Abstract
Structured single-stranded nucleic acids, or aptamers, bind target molecules with high affinity and specificity, which translates into unique therapeutic possibilities. Currently, aptamers can be identified to most proteins, including blood-clotting factors, cell-surface receptors, and transcription factors. Chemical modifications to the oligonucleotides enhance their pharmacokinetics and pharmacodynamics, thus extending their therapeutic potential. Several aptamers have entered the clinical pipeline for applications and diseases such as macular degeneration, coronary artery bypass graft surgery, and various types of cancer. Furthermore, the functional repertoire of aptamers has expanded with the descriptions of multivalent agonistic aptamers and aptamers-siRNA chimeras. This review highlights those aptamers and aptamer-based approaches with particular likelihood of achieving therapeutic application.
Introduction
The first generation of targeted therapeutics has had a notable impact on the treatment of human pathologies. These reagents, such as imatinib (STI571/Gleevec, Novartis), a receptor tyrosine kinase inhibitor (TKI) that targets the kinase domains of abl, c-kit, and platelet-derived growth factor receptor (PDGF-R), treat the diseased tissue and typically avoid harmful side effects that arise from nonspecifically targeting healthy tissue. The first wave of targeted pharmaceuticals, antibodies and small-molecule inhibitors, directly interfere with the function/activity of the disease-driving proteins. An emerging wave of targeted therapeutic molecules is composed of nucleic acids. Generally, these molecules interfere with disease processes at steps preceding protein activity. These nucleic acid-based reagents range from small-interfering RNAs (siRNAs) and antisense oligodeoxynucleotides (ODNs) to viral vectors for gene delivery. Each of these approaches offers unique and creative ways to accomplish the ultimate therapeutic goal, which is to selectively target cells involved in the disease and leave nontargeted tissues unaffected.
DNA and RNA aptamers are distinct from other nucleic acid-based therapies because they usually do not directly affect steps preceding protein function (such as transcription, splicing, RNA processing, translation). Typically, aptamers directly modulate the function of their target proteins, similar to antibodies or small-molecule inhibitors by binding to and regulating the target. In addition to high specificity and affinity for targets, aptamers offer unique advantages as therapeutic reagents, such as amenability to chemical modifications, ease of production, and low immunogenicity. Initial skepticism about using nucleic acids as therapeutic agents has been partially addressed with modifications that increase serum stability and circulating half-life. These modifications are enabling aptamer-based systemic therapeutic applications.
This review focuses on the use of aptamers as therapeutic agents to treat human pathologies. The first section outlines the basic methodologies for identifying aptamers specific for their targets and the types of proteins that aptamers have been successfully selected against. The second section discusses alterations that have been made to aptamers to increase their therapeutic potential, such as modifications that promote resistance to serum nucleases. The third section explores the different types of aptamers reported to date, including inhibitory, agonistic, and regulatable aptamers, as well as aptamers that are used to deliver secondary reagents to specific cell types. The final section gives a perspective on roadblocks that must be overcome for aptamer technology to reach its full clinical potential.
Overview of Aptamer Selection
Aptamers are synthetic, highly structured, single-stranded DNA or RNA ligands. The term “aptamer” literally means “to fit” (aptus) in Latin (Ellington and Szostak, 1990), which reflects 2 important properties of aptamers: the ability to fold into complex tertiary structures and to bind with high affinity (low nM to high pM equilibrium dissociation constants) and specificity to their targets. Two separate groups in the early 1990s recognized the therapeutic value of using nucleic acids to modulate protein function. These groups pioneered methods to isolate aptamers using combinatorial RNA libraries (Ellington and Szostak, 1990; Tuerk and Gold, 1990).
In vitro SELEX
Isolation of aptamers specific for a target of interest involves iterative rounds of a process termed SELEX (systematic evolution of ligands by exponential enrichment; Tuerk and Gold, 1990) (Fig. 1). For the SELEX process, an aptamer library is incubated with a protein target. The protein-bound aptamers are then specifically recovered. These sequences are amplified with PCR or RT-PCR. Single-stranded RNA or DNA sequences representing the recovered sequences are then generated from these PCR products, and used in the subsequent selection round. As we near the 20-year mark for the description of SELEX, most aptamers are still selected by this traditional in vitro methodology using recombinant proteins as the target, though technologies such as capillary electrophoresis, microfluidic channels, and automation are being applied to SELEX (Cox et al., 1998; Mendonsa and Bowser, 2004; Berezovski et al., 2005; Eulberg et al., 2005; Lou et al., 2009). One goal of these alternative selection approaches is to streamline the selection process, thereby decreasing it from many iterative rounds (months) to a few or even one single round of selection while maintaining the stringent criteria of specificity and high affinity for targets.
FIG. 1.

Schematic of (in vitro) SELEX technology. The RNA/DNA aptamer library contains a variable region of 20–40 bases flanked by 2 constant regions. These constant regions include primer sites for PCR/RT-PCR amplification. The library is incubated with the target (typically recombinant protein) and then bound aptamers are recovered and amplified. Iterative rounds are carried out to obtain a focused library of aptamers with high affinity and specificity for the target of interest.
Cell-based SELEX
Isolating aptamers against cell-surface receptors presents other challenges when performing selections in vitro with recombinant proteins, such as replicating the native conformation and glycosylation pattern of the extracellular regions of proteins. Recently, multiple groups have reported selections using living cells as the target to identify receptor-specific aptamers and aptamers that bind to a specific cell type (Blank et al., 2001; Daniels et al., 2003; Cerchia et al., 2005; Shamah et al., 2008) (Fig. 2). The cell-based approach might prove valuable in identifying new markers for different types of cancer and other diseases. Some of the aptamers that have arisen from these types of selections, such as the tenascin-C and RET tyrosine kinase receptor aptamers (Daniels et al., 2003; Cerchia et al., 2005), are discussed in detail below. These methods, however, are still in their infancy relative to the original in vitro SELEX method, and there is some concern, recently presented by Ellington and colleagues, regarding the target specificities of some of the aptamers found with these cell-based selections (Li et al., 2009). Regardless, any new methods to yield aptamers with high specificity and affinity for their targets will greatly augment the SELEX technology.
FIG. 2.
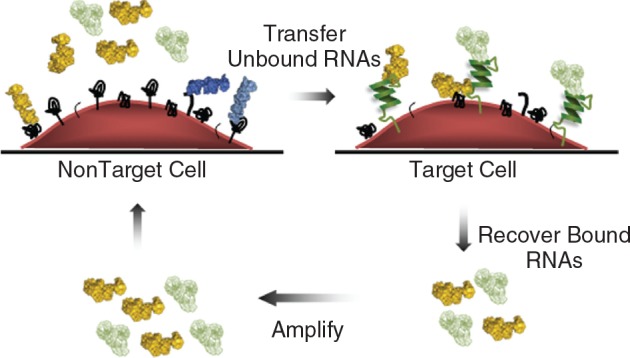
Cell-SELEX. The RNA/DNA library is generated as in Figure 1. In general, the library is first incubated with a nontarget cell in a preclear/counter-selection step, reviewed by Shamah et al. (2008). Unbound aptamers are then recovered and transferred to the target cell, which may either express a particular target or be a target cell type (eg, cancer cells). Bound aptamers are recovered and amplified as in traditional SELEX.
In addition to cell-based selections, several groups have attempted selections against unicellular organism such as parasites and bacteria. For example, Goringer and colleagues successfully isolated RNA aptamers that recognize African trypanosomes using live parasites (Homann and Goringer, 1999). This represents the first selection against a whole-cell organism. While the anti-African trypanosome aptamer has been chemically modified to increase stability (Adler et al., 2008), the activity of this reagent in an animal model has not yet been evaluated. An aptamer that recognizes Mycobacterium tuberculosis has also been recently identified using whole-organism SELEX (Chen et al., 2007). These aptamers have the potential to function as markers on the surface of the target parasite or bacterium, and as such might be modified to function as novel drugs against these unicellular organisms. Whether these selections can be easily adapted to isolate aptamers targeting multicellular organisms or specific tissue types/organs remains to be determined.
Modifications of Aptamers for Clinical Applications
DNA and RNA aptamers intended for clinical applications are usually modified in order to optimize their pharmacokinetic (PK) and pharmacodynamic (PD) profiles and to promote their safety (eg, render them nonimmunogenic). The modifications used to optimize the PK and PD profiles of oligonucleotides have been extensively characterized and validated for antisense ODNs (Matteucci, 1997; Manoharan, 2002; Jason et al., 2004). Many of these chemical modifications have also been applied to synthetic siRNAs without significant loss of function. These include alterations to the backbone and to the side chains of the nucleic acids. A recent review by Behlke describes, in depth, the various chemical modifications, including successes and failures, that have been explored with siRNAs (Behlke, 2008). Another review by Keefe and Cload discusses the use of modified nucleotides in aptamers (Keefe and Cload, 2008). For brevity, only a handful of modifications are covered in this review.
The most typical modifications are the substitution of either a fluoro or an O-methyl (O-Me) group for the 2′-hydroxyl of the ribose moiety (Pieken et al., 1991; Burmeister et al., 2005). These modifications are designed to increase resistance to nucleases, which serves to enhance the PK profile of aptamers in vivo. In fact, the first bench-to-bedside FDA-approved aptamer, pegaptanib (Macugen, Eyetech Pharmaceuticals/Pfizer) contains 2′-fluoro-modified pyrimidines and 2′-O-Me-modified purines (Ruckman et al., 1998). Most groups that use SELEX to identify aptamers from combinatorial RNA libraries rely on in vitro transcription to generate each pool of RNA aptamers but, unfortunately, T7 RNA polymerase, the viral polymerase most commonly used for these transcriptions, inefficiently incorporates such modified nucleotides. Importantly, mutations have been engineered into T7 polymerase that promote incorporation of 2′-fluoro and 2′-O-Me nucleotides more efficiently than the wild-type polymerase (Huang et al., 1997; Chelliserrykattil and Ellington, 2004). With these tools in hand, many groups include 2′-fluoro or 2′-O-Me nucleotides into their RNA libraries and each round of SELEX. In contrast, chemical synthesis of aptamers allows for addition of a wider variety of modified nucleotides, which can be introduced during the post-selection optimization process. For example, the pegaptanib aptamer, which targets vascular endothelial growth factor (VEGF), was originally selected using a 2′-fluoro pyrimidine RNA library. This aptamer was subsequently modified to include 2′-O-Me purines during post-selection chemical synthesis (Ruckman et al., 1998). However, each post-selection modification requires additional characterization as many modifications can alter the critical properties of aptamers.
