Abstract
Objective: Metformin was assessed as an interventional medication for weight gain in children and adolescents taking atypical antipsychotic agents.
Method: A 12-week open-label trial was conducted to evaluate metformin's effectiveness and safety for weight management. Eleven subjects, ages 10–18 years, participated in the study. Each subject received metformin orally up to 2000 mg/day. Primary outcome measures included weight, body mass index (BMI), and waist circumference. Secondary outcome measures included serum glucose, insulin, and fasting lipid profile. Changes in weight, BMI, waist, and metabolic profile were obtained by using repeated measures of covariance.
Results: The mean reduction in weight, waist, BMI, serum glucose, and serum insulin was not statistically significant. However, 5 out of 11 patients lost weight (mean, −2.82 kg ±7.25), and overall the sample did not continue to gain weight. There was a significant decrease in triglyceride levels. Metformin was fairly well tolerated.
Conclusion: Preliminary data suggests that metformin may safely and effectively improve the triglyceride profile. However, contrary to study hypotheses, weight, waist, and BMI reduction were not statistically significant. Future double-blind studies with larger sample sizes and of longer duration are warranted to assess more fully the safety and efficacy of this intervention.
Introduction
The use of atypical antipsychotic agents to treat pediatric mental illness, including bipolar disorder (BD), schizophrenia, and pervasive developmental disorder (PDD) has been increasing in prevalence (Findling et al. 2005). Their use is often associated with weight gain and abnormalities in metabolic profiles. For example, antipsychotics that produce the greatest weight gain are associated with higher rates of diabetes (Jin et al. 2002).
Metformin, which is a biguanide oral hypoglycemic, decreases endogenous hepatic glucose production and intestinal glucose absorption, while it increases insulin sensitivity (Webb and Viner 2006). Metformin is approved for the treatment of diabetes mellitus II in pediatric patients aged 10 and older (Jones et al. 2002). It has been prescribed for patients with noninsulin-dependent diabetes to control serum glucose levels, and it has been reported to cause weight loss in several groups of patients, including women with polycystic ovary disease, obese men, and women with elevated lipid profiles with and without noninsulin-dependent diabetes (Paolisso et al. 1998; Kay et al. 2001; Bridger et al. 2006; Glueck et al. 2006; Srinivasan et al. 2006). In 2001, Freemark and associates conducted a randomized, placebo-controlled, double-blind trial in 29 obese adolescents. They concluded that metformin, when given 500 mg twice daily, may be considered as an adjunct to diet and exercise in teens at risk for diabetes mellitus II (Freemark and Bursey 2001). Morrison and colleagues published a 12-week open-label study of metformin on 19 pediatric patients on psychotropic drugs who demonstrated significant weight loss (15 of 19; mean, −2.93 ± 3.13) on metformin (Morrison et al. 2002). Klein and colleagues, in their randomized, double-blind, placebo-controlled trial of metformin in children and adolescents taking atypical antipsychotics, found arrested weight gain, as well as improved insulin resistance (Klein et al. 2006). Whereas Baptista and associates found that metformin did not prevent olanzapine-induced weight gain in a group of adult patients with schizophrenia (Baptista et al. 2006), Wu and colleagues published two studies indicating that metformin, administered to a group of adult patients with schizophrenia on antipsychotics, including olanzapine, was effective in attenuating weight gain and improving insulin sensitivity (Wu et al. 2008a; Wu et al. 2008b).
This study is an open-label, 12-week trial of metformin in the treatment of weight gain and metabolic profiles in pediatric patients who had gained weight on atypical antipsychotics. We hypothesized that metformin would manage weight gain, leading to weight loss, and improve metabolic profile as conforming to the studies preceding this one.
Method
The Cambridge Health Alliance Institutional Review Board approved the protocol. Written informed consent was obtained from the child's legal guardian and assent was obtained from the child prior to participating in the study.
Patients were recruited from Cambridge Health Alliance in Cambridge, Massachusetts. Patients with a diagnosis of BD, schizophrenia spectrum disorder, and PDD, who were taking risperidone, aripiprazole, or clozapine and had experienced weight gain of more than 10% above baseline, were included in the study. Subjects were excluded from participation if they met criteria for an eating disorder, substance abuse/dependence, significant medical or neurological illness, intelligence quotient (IQ) <70, inability to tolerate blood draws, allergy to metformin, pregnancy, recent (in the past 3 months) history of suicidality, clinically unstable on their current medication regimen, and patients on their current antipsychotic medication regimen for under 1 month.
