Abstract
Allylmethylsulfide (AMS), a volatile organosulfur derivative from garlic, has been shown to have radioprotective effects in radiation-challenged cell and animal models, but the mechanism of radioprotection is not well understood. To determine the mechanism of radioprotection in an in vivo model, we first verified the antioxidant capacity of AMS using 2,2′-azobis(2-amidinopropane) dihydrochloride-induced human embryonic kidney 293T cells by measuring reactive oxygen species generation, reduced glutathione, protein tyrosine kinase/protein tyrosine phosphatase balance, and nuclear factor-κB (NF-κB) protein levels. We then investigated the protective effects of AMS (55 and 275 μmol/kg, intraperitoneal treatment) on 15 Gy X-ray-irradiated mouse kidney. The results showed that AMS decreased the free radical-induced lipid peroxidation in mice exposed to X-rays. Moreover, the antioxidative AMS suppressed the activation of NF-κB and its dependent genes such as vascular cell adhesion molecule-1, inducible nitric oxide synthase, and cyclooxygenase-2 through inhibition of IκBα phosphorylation and activation of IκB kinase α/β and mitogen-activated protein kinases (MAPKs). Based on these results, AMS may be a useful radioprotective agent by down-regulating the MAPKs and NF-κB signaling pathway that can be induced via X-ray irradiation.
Key Words: allylmethylsulfide, mitogen-activated protein kinases, nuclear factor κB signaling, oxidative stress, radioprotection, X-rays
Introduction
Ionizing radiation has been known to cause harmful effects on cells and biological systems. First, it can increase the generation of highly reactive oxygen species (ROS), which can induce a number of detrimental biological changes including disruption of DNA,1 lipid peroxidation,2 and inactivation of enzymes.3 Second, ionizing events through large amounts of ROS promote activation of receptors and intracellular signaling pathways via activating protein tyrosine kinase (PTK)4 and inhibiting protein tyrosine phosphatase (PTP) activities.5
X-rays are ionizing radiation that carries enough energy to ionize an atom or molecule. These ionizations can be very destructive to living tissues6 and may destroy the genetic material of the cell, which can induce an inflammatory response within irradiated tissues.7,8 Reports also show that the inflammatory response induced through radiation occurs through a series of cascades, such as activation of mitogen-activated protein kinase (MAPK), and up-regulation of redox-sensitive transcription factors, including nuclear factor-κB (NF-κB).9 The radiation-induced activation of MAPKs, which associates the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 in mammalian cells, plays a major role in mediating cellular proliferation, differentiation, inflammatory responses, and ultimately cell death.10–12 Moreover, MAPKs are an important upstream regulator of the NF-κB signaling pathway, and activation of NF-κB via MAPKs results in the production of pro-inflammatory genes including tumor necrosis factor-α, interleukin, cyclooxygenase-2 (COX-2), and vascular cell adhesion molecule-1 (VCAM-1).13 Cytosolic NF-κB is found predominantly as a heterodimer of p65 and p50 subunits, complexed by an inhibitory subunit, IκBα. The exposure of cells to various stimuli results in the activation of IκB kinase (IKK), leading to the phosphorylation and degradation of IκBα. Then, NF-κB translocates to the nucleus, where it binds to the promoter region of the target gene and induces transcription.14 Thus, inhibition of NF-κB activation is a useful strategy for attenuating the radiation-induced inflammatory response,9,15 and the signaling pathways leading to activation of NF-κB are important targets for radioprotection.
Allylmethylsulfide (AMS) is an organosulfur derivative of garlic that has been shown to have radioprotective properties in radiation-induced cell and animal models. Previous studies have shown that garlic plays important pharmacological role for cancer, heart disease, thrombosis, hypertension and hyperlipidemia.16–18 The potential mechanisms through which garlic induces these beneficial effects include its potent antioxidant action, activation of the immune system, and inhibition of prostaglandin production.19–21 However, the mechanism through which AMS exerts its radioprotective effects is not well studied. In addition, recent studies demonstrated that the antioxidant effects of allicin, a garlic component, inhibited intercellular adhesion molecule expression in gamma-irradiated human vascular endothelial cells.22
In the present study, fundamental data on the antioxidative effect of AMS were obtained using human embryonic kidney (HEK) 293T cells. Based on the in vitro antioxidative data for AMS, the present study was designed to determine whether AMS exhibited radioprotective capacity through modulation of inflammation in X-ray-irradiated mice. We also examined the effect of AMS on NF-κB signaling pathways via MAPKs and IKKα/β activations in X-ray-exposed mice.