Examples of other types of modified nucleic acids include locked nucleic acids (LNA) and spiegelmers. LNAs contain a methylene bridge connecting the 2′-O to the 4′-C, and substitution of LNAs into oligonucleotides increases the stability of base pairing, which in turn increases the stability of the duplex and resistance to nucleases (Schmidt et al., 2004). Spiegelmers do not contain additional groups added to the sugar moieties but instead are enantiomers of natural nucleic acids (reviewed by (Eulberg and Klussmann, 2003)). In other words, the natural d-nucleic acids are substituted with enantiomeric l-nucleic acids. This property prevents recognition by nucleases, thereby increasing the stability of the aptamer.
A hurdle to administering aptamers to patients for many therapeutic applications is a short circulating half-life due to the small size of RNA and DNA aptamers. While a low molecular weight can be an advantage as it allows economical chemical synthesis and better target accessibility of aptamers for therapeutic purposes, it promotes rapid clearance by the renal system. By simply increasing the molecular weight of the aptamer, the circulating half-life can be extended significantly (Healy et al., 2004; Boomer et al., 2005). The most common method to increase the size of the aptamer is to add a polyethylene glycol (PEG) moiety. For example, pegaptanib is conjugated to a 40-kDa PEG to decrease renal clearance (Ruckman et al., 1998). Addition of cholesterol to aptamers is another approach to enhance the PK profile of aptamers (Willis et al., 1998), and this moiety has been appended to REG-1, an aptamer against factor IXa, a component of the coagulation cascade (Rusconi et al., 2004). However, 1 study reported rapid plasma clearance of a cholesterol-conjugated aptamer compared to an unconjugated aptamer (Healy et al., 2004). The specific modifications to individual aptamers that improve PK and PD are highlighted in the following sections.
Functional Classes of Aptamers
To date, many groups have successfully identified aptamers with a variety of functions, including inhibitory and decoy-like aptamers, regulatable aptamers, multivalent/agonistic aptamers, and aptamers that act as delivery vehicles. Each of these classes of aptamers has potential applications in therapeutics and/or diagnostics. In the case of agonistic aptamers, regulatable aptamers, and aptamers for delivery, alterations were made to the original aptamers to achieve the desired function. For the purpose of this review, special focus will be placed on aptamers that have entered the clinical pipeline or demonstrate exceptional therapeutic potential based on preclinical studies (Table 1).
Table 1.
Aptamers in the Clinical Pipeline
| Aptamer, target | Company | Disease(s) | Stage of clinical development |
|---|---|---|---|
| Pegaptanib (Macugen), VEGF-165 | Eyetech Pharmaceuticals/ Pfizer | AMD, diabetic retinopathy | FDA-approved AMD 2004; Phase II for diabetic retinopathy: improved vision, decreased edema |
| REG-1 (RB006/RB007), Factor IXa | Regado Biosciences | PCI, CABG | Phase I: Reversible anti coagulation; Phase II trial completed (results not released) |
| AS1411, Nucleolin | Antisoma | RCC, AML | Phase I: Tumor regression; Phase II underway |
| NU172, Thrombin | Nuvelo/Archemix | PCI, CABG | Phase I: No adverse safety profile; Phase II planned |
| ARC1779, von Willebrand factor | Archemix | TMA, TTP, CEA | Phase I: No adverse effects in healthy patients; Phase II underway |
| ARC183, Thrombin | Archemix | CABG | Phase I: Suboptimal dose profile; withdrawn |
Abbreviations: AMD, age-related macular degeneration; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; RCC, renal cell carcinoma; AML, acute myeloid leukemia; TMA, thrombotic microangiopathies; TTP, thrombocytopenic purpura; CEA, carotid endarterectomy.
Inhibitory aptamers
The list of inhibitory aptamers is growing rapidly, and 1 aptamer has been approved by the FDA for the treatment of a human disease. The catalog of targets is particularly diverse, including soluble proteins, transcription factors, cell-surface receptors, and other intracellular proteins. The most extensively characterized inhibitory aptamer is the RNA aptamer that targets VEGF. This aptamer (also referred to as pegaptanib) was approved by the FDA in December 2004, for the treatment of wet age-related macular degeneration (AMD) and is currently being tested in patients with diabetic retinopathy (Table 1). Ng et al. have published a comprehensive review of the path leading to the therapeutic application of pegaptanib in ocular disease (Ng et al., 2006).
Pegaptanib specifically targets the heparin-binding domain of VEGF-165, a growth factor that promotes the blood vessel formation (vascularization) associated with AMD. In order to translate this aptamer into the clinic, the VEGF-165 aptamer was chemically modified with 2′-fluoro pyrimidines, 2′-O-Me purines, and PEG to generate a better therapeutic agent (Ruckman et al., 1998; Ng et al., 2006). Though PEGylation increases the efficacy of pegaptanib upon intraocular injection, its applicability to additional diseases that would require systemic administration is unclear. In addition to AMD, a Phase II clinical trial also investigated pegaptanib in the treatment of diabetic retinopathy and found improved vision and reduced macular edema (Cunningham et al., 2005). Given that inhibiting vascularization is a major focus for anticancer drugs, pegaptanib is a potential candidate for treatment of solid tumors that are extensively vascularized. Another aptamer that targets abnormal blood vessel growth is the platelet-derived growth factor-B (PDGF-B) aptamer (Green et al., 1996). Data in animal models suggest the PDGF-B aptamer may reduce angiogenesis in a variety of diseases (Akiyama et al., 2006; Sennino et al., 2007).
Following the footsteps of the first FDA-approved aptamer is AS1411 (Antisoma), a DNA aptamer that binds to nucleolin. This DNA aptamer is part of the guanine-rich oligonucleotide (GRO) class of aptamers that form G-quartets, a structural element that exhibits antiproliferative activity (Bates et al., 1999). The selection of AS1411 is distinct from that of other aptamers covered in this review article. A panel of GROs was exposed to cancer cells and screened for anti-proliferative effects (Bates et al., 1999). The aptamer that produced the antiproliferative result was found to associate with nucleolin, a protein that is often overexpressed on the surface of cancer cells. Nucleolin has many functions, so inhibiting this protein with AS1411 affects a variety of signaling pathways, including NF-κB (Girvan et al., 2006) and Bcl-2 (Soundararajan et al., 2008). Quite interestingly, the AS1411 aptamer, whose only modification is a 3′ propyl amino group (Bates et al., 1999), is more resistant to nucleases in serum-containing media compared to phosphorothioate and mixed DNA/2′-O-Me RNA counterparts (Dapic et al., 2002). A Phase I clinical trial of AS1411 in 30 patients with various advanced carcinomas resulted in no serious toxicity and led to tumor regression in 2 patients with renal cell carcinoma (Ireson and Kelland, 2006) and Antisoma Web site (Table 1). Phase II trials of renal cell carcinoma and acute myeloid leukemia are currently underway (see Antisoma Web site).
In addition, showing promise as an anticancer therapeutic is the tenascin-C aptamer (TTA1) that was identified using a cell-based SELEX approach with U251 human glioblastoma cells (Hicke et al., 2001; Daniels et al., 2003). Tenascin-C is expressed on many types of solid tumors, such as glioblastoma, breast, lung, and colorectal cancers, which lend this aptamer wide therapeutic application. The tenascin-C RNA aptamer TTA1 has been extensively modified to increase stability and resistance to nucleases (Schmidt et al., 2004). In preclinical studies evaluating tumor uptake and biodistribution in a human tumor xenograft model, the TTA1 aptamer demonstrated rapid uptake by the tumor (Hicke et al., 2006). The TTA1 aptamer was rapidly cleared by the renal system with a half-life of <2 minutes (Hicke et al., 2006), and thus may be well suited for use as an imaging agent or as a delivery tool. Modifications that would increase its circulating half-life would likely be beneficial for other therapeutic applications.
Aptamers have been identified for several blood-clotting factors, such as von Willebrand factor, thrombin, factor VII, and factor IXa (Bock et al., 1992; Rusconi et al., 2000; White et al., 2001; Rusconi et al., 2002; Muller et al., 2007; Oney et al., 2007). Several of these aptamers, particularly the factor IXa regulatable aptamer, show particular promise in preclinical and Phase I clinical trials (Table 1). The factor IXa aptamer is discussed in detail in the “Regulatable Aptamers” section. The goal of a majority of these aptamers is to prevent coagulation during acute cardiovascular procedures. In this scenario, the short half-life of aptamers is ideal as a therapeutic compared to antibodies or small-molecule inhibitors. For example, a thrombin-specific DNA aptamer, NU172 (Nuvelo/Archemix) has been evaluated in Phase I clinical trials for intravenous administration during acute cardiovascular surgery and a Phase II trial is being planned (see Archemix Web site). A second thrombin-specific DNA aptamer, ARC183 (Archemix), that also promotes anticoagulation has been studied extensively in preclinical trials and has undergone a Phase I clinical trial. Unfortunately, the dose required for efficacious anticoagulation was suboptimal for administration during coronary artery bypass graft surgery (see Archemix Web site). ARC1779 (Archemix), which targets von Willebrand factor, is designed to inhibit coagulation in acute coronary syndromes. Phase I clinical trials of healthy patients resulted in no adverse effects (Gilbert et al., 2007) and Phase II trials are ongoing (Archemix Web site).
Activation of many cell-surface signaling receptors in tumorigenesis promotes proliferation and/or survival of tumor cells, and inactivation of these receptors using inhibitory or neutralizing aptamers represents 1 method to halt this aberrant growth. Antagonistic aptamers targeting the cell-surface receptors HER3/ErbB-3 (epidermal growth factor receptor 3) and transforming growth factor β type III receptor (TGFβRIII) have been described (Chen et al., 2003; Ohuchi et al., 2006; Sennino et al., 2007). The aptamers against TGFβRIII and HER3 possess inhibitory activity in vitro. These aptamers have not yet been evaluated in animals. Also, the HER3 aptamer is not protected from nuclease digestion by chemical modifications (Chen et al., 2003), so its use may be limited to the laboratory.
Often in the setting of disease, mutant proteins structurally distinct from their wild-type nonpathogenic counterparts drive the disease pathology. One such example is the tyrosine kinase receptor RET (rearranged during transfection), a receptor that is mutated in inherited multiple endocrine neoplasia (MEN) syndromes (eg, MEN2A and MEN2B) and in familial medullary thyroid carcinoma (FMTC). The extracellular MEN2A mutation promotes constitutive dimerization and activation of the receptor. Cerchia et al. isolated an RNA aptamer that inhibits dimerization and subsequent activation of both the constitutively dimerized MEN2A receptor and the ligand-activated wild-type receptor but not the MEN2B receptor (Cerchia et al., 2005). This aptamer has potential promise as an anticancer therapeutic based on receptor inhibition in cells (Cerchia et al., 2005; Pestourie et al., 2006) and three-dimensional collagen cultures (Vento et al., 2008), but has yet to be evaluated in animal models. This aptamer is 2′-fluoro-modified to promote resistance to nucleases (Cerchia et al., 2005), thus making it more suitable for clinical development.