Subjects were given metformin starting at 500 mg orally/day, which was titrated up by 500 mg/week as tolerated without side effects up to a target dose of 2000 mg per os/day. Determination of past weight gain was based on clinical records and the patient's weight upon enrollment into the study. Patients were seen over a 12-week period with weekly visits to monitor psychiatric symptoms, weight changes, side effects, and metabolic profiles. Patients were instructed not to change their baseline diet or activity level during the study. Clinical measures and safety monitoring questionnaires were conducted at all visits. The Brief Psychiatric Rating Scale (BPRS) (Ventura et al. 2000) and Monitoring of Side Effects System (MOSES) (Washington State, 2003) were used. Laboratory tests for metabolic profile and liver function were conducted at weeks 0, 4, 8, and 12. Fasting glucose was measured every other week. Menarchal girls took a urine pregnancy test prior to initiating the study medication.
Descriptive statistics were obtained for all variables. A total of 46% of subjects completed all 12 weeks of the medication trial (2006–2007 rolling admission, n = 24). In addition to metformin, 60% of subjects were being treated with risperidone, 30% were being treated with aripiprazole, and 10% were being treated with clozapine.
The study's primary outcome measures were change in weight, body mass index (BMI), and waist circumference at 12 weeks of metformin treatment. Secondary outcome measures included serum glucose, insulin, low-density lipoproteins (LDL), high-density lipoproteins (HDL), cholesterol, and triglycerides levels.
To evaluate the time course of improvement while being treated with metformin, a linear mixed-model analysis was fitted on the change in visit scores from baseline to completion, with sex, diagnosis, week, and age in the model. Exploratory analyses also included race in the model, but results only showed a trend toward significance for insulin and cholesterol; thus, race was only included in further analyses with insulin and cholesterol. A Toeplitz covariance structure was fit to the within-patient repeated measures using the Kenward–Roger method (Kenward and Roger 1997) to estimate degrees of freedom. Patients were analyzed on an intent-to-treat (ITT) basis for all analyses. Patients with a baseline and at least one post-baseline measurement were included in the analyses. All p values were based on two-tailed tests with significance level of 0.05.
Results
Table 1 lists the demographic characteristics of the patients enrolled in the study. Duration of participation (weeks) in the study was a significant predictor of triglyceride levels and showed a trend toward significance to cholesterol and LDL (F = 4.48, df = 3, 9.05; p < 0.05; F = 3.10, df = 3, 10.12; p < 0.10; F = 3.18, df = 3, 7.75; p < 0.10). The longer the patient was in the study, the lower the triglyceride levels. However, duration in weeks did not significantly predict a change in weight, waist circumference, BMI, serum insulin, serum glucose, and HDL levels. Diagnosis significantly predicted glucose and insulin (p < 0.05) and showed a trend toward significance for BMI, LDL, and triglycerides (p < 0.10). In general, patients with bipolar disorder (BD) showed higher glucose levels and greater BMI than patients with schizophrenia, who showed higher levels than patients with PDD, whereas the opposite pattern was true for insulin levels. Subjects with BD showed higher levels of LDL than subjects with schizophrenia and PDD, who showed similar levels of LDL. Subjects with PDD showed the highest levels of triglycerides, followed by BD, and then schizophrenia. Age also predicted weight and waist measurements (p < 0.05, p < 0.01), and a trend toward significance for BMI and triglycerides (p < 0.10). Younger subjects showed lower triglycerides levels, and there was an increase in level with age. Both weight and waist circumference increased with age. Sex showed statistical significance with regard to glucose (p < 0.05), and race showed a trend toward significance for cholesterol (p < 0.10). Males showed slightly higher levels of glucose than females, whereas Caucasians showed higher levels of cholesterol, followed by other, African-American, and Hispanic subjects.
Table 1.
Demographics
| Mean age | 14 (SD 2.6) |
| Gender | |
| Male | 64% |
| Female | 36% |
| Race | |
| Caucasian | 46% |
| African-American | 18% |
| Hispanic | 18% |
| Other/mixed | 18% |
| Psychiatric diagnosis | |
| Bipolar disorder | 73% |
| Schizophrenia spectrum | 9% |
| Ppervasive developmental disorder | 18% |
Abbreviations: SD, standard deviation.