Materials and Methods
Mice, radiation, and AMS treatment
The 8–10-week-old male C57/BL6 mice used in this study were obtained from Samtako (Osan, Republic of Korea). The research protocol was approved by the Pusan National University (Busan, Republic of Korea) Institutional Animal Care and Use Committee. Mice were housed five per cage and acclimatized for at least 1 week before starting the experiment. The mice were divided into four groups of five animals each: normal, irradiation (control), and irradiation with AMS (55 or 275 μmol/kg) treatment. The mice in AMS-treated groups were injected intraperitoneally for 3 days before X-ray irradiation, and those in the other groups were injected with saline. For whole-body irradiation, anesthetized mice were exposed to 15 Gy X-irradiation using a Clinac 21 EX (Varian Medical System, Palo Alto, CA) X-ray generator operating at an exposure rate of 6 Gy/minute. The mice were killed 24 hours after 15 Gy X-ray irradiation, and the kidneys were quickly removed and rinsed in ice-cold buffer (100 mM Tris [pH 7.4], 1 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), 1 μM pepstatin, 2 μM leupeptin, 80 mg/L trypsin inhibitor, 20 mM β-glycerophosphate, 20 mM NaF, and 2 mM sodium orthovanadate). The tissue was immediately frozen in liquid nitrogen and stored at −80°C. For the current study, we chose to examine the kidney because it is the most radio-sensitive of the abdominal organs.23
Reagents
AMS was purchased from Sigma (St. Louis, MO). 2′,7′-Dichlorofluorescin diacetate (DCFDA), 3,6-fluorescein diphosphate, and the Antibody Beacon™ tyrosine kinase assay kit were obtained from Molecular Probes, Inc. (Eugene, OR). The LPO-586™ assay kit was obtained from Oxis Health, Inc. (Foster, CA). 2,2′-Azobis(2-amidinopropane) dihydrochloride (AAPH) was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). A horseradish peroxide-conjugated secondary antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Polyvinylidene difluoride membranes were obtained from Millipore Corp. (Bedford, MA). All other chemicals were of the highest purity available from Sigma.
Cell culture conditions and cell lysis
HEK293T cells were obtained from American Type Culture Collection (Rockville, MD). The cells were cultured in Dulbecco's Modified Eagle's Medium (Nissui Co., Tokyo, Japan) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY), glutamine at 233.6 mg/mL, penicillin-streptomycin at 100 μg/mL, and amphotericin B at 0.25 μg/mL adjusted to pH 7.4–7.6 with NaHCO3 in an atmosphere of 5% CO2. The fresh medium was replaced after 1 day to remove nonadherent cells or cell debris.
Cells were washed with phosphate-buffered saline, and then 1 mL of ice-cold phosphate-buffered saline (PBS) was added. Pellets were harvested by centrifugation at 950 g at 4°C for 5 minutes. The pellets were suspended in 10 mM Tris (pH 8.0), 1.5 mM MgCl2, 1 mM dithiothreitol (DTT), 0.1% Nonidet (N) P-40, and protease inhibitors and incubated on ice for 15 minutes. Nuclear proteins were separated from cytosol by centrifugation at 14,000 g at 4°C for 15 minutes. The supernatants were used as the cytosolic fraction, and the pellets were resuspended in 10 mM Tris (pH 8.0), 50 mM KCl, 100 mM NaCl, and protease inhibitors, incubated on ice for 30 minutes, and then centrifuged at 14,000 g at 4°C for 30 minutes. The resultant supernatants were used as the nuclear fraction.
