, the concentration was adjusted to 1.5 ×108 CFU/ml by microscopic cell counter before dilution and spiking.
2.2. PCR validation of captured DNA on the PEPS surface
The captured tDNA on the sensor surface was eluted after detection by immersing the sensor in a close-loop flow of 80°C de-ionized (DI) water. The eluent was collected and freeze-dried for PCR validation. PCR was carried out with reagents from Qiagen to prepare the mastermix. For each reaction, 10 μL of freeze-dried sample was added to a total of 25 μL final PCR mix. For positive control, 1 μL of tDNA was added as template. The forward primer, 5’- GCCGGGTTCGTTAATACGGCA, the reverse primer, 5’- GAACGTTCCAGCGCTGCGACA and the PCR conditions were adopted from Kai et al (Kai et al. 2000).
2.3. Probes
Amine-(CH2)12-5’-GCC GGG TTC GTT AAT ACG GCA-3’ (Sigma) was the probe used for the stx2 gene of EC. It was a 21-nt long sense strand, targeting the anti-sense strand of the stx2 gene and amine-activated with a 12-carbon spacer at the 5’ end. The probe for the tcdB gene of CD was amine-(CH2)12-5’-CCA AAA TGG AGT GTT ACA AAC AGG TG-3’ (Sigma). It was a 26-nt long sense strand, targeting the anti-sense strand of the tcdB gene and amine-activated with a 12-carbon spacer at the 5’ end. Both probes were blasted(http://blast.ncbi.nlm.nih.gov/) against human genome and the genomes of the bacteria in the common gut flora. The probe for EC had a melting temperature, Tm =71°C for the binding to the stx2 gene and a Tm =35°C for binding to the human genome and no binding to the genomes of the bacteria in the common gut flora. The probe for CD had a Tm = 68°C for the binding to the tcdB gene and a Tm=50°C for the binding to the human genome and no binding to the genomes of common gut-flora bacteria.
2.4. Detection temperature
The detection temperature of the stx2 gene of EC was 50°C and that of tcdB gene of CD was 58°C. Both detection temperatures were roughly the midpoint between the melting temperature of the probe to tDNA and that of the probe to the human gene. This ensured that at the detection temperatures only the tDNA could strongly bind to the probe but not the human gene to minimize nonspecific binding by the human gene.
2.5. PEPS fabrication
PEPS was fabricated from 8 μm-thick PMN-PT freestanding film with the top and bottom surfaces coated with 200 nm think Au/Cr electrodes by thermal evaporation (Thermionics VE 90) and cut into 2 mm by 0.6-0.8 mm strips with a wire saw (Princeton Scientific Precision, Princeton, NJ). The top and bottom sides of the strips were attached with gold wires with 10 μm diameter using a conductive glue (XCE3104XL, Emerson and Cuming Company, Billerica, MA). The rear end of the strip was fixed on a glass substrate with a nonconductive glue (Loctite 1C Hysol Epoxy Adhesive). The PEPS was then poled at 15 kV/cm at 90°C for 30 min in an incubator (Digital Control Steel Door Incubator 10–180E, Quincy Lab).
2.6. PEPS insulation
A PEPS was electrically insulated to stabilize the resonance peaks for in-liquid detection by an optimized 3-mercaptopropyltrimethoxysilane (MPS) (Sigma) solution deposition method.(Kirimli et al. 2014, 2015) First, a PEPS was cleaned in a 1-in-100 diluted Piranha solution (two parts of 98% sulfuric acid (Fisher) with one part of 30% hydrogen peroxide (Fisher)) for 1 min followed by DI water and ethanol rinsing. It was then soaked in a 0.1 mM MPS solution in ethanol with 0.1% DI water for 30 min followed by soaking in a 0.1% MPS solution in ethanol with 0.5% DI water at pH 9. The MPS solution was replaced with a fresh one every 12 h for 120 h or until the PEPS achieved a standard deviation of the WEM resonance frequency of < 20 Hz and a downshift of the WEM resonance frequency of < 20 Hz in phosphate buffer saline (PBS) for 30 min. For each MPS solution replacement, the PEPS was first rinsed with DI water and followed by rinsing in ethanol before immersing in a fresh MPS solution.
2.7. PEPS regeneration and repeatability
There were 3 PEPSs used in total for all the detection tests conducted in this study: two used for EC and one for CD. After each detection, a PEPS was regenerated for reuse. First, a PEPS was cleaned in a 1-in-100 diluted Piranha solution for 1 min followed by DI water and ethanol rinsing. It was then soaked in a 0.1% MPS solution in ethanol with 0.5% DI water at pH 9 for MPS coating. The MPS solution was replaced with a fresh one every 12 h for 24 h or until the PEPS achieved a standard deviation of the WEM resonance frequency of < 20 Hz and a downshift of the WEM resonance frequency of < 20 Hz in PBS for 30 min.