A further increase in weight experienced from the atypical antipsychotics during the course of the trial was arrested in all patients. Five of 11 patients lost weight. Mean changes in weight, waist, and BMI by length of follow up and overall, as well as the mean serum glucose, insulin, LDL, HDL, cholesterol, and triglycerides levels are summarized in Tables 2 and 3. The most common side effects reported by subjects are listed in Table 4. Six subjects dropped out of the study prior to completion due to a variety of reasons, including inability to attend study visits, aggression, stomach aches, inability to tolerate blood draws, and medication change.
Table 2.
Means of Study Variables at Baseline, Week 4, Week 8, and Week 12
| Weight (pounds) | BMI (kg/m2) | Waist (cm) | Insulin | Glucose | LDL | HDL | Cholesterol | Triglycerides | |
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD), baseline | 207.60 (63.43) | 33.64 (5.71) | 110.40 (18.66) | 21.20 (11.57) | 86.22 (16.11) | 106.27 (24.63) | 47.36 (21.76) | 177.00 (29.23) | 129.82 (63.70) |
| Mean (SD), week 4 | 209.49 (68.75) | 32.30 (5.66) | 106.60 (15.88) | 20.80 (16.22) | 88.88 (6.94) | 106.75 (17.18) | 41.12 (9.33) | 171.25 (16.87) | 115.88 (68.23) |
| Mean (SD), week 8 | 199.28 (51.87) | 32.56 (5.72) | 103.40 (13.69) | 13.00 (5.83) | 85.00 (10.02) | 110.67 (17.74) | 55.17 (24.73) | 185.83 (30.04) | 99.67 (32.05) |
| Mean (SD), week 12 | 187.84 (69.37) | 31.78 (6.43) | 100.20 (16.45) | 20.00 (23.57) | 89.80 (8.98) | 119.20 (17.54) | 56.20 (24.40) | 196.20 (34.81) | 103.20 (37.53) |
Abbreviations: BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SD, standard deviation.
Table 3.
Overall Mean Changes in Study Variables
| Weight change (kg) | BMI (kg/m2) | Waist (cm) | Insulin | Glucose | LDL | HDL | Cholesterol | Triglycerides | |
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD),LOCF | −0.33 (6.60) | −0.62 (1.04) | −0.78 (9.04) | −2.71 (11.19) | 0.60 (10.26) | 4.57 (15.8) | −0.71 (5.59) | 2.14 (10.29) | −9.00 (37.39) |
| Mean (SD), only completersa | −2.82 (7.25) | 1.14 (1.18) | 1.75 (7.63) | −2.60 (13.54) | 0.50 (11.85) | 9.40 (14.38) | −1.00 (6.82) | 4.60 (10.38) | −19.80 (35.70) |
Completers: week 12, baseline.
Abbreviations: BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; SD, standard deviation; LOCF, last observation carried forward, baseline.
Table 4.
Side Effects Profile
| Symptom | Percentage |
|---|---|
| Decreased appetite | 30.0 |
| Irritability | 27.8 |
| Constipation | 22.5 |
| Decreased attention | 19.4 |
| Drowsiness | 19.4 |
| Anxiety | 16.7 |
| Abnormal taste | 15.0 |
| Musculoskeletal pain | 15.0 |
Despite lack of significance, Figs. 1 and 2 show the decreases in weight and waist measurements from baseline to end point. In addition, effect size is at least moderate, indicating that there was a notable change in waist.
FIG. 1.