Assessment of ROS and reduced glutathione (GSH) assay in HEK293T cells
To determine the intracellular ROS scavenging activity of HEK293T cells, cells were seeded in a 96-well plate. After 1 day, the medium was changed to a fresh serum-free medium. The cells were treated with or without AMS and were preincubated for 6 hours. After a 1-hour treatment with AAPH (100 μM), an ROS generator, the medium was replaced with fresh serum-free medium, and then 2′,7′-DCFDA (20 μM) was added. Total ROS were measured at a fluorescence intensity of dichlorofluorescin for 30 minutes. The fluorescence was used at excitation and emission wavelengths of 485 and 530 nm, respectively.24
For detection of GSH levels, 25% meta-phosphoric acid was added to cell lysates and then centrifuged at 12,000 g for 10 minutes. The supernatants were taken for assay. Buffer containing 1 mM EDTA and 50 mM phosphate was added to the samples followed by addition of o-phthaladehyde (0.1 mg/mL). After 20 minutes at room temperature, the fluorescence was measured at an excitation of 360 nm and emission of 485 nm.25
Measurement of PTK and PTP in HEK293T cells
PTK activity
PTK activities in cell lysates were assayed with the Antibody Beacon tyrosine kinase assay kit. To detect the tyrosine kinase activity, samples were prepared in 1 × kinase buffer (100 mM Tris-HCl [pH 7.5], 20 mM MgCl2, 2 mM EGTA, 2 mM DTT, and 0.02% Brij 35) and mixed with the Antibody Beacon detection complex plus substrate in the 96-well microplate. Then, ATP reagent was added to the plate and continuously incubated at the reaction temperature. Fluorescence was measured at multiple time points on a GENios (Tecan Systems, Inc., San Jose, CA) with excitation and emission wavelengths set at 485 and 535 nm, respectively.
PTP activity
3,6-Fluorescein diphosphate is a very sensitive fluorogenic substrate for assaying PTP activity. To detect the tyrosine phosphatase activity, samples were prepared in PTP assay buffer (50 mM Tris-HCl [pH 6.3], 2 mM EGTA, 5 mM DTT, and 100 μM CaCl2) and mixed with 100 μM 3,6-fluorescein diphosphate in the 96-well microplate. The plate was incubated at the reaction temperature for 5 minutes, and then fluorescence was measured at multiple time points on the GENios with excitation and emission wavelengths set at 485 and 535 nm, respectively.
Preparation of kidney tissue
Three hundred milligrams of frozen kidney tissue was washed with PBS and homogenized in 2 mL of hypotonic lysis buffer (buffer A; 10 mM HEPES [pH 7.8], 10 mM KCl, 2 mM MgCl2, 1 mM DTT, 0.1 mM EDTA, and 0.1 mM PMSF) using a tissue homogenizer for 20 seconds. Homogenates were kept on ice for 15 minutes, 125 μL of 10% N P-40 solution was added and mixed for 15 seconds, and the mixture was centrifuged for 2 minutes at 14,000 g. The pelleted nuclei was washed once with 400 μL of buffer A plus 25 μL of 10% N P-40, centrifuged, suspended in 200 μL of buffer C (50 mM HEPES [pH 7.8], 50 mM KCl, 300 mM NaCl, 0.1 mM PMSF, and 10% [vol/vol] glycerol), kept on ice for 30 minutes, and centrifuged for 10 minutes at 14,000 g. The supernatant (nuclear protein) was harvested and then stored at −80°C.24
Assessment of malondialdehyde (MDA)/4-hydroxyalkenals (HAE) in mice kidney
MDA/HAE concentrations were determined by using an LPO-586 assay kit for evaluating lipid peroxidation. The kit uses a chromatogenic reagent that reacts with the lipid peroxidation products MDA and HAE, yielding a stable chromophore with maximum absorbance at 586 nm.
Protein measurement by western blot
NF-κB protein expression in kidney tissue or cell lysates was confirmed by western blot analysis. Aliquots (30 μg) of nuclear fraction were separated on sodium dodecyl sulfate-polyacrylamide mini-gel using a Laemmli buffer system26 and transferred to a polyvinylidene difluoride membrane at 100 V for 1.5 hours. The membrane was blocked using 5% nonfat milk in 10 mM Tris (pH 7.5), 100 mM NaCl, and 0.1% Tween-20 at room temperature for 1 hour. The blot was incubated with anti-p-p65 (1:1,000, Cell Signaling, Danvers, MA) and p65 and p50 (1:500; Santa Cruz Biotechnology) antibody, followed by anti-rabbit IgG secondary antibody (1:5,000; Santa Cruz Biotechnology), at 25°C for 2 hours, respectively. Antibody labeling was detected using enhanced chemiluminescence (Amersham Pharmacia Biotech, Little Chalfont, UK) following the manufacturer's instructions and exposed to Hyperfilm™ (Amersham Pharmacia Biotech).
Statistical analysis
The statistical significance of the difference between the groups was determined by one-factor analysis of variance (ANOVA) followed by Fisher's Protected Least Significant Difference (LSD) post hoc test. Values of P < .05 were considered statistically significant.