2.8. Probe immobilization
Once both the standard deviation and the downshift of a PEPS’s WEM resonance frequency in PBS were less than 20 Hz for 30 min it was ready for probe immobilization. The probe was covalently immobilized on a PEPS surface using sulfosuccinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (Sulfo-SMCC) (Pierce) as a bi-functional linker (Kirimli et al. 2015)--The N-hydroxysuccinimide (NHS) on one end of the SMCC reacted with the amine group at the 5’ end of the probe while the maleimide on the other end of the SMCC reacted with the thiol group of the MPS insulation layer on the PEPS surface thereby covalently immobilize the probe on the PEPS surface (Kirimli et al. 2015). This was achieved by first dipping the PEPS in 300 μl of PBS with 1 mg of Sulfo-SMCC for 30 min followed by washing in PBS solution for 2 min to immobilize SMCC on the PEPS surface. It was then dipped in a 10−8 M probe solution in PBS for 30 min followed by washing in PBS solution for 2 min to complete the probe immobilization.
2.9. Bacterial detection in diarrheal stool
The stool samples—including both CD patient stools and simulated diarrheal (SD) stools spiked with EC--were first sieved with a 1-mm sieve (Fisher) to remove chunks 1 mm or larger that might clog the tubing. The detection was carried out with a SD stool volume of 10 mL spiked with 3% sodium dodecyl sulfate (SDS) (Sigma) and 3% bovine serum albumin (BSA) for EC detection and 10% SDS and 3% BSA for CD detection. The reason CD stool was spiked with more SDS was that CD formed spores and was harder to break open to expose the DNA. The SD stool sample was loaded in the reservoir at 95°C for 10 min to lyse the bacteria and denature the double-stranded DNA. The SD stool was then passed in 0.5-1 m ethyl vinyl acetate (EVA) tubing of a 0.8 mm inner diameter and a 2.4 mm outer diameter (McMaster Carr) immersed in room-temperature water at a flow rate of 1.5 ml/min to arrive at the detection cell where the probe-coated PEPS was placed at center of the flow with its major faces parallel to the flow. The detection was carried out for 30 min using an AIM 4170C impedance analyzer (Array Solutions) to monitor the WEM resonance spectrum of the PEPS with time. The detection resonance frequency shift with time was obtained as described previously (Kirimli et al. 2014).
2.10. Upstream reporter DNA (urDNA) and downstream reporter DNA (drDNA) for EC
Both urDNA and drDNA were 21-nt long. urDNA was complementary to the tDNA sequence upstream of what was complementary to the probe while drDNA was complementary to the tDNA sequence downstream of what was complementary to the probe. urDNA, amine-(CH2)12-5’-GAG CAA AAT TTA TAT GTG-3’ (Sigma) was amine-activated with a 12-carbon spacer at the 5’ end and drDNA, 5’-ACA AAT ACT TTC TAC CGT TTT-3’-(CH2)7-Amine (Sigma) was amine-activated with a 7-carbon spacer at the 3’ end. Tm for the binding of urDNA to tDNA was 51°C and that for drDNA to tDNA was 54°C, both of which were much higher than room temperature at which FRMs detections were carried out.
2.11. FRMs validation
FRMs were carboxylated microspheres 6 μm in size emitting blue light (Bright Blue (BB) (≅ Coumarin, Polysciences) with the excitation maximum at 360 nm and the emission maximum at 407 nm. For EC genetic detection validation, FRMs were covalently conjugated with 50% urDNA and 50% drDNA by reacting the amine group of the urDNA and drDNA to the carboxyl group on the FRM surface in the presence of N-(3-dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC) and NHS as described before (Kirimli et al. 2015). After the detection of stx2 of EC in SD stool spiked with 3% SDS + 3% BSA, 10 ml of FRMs at 105/ml in PBS with 3% SDS+3% BSA at room temperature was circulated with a flow rate of 2 ml/min for 30 min for FRMs detection. The fluorescent images of the FRMs captured on the PEPS surface after detection was obtained with an Olympus BX51 microscope.