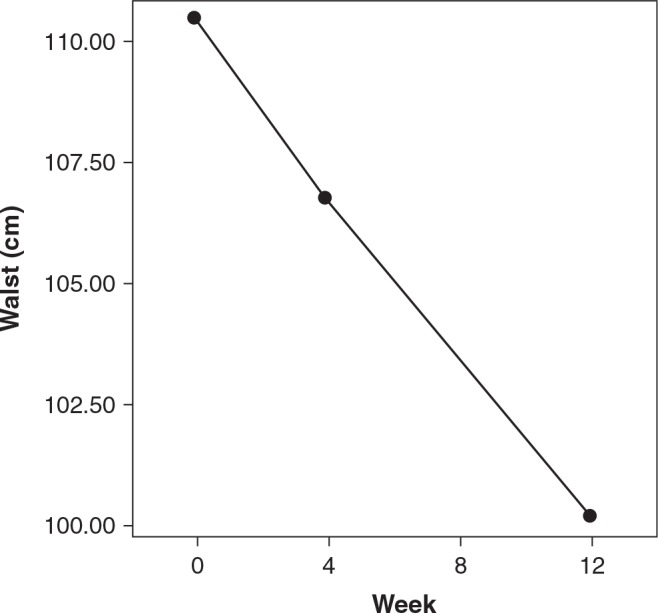
Change in waist measurement from baseline to week 12. Dot/lines show means. P = nonsignificant; Cohen's d = 0.58; effect size r = 0.28.
FIG. 2.
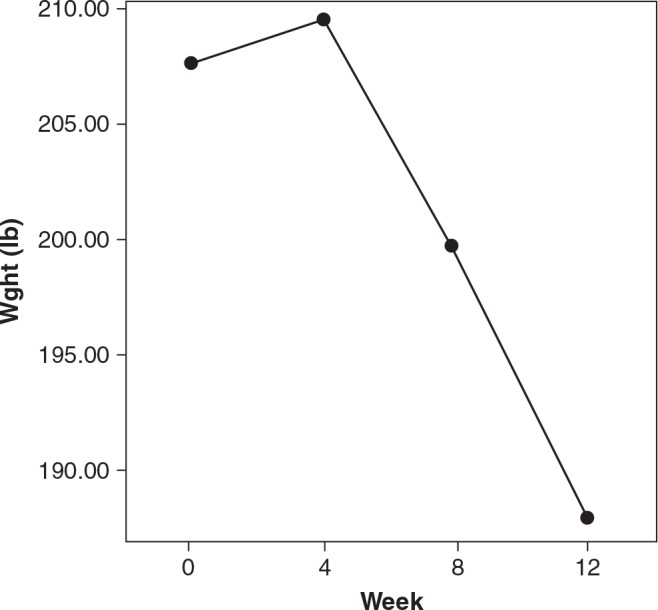
Change in weight from baseline to week 12. Dot/lines show means. P = nonsignificant; Cohen's d = 0.30; effect-size r = 15.
Discussion
In this preliminary evaluation of metformin as a treatment for weight gain associated with atypical antipsychotics, a further increase in weight experienced from these medications was attenuated in all patients. In addition, 5 of 11 patients lost weight. There was also a moderate effect size indicating a decrease in waist circumference over time. However, metformin did not improve insulin sensitivity and showed a trend toward increasing both LDL and cholesterol. Triglyceride levels improved. In this sample of 11 patients, metformin was generally well tolerated.
This study has several limitations. First, it was an open-label study and hence is subject to the placebo effect. However, the pattern of continued weight loss suggests that the weight loss was not entirely due to the placebo effect. Second, only a small number of patients was enrolled in this study, so comparisons of weight loss between the various atypicals were not possible. Similarly, the effect of different doses of metformin was not evaluated. The effect of different psychiatric diagnoses on metformin response was not addressed in this study. Third, although the patients were instructed not to change energy intake or physical activity, some may have due to increased motivation to lose weight. We did not obtain hard data on dietary intake and/or physical activity, which may themselves have become an intervention. Such information would be essential in future studies. Fourth, the study may not have been long enough to capture the full effect of metformin. Long-term follow-up studies are necessary to determine if further weight loss would occur over time and if, for those who lost weight, weight loss is sustained.
In conclusion, metformin holds promise as a treatment for weight gain in pediatric patients taking atypical antipsychotic medications. Further studies, including randomized, controlled trials and longer follow-up periods with larger sample sizes, are warranted to determine the safety and efficacy of this agent in these patients.
Disclosures
Dr. Frazier has served as a consultant and/or received grant support from the following: Bristol Myers Squibb, Eli Lily, Glaxo Smith Kline, Johnson & Johnson, Neuropharm, Otsuka Americal Pharmaceutical, and Pfizer. Dr. Shin, Ms. Bregman, Ms. Breeze, and Ms. Noyes have no conflicts of interest or financial ties to disclose.