Results
Antioxidant effects of AMS on AAPH-induced oxidative stress in HEK293T cells
The antioxidant effects of AMS on ROS generation and GSH depletion are demonstrated in cultured HEK293T cells. In this experiment, AAPH is known to mediate free radical-induced oxidation,27 and it results in ROS formation and GSH depletion in HEK293T cells. Figure 1A clearly revealed that AMS significantly decreased AAPH-induced ROS generation at a level similar to N-acetylcysteine (NAC), a positive control in HEK293T cells. GSH depletion in response to AAPH was restored to control levels by AMS treatment (1 μM and 5 μM), respectively (Fig. 1B). Moreover, the GSH boosting at 5 μM AMS was higher than that of NAC. The results indicated therefore that AMS can act as an antioxidant agent.
FIG. 1.
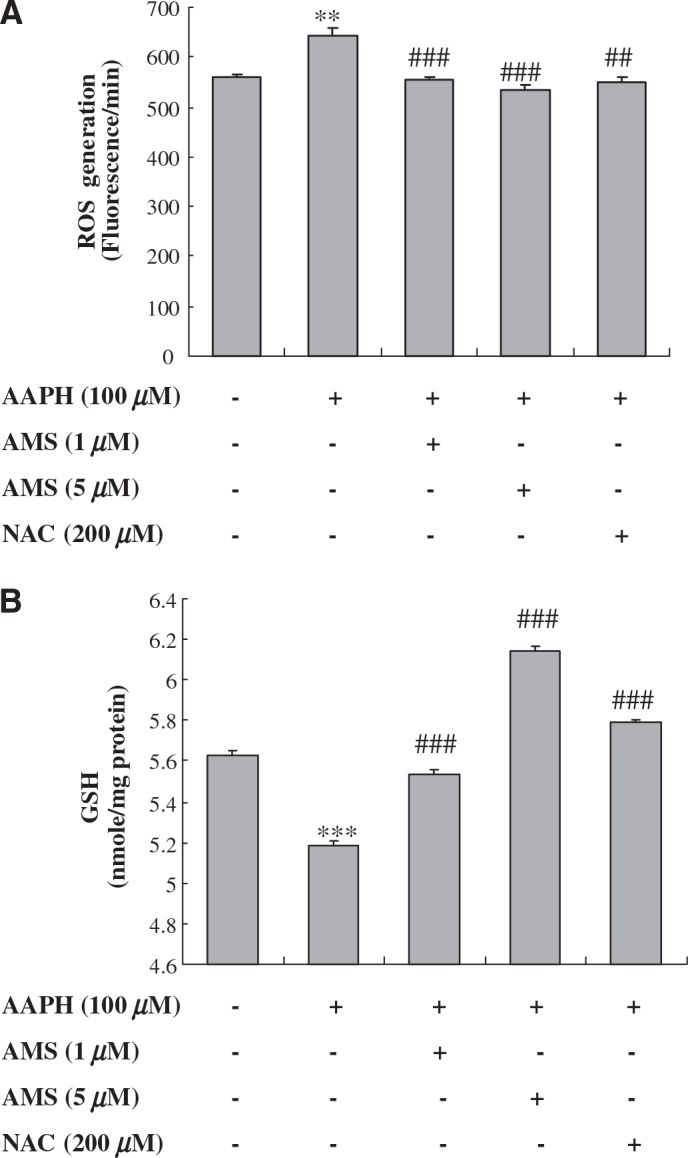
Effect of AMS on AAPH-induced (A) ROS generation and (B) GSH depletion in HEK293T cells. Cells were preincubated with or without AMS (1 μM or 5 μM) or N-acetylcysteine (NAC) (200 μM) for 6 hours prior to AAPH treatment. ROS and GSH levels were measured 1 hour after AAPH treatment. Three independent experiments were performed with quintuple assays. Results of one-factor ANOVA followed by Fisher's protected LSD post hoc test were used: **P < .01, ***P < .001 versus untreated control; ##P < .01, ###P < .001 versus AAPH-treated cells, respectively.
Modulatory effects of AMS on AAPH-stimulated PTK/PTP status in HEK293T cells
To elucidate the modulatory effects of AMS on PTK/PTP imbalance in oxidative conditions induced by AAPH, PTP and PTK were measured using fluorometric assays in HEK 293T cell lysates (Fig. 2). As shown in Figure 2A, the AAPH-induced increase of PTK activity was suppressed in the AMS-treated group at a level similar to the control group. On the other hand, decreased PTP activity in AAPH-treated lysate was increased to be significant in the AMS-treated group (Fig. 2B). From these results, the PTK/PTP ratio was obtained as depicted in Figure 2C. Data in Figure 2C showed an increase in the PTK/PTP ratio for AAPH, whereas AMS treatment showed the reversed ratio in a dose-dependent manner. These data suggest that AMS modulates PTK/PTP imbalance.