2.12. TC testing of CD
Stool culture followed by enzyme immunoassay (EIA) was the standard criterion for determining the existence of C. difficile and its toxigenicity in stool. 500 μl of a thawed fecal specimen were placed directly into 5 ml of CCMB-TAL broth (cycloserine-cefoxitin-mannitol broth with taurocholate and lysozyme; AS-8216; Anaerobe Systems). 1 ml of the stool-containing CCMB-TAL broth was then mixed with an equal volume of 95% ethanol for 1 hr. 100 μl of the alcohol-shocked CCMB-TAL broth suspension was then plated on a CCFA-HT agar plate (cycloserine-cefoxitin-fructose agar with horse blood and taurocholate; AS-2136; Anaerobe Systems) to incubate anaerobically at 37°C for up to 7 days and observed daily for growth. AnaeroPack Rectangular Jar (Thermo Scientific; R685070) and AnaeroPack-Anaero (Thermo Scientific; R681001) were used to generate the anaerobic environment. Colonies on the CCFA-HT agar plate were subcultured in chopped meat (CM) broth (Anaerobe Systems) anaerobically at 37°C for 24 h for EIA and plated on a CDC plate (CDC Anaerobic Blood Agar; AS-646; Anaerobe Systems) anaerobically at 37°C for 4 days for microbiological identification. If multiple types of colonies were found on the CCFA-HT agar plate, each type of colonies was Gram stained to identify the Gram-positive types. Each Gram-positive type of colonies was subcultured in a separate CM broth anaerobically at 37°C for 24 h for separate EIA testing of each Gram-positive type of colonies. Each Gram-positive type of colonies was also plated on a separate CDC plate anaerobically at 37°C for 4 days for microbiological identification.
2.13. EIA
Each subculture in a CM broth was first filtered using a syringe with a filter with 0.45-μm pores (Fisher Scientific, 09-719B). The filtrate was then tested with the commercial C. diff Quik Chek Complete (Alere) EIA test per Vendor’s instructions to determine the presence of CD by detecting GDH antigen and its toxigenicity by detecting toxins A and B.
2.14. qPCR for CD patient samples
CD qPCR (Cepheid) were conducted by the Microbiology Laboratory of Hahnemann University Hospital as part of the patient care. The qPCR status of the clinical samples were unblended to the personnel conducting the PEPS tests only after all the PEPS predictions were made public.
3. RESULTS and DISCUSSIONS
3.1. In Situ Genetic Detection of EC Spiked in Stool
To create simulate diarrheal stool for all detections described below, we diluted solid stool of a healthy individual 10-fold in PBS solution as simulated diarrhea stool (stool thereafter unless otherwise specified). For model detection studies, we spiked EC (ATCC 43895) as a model organism in simulated diarrheal stool and detected the spiked EC by its Shinga-like toxin 2 (stx2) gene as the tDNA. We detected spiked extracted baDNA from EC as well as spiked 100-nucleotide (100-nt) synthetic single-stranded tDNA (Sigma) for comparison.
3.1.1. Detection of spiked ssDNA and baDNA in stool for comparison
Before detecting the tDNA directly from EC bacteria spiked in stool we carried out detection of spiked ssDNA and spiked EC baDNA in stool first for comparison to determine the effectiveness of the bacteria lysing scheme and that of the de-hybridization scheme. For ssDNA and baDNA detection, similarly, a probe-coated PEPS was pre-treated and blocked with 5% BSA in PBS and the stool to be tested was also spiked with 5% BSA to block non-specific binding. The ssDNA or baDNA-added, BSA-spiked stool was similarly first loaded in the 95°C reservoir for 10 min to de-hybridize the baDNA followed by flowing the stool through a 50-cm long tubing of a 0.8-mm inner diameter surrounded by room-temperature water. Note that the time it took for the stool to reach the detection cell was 15 s. A probe-coated PEPS was pre-treated with 5% BSA for 30 min and was vertically situated at the center of the flow with its two major faces parallel to the flow (Fig. 1(b)) to detect the ssDNA or the de-hybridized baDNA. After exiting the detection cell, the stool continues to flow back to the reservoir in narrow tubing to form a close loop. Note at a clinically relevant concentration of 10−18M (or 600 copies/ml), there would only be 3 copies of DNA within the 50-cm long tubing. This indicates that most of the DNA would most likely be in single file within the 50-cm long tubing thus, remaining single-stranded when reaching the detection cell and thus permitting detection by the probe-coated PEPS. Indeed, the detection resonance frequency shift of baDNA (ΔfbaDNA) at t = 30 min at 600 copies/ml was found to be 80% of the detection frequency shift of 100-nt long ssDNA (ΔfssDNA) at the same concentration and flow conditions (see the supplemental information), confirming that the above de-hybridization scheme was indeed effective. Using the above detection scheme, the detection Δf/f versus time at various baDNA concentrations and the corresponding −Δf/f at t = 30 min are shown in Figs. 2(a) and 2(b), respectively. Clearly, the −Δf/f at t = 30 min of 60-600,000 copies/ml were all different from that of 0 copies/ml. Also shown in Fig. 2(a) was the detection result of the baDNA of Escherichia coli (Migula) (ATCC25922) (wild type) in stool at 6×107 copies/ml and human gene in stool at 6×105 copies/ml. Note that there was no observable Δf/f for the wild type or the human gene at these high concentrations. The standard deviation of Δf/f at 0 copies/ml was 1.2×10−6. The convention is to use the standard deviation at 0 copies/ml as noise (N). Using the average −Δf/f at t = 30 min for each concentration as signal (S), we obtained S/N = 11, 24, 56, and 139 at 60, 600, 6000, and 60,000 copies/ml, respectively, all well above the commonly accepted threshold of S/N=3.