Aptamers that inhibit their target's function have been selected for diseases other than cancer, such as viral infections. One area of active aptamer development is human immunodeficiency virus (HIV), and aptamers have been identified that either block the function of the HIV reverse transcriptase (Tuerk et al., 1992; Schneider et al., 1995; Burke et al., 1996; Li et al., 2008) or inhibit the glycoprotein gp120, which in turn decreases the infectivity of the virus (Khati et al., 2003; Dey et al., 2005). Other HIV-targeted aptamers that have been used as delivery vehicles are described in detail in “Aptamers as Delivery Tools” section below. An aptamer that recognizes and inhibits the HA1 protein of H5N1 influenza virus has also been reported (Cheng et al., 2008).
Additional potential applications of aptamers include the detection and disruption of the formation of amyloid fibrils that arise in neurodegenerative diseases and renal failure, such as the aptamer that inhibits β-secretase BACE-1 (Rentmeister et al., 2006) and those that recognize amyloid fibril constituents Aβ (Ylera et al., 2002), prion protein PrP (Weiss et al., 1997; Proske et al., 2002; Rhie et al., 2003; Sayer et al., 2004), and β2-microglobulin (Bunka et al., 2007). Each of these aptamers is in early stages of preclinical development as a therapeutic or potential diagnostic tool.
Decoy-like aptamers
One obvious therapeutic application of aptamers is to target DNA- or RNA-binding proteins because these proteins have a natural propensity to bind nucleic acids. By mimicking the target sequence of the proteins, aptamers can act as decoys to inhibit binding of proteins such as HIV-tat, NF-κB, and E2F to their cognate sequences on DNA and thus prevent transcription of target genes. One important distinction is that aptamer decoys are single-stranded ligands in contrast to many decoy ODNs, which are double-stranded duplexes. With some exceptions, the majority of the decoy aptamers were not identified by SELEX. The first aptamer decoy described acts on HIV-tat, a transcriptional co-activator expressed by HIV (Sullenger et al., 1990). A sequence representing TAR, the trans-activation response region responsible for HIV replication, was used as a decoy to sequester HIV-tat, which resulted in a significant decrease in viral replication (Sullenger et al., 1990). Subsequent studies revealed that structural elements within the TAR decoys dictate binding to and inhibition of HIV-tat (Sullenger et al., 1991), and additional TAR decoys that are effective in preventing HIV replication have been identified by several groups (Browning et al., 1999; Yamamoto et al., 2000; Michienzi et al., 2002). In particular, one TAR decoy RNA recognizes HIV-1 and HIV-2-tat isoforms (Browning et al., 1999), thus enhancing its therapeutic possibilities. A second HIV-targeted decoy aptamer that mimics the rev-responsive element to reduce HIV replication was transduced into bone marrow of HIV-1 pediatric patients using a retroviral vector (Lee et al., 1992; Kohn et al., 1999). This clinical trial revealed low levels of the transduced decoy gene in patients after 1 year (Kohn et al., 1999), illustrating that the primary hurdle for the clinical application of this decoy and others is formulating effective delivery methods.
Two RNA aptamers that sequester E2F isoforms have been identified using SELEX (Ishizaki et al., 1996; Giangrande et al., 2007). These aptamers prevent E2F from binding its cognate DNA response element. The mechanisms by which this inhibition occurs have not been elucidated, so it is possible that the RNA aptamers do not directly interfere with the DNA-binding site, but instead alter the conformation of the E2Fs to prevent DNA binding. The 2′-fluoro-modified RNA aptamer developed by Giangrande et al. primarily targets the growth-promoting E2F isoforms (E2F1 and E2F3) without affecting E2F isoforms implicated in vascular smooth muscle cell (VSMC) differentiation and growth arrest (E2F4). In a mouse model of venous bypass graft, the E2F1/3 aptamer effectively inhibited neointimal formation (restenosis) (Giangrande et al., 2007). Interestingly, the authors showed that the E2F aptamer was effective at blocking VSMC proliferation only in the presence of E2F4 activity. This is not surprising in light of the results obtained with edifoligide (Corgentech/Anesiva), a double-stranded DNA decoy with sequence identity to the E2F DNA-binding site that acts as a pan inhibitor of E2F activity (Ehsan et al., 2001; Alexander et al., 2005; PREVENT IV Investigators, 2005; Conte et al., 2006). Indeed, despite initial encouraging safety data, the results from later PREVENT III/IV trials revealed no benefit in patients treated with edifoligide compared to placebo (Alexander et al., 2005; PREVENT IV Investigators, 2005; Conte et al., 2006). Thus, the E2F1/3 RNA aptamer highlights an important advantage of aptamers over other reagents (eg, small-molecule inhibitors, DNA decoys), that is their binding specificity.
Finally, several RNA aptamers have been generated that inhibit the transcription factor NF-κB (Lebruska and Maher, 1999; Cassiday and Maher, 2003; Bassett et al., 2004; Wurster and Maher, 2008). Though no clinical trials have been conducted with these RNA aptamer inhibitors of NF-κB activity, a dsDNA decoy that targets the DNA-binding domain of NF-κB (Averina, Anesiva/Corgentech) has shown promise in a Phase I/II clinical trial for the treatment of eczema/ atopic dermatitis, one of many inflammatory diseases modulated by NF-κB. Given that NF-κB is a key regulatory factor in many human diseases, the NF-κB RNA aptamers may result in powerful therapeutics for treating many human pathologies.
Regulatable aptamers
In some clinical applications, rapid inactivation of a drug would reduce the likelihood of harmful long-term effects. Though unmodified aptamers are rapidly cleared, it is still advantageous, in some cases, to modulate the activity of aptamers. The first so-called regulatable or modulator-controlled aptamer, REG-1 (RB006/RB007, Regado Biosciences), is a 2-part therapeutic agent consisting of an RNA aptamer specific for the coagulation factor IXa (RB006) and a single-stranded RNA oligonucleotide complementary to the RB006 aptamer (RB007) (Rusconi et al., 2002; Rusconi et al., 2004). Aptamer inhibition of factor IXa by RB006 is structurally disrupted by administration of the antidote complementary strand RB007 (Fig. 3).
FIG. 3.

Regulatable aptamer, REG-1. A schematic of the predicted secondary structure of REG-1 aptamer is shown in the left panel. The antidote oligo (middle), is complementary to the 3′ end of the REG-1 aptamer and, when combined with the REG-1 aptamer, promotes unfolding of the aptamer, thus abrogating aptamer interaction with the target, factor IXa. Adapted from Rusconi et al. (2002).
The REG-1 aptamer:antidote therapy has been tested in Phase I and II clinical trials with promising results (Dyke et al., 2006; Chan et al., 2008a, 2008b). Phase Ia and Ib trials demonstrated no adverse effects from the aptamer or antidote alone in healthy patients and patients with stable cardiovascular disease receiving antiplatelet therapy, respectively (Dyke et al., 2006; Chan et al., 2008a). Importantly, these trials reported reversible anticoagulation in both patient groups. A Phase Ic trial revealed that varying doses of the antidote can be used to modulate anticoagulation (Chan et al., 2008b). A Phase II trial of percutaneous coronary intervention, termed REVERSAL-PCI, compared REG-1 with standard heparin therapy during cardiac procedures (Regado Biosciences Web site). The REVERSAL-PCI trial has closed, but results have not yet been released. REG-1 represents a significant advancement as an anticoagulation therapy to prevent clot formation during cardiac surgery, and the aptamer:antidote combination is likely to serve as a model for the development of other regulatable therapies.
Multivalent aptamers
Studies with antibodies revealed that oligomerization of the antibodies can (1) increase their avidity and thus their potency, (2) promote receptor activation, and (3) facilitate receptor internalization. Lis and colleagues first demonstrated multivalent assembly of aptamers as a way to increase the avidity and potency of an antagonist aptamer against B52 in Drosophila (Shi et al., 1999). The first potential therapeutic multivalent aptamer targets cytotoxic T-cell antigen-4 (CTLA-4), a receptor expressed on T cells that negatively regulates T-cell activation (Santulli-Marotto et al., 2003). Gilboa and colleagues postulated that inhibition of CTLA-4 with an aptamer would promote T-cell activation to generate an antitumor immune response. More importantly, by making the CLTA-4 aptamer tetrameric, the immune response was enhanced both in cell culture and in vivo (Santulli-Marotto et al., 2003).
A bivalent aptamer targeting HIV has also been described and consists of 2 separate RNA aptamers that bind to 2 distinct stem-loop structures within the HIV 5′ UTR: the HIV-1 TAR element and the dimerization initiation site (Boucard et al., 2006). Linking the 2 aptamers resulted in a more stable interaction of the dimeric molecule with the target sequences in vitro compared to the binding affinities of the aptamers as monomers (Boucard et al., 2006). Similarly, bivalent aptamers targeting thrombin have been engineered as a way to increase the avidity of the aptamer for its target and enhance the anticoagulation effect (Muller et al., 2007; Kim et al., 2008; Mayer et al., 2009; Tian and Heyduk, 2009).
In the setting of some diseases, however, it may be advantageous to activate a particular receptor. All of the aptamers described thus far are either inert or act in some inhibitory capacity as monomeric molecules. Agonistic aptamers represent a unique substitute for ligand-mediated receptor activation. To date, 2 agonistic aptamers have been described in the literature (Dollins et al., 2008; McNamara et al., 2008). The experiments by McNamara et al. represent the first description of an agonistic aptamer (McNamara et al., 2008). Both reports identify aptamers that activate costimulatory T-cell receptors (4–1BB and OX40) to activate antitumor immune responses, a goal similar to that of the CTLA-4 inhibitory aptamer. In the study of 4–1BB agonistic aptamers, the monomeric 4–1BB aptamers displayed no co-stimulation of T cells in vitro, which led the investigators to explore dimerization as a mode to simulate a 2:2 receptor:ligand stoichiometry (McNamara et al., 2008). The dimerized 4–1BB aptamer led to co-stimulatory activity in vitro as demonstrated by increased proliferation of CD8+ T cells and interferon γ (IFNγ) secretion. Importantly, intratumoral injection of the 4–1BB agonistic aptamer led to complete regression of mastocytoma tumors in almost half of the mice treated. Furthermore, a secondary tumor challenge to surviving mice indicates the aptamer-mediated tumor response is due to specific immune activation. Hence, bivalent 4–1BB aptamers represent a potential therapeutic tool to activate a T-cell response against tumors and prevent recurrence of disease after withdrawal of treatment. In order to translate this technology to the clinic however, 4–1BB aptamers specific for the human 4–1BB ortholog must be developed.
Similar to results with the 4–1BB monomeric aptamers, the OX40 aptamers were only active when dimerized (Dollins et al., 2008). The bivalent OX40 aptamer co-stimulated lymph node cells as demonstrated by increased IFNγ secretion and nuclear localization of NF-κB in vitro. To assess the in vivo potential of the OX40 bivalent aptamer, Dollins et al. used a combination dendritic cell (DC) vaccine/OX40 bivalent aptamer in an immunotherapeutic model for melanoma. The data suggest that co-stimulation of T cells by the OX40 dimeric aptamers enhances tumor immunity in this model (Dollins et al., 2008).