References
- Baptista T, Martinez J, Lacruz A, Rangel N, Beaulieu S, Serrano A, Arape Y, Martinez M, de Mendoza S, Teneud L, Hernandez L: Metformin for prevention of weight gain and insulin resistance with olanzapine: A double-blind placebo-controlled trial. Can J Psychiatry 51:192–196, 2006 [DOI] [PubMed] [Google Scholar]
- Bridger T, MacDonald S, Baltzer F, Rodd C: Randomized placebo-controlled trial of metformin for adolescents with polycystic ovary syndrome. Arch Pediatr Adolesc Med 160: 241–246, 2006 [DOI] [PubMed] [Google Scholar]
- Findling RL, Steiner H, Weller EB: Use of antipsychotics in children and adolescents. J Clin Psychiatry 66:S29–S40, 2005 [PubMed] [Google Scholar]
- Freemark M, Bursey D: The effects of metformin on body mass index and glucose tolerance in obese adolescents with fasting hyperinsulinemia and a family history of type 2 diabetes. Pediatrics 107:E55, 2001 [DOI] [PubMed] [Google Scholar]
- Glueck CJ, Aregawi D, Winiarska M, Agloria M, Luo G, Sieve L, Wang P: Metformin-diet ameliorates coronary heart disease risk factors and facilitates resumption of regular menses in adolescents with polycystic ovary syndrome. J Pediatr Endocrinol Metab 19:831–842, 2006 [DOI] [PubMed] [Google Scholar]
- Jin H, Meyer JM, Jeste DV: Phenomenology of and risk factors for new-onset diabetes mellitus and diabetic ketoacidosis associated with atypical antipsychotics: An analysis of 45 published cases. Ann Clin Psychiatry 14:59–64, 2002 [DOI] [PubMed] [Google Scholar]
- Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ: Effect of metformin in pediatric patients with type 2 diabetes: A randomized controlled trial. Diabetes Care 25:89–94, 2002 [DOI] [PubMed] [Google Scholar]
- Kay JP, Alemzadeh R, Langley G, D'Angelo L, Smith P, Holshouser S: Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism 50:1457–1461, 2001 [DOI] [PubMed] [Google Scholar]
- Kenward MG, Roger JH: Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 53:983–997, 1997 [PubMed] [Google Scholar]
- Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA: A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. Am J Psychiatry 163:2072–209, 2006 [DOI] [PubMed] [Google Scholar]
- Morrison JA, Cottingham EM, Barton BA: Metformin for weight loss in pediatric patients taking psychotropic drugs. Am J Psychiatry 159:655–657, 2002 [DOI] [PubMed] [Google Scholar]
- Paolisso G, Amato L, Eccellente R, Gambardella A, Tagliamonte MR, Varricchio G, Carella C, Giugliano D, D'Onofrio F: Effect of metformin on food intake in obese subjects. Eur J Clin Invest 28:441–446, 1998 [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Ambler GR, Baur LA, Garnett SP, Tepsa M, Yap F, Ward GM, Cowell CT: Randomized, controlled trial of metformin for obesity and insulin resistance in children and adolescents: Improvement in body composition and fasting insulin. J Clin Endocrinol Metab 91:2074–2080, 2006 [DOI] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA: Symptom dimensions in recent-onset schizophrenia and mania: A principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res 97: 129–135, 2000 [DOI] [PubMed] [Google Scholar]
- Washington State Department of Social and Health Services. Monitoring Of Side Effects Scale (MOSES). 2003. www.dshs.wa.gov/pdf/ms/forms/10_334.pdf Last accessed May25, 2009
- Webb E, Viner R: Should metformin be prescribed to overweight adolescents in whom dietary/behavioural modifications have not helped? Arch Dis Child 91:793–794, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Zhao J, Jin H, Shao P, Fang M, Guo X: Life-style intervention and metformin for treatment of antipsychotic-induced weight gain. JAMA 299:185–193, 2008a [DOI] [PubMed] [Google Scholar]
- Wu RR, Zhao JP, Guo XF, He YQ, Fang MS, Guo WB, Chen JD, Li LH: Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: A double-blind, placebo-controlled study. Am J Psychiatry 165:352–358, 2008b [DOI] [PubMed] [Google Scholar]