Fig. 2:
(a) Δf/f versus time of double-stranded bacterial DNA detection in stool at 50°C at various concentrations—also included are the negative control (blank stool with 5% BSA) (full circles) and the wild type at 6×107 copies/ml (open circles), and (b) −Δf/f and S/N versus bacterial DNA concentration in stool. The insert in (b) shows the ratio (−Δf/f)Ec/(−Δf/f)BaDNA versus concentration.
3.1.2. In situ lysing EC and detecting stx2 in stool at 150 CFU/ml
Directly spiking EC in stool, simply heating the stool with 5% BSA was insufficient to lyse the bacteria and release the DNA. Following DNA extraction strategies, SDS was added in the stool in addition to BSA to help lyse the bacteria and release the DNA at 95°C. It was found that treating the stool with 3% SDS and 3% BSA could effectively lyse the EC, release and de-hybridize the DNA using the same heating and cooling scheme described above. In addition, after blocking with 3% SDS and 5% BSA in PBS post probe immobilization, a PEPS would exhibit negligible resonance frequency shift in stool treated with 3% SDS and 3% BSA (see supplemental information). Thus, all EC detections were carried out with the stool spiked with 3% BSA and 3% SDS and the PEPS blocked with 5% BSA and 3% SDS in PBS. The resultant −Δf/f at t = 30 min versus EC concentration is also plotted in Fig. 2(b) to compare with that obtained with baDNA. Clearly, the detection −Δf/f obtained from EC spiked in stool closely followed that from the baDNA spiked in stool down to 150 CFU/ml. Note the S/N shown as the right-hand y axis still applied to EC as it shared the same N, which was the standard deviation of average −Δf/f at 30 min of the blank stools. Note in all the detections in Fig. 2(b), the error bars are not shown because they are all smaller than the size of the symbols. The small error bars were correlated with the high S/N values of these detections due to the stringent sensor stability criteria described in Sec. 2.7 before the detection.
3.1.3. In situ validation of EC detection with fluorescent reporter microspheres (FRMs)
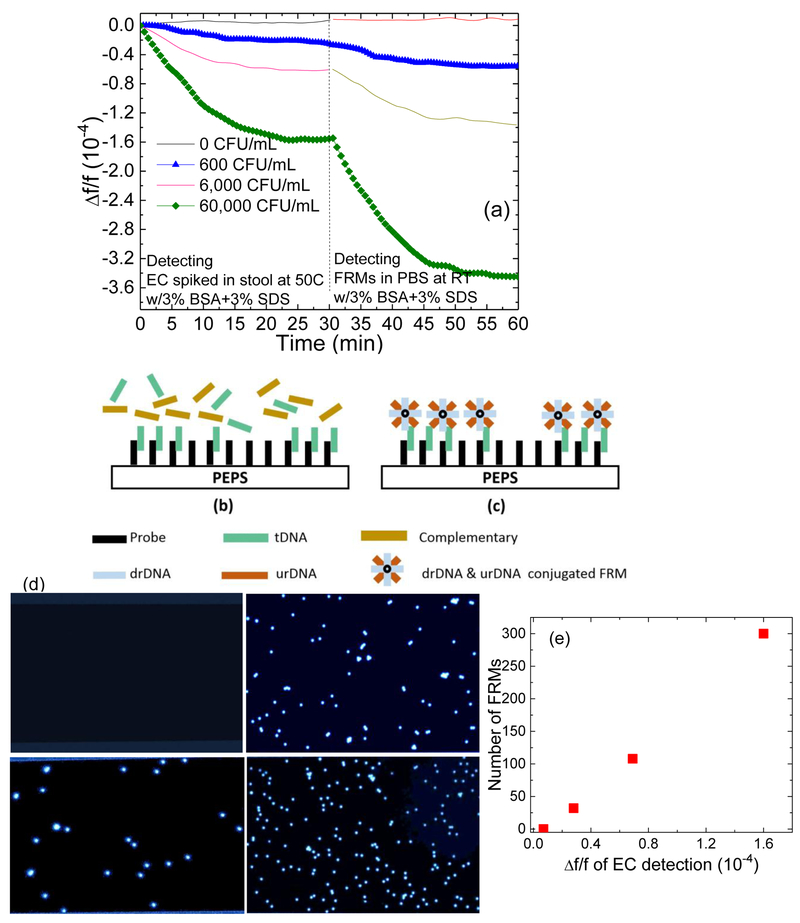
The direct stx2 detection of EC in stool was validated by in situ detecting fluorescent reporter microspheres 6-μm in diameter (Polysciences) following the EC detection. Each FRM was covalently coated with two types of reporter DNAs (rDNAs) 30-nt long complementary to stx2 but different from pDNA (Kirimli et al. 2014, 2015). The urDNA, and the drDNA were respectively complementary to the sequences upstream and downstream. The sequence of the tDNA that was complementary to the probe. After the detection of stx2 of EC in stool with 3% SDS+3% BSA, 10 ml of FRMs at 105/ml in PBS with 3% SDS+3% BSA at room temperature was circulated with a flow rate of 2 ml/min for 30 min. In Fig. 3(a), we show the Δf/f versus time of such EC detection at 0, 600, 6,000 and 60,000 CFU/ml at 50°C followed by FRMs detection in PBS at room temperature. Clearly, −Δf/f of FRMs detection increased with an increasing EC concentration and remained negligible following the detection in blank stool, validating that the Δf/f during EC detection in stool was indeed due to the specific binding of stx2 to the probe on the PEPS surface. A schematic of the tDNA detection by the probe coated on the PEPS surface during the detection in stool at t = 0-30 min in Fig. 3(a) is shown in Fig. 3(b), and a schematic of the following FRMs detection by the captured tDNA on the PEPS surface at t = 30 −60 min of Fig. 3(a) is shown in Fig. 3(c). In an earlier study (Kirimli et al. 2014) we