In a recent report, bivalent aptamers against the prostate-specific membrane antigen (PSMA) were used to enhance receptor internalization, thereby facilitating delivery of cytotoxic siRNAs specifically to PSMA-positive prostate cancer cells (Wullner et al., 2008). In this report, the bivalent aptamer:siRNA chimera was evaluated only as a delivery tool, so it is not clear whether aptamer-mediated multimerization of the PSMA receptor also affects receptor function. Interestingly, the data indicate a 2-fold increase in avidity for the bivalent aptamer:siRNA chimeras compared to monovalent aptamer:siRNA chimeras (Wullner et al., 2008).
Approaches used to multimerize aptamers
While the overall construction of the various multivalent aptamers differs from group to group, most groups used noncovalent approaches to assemble the dimeric or tetrameric aptamers (Santulli-Marotto et al., 2003; Dollins et al., 2008; McNamara et al., 2008). The CTLA-4 tetravalent aptamer was generated by first constructing a DNA linker that would simultaneously anneal to 4 aptamers (Santulli-Marotto et al., 2003). As depicted in Figure 4A, the center of the DNA linker contains a double-stranded (ds) region, while the flanking regions contain sequences complementary to the 5′ end of the CTLA-4 aptamer. The length of the dsDNA center sequences was designed to allow 2 helical turns. This rotation should allow binding of the annealed aptamers to adjacent CTLA-4 receptors in the proper orientation.
FIG. 4.
Multivalent aptamers. (A) The CTLA-4 tetravalent aptamer was assembled by annealing the 5′ end of each aptamer to a dsDNA linker (grey) with regions complementary to the aptamer. Adapted from Santulli-Marotto et al. (2003). (B) The 4–1BB bivalent aptamer was generated by adding 21 complementary nucleotides to the 3′ end of each aptamer. The 2 complementary sequences base pair (grey) to link the 2 aptamers. Adapted from McNamara et al. (2008). (C) To assemble the OX40 bivalent aptamer, a single-stranded DNA scaffold (light grey) complementary to the 3′ tail of each aptamer was generated. Two DNA scaffolds were connected by an 18-carbon PEG linker (dark grey), which provides flexibility to the structure. Adapted from Dollins et al. (2008). (D) Covalently linked aptamers, such as the thrombin aptamers depicted here, have been joined with flexible PEG-based phosphoramidite linkers (grey) of varying lengths. Adapted from Kim et al. (2008) and Tian and Heyduk (2009).
The approach taken by McNamara et al. to generate the bivalent 4–1BB aptamers is relatively simple in design. As shown in Figure 4B, complementary sequences were added to the 3′ end of each 4–1BB aptamer to yield a 21-bp RNA linker region (McNamara et al., 2008). The 2 aptamers were then annealed to each other similarly to the way in which the 3′ ends of the CTLA-4 aptamers anneal to the dsDNA linker. Consistent with the design of the dsDNA linker, the 4–1BB RNA linker is an optimal length (21 nt) to position each aptamer with the binding interface in the same orientation. One advantage of this simple design is the ease of production of an all-RNA molecule.
To generate the OX40 dimeric aptamer, a DNA scaffold was created as shown in Figure 4C. The scaffold is similar to the CTLA-4 scaffold (Fig. 4A) with the use of dsDNA and a 5′ single-stranded flanking region with sequences that anneal to the 3′ end of the OX40 aptamer. However, the OX40 scaffold contains 2 dsDNA regions separated by a PEG spacer (Dollins et al., 2008). This linker structurally separates the annealing sites of the 2 aptamers to increase flexibility of the molecule, which allows simultaneous binding of each aptamer to its target receptor.
Wullner et al. evaluated 2 different approaches to generating bivalent PSMA aptamer:siRNA chimeras, and showed similar results with both approaches (Wullner et al., 2008). In one, the siRNA served as a dsRNA linker to join the 2 PSMA aptamers. The second design used a dsRNA (siRNA) to link the 2 aptamers, and a second siRNA was appended to the 3′ end of 1 aptamer to deliver 2 different siRNAs to the target cells.
In contrast to the noncovalent approaches to multimerization, covalent approaches have also been investigated (Boucard et al., 2006; Muller et al., 2007; Kim et al., 2008; Tian and Heyduk, 2009). In a recent study by Tan and colleagues, 2 different thrombin-specific aptamers were joined by a flexible phosphoramidite linker (Fig. 4D) (Kim et al., 2008). While only one of the aptamers effectively inhibits thrombin activity, binding of the second “inert” aptamer serves to increase the association of the first aptamer with the target, thus increasing the potency of the first inhibitory aptamer (Kim et al., 2008). This study also described an antidote to reverse the anticoagulation effect using a DNA strand complementary to the inhibitory aptamer, comparable to the design of REG-1 (Kim et al., 2008). Similar results were obtained by a second group that used a phosphoramidite-PEG linker to assemble the thrombin bivalent aptamer (Tian and Heyduk, 2009).
The HIV bivalent aptamers were also conjugated together using 2 hexaethylene glycol phosphate units (Boucard et al., 2006). For therapeutic purposes, the flexible linker approach has important advantages over nucleic acid-based linkers, including (1) a covalent attachment, (2) flexible linker, and (3) minimization of nucleic acid sequences that may give rise to nonspecific targeting and/or immunostimulation.
Aptamers as delivery tools
Several groups have reported linking siRNAs to aptamers as a way to specifically deliver siRNAs to target cells (Fig. 5C). Since delivery of siRNAs represents the major hindrance in translating this powerful technology into the clinic, such targeted delivery approaches may have an important impact on this field. Aptamers are also being utilized to deliver toxins, radioisotopes, and chemotherapeutic agents encapsulated in nanoparticles. Specific delivery of these therapeutic agents offers several benefits over nontargeted treatment. First, targeted delivery will reduce harmful side effects associated with injury to normal cells and tissues. Second, the amount of drug required to elicit a response may also be significantly decreased with targeted delivery. This could translate to lower costs for production and treatment as well as a reduction in off-target effects.
FIG. 5.
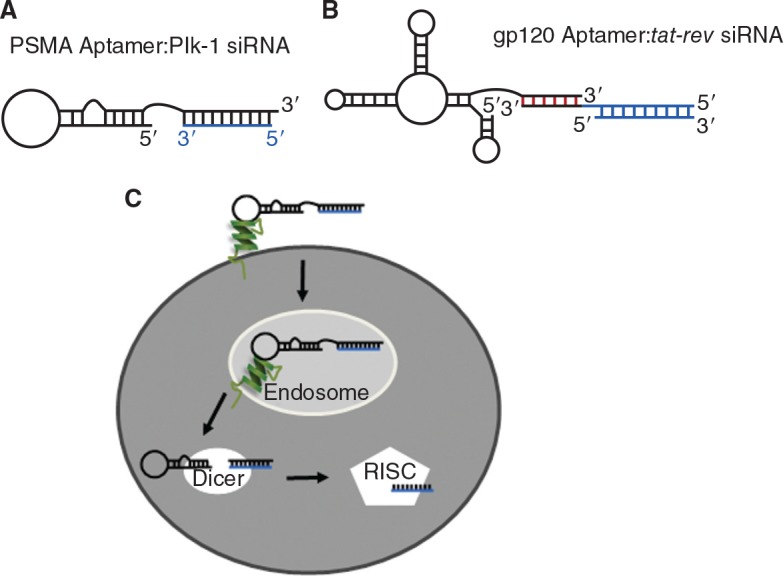
Aptamer:siRNA chimeras. (A) The PSMA aptamer:Plk-1 siRNA chimera was generated by extending the 3′ end of the PSMA aptamer to contain 21 nucleotides complementary to the antisense strand of the Plk-1 siRNA (blue). The chimera was formed by annealing the PSMA aptamer to the Plk-1 siRNA antisense strand. Adapted from McNamara et al. (2006). (B) To create the HIVgp120: tat-rev siRNA chimera, a 16-nt dsRNA “stick” (red) was used as the scaffold to link the gp120 aptamer and tat-rev siRNAs (blue). Adapted from Zhou et al. (2009). (C) Schematic of aptamer:siRNA chimera entry into cells and processing by Dicer and RISC (RNA-induced silencing complex). First, the aptamer portion of the chimera binds to its target and is internalized. The complex escapes the endosome, where it is then loaded into Dicer and processed. The antisense strand is then presented to RISC resulting in silencing of the target mRNA. Adapted from McNamara et al. (2006).
Two aptamers specific for PSMA have been used extensively as a delivery vehicle for siRNAs, drug, and toxins. PSMA is a cell-surface protein that is overexpressed on a majority of primary and metastatic prostate cancer cells. Importantly, PSMA is continually recycled from the plasma membrane, making it an attractive conduit to deliver molecules intracellularly. Lupold et al. selected 2′-fluoro-modified RNA aptamers (A9 and A10) against PSMA using SELEX (Lupold et al., 2002). Three groups have since linked the PSMA aptamers to siRNAs against prosurvival genes in proof-of-concept studies to demonstrate intracellular delivery of siRNAs via aptamer-mediated endocytosis (Fig. 5; Chu et al., 2006b; McNamara et al., 2006; Wullner et al., 2008). The first report of aptamer-mediated siRNA delivery was a proof-of-concept study using 27-mer siRNAs against lamin A/C, GAPDH1, and GAPDH2 (Chu et al., 2006b). A streptavidin module was used to link a 5′-biotinylated A9 aptamer and a 5′-biotinylated siRNA sense strand. The data with the aptamer:siRNA chimeras demonstrate internalization-dependent silencing of lamin A/C and GAPDH in PSMA-positive prostate cancer cells but not PSMA-negative cells (Chu et al., 2006b).
A study by Giangrande and colleagues revealed for the first time therapeutic delivery of siRNAs via an RNA aptamer in an animal model of prostate cancer (McNamara et al., 2006) (Fig. 5A). PSMA aptamers were used to deliver siRNAs against the prosurvival factors Plk-1 and Bcl-2, which are overexpressed at the protein level in prostate cancer. This study found that aptamer:siRNA chimeras promoted death and regression of PSMA-positive cells and tumors, respectively, but not their PSMA-negative counterparts (McNamara et al., 2006). Furthermore, silencing required Dicer, indicating these results were due to RNAi-mediated gene silencing. This approach has served as a platform technology to deliver siRNAs to target cells in other diseases, including HIV (Fig. 5B and C).
Another PSMA:siRNA chimera was designed to silence eukaryotic elongation factor 2 (EEF2), a protein involved in protein synthesis that, when inhibited, results in apoptosis (Wullner et al., 2008). The design of this chimera differs from that of McNamara et al. in that bivalent PSMA aptamers were used to specifically deliver the EEF2 siRNA to PSMA-positive prostate cancer cells. Delivery of the EEF2 siRNA with bivalent aptamers resulted in a near complete loss of cell viability of PSMA-positive cells compared to a 55% decrease with the monovalent aptamer:siRNA chimera (Wullner et al., 2008).
The HIV glycoprotein gp120 represents a second attractive target for aptamer-mediated siRNA delivery. The work by Rossi and colleagues expands on the chimera idea and delivers a double insult to the HIV-infected cells (Zhou et al., 2008; Zhou et al., 2009). Instead of using a relatively inert aptamer like the PSMA aptamers as a delivery vehicle, an inhibitory aptamer against the envelope glycoprotein gp120 (Khati et al., 2003) was selected to deliver an anti-tat-rev siRNA (Zhou et al., 2008). This aptamer:siRNA chimera simultaneously decreases infection by blocking gp120 and prevents replication by silencing tat-rev (Zhou et al., 2008) (Fig. 5B). This dual-function chimera represents a novel therapeutic strategy to halt HIV infection and replication. A subsequent study by the same group selected for additional gp120-specific inhibitory aptamers with higher affinity for the target as well as a diverse binding profile given the variability associated with gp120 in HIV (Zhou et al., 2009). A novel method was introduced to link the siRNA to the aptamer. A “stick” sequence was added to the 3′ end of the aptamer sequence to allow attachment of different and multiple siRNAs to the aptamer (Fig. 5B). The siRNA antisense or sense strand contains a complementary 3′ “stick” sequence to facilitate annealing to the aptamer. Addition of this “stick” sequence enhanced Dicer processing compared to a chimera with a 2 nucleotide UU-linker, and processing was more efficient when the “stick” sequence was added to the 3′ end of the antisense siRNA strand (Zhou et al., 2009). Importantly, the aptamer:siRNA chimera suppressed HIV replication in cultured CEM T cells and primary blood mononuclear cells (Zhou et al., 2009), thus validating this chimera design as a prospective candidate for anti-HIV therapy.
Cardiotoxicity is associated with several types of chemotherapy, particularly anthracyclines. Targeted delivery of chemotherapies is one approach to avoid damage to healthy tissues. Several chemotherapies have been conjugated to aptamers, including doxorubicin (Dox) and cisplatin. Dox contains flat aromatic rings that intercalate into dsDNA, a property that was exploited to form a Dox:PSMA aptamer conjugate (Bagalkot et al., 2006). Studies using PSMA-positive and -negative prostate cancer cell lines demonstrated specific delivery of the Dox:aptamer conjugate to PSMA-positive cells and a significant decrease in cell viability comparable to unconjugated Dox treatment (Bagalkot et al., 2006). Another group covalently conjugated Dox to an aptamer against sgc8c, a protein expressed on the surface of T-cell acute lymphoblastic leukemia cells (Huang et al., 2009). This study also reported a decrease in cell survival in response to specific delivery of Dox to the leukemia cells.
Aptamer-conjugated nanoparticles are a quickly emerging field of therapeutic interest. For example, 2 reports in the literature demonstrate PSMA aptamer-mediated delivery of chemotherapeutics encapsulated in nanoparticles (Farokhzad et al., 2006; Dhar et al., 2008). In the first example, Farokzhad et al. assembled a poly(d,l-lactic-co-glycolic acid)-block-PEG (PLGA-b-PEG) copolymer with the PSMA aptamer attached to the surface, and then used this aptamer:nanoparticle to encapsulate the chemotherapeutic Dox (Farokhzad et al., 2006). When applied intratumorally to human xenograft models of prostate cancer, the biomolecule caused tumor regression of PSMA-positive tumors with no apparent adverse effects (Farokhzad et al., 2006). In a second study, an optimized PSMA aptamer-directed nanoparticle entered target cells in vivo, and the nanoparticle was time-released, an important property for prolonged drug delivery (Cheng et al., 2007). This group then encapsulated cisplatin within the nanoparticle portion of the aptamer:nanoparticle biomolecule and showed decreased cell viability in PSMA-positive prostate cancer cells (Dhar et al., 2008).
Another application of aptamers as delivery tools is for patients with lysosomal storage diseases, such as Gaucher disease. These patients have deficiencies in lysosomal enzymes, and delivery of replacement enzymes via endocytosis is one method to treat these patients. Chen et al. first isolated RNA and DNA aptamers against the transferrin receptor, which is also subject to rapid internalization by endocytosis (Chen et al., 2008). Next, a chimeric molecule was generated by linking the transferrin receptor DNA aptamer to a dephosphorylated α-l-iduronidase enzyme (GS24-dePIdu), and the aptamer:enzyme chimera demonstrated a rescue of activity in α-l-iduronidase-null fibroblasts (Chen et al., 2008). This study is the first to report the rescue of an enzymatic cellular function through aptamer-mediated enzyme delivery.
Aptamers also might serve as a vehicle to deliver toxins to diseased cells and tissues. The PSMA aptamer has been used to expose PSMA-positive prostate cancer cells to gelonin, a ribosomal N-glycosidase that cleaves a glycosidic bond in rRNA, thus blocking protein synthesis (Chu et al., 2006a). Gelonin:PSMA aptamer chimeras were synthesized by adding a cross-linker (N-succinimidyl-3-(2-pyridyldithio)propionate) to the aptamer. The linker contains an amine-reactive NHS ester that forms disulfide bonds with free cysteines in gelonin. In cytotoxic assays using PSMA-positive and -negative prostate cancer cells, the aptamer:toxin conjugate promoted greater toxicity to the PSMA-positive cells (Chu et al., 2006a). A recent publication describes phototoxic aptamers in which the toxin chlorin e6, a heme-like photodynamic agent, is released after activation by light (Ferreira et al., 2009). The aptamer portion is specific for the O-glycan-peptide antigens present on cancer cells of epithelial origin (breast, lung, colon, ovarian, and pancreatic cancers) but not normal epithelial cells. This strategy expands on the idea of toxin delivery first described by Chu et al. and inserts an additional layer of control. By directing the light to a particular organ or region of the body, the off-target effects would potentially be minimized further.
Using aptamers as a mode to deliver radioisotopes to cancer cells presents one method to reduce off-target effects associated with radio-imaging/therapy. Conjugation of the radioisotope 99mTc to an aptamer targeting tenascin-C was characterized for diagnostic purposes in human xenograft models of glioblastoma and breast cancer (Hicke et al., 2006). Importantly, no uptake was observed in tumors negative for tenascin-C. Studies are ongoing to develop similar agents for therapeutic use. The advantages of using aptamers for radio-imaging/therapy are their favorable PK properties (rapid renal clearance), and their exquisite affinity/specificity for their targets. These properties of aptamers promise to deliver high-resolution images (with high signal-to-noise ratios) and decrease the likelihood of nonspecific targeting (reduce toxicity to nontarget tissues).
To date, no group has reported using an aptamer to deliver another aptamer intracellularly, but with the new focus on using receptor-specific aptamers as delivery vehicles, those studies are likely underway. Aptamers selected against intracellular targets (eg, E2F1/3 and NF-κB) as well as decoy aptamers (eg, HIV-TAR decoy) are ideal for cell-specific delivery since this issue has proven to be a road-block for translating them into the clinic (Kohn et al., 1999).
Perspective
Since the first reports of SELEX-identified aptamers in the early 1990s (Ellington and Szostak, 1990; Tuerk and Gold, 1990), the toolbox of aptamers has significantly expanded with targets as diverse as cytokines, cell-surface receptors, and whole organisms. Since aptamers can be selected against most protein targets, the possible therapeutic applications range far and wide. Several of the modifications to aptamers discussed in this article, such as aptamer:siRNA chimeras, multivalent, agonistic, and modulatable aptamers, represent platform technologies that will be applied to many other aptamers in the future.
Great advances have been made to optimize the PK and PD of aptamers to enable delivery in humans. However, most studies to date rely on local administration of the aptamer, such as intraocular injection of pegaptanib for the treatment of AMD, and future optimization studies should focus on chemical modifications that facilitate systemic delivery. Although aptamers are less likely to illicit an immune response compared to protein-based therapeutics, the safety profile of each individual aptamer must be scrutinized. This includes examination of off-target effects due to inhibition/ activation of other proteins with similar structural conformations as the target protein. As is the case for antibodies and small-molecule inhibitors, these studies must be conducted empirically and will differ for each aptamer.
Aptamers also have excellent potential for scientific applications beyond treating patients, including diagnostics and basic science research into the mechanisms of disease. For example, the aptamers that have been identified that recognize specific cell types, such as B-cell lymphoma and T-cell acute lymphoblastic leukemia, would be ideal for diagnostic purposes (Shangguan et al., 2007; Tang et al., 2007). Aptamers may also serve as replacements for antibodies in immuno-histochemistry or enzyme-linked immunosorbent assays (ELISA) to analyze tissue and blood samples. For example, Somalogic has described the development of aptamer arrays for diagnostic applications (Bock et al., 2004; Kirby et al., 2004; Collett et al., 2005). However, the wide applicability of aptamer technology will require advances in aptamer selection methodologies that would enable efficient identification of aptamers for a target of interest. Although aptamers are relatively new to the clinic, their versatility and the many aptamer-based therapeutic approaches discussed suggest this technology will have a substantial impact in patient care in the coming years.
Acknowledgments
The authors thank Drs. James O. McNamara, II, and William Thiel for helpful discussions and assistance with figures.
Author Disclosure Statement
No competing financial interests exist.