had used a 16 nt pDNA to carry out tDNA detection and the subsequent FRMs detection up to a tDNA concentration of 1−8 M where the detection Δf/f saturated above 10−10 M. Using the method published earlier (Capobianco et al. 2011), the KD for the pDNA-tDNA binding in Fig. 8b of (Kirimli et al. 2014) was estimated as the tDNA concentration at half of the saturated detection Δf/f, which was about 3×10−16 M. Similarly, the KD for the FRMs detection was also estimated to be about 3×10−16M tDMA concentration. The reason that the KD of the tDNA detection and that of the FRMs detection were both 3×10−16M tDNA concentration is that the FRMs detection at a fixed FRMs concentration was only used to illustrate how the amount of tDNA captured by the pDNA on the sensor surface increased with an increasing tDNA concentration. As such, the detection kinetics of the tDNA detection and that of the FRMs detection are both governed by the binding of the tDNA to the pDNA on the sensor surface. Furthermore, for the current tDNA detection and FRMs detection in Figs 3b and 3c the pDNA was much longer than 16-nt. It is known that a longer pDNA is correlated with a lower KD. Therefore, we can reasonably expect the KD for Fig. 3b and 3c should be lower than 3×10−16M.
Fig.3:
Verification of target DNA detection in stool: (a) Δf/f versus time of 30 min genetic detection of EC spiked in stool followed by 30 min of in situ validation FRMs detection at 0, 600, 6,000, and 60,000 CFU/mL, (b) a schematic of the capturing of the targeted DNA (tDNA) by the probe on the PEPS surface during the tDNA detection in stool at t = 0-30 min of (a), (c) the subsequent capturing of the FRMs by the captured tDNA on the PEPS surface at t = 30-60 min of (a), (d) fluorescent micrographs of FRMs captured on the PEPS surface after detection, (e) number of FRMs captured on the PEPS surface versus average Δf/f at 25-30 min of EC detection in stool shown in (a). Note error bars in (e) are not visible because they are smaller the symbols.
3.1.4. Visual validation of PEPS detection of FRMs:
Optical micrographs of the FRMs captured on the PEPS surfaces were visualized under a fluorescent microscope as shown in Fig. 3(d). 0, 32, 108, and 300 FRMs were captured on the PEPS surfaces following the detection of EC at 0, 600, 6,000 and 60,000 CFU/ml in stool, respectively. Note that the number of the FRMs captured on the PEPS surface was proportional to −Δf/f of the EC detection prior to the FRMs detection as shown in Fig. 3(e), further validating that the stx2 detection was specific. Note error bars were not shown in Fig. 3(e) because they were smaller than the size of the symbol.
3.1.5. PCR validation of PEPS EC detection.
In addition, we eluted the captured baDNA by heating the detection cell at 80°C after washing with DI water twice. The eluate was freeze-dried and examined with PCR. In Fig. 4 the PCR products of the eluates obtained with 1000, 10,000, and 100,000 CFU/ml are shown in channels 4, 5, and 6. As can be seen, channels 4, 5, and 6 all showed a band at the amplicon’s length of 200 bp as similar to the positive control (channel 3) and the fluorescent intensity of the band increased with an EC concentration, validating the PEPS detection signals were indeed due to the binding of the EC DNA on the sensor surface.
Fig.4:
Image of PCR product of (1) water and (2) ATCC25922 as negative controls, (3) EC O157:H7 (100ng) as positive control, (4) eluate of detection at 1000 cfu/ml, (5) eluate of detection at 10,000 cfu/ml, and (6) eluate of detection at 100,000 cfu/ml, (7) DNA ladder.
3.1.6. EC quantification in blinded stool samples
The detection protocol was tested with 9 blinded stool samples spiked with extracted EC DNA at various concentrations including two samples containing no DNA (samples 2 and 5) followed by PCR validation as described above. PEPS correctly quantify all bacterial DNA concentrations over the range 102-106 copies/ml as shown in Fig. 5. Note error bars are not shown here because each sample was tested only once. It is worth noting that the nine samples were tested by two individuals. That the bacterial DNA concentrations were obtained correctly by both individuals indicates that PEPS bacterial DNA detection was operator-independent.
Fig.5:
Measured bacterial DNA concentrations compared to the actual concentrations of 9 blinded stool samples spiked with EC. Note samples 2 and 5 contained no baDNA.
3.2. Testing of 40 CDI Patient Stool Samples
For sample availability reasons, the same methodology was applied to detect CD in patient stools. The test was conducted by targeting the tcdB gene in 40 blinded discarded archived patient stools and compared PEPS detection results to qPCR and TC. The CDI patient samples were obtained from archive of the Clinical Microbiology Laboratory of Hahnemann University Hospital. Amine-(CH2)12-5’-CCA AAA TGG AGT GTTA CAA ACA GGT G-3’ was used as the probe to target tcdB of CD which has a melting temperature, Tm=68°C for the binding to tcdB and a Tm=50°C for the binding to the human genome. There was no binding of the probe with the genomes of common gut-flora bacteria. Therefore, we carried out the detection at 58°C to avoid potential binding to human genes. The patient sample was first sieved to remove large chunks to prevent blocking of the narrow tubing. The detection was carried out with a stool volume of 10 ml and with 10% SDS and 3% BSA. Before unblinding, we used a −Δf = 25 Hz as a cutoff. The detection −Δf of the 40 samples are shown in Fig. 6(a) in comparison of the clinical qPCR (Cepheid) predictions with full triangles denoting qPCR positives and open diamonds denoting qPCR negatives. As can be seen, of the 40 PEPS tests, 36 had results agreed with those of qPCR and 4 (samples 17, 22, 28, and 34) had results disagreed with qPCR. Of the samples for which PEPS and qPCR agreed with each other we took the qPCR results as the standard: i.e., qPCR positives as true positives and qPCR negatives as true negatives. Of the four samples for which PEPS and pPCR disagreed each other, we carried out TC tests as the gold standard in which stool samples were cultured and subcultured followed by toxin EIA testing on the cultured samples. The results of the TC tests of these four samples are shown in Fig. 6(b). As can be seen, samples 17 and 22 were positive (as indicated by the presence of the two vertical lines in the EIA test results of these two samples) and samples 28 and 34 were TC negatives (as indicated by the absence of two vertical lines in the EIA test results of these two samples). For these four samples, we took TC positives as true positives and TC negatives as true negatives. For the rest of the samples we took qPCR positives as true positives and qPCR negatives as true negatives. Using such a qPCR/TC combination as the gold standard we plotted the −Δf of the 40 samples in Fig. 6(c) with full circles denoting true qPCR/TC combination positive and open squares denoting true qPCR/TC combination negative. With the stated 25 Hz cutoff, there was only 1 false negative (sample 22) and 1 false positive (sample 28) out of 40 blinded samples, i.e., 95% sensitivity and 95% specificity. For the same 40 stools, qPCR also had one false negative (sample 17) and 1 false positive (sample 34), i.e., also 95% sensitivity and 95% specificity, suggesting that the rapid, label-free PEPS stool genetic test without gene isolation and amplification could achieve similar sensitivity and specificity as qPCR tests.
Fig. 6:
(a) Detection resonance frequency shift, −Δf, of 40 blinded patient stool samples in comparison with qPCR: full triangles as qPCR positives and open diamonds as qPCR negatives where arrows indicate samples 17, 22, 28, and 34 for which PEPS and qPCR results did not agree; (b) toxigenic culture (TC) results of samples 17 and 22 were TC positives as indicated by the presence of 2 vertical lines and samples 28 and 34 were TC negatives as indicated by the absence of two vertical lines; and (c) −Δf of the 40 blinded patient stool samples: full circles as qPCR/TC combination positives and open squares as qPCR/TC combination negatives where qPCR/TC combination means that the positive/negative status of samples 17, 22, 28, and 34 was according to the TC test if a TC tests carried out on these samples and that of the rest of the samples was according to the qPCR tests.