References
- ADLER A., FORSTER N., HOMANN M., and GORINGER H.U. (2008). Post-SELEX chemical optimization of a trypanosome-specific RNA aptamer. Comb. Chem. High Throughput Screen. 11, 16–23 [DOI] [PubMed] [Google Scholar]
- AKIYAMA H., KACHI S., SILVA R.L., UMEDA N., HACKETT S.F., MCCAULEY D., MCCAULEY T., ZOLTOSKI A., EPSTEIN D.M., and CAMPOCHIARO P.A. (2006). Intraocular injection of an aptamer that binds PDGFB: a potential treatment for proliferative retinopathies. J. Cell Physiol. 207, 407–412 [DOI] [PubMed] [Google Scholar]
- ALEXANDER J.H., FERGUSON J.T.B., JOSEPH D.M., MACK M.J., WOLF R.K., GIBSON C.M., GENNEVOIS D., LORENZ T.J., HARRINGTON R.A., PETERSON E.D., LEE K.L., CALIFF R.M., and KOUCHOUKOS N.T. (2005). The PRoject of Ex-vivo Vein graft ENgineering via Transfection IV (PREVENT IV) trial: Study rationale, design, and baseline patient characteristics. Am. Heart J. 150, 643–649 [DOI] [PubMed] [Google Scholar]
- BAGALKOT V., FAROKHZAD O.C., LANGER R., and JON S. (2006). An aptamer-doxorubicin physical conjugate as a novel targeted drug-delivery platform. Angew. Chem. Int. Ed. Engl. 45, 8149–8152 [DOI] [PubMed] [Google Scholar]
- BASSETT S.E., FENNEWALD S.M., KING D.J., LI X., HERZOG N.K., SHOPE R., ARONSON J.F., LUXON B.A., and GORENSTEIN D.G. (2004). Combinatorial selection and edited combinatorial selection of phosphorothioate aptamers targeting human nuclear factor-kappa B RelA/p50 and RelA/RelA. Biochemistry 43, 9105–9115 [DOI] [PubMed] [Google Scholar]
- BATES P.J., KAHLON J.B., THOMAS S.D., TRENT J.O., and MILLER D.M. (1999). Antiproliferative activity of G-rich oligo-nucleotides correlates with protein binding. J. Biol. Chem. 274, 26369–26377 [DOI] [PubMed] [Google Scholar]
- BEHLKE M.A. (2008). Chemical modification of siRNAs for in vivo use. Oligonucleotides. 18, 305–320 [DOI] [PubMed] [Google Scholar]
- BEREZOVSKI M., DRABOVICH A., KRYLOVA S.M., MUSHEEV M., OKHONIN V., PETROV A., and KRYLOV S.N. (2005). Nonequilibrium capillary electrophoresis of equilibrium mixtures: a universal tool for development of aptamers. J. Am. Chem. Soc. 127, 3165–3171 [DOI] [PubMed] [Google Scholar]
- BLANK M., WEINSCHENK T., PRIEMER M., and SCHLUESENER H. (2001). Systematic evolution of a DNA aptamer binding to rat brain tumor microvessels: selective targeting of endothelial regulatory protein pigpen. J. Biol. Chem. 276, 16464–16468 [DOI] [PubMed] [Google Scholar]
- BOCK C., COLEMAN M., COLLINS B., DAVIS J., FOULDS G., GOLD L., GREEF C., HEIL J., HEILIG J.S., HICKE B., HURST M.N., HUSAR G.M., MILLER D., OSTROFF R., PETACH H., SCHNEIDER D., VANT-HULL B., WAUGH S., WEISS A., WILCOX S.K., and ZICHI D. (2004). Photoaptamer arrays applied to multiplexed proteomic analysis. Proteomics. 4, 609–618 [DOI] [PubMed] [Google Scholar]
- BOCK L.C., GRIFFIN L.C., LATHAM J.A., VERMAAS E.H., and TOOLE J.J. (1992). Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature. 355, 564–566 [DOI] [PubMed] [Google Scholar]
- BOOMER R.M., LEWIS S.D., HEALY J.M., KURZ M., WILSON C., and MCCAULEY T.G. (2005). Conjugation to polyethylene glycol polymer promotes aptamer biodistribution to healthy and inflamed tissues. Oligonucleotides. 15, 183–195 [DOI] [PubMed] [Google Scholar]
- BOUCARD D., TOULME J.J., and DI PRIMO C. (2006). Bimodal loop-loop interactions increase the affinity of RNA aptamers for HIV-1 RNA structures. Biochemistry. 45, 1518–1524 [DOI] [PubMed] [Google Scholar]
- BROWNING C.M., CAGNON L., GOOD P.D., ROSSI J., ENGELKE D.R., and MARKOVITZ D.M. (1999). Potent inhibition of human immunodeficiency virus type 1 (HIV-1) gene expression and virus production by an HIV-2 tat activation-response RNA decoy. J. Virol. 73, 5191–5195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUNKA D.H.J., MANTLE B.J., MORTEN I.J., TENNENT G.A., RADFORD S.E., and STOCKLEY P.G. (2007). Production and characterization of RNA aptamers specific for amyloid fibril epitopes. J. Biol. Chem. 282, 34500–34509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKE D.H., SCATES L., ANDREWS K., and GOLD L. (1996). Bent pseudoknots and novel RNA inhibitors of type 1 human immunodeficiency virus (HIV-1) reverse transcriptase. J. Mol. Biol. 264, 650–666 [DOI] [PubMed] [Google Scholar]
- BURMEISTER P.E., LEWIS S.D., SILVA R.F., PREISS J.R., HORWITZ L.R., PENDERGRAST P.S., MCCAULEY T.G., KURZ J.C., EPSTEIN D.M., WILSON C., and KEEFE A.D. (2005). Direct in vitro selection of a 2′-O-methyl aptamer to VEGF. Chem. Biol. 12, 25–33 [DOI] [PubMed] [Google Scholar]
- CASSIDAY L.A., and MAHER L.J. (2003). Yeast genetic selections to optimize RNA decoys for transcription factor NF-kappaB. Proc. Natl. Acad. Sci. USA 100, 3930–3935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERCHIA L., DUCONGE F., PESTOURIE C., BOULAY J., AISSOUNI Y., GOMBERT K., TAVITIAN B., DE FRANCISCIS V., and LIBRI D. (2005). Neutralizing aptamers from whole-cell SELEX inhibit the RET receptor tyrosine kinase. PLoS Biol. 3, e123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- CHAN M.Y., COHEN M.G., DYKE C.K., MYLES S.K., ABERLE L.G., LIN M., WALDER J., STEINHUBL S.R., GILCHRIST I.C., KLEIMAN N.S., VORCHHEIMER D.A., CHRONOS N., MELLONI C., ALEXANDER J.H., HARRINGTON R.A., TONKENS R.M., BECKER R.C., and RUSCONI C.P. (2008a). Phase 1b randomized study of antidote-controlled modulation of factor IXa activity in patients with stable coronary artery disease. Circulation. 117, 2865–2874 [DOI] [PubMed] [Google Scholar]
- CHAN M.Y., RUSCONI C.P., ALEXANDER J.H., TONKENS R.M., HARRINGTON R.A., and BECKER R.C. (2008b). A randomized, repeat-dose, pharmacodynamic and safety study of an antidote-controlled factor IXa inhibitor. J. Thromb. Haemost. 6, 789–796 [DOI] [PubMed] [Google Scholar]
- CHELLISERRYKATTIL J., and ELLINGTON A.D. (2004). Evolution of a T7 RNA polymerase variant that transcribes 2′-O-methyl RNA. Nat. Biotechnol. 22, 1155–1160 [DOI] [PubMed] [Google Scholar]
- CHEN C.-H.B., CHERNIS G.A., HOANG V.Q., and LANDGRAF R. (2003). Inhibition of heregulin signaling by an aptamer that preferentially binds to the oligomeric form of human epidermal growth factor receptor-3. Proc. Natl. Acad. Sci. USA. 100, 9226–9231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN C.-H.B., DELLAMAGGIORE K.R., OUELLETTE C.P., SEDANO C.D., LIZADJOHRY M., CHERNIS G.A., GONZALES M., BALTASAR F.E., FAN A.L., MYEROWITZ R., and NEUFELD E.F. (2008). Aptamer-based endocytosis of a lysosomal enzyme. Proc. Natl. Acad. Sci. USA. 105, 15908–15913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN F., ZHOU J., LUO F., MOHAMMED A.-B., and ZHANG X.-L. (2007). Aptamer from whole-bacterium SELEX as new therapeutic reagent against virulent Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun. 357, 743–748 [DOI] [PubMed] [Google Scholar]
- CHENG C., DONG J., YAO L., CHEN A., JIA R., HUAN L., GUO J., SHU Y., and ZHANG Z. (2008). Potent inhibition of human influenza H5N1 virus by oligonucleotides derived by SELEX. Biochem. Biophys. Res. Commun. 366, 670–674 [DOI] [PubMed] [Google Scholar]
- CHENG J., TEPLY B.A., SHERIFI I., SUNG J., LUTHER G., GU F.X., LEVY-NISSENBAUM E., RADOVIC-MORENO A.F., LANGER R., and FAROKHZAD O.C. (2007). Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials. 28, 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU T.C., MARKS J.W., III LA.VERY L.A., FAULKNER S., ROSENBLUM M.G., ELLINGTON A.D., and LEVY M. (2006a). Aptamer:toxin conjugates that specifically target prostate tumor cells. Cancer Res. 66, 5989–5992 [DOI] [PubMed] [Google Scholar]
- CHU T.C., TWU K.Y., ELLINGTON A.D., and LEVY M. (2006b). Aptamer mediated siRNA delivery. Nucleic Acids Res. 34, e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLETT J.R., CHO E.J., and ELLINGTON A.D. (2005). Production and processing of aptamer microarrays. Methods. 37, 4–15 [DOI] [PubMed] [Google Scholar]
- CONTE M.S., BANDYK D.F., CLOWES A.W., MONETA G.L., SEELY L., LORENZ T.J., NAMINI H., HAMDAN A.D., RODDY S.P., BELKIN M., BERCELI S.A., DEMASI R.J., SAMSON R.H., and BERMAN S.S. (2006). Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J. Vasc. Surg. 43, 742–751; discussion 751. [DOI] [PubMed] [Google Scholar]
- COX J.C., RUDOLPH P., and ELLINGTON A.D. (1998). Automated RNA selection. Biotechnol. Prog. 14, 845–850 [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM E.T., Jr, ADAMIS A.P., ALTAWEEL M., AIELLO L.P., BRESSLER N.M., D'AMICO D.J., GOLDBAUM M., GUYER D.R., KATZ B., PATEL M., and SCHWARTZ S.D. (2005). A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 112, 1747–1757 [DOI] [PubMed] [Google Scholar]
- DANIELS D.A., CHEN H., HICKE B.J., SWIDEREK K.M., and GOLD L. (2003). A tenascin-C aptamer identified by tumor cell SELEX: systematic evolution of ligands by exponential enrichment. Proc. Natl. Acad. Sci. USA. 100, 15416–15421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAPIC V., BATES P.J., TRENT J.O., RODGER A., THOMAS S.D., and MILLER D.M. (2002). Antiproliferative activity of G-quartet-forming oligonucleotides with backbone and sugar modifications. Biochemistry. 41, 3676–3685 [DOI] [PubMed] [Google Scholar]