4. CONCLUSION
We have demonstrated rapid, label-free bacterial genetic signature detection directly from stool without DNA isolation or amplification. The methodology was validated by (i) follow-up FRMs detection, (ii) microscopic visual verification of the captured FRMs, (iii) PCR confirmation of the captured tDNA, and (iv) correct quantification of EC in blind stools spiked with EC. The same methodology was applied to detect CD in 40 blind patient stools with the same sensitivity (95%) and specificity (95%) as the comparing commercial qPCR. Even though in this initial study, the laboratory flow setup may seem cumbersome it can be easily miniaturized and automated. In the future, we will publish results with a custom-built automated flow system. This coupled with the high sensitivity and specificity of the PEPS will further illustrate the potential of a PEPS genetic test as a rapid and inexpensive diagnostic tool at point of care for a wide range of applications.
Supplementary Material
Highlights.
Piezoelectric sensor contains a flow system that lyses bacteria, exposes and de-hybridizes the genetic signature of the bacteria.
The genetic signature of bacteria is detected in situ in stool without gene isolation or amplification
The detection is label-free with 150 CFU/ml sensitivity and in 40 min
Toxin B gene of Clostridium difficile was detected in 40 patient stools with 95% sensitivity and 95% specificity
ACKNOWLEDGMENT
This work was supported in part by the National Institute of Health Grants No. 1R41AI112224 and 1R41AI120445 and Pennsylvania Department of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andreu A, Merkert JW, Lecaros LA, Broglin BL, Brazell JT, El-Kouedi M, 2006. Sensors and Actuators B: Chemical 114(2), 1116–1120. [Google Scholar]
- Bartlett JG, Gerding DN, 2008. Clinical Infectious Diseases 46, S12–S18. [DOI] [PubMed] [Google Scholar]
- Cagle PT, Zhai QH, Murphy L, Low PS, 2013. Archives of Pathology & Laboratory Medicine 137(2), 241–244. [DOI] [PubMed] [Google Scholar]
- Capobianco JA, Shih WY, Adams GP, Shih W-H, 2011. Sensors and Actuators B-Chemical 160(1), 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico RM, et al. , 2008. Guide to the Elimination of Clostridium difficile in Healthcare Settings. ASSOCIATION FOR PROFESSIONALS IN INFECTION CONTROL AND EPIDEMIOLOGY. [DOI] [PubMed] [Google Scholar]
- Caruso F, Rodda E, Furlong DF, Niikura K, Okahata Y, 1997. Analytical Chemistry 69(11), 2043–2049. [DOI] [PubMed] [Google Scholar]
- CDC, 2016. [Google Scholar]
- Chang H, Yuan Y, Shi N, Guan Y, 2007. Anal Chem 79(13), 5111–5115. [DOI] [PubMed] [Google Scholar]
- Chapin KC, Dickenson RA, Wu FM, Andrea SB, 2011. Journal of Molecular Diagnostics 13(4), 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-P, Ganguly A, Lu C-Y, Chen T-Y, Kuo C-C, Chen R-S, Tu W-H, Fischer WB, Chen K-H, Chen L-C, 2011. Anal Chem 83(6), 1938–1943. [DOI] [PubMed] [Google Scholar]
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH, 2010. Infection Control and Hospital Epidemiology 31(5), 431–455. [DOI] [PubMed] [Google Scholar]
- Culbreath K, Ager E, Nemeyer RJ, Kerr A, Gilligan PH, 2012. Journal of Clinical Microbiology 50(9), 3073–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmee M, Van Broeck J, Simon A, Janssens M, Avesani V, 2005. Journal of Medical Microbiology 54(2), 187–191. [DOI] [PubMed] [Google Scholar]
- Feng K, Li J, Jiang JH, Shen GL, Yu RQ, 2007. Biosens Bioelectron 22(8), 1651–1657. [DOI] [PubMed] [Google Scholar]
- Gao W, Dong H, Lei J, Ji H, Ju H, 2011. Chem Commun (Camb) 47(18), 5220–5222. [DOI] [PubMed] [Google Scholar]
- Gao Z, Agarwal A, Trigg AD, Singh N, Fang C, Tung CH, Fan Y, Buddharaju KD, Kong I, 2007. Anal Chem 79(9), 3291–3297. [DOI] [PubMed] [Google Scholar]
- Gasparac R, Taft BJ, Lapierre-Devlin MA, Lazareck AD, Xu JM, Kelley SO, 2004. Journal of the American Chemical Society 126(39), 12270–12271. [DOI] [PubMed] [Google Scholar]
- Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J, 1995. Infection Control and Hospital Epidemiology 16(8), 459–477. [DOI] [PubMed] [Google Scholar]