- DEY A.K., KHATI M., TANG M., WYATT R., LEA S.M., and JAMES W. (2005). An aptamer that neutralizes R5 strains of human immunodeficiency virus type 1 blocks gp120-CCR5 interaction. J. Virol. 79, 13806–13810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHAR S., GU F.X., LANGER R., FAROKHZAD O.C., and LIPPARD S.J. (2008). Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles. Proc. Natl. Acad. Sci. USA. 105, 17356–17361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLLINS C.M., NAIR S., BOCZKOWSKI D., LEE J., LAYZER J.M., GILBOA E., and SULLENGER B.A. (2008). Assembling OX40 aptamers on a molecular scaffold to create a receptor-activating aptamer. Chem. Biol. 15, 675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYKE C.K., STEINHUBL S.R., KLEIMAN N.S., CANNON R.O., ABERLE,L.G.,LIN,M.,MYLES,S.K.,MELLONI,C.,HARRINGTON R.A., ALEXANDER J.H., BECKER R.C., and RUSCONI C.P. (2006). First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: A Phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor IXa activity. Circulation. 114, 2490–2497 [DOI] [PubMed] [Google Scholar]
- EHSAN A., MANN M.J., DELL'ACQUA G., and DZAU V.J. (2001). Long-term stabilization of vein graft wall architecture and prolonged resistance to experimental atherosclerosis after E2F decoy oligonucleotide gene therapy. J. Thorac. Cardiovasc. Surg. 121, 714–722 [DOI] [PubMed] [Google Scholar]
- ELLINGTON A.D., and SZOSTAK J.W. (1990). In vitro selection of RNA molecules that bind specific ligands. Nature. 346, 818–822 [DOI] [PubMed] [Google Scholar]
- EULBERG D., BUCHNER K., MAASCH C., and KLUSSMANN S. (2005). Development of an automated in vitro selection protocol to obtain RNA-based aptamers: identification of a biostable substance P antagonist. Nucleic Acids Res. 33, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EULBERG D., and KLUSSMANN S. (2003). Spiegelmers: biostable aptamers. Chembiochem. 4, 979–983 [DOI] [PubMed] [Google Scholar]
- FAROKHZAD O.C., CHENG J., TEPLY B.A., SHERIFI I., JON S., KANTOFF P.W., RICHIE J.P., and LANGER R. (2006). Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. USA. 103, 6315–6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERREIRA C.S.M., CHEUNG M.C., MISSAILIDIS S., BISLAND S., and GARIEPY J. (2009). Phototoxic aptamers selectively enter and kill epithelial cancer cells. Nucleic Acids Res. 37, 866–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANGRANDE P.H., ZHANG J., TANNER A., ECKHART A.D., REMPEL R.E., ANDRECHEK E.R., LAYZER J.M., KEYS J.R., HAGEN P.O., NEVINS J.R., KOCH W.J., and SULLENGER B.A. (2007). Distinct roles of E2F proteins in vascular smooth muscle cell proliferation and intimal hyperplasia. Proc. Natl. Acad. Sci. USA. 104, 12988–12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILBERT J.C., DEFEO-FRAULINI T., HUTABARAT R.M., HORVATH C.J., MERLINO P.G., MARSH H.N., HEALY J.M., BOUFAKHREDDINE S., HOLOHAN T.V., and SCHAUB R.G. (2007). First-in-human evaluation of anti von Willebrand factor therapeutic aptamer ARC1779 in healthy volunteers. Circulation. 116, 2678–2686 [DOI] [PubMed] [Google Scholar]
- GIRVAN A.C., TENG Y., CASSON L.K., THOMAS S.D., JULIGER S., BALL M.W., KLEIN J.B., PIERCE W.M., Jr, BARVE S.S., and BATES P.J. (2006). AGRO100 inhibits activation of nuclear factor-kappaB (NF-kappaB) by forming a complex with NF-kappaB essential modulator (NEMO) and nucleolin. Mol. Cancer Ther. 5, 1790–1799 [DOI] [PubMed] [Google Scholar]
- GREEN L.S., JELLINEK D., JENISON R., OSTMAN A., HELDIN C.-H., and JANJIC N. (1996). Inhibitory DNA ligands to platelet-derived growth factor B-chain. Biochemistry. 35, 14413–14424 [DOI] [PubMed] [Google Scholar]
- HEALY J.M., LEWIS S.D., KURZ M., BOOMER R.M., THOMPSON K.M., WILSON C., and MCCAULEY T.G. (2004). Pharmacokinetics and biodistribution of novel aptamer compositions. Pharm. Res. 21, 2234–2246 [DOI] [PubMed] [Google Scholar]
- HICKE B.J., MARION C., CHANG Y.-F., GOULD T., LYNOTT C.K., PARMA D., SCHMIDT P.G., and WARREN S. (2001). Tenascin-C aptamers are generated using tumor cells and purified protein. J. Biol. Chem. 276, 48644–48654 [DOI] [PubMed] [Google Scholar]
- HICKE B.J., STEPHENS A.W., GOULD T., CHANG Y.-F., LYNOTT C.K., HEIL J., BORKOWSKI S., HILGER C.-S., COOK G., WARREN S., and SCHMIDT P.G. (2006). Tumor targeting by an aptamer. J. Nucl. Med. 47, 668–678 [PubMed] [Google Scholar]
- HOMANN M., and GORINGER H. (1999). Combinatorial selection of high affinity RNA ligands to live African trypanosomes. Nucleic Acids Res. 27, 2006–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG Y., ECKSTEIN F., PADILLA R., and SOUSA R. (1997). Mechanism of ribose 2′-group discrimination by an RNA polymerase. Biochemistry. 36, 8231–8242 [DOI] [PubMed] [Google Scholar]
- HUANG Y.F., SHANGGUAN D., LIU H., PHILLIPS J.A., ZHANG X., CHEN Y., and TAN W. (2009). Molecular assembly of an aptamer-drug conjugate for targeted drug delivery to tumor cells. Chembiochem. 10, 862–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRESON C.R., and KELLAND L.R. (2006). Discovery and development of anticancer aptamers. Mol. Cancer Ther. 5, 2957–2962 [DOI] [PubMed] [Google Scholar]
- ISHIZAKI J., NEVINS J.R., and SULLENGER B.A. (1996). Inhibition of cell proliferation by an RNA ligand that selectively blocks E2F function. Nat. Med. 2, 1386–1389 [DOI] [PubMed] [Google Scholar]
- JASON T.L., KOROPATNICK J., and BERG R.W. (2004). Toxicology of antisense therapeutics. Toxicol. Appl. Pharmacol. 201, 66–83 [DOI] [PubMed] [Google Scholar]
- KEEFE A.D., and CLOAD S.T. (2008). SELEX with modified nucleotides. Curr. Opin. Chem. Biol. 12, 448–456 [DOI] [PubMed] [Google Scholar]
- KHATI M., SCHUMAN M., IBRAHIM J., SATTENTAU Q., GORDON S., and JAMES W. (2003). Neutralization of infectivity of diverse R5 clinical isolates of human immunodeficiency virus type 1 by gp120-binding 2′F-RNA aptamers. J. Virol. 77, 12692–12698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM Y., CAO Z., and TAN W. (2008). Molecular assembly for high-performance bivalent nucleic acid inhibitor. Proc. Natl. Acad. Sci. USA. 105, 5664–5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBY R., CHO E.J., GEHRKE B., BAYER T., PARK Y.S., NEIKIRK D.P., MCDEVITT J.T., and ELLINGTON A.D. (2004). Aptamer-based sensor arrays for the detection and quantitation of proteins. Anal. Chem. 76, 4066–4075 [DOI] [PubMed] [Google Scholar]
- KOHN D.B., BAUER G., RICE C.R., ROTHSCHILD J.C., CARBONARO D.A., VALDEZ P., HAO Q., ZHOU C., BAHNER I., KEARNS K., BRODY K., FOX S., HADEN E., WILSON K., SALATA C., DOLAN C., WETTER C., AGUILAR-CORDOVA E., and CHURCH J. (1999). A clinical trial of retroviral-mediated transfer of a rev-responsive element decoy gene into CD34(+) cells from the bone marrow of human immunodeficiency virus-1-infected children. Blood. 94, 368–371 [PubMed] [Google Scholar]
- LEBRUSKA L.L., and MAHER L.J. (1999). Selection and characterization of an RNA decoy for transcription factor NF-kappaB. Biochemistry 38, 3168–3174 [DOI] [PubMed] [Google Scholar]
- LEE T.C., SULLENGER B.A., GALLARDO H.F., UNGERS G.E., and GILBOA E. (1992). Overexpression of RRE-derived sequences inhibits HIV-1 replication in CEM cells. New Biol. 4, 66–74 [PubMed] [Google Scholar]
- LI N., EBRIGHT J., STOVALL G., CHEN X., NGUYEN H., SINGH A., SYRETT H., and ELLINGTON A. (2009). Technical and biological issues relevant to cell typing by aptamers. J. Proteome Res. 8:2438–2448 [DOI] [PubMed] [Google Scholar]
- LI N., WANG Y., POTHUKUCHY A., SYRETT A., HUSAIN N., GOPALAKRISHA S., KOSARAJU P., and ELLINGTON A.D. (2008). Aptamers that recognize drug-resistant HIV-1 reverse transcriptase. Nucleic Acids Res. 36, 6739–6751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOU X., QIAN J., XIAO Y., VIEL L., GERDON A.E., LAGALLY E.T., ATZBERGER P., TARASOW T.M., HEEGER A.J., and SOH H.T. (2009). Micromagnetic selection of aptamers in microfluidic channels. Proc. Natl. Acad. Sci. USA. 106, 2989–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUPOLD S.E., HICKE B.J., LIN Y., and COFFEY D.S. (2002). Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res. 62, 4029–4033 [PubMed] [Google Scholar]
- MANOHARAN M. (2002). Oligonucleotide conjugates as potential antisense drugs with improved uptake, biodistribution, targeted delivery, and mechanism of action. Antisense Nucleic Acid Drug Dev. 12, 103–128 [DOI] [PubMed] [Google Scholar]
- MATTEUCCI M. (1997). Oligonucleotide analogues: an overview. Ciba Found. Symp. 209, 5–14; discussion 14–18. [PubMed] [Google Scholar]
- MAYER G., MULLER J., MACK T., FREITAG D.F., HOVER T., POTZSCH B., and HECKEL A. (2009). Differential regulation of protein subdomain activity with caged bivalent ligands. Chembiochem. 10, 654–657 [DOI] [PubMed] [Google Scholar]
- MCNAMARA J.O., II AN.DRECHEK E.R., WANG Y., VILES K.D., REMPEL R.E., GILBOA E., SULLENGER B.A., and GIANGRANDE P.H. (2006). Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 24, 1005–1015 [DOI] [PubMed] [Google Scholar]
- MCNAMARA J.O., KOLONIAS D., PASTOR F., MITTLER R.S., CHEN L., GIANGRANDE P.H., SULLENGER B., and GILBOA E. (2008). Multivalent 4–1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J. Clin. Invest. 118, 376–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENDONSA S.D., and BOWSER M.T. (2004). In vitro evolution of functional DNA using capillary electrophoresis. J. Am. Chem. Soc. 126, 20–21 [DOI] [PubMed] [Google Scholar]
- MICHIENZI A., LI S., ZAIA J.A., and ROSSI J.J. (2002). A nucleolar TAR decoy inhibitor of HIV-1 replication. Proc. Natl. Acad. Sci. USA. 99, 14047–14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER J., WULFFEN B., POTZSCH B., and MAYER G. (2007). Multidomain targeting generates a high-affinity thrombin-inhibiting bivalent aptamer. Chembiochem. 8, 2223–2226 [DOI] [PubMed] [Google Scholar]
- NG E.W.M., SHIMA D.T., CALIAS P., CUNNINGHAM E.T., GUYER D.R., and ADAMIS A.P. (2006). Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug Discov. 5, 123–132 [DOI] [PubMed] [Google Scholar]
- OHUCHI S.P., OHTSU T., and NAKAMURA Y. (2006). Selection of RNA aptamers against recombinant transforming growth factor-beta type III receptor displayed on cell surface. Biochimie. 88, 897–904 [DOI] [PubMed] [Google Scholar]
- ONEY S., NIMJEE S.M., LAYZER J., QUE-GEWIRTH N., GINSBURG D., BECKER R.C., AREPALLY G., and SULLENGER B.A. (2007). Antidote-controlled platelet inhibition targeting von Willebrand factor with aptamers. Oligonucleotides. 17, 265–274 [DOI] [PubMed] [Google Scholar]
- PESTOURIE C., CERCHIA L., GOMBERT K., AISSOUNI Y., BOULAY J., DE FRANCISCIS V., LIBRI D., TAVITIAN B., and DUCONGE F. (2006). Comparison of different strategies to select aptamers against a transmembrane protein target. Oligonucleotides. 16, 323–335 [DOI] [PubMed] [Google Scholar]
- PIEKEN W.A., OLSEN D.B., BENSELER F., AURUP H., and ECKSTEIN F. (1991). Kinetic characterization of ribonuclease-resistant 2′-modified hammerhead ribozymes. Science. 253, 314–317 [DOI] [PubMed] [Google Scholar]
- PREVENT IV INVESTIGATORS. (2005). Efficacy and safety of Edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: A randomized controlled trial. JAMA. 294, 2446–2454 [DOI] [PubMed] [Google Scholar]
- PROSKE D., GILCH S., WOPFNER F., SCHATZL H.M., WINNACKER E.L., and FAMULOK M. (2002). Prion-protein-specific aptamer reduces PrPSc formation. Chembiochem. 3, 717–725 [DOI] [PubMed] [Google Scholar]
- RENTMEISTER A., BILL A., WAHLE T., WALTER J., and FAMULOK M. (2006). RNA aptamers selectively modulate protein recruitment to the cytoplasmic domain of beta-secretase BACE1 in vitro. RNA. 12, 1650–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHIE A., KIRBY L., SAYER N., WELLESLEY R., DISTERER P., SYLVESTER I., GILL A., HOPE J., JAMES W., and TAHIRI-ALAOUI A. (2003). Characterization of 2′-fluoro-RNA aptamers that bind preferentially to disease-associated conformations of prion protein and inhibit conversion. J. Biol. Chem. 278, 39697–39705 [DOI] [PubMed] [Google Scholar]
- RUCKMAN J., GREEN L.S., BEESON J., WAUGH S., GILLETTE W.L., HENNINGER D.D., CLAESSON-WELSH L., and JANJIC N. (1998). 2′-fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165): inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J. Biol. Chem. 273, 20556–20567 [DOI] [PubMed] [Google Scholar]
- RUSCONI C.P., ROBERTS J.D., PITOC G.A., NIMJEE S.M., WHITE R.R., QUICK G., Jr., SCARDINO E., FAY W.P., and SULLENGER B.A. (2004). Antidote-mediated control of an anticoagulant aptamer in vivo. Nat. Biotechnol. 22, 1423–1428 [DOI] [PubMed] [Google Scholar]
- RUSCONI C.P., SCARDINO E., LAYZER J., PITOC G.A., ORTEL T.L., MONROE D., and SULLENGER B.A. (2002). RNA aptamers as reversible antagonists of coagulation factor IXa. Nature. 419, 90–94 [DOI] [PubMed] [Google Scholar]
- RUSCONI C.P., YEH A., LYERLY H.K., LAWSON J.H., and SULLENGER B.A. (2000). Blocking the initiation of coagulation by RNA aptamers to factor VIIa. Thromb. Haemost. 84, 841–848 [PubMed] [Google Scholar]
- SANTULLI-MAROTTO S., NAIR S.K., RUSCONI C., SULLENGER B., and GILBOA E. (2003). Multivalent RNA aptamers that inhibit CTLA-4 and enhance tumor immunity. Cancer Res. 63, 7483–7489 [PubMed] [Google Scholar]
- SAYER N.M., CUBIN M., RHIE A., BULLOCK M., TAHIRI-ALAOUI A., and JAMES W. (2004). Structural determinants of conformationally selective, prion-binding aptamers. J. Biol. Chem. 279, 13102–13109 [DOI] [PubMed] [Google Scholar]
- SCHMIDT K.S., BORKOWSKI S., KURRECK J., STEPHENS A.W., BALD R., HECHT M., FRIEBE M., DINKELBORG L., and ERDMANN V.A. (2004). Application of locked nucleic acids to improve aptamer in vivo stability and targeting function. Nucleic Acids Res. 32, 5757–5765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER D.J., FEIGON J., HOSTOMSKY Z., and GOLD L. (1995). High-affinity ssDNA inhibitors of the reverse transcriptase of type 1 human immunodeficiency virus. Biochemistry. 34, 9599–9610 [DOI] [PubMed] [Google Scholar]
- SENNINO B., FALCON B.L., MCCAULEY D., LE T., MCCAULEY T., KURZ J.C., HASKELL A., EPSTEIN D.M., and MCDONALD D.M. (2007). Sequential loss of tumor vessel pericytes and endothelial cells after inhibition of platelet-derived growth factor B by selective aptamer AX102. Cancer Res. 67, 7358–7367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAMAH S.M., HEALY J.M., and CLOAD S.T. (2008). Complex target SELEX. Acc. Chem. Res. 41, 130–138 [DOI] [PubMed] [Google Scholar]
- SHANGGUAN D., CAO Z.C., LI Y., and TAN W. (2007). Aptamers evolved from cultured cancer cells reveal molecular differences of cancer cells in patient samples. Clin. Chem. 53, 1153–1155 [DOI] [PubMed] [Google Scholar]
- SHI H., HOFFMAN B.E., and LIS J.T. (1999). RNA aptamers as effective protein antagonists in a multicellular organism. Proc. Natl. Acad. Sci. USA. 96, 10033–10038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUNDARARAJAN S., CHEN W., SPICER E.K., COURTENAY-LUCK N., and FERNANDES D.J. (2008). The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 68, 2358–2365 [DOI] [PubMed] [Google Scholar]
- SULLENGER B.A., GALLARDO H.F., UNGERS G.E., and GILBOA E. (1990). Overexpression of TAR sequences renders cells resistant to human immunodeficiency virus replication. Cell. 63, 601–608 [DOI] [PubMed] [Google Scholar]
- SULLENGER B.A., GALLARDO H.F., UNGERS G.E., and GILBOA E. (1991). Analysis of trans-acting response decoy RNA-mediated inhibition of human immunodeficiency virus type 1 transactivation. J. Virol. 65, 6811–6816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANG Z., SHANGGUAN D., WANG K., SHI H., SEFAH K., MALLIKRATCHY P., CHEN H.W., LI Y., and TAN W. (2007). Selection of aptamers for molecular recognition and characterization of cancer cells. Anal. Chem. 79, 4900–4907 [DOI] [PubMed] [Google Scholar]
- TIAN L., and HEYDUK T. (2009). Bivalent ligands with long nanometer-scale flexible linkers. Biochemistry. 48, 264–275 [DOI] [PubMed] [Google Scholar]
- TUERK C., and GOLD L. (1990). Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 249, 505–510 [DOI] [PubMed] [Google Scholar]
- TUERK C., MACDOUGAL S., and GOLD L. (1992). RNA pseudo-knots that inhibit human immunodeficiency virus type 1 reverse transcriptase. Proc. Natl. Acad. Sci. USA. 89, 6988–6992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENTO M.T., IUORIO M., NETTI P.A., DUCONGE F., TAVITIAN B., FRANCISCIS V., and CERCHIA L. (2008). Distribution and bioactivity of the Ret-specific D4 aptamer in three-dimensional collagen gel cultures. Mol. Cancer Ther. 7, 3381–3388 [DOI] [PubMed] [Google Scholar]
- WEISS S., PROSKE D., NEUMANN M., GROSCHUP M., KRETZSCHMAR H., FAMULOK M., and WINNACKER E. (1997). RNA aptamers specifically interact with the prion protein PrP. J. Virol. 71, 8790–8797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., RUSCONI C., SCARDINO E., WOLBERG A., LAWSON J., HOFFMAN M., and SULLENGER B. (2001). Generation of species cross-reactive aptamers using “toggle” SELEX. Mol. Ther. 4, 567–573 [DOI] [PubMed] [Google Scholar]
- WILLIS M.C., COLLINS B., ZHANG T., GREEN L.S., SEBESTA D.P., BELL C., KELLOGG E., GILL S.C., MAGALLANEZ A., KNAUER S., BENDELE R.A., GILL P.S., and JANJIC N. (1998). Liposome-anchored vascular endothelial growth factor aptamers. Bioconjug. Chem. 9, 573–582 [DOI] [PubMed] [Google Scholar]
- WULLNER U., NEEF I., ELLER A., KLEINES M., TUR M.K., and BARTH S. (2008). Cell-specific induction of apoptosis by rationally designed bivalent aptamer-siRNA transcripts silencing eukaryotic elongation factor 2. Curr. Cancer Drug Targets 8, 554–565 [DOI] [PubMed] [Google Scholar]
- WURSTER S.E., and MAHER L.J. (2008). Selection and characterization of anti-NF-kappaB p65 RNA aptamers. RNA 14, 1037–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO R., KATAHIRA M., NISHIKAWA S., BABA T., TAIRA K., and KUMAR P.K. (2000). A novel RNA motif that binds efficiently and specifically to the Ttat protein of HIV and inhibits the trans-activation by Tat of transcription in vitro and in vivo. Genes Cells 5, 371–388 [DOI] [PubMed] [Google Scholar]
- YLERA F., LURZ R., ERDMANN V.A., and FÜRSTE J.P. (2002). Selection of RNA aptamers to the Alzheimer's disease amyloid peptide. Biochem. Biophys. Res. Commun. 290, 1583–1588 [DOI] [PubMed] [Google Scholar]
- ZHOU J., LI H., LI S., ZAIA J., and ROSSI J.J. (2008). Novel dual inhibitory function aptamer-siRNA delivery system for HIV-1 therapy. Mol. Ther. 16, 1481–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU J., SWIDERSKI P., LI H., ZHANG J., NEFF C.P., AKKINA R., and ROSSI J.J. (2009). Selection, characterization and application of new RNA HIV gp 120 aptamers for facile delivery of Dicer substrate siRNAs into HIV infected cells. Nucleic Acids Res. 37, 3094–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]