- Ghantoji SS, Sail K, Lairson DR, DuPont HL, Garey KW, 2010. Journal of Hospital Infection 74(4), 309–318. [DOI] [PubMed] [Google Scholar]
- Gifford LK, Sendroiu IE, Corn RM, Luptak A, 2010. J Am Chem Soc 132(27), 9265–9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grys TE, Sloan LM, Rosenblatt JE, Patel R, 2009. Journal of Clinical Microbiology 47(7), 2008–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant RL, Kosek M, Moore S, Lorntz B, Brantley R, Lima AAM, 2002. Arch Med Res 33(4), 351–355. [DOI] [PubMed] [Google Scholar]
- Hahm J.-i., Lieber CM, 2003. Nano Letters 4(1), 51–54. [Google Scholar]
- Hammond DM, Manetto A, Gierlich J, Azov VA, Gramlich PM, Burley GA, Maul M, Carell T, 2007. Angew Chem Int Ed Engl 46(22), 4184–4187. [DOI] [PubMed] [Google Scholar]
- He L, Musick MD, Nicewarner SR, Salinas FG, Benkovic SJ, Natan MJ, Keating CD, 2000. Journal of the American Chemical Society 122(38), 9071–9077. http://blast.ncbi.nlm.nih.gov/. [Google Scholar]
- Husale S, Persson HHJ, Sahin O, 2009. Nature 462(7276), 1075–U1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai E, Ikebukuro K, Hoshina S, Watanabe H, Karube I, 2000. Fems Immunology and Medical Microbiology 29(4), 283–288. [DOI] [PubMed] [Google Scholar]
- Kirimli CE, Shih WH, Shih WY, 2014. Analyst 139(11), 2754–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirimli CE, Shih WH, Shih WY, 2015. Analyst 140(5), 1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkina T, Vlandas A, Ahmad A, Kern K, Balasubramanian K, 2011. Angew Chem Int Ed Engl 50(16), 3710–3714. [DOI] [PubMed] [Google Scholar]
- Loaiza OA, Campuzano S, Pedrero M, Pividori MI, Garcia P, Pingarron JM, 2008. Anal Chem 80(21), 8239–8245. [DOI] [PubMed] [Google Scholar]
- Mao X, Yang L, Su XL, Li Y, 2006. Biosens Bioelectron 21(7), 1178–1185. [DOI] [PubMed] [Google Scholar]
- Moody J, Cosgrove SE, Olmsted R, Septimus E, Aureden K, Oriola S, Patel GW, Trivedi KK, 2012a. Am J Infect Control 40(2), 94–95. [DOI] [PubMed] [Google Scholar]
- Moody J, Cosgrove SE, Olmsted R, Septimus E, Aureden K, Oriola S, Patel GW, Trivedi KK, 2012b. Infection Control and Hospital Epidemiology 33(4), 328–330. [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Lahue BJ, Caro JJ, Davidson DM, 2007. Infection Control and Hospital Epidemiology 28(11), 1219–1227. [DOI] [PubMed] [Google Scholar]
- Pancholi P, Kelly C, Raczkowski M, Balada-Llasat JM, 2012. Journal of Clinical Microbiology 50(4), 1331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Taton TA, Mirkin CA, 2002. Science 295(5559), 1503–1506. [DOI] [PubMed] [Google Scholar]
- Passamano M, Pighini M, 2006. Sensors and Actuators B: Chemical 118(1–2), 177–181. [Google Scholar]
- Rijal K, Mutharasan R, 2007. Analytical chemistry 79(19), 7392–7400. [DOI] [PubMed] [Google Scholar]
- Shih WY, Luo H, Li H, Martorano C, Shih W-H, 2006. Applied Physics Letters 89(24), 242913. [Google Scholar]
- Soleymani L, Fang Z, Kelley SO, Sargent EH, 2009. Applied Physics Letters 95(14), 143701–143703. [Google Scholar]
- Su M, Li SU, Dravid VP, 2003. Applied Physics Letters 82(20), 3562–3564. [Google Scholar]
- Wang J, Kawde AN, Musameh M, 2003a. Analyst 128(7), 912–916. [DOI] [PubMed] [Google Scholar]
- Wang J, Polsky R, Merkoci A, Turner KL, 2003b. Langmuir : the ACS journal of surfaces and colloids 19(4), 989–991. [Google Scholar]
- Wu W, Shih WH, Shih WY, 2016. Journal of Applied Physics 119(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Zhou N, Zhang Y, Zhang W, Jiao K, Li G, 2009. Biosens Bioelectron 24(7), 2165–2170. [DOI] [PubMed] [Google Scholar]
- Zhang GJ, Luo ZH, Huang MJ, Tay GK, Lim EJ, 2010. Biosens Bioelectron 25(11), 2447–2453. [DOI] [PubMed] [Google Scholar]
- Zheng G, Patolsky F, Cui Y, Wang WU, Lieber CM, 2005. Nat Biotechnol 23(10), 1294–1301. [DOI] [PubMed] [Google Scholar]
- Zheng S, Choi JH, Lee SM, Hwang KS, Kim SK, Kim TS, 2011. Lab on a Chip 11(1), 63–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.