FIG. 2.
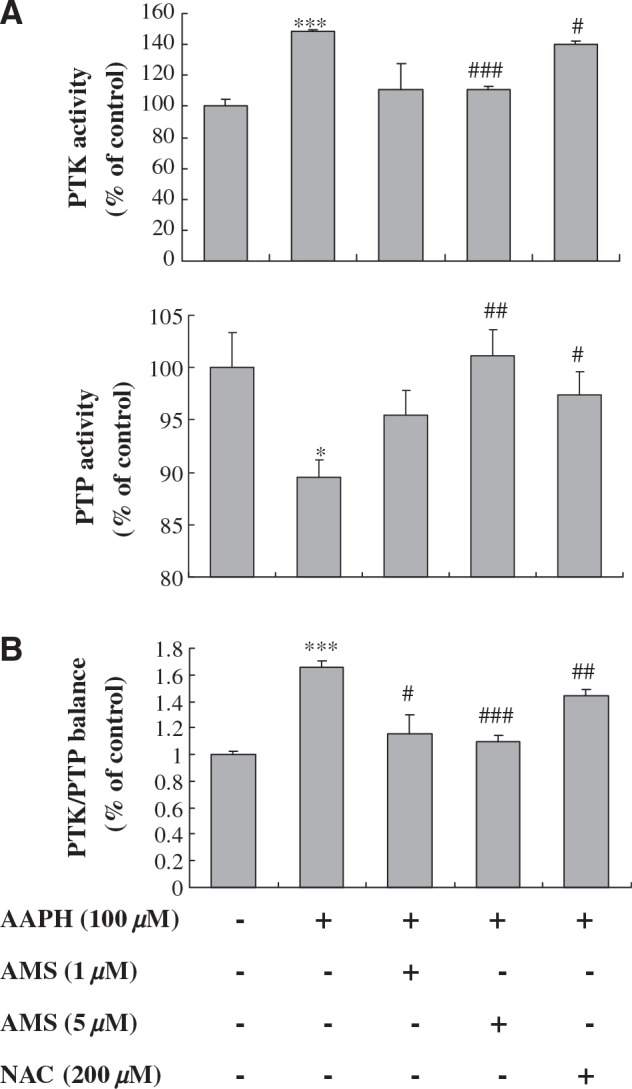
Effect of AMS on AAPH-induced PTK/PTP imbalance in HEK293T cells: (A) PTK and PTP activities and (B) PTK/PTP ratio. Cells were preincubated with or without AMS (1 μM or 5 μM) or N-acetylcysteine (NAC) (200 μM) for 6 hours prior to AAPH treatment. PTK and PTP activities were measured 1 hour after AAPH treatment and applied to measure the PTK/PTP ratio in cell lysate. The balance point is set to the value that PTK/PTP ratio is 1.0 for the control group. Data are mean ± SE values of triplicate assays in five separate experiments. Results of one-factor ANOVA followed by Fisher's protected LSD post hoc test were used: *P < .05, ***P < .001 versus untreated control; #P < .05, ##P < .01, ###P < .001 versus AAPH-treated cells, respectively.
Inhibitory effects of AMS on NF-κB activation in HEK293T cells
The nuclear translocation of NF-κB is facilitated as a heterodimer of a p50 and a p65 subunit that function predominantly as transcriptional activators. The subunit p65 is known to undergo phosphorylation, leading to nuclear translocation and binding to a specific DNA sequence.28 Because ROS are responsible for regulation on the transcriptional pathways of NF-κB29 and are known to elevate NF-κB, we evaluated the effect of AAPH on the activation of NF-κB in the HEK293T cell line. In the current study, to determine p-p65, p65, and p50 activation, we examined protein levels by western blot using p-p65-, p65-, and p50-specific antibodies. As shown in Figure 3, nuclear translocation of p-p65 and p65 significantly increased in AAPH-stimulated nuclear lysate, whereas the AMS-treated group showed lower levels of p-p65 and p65. On the other hand, we found that AAPH did not influence p50 activation. AMS treatment inhibited AAPH-induced NF-κB activation in cultured cells.
FIG. 3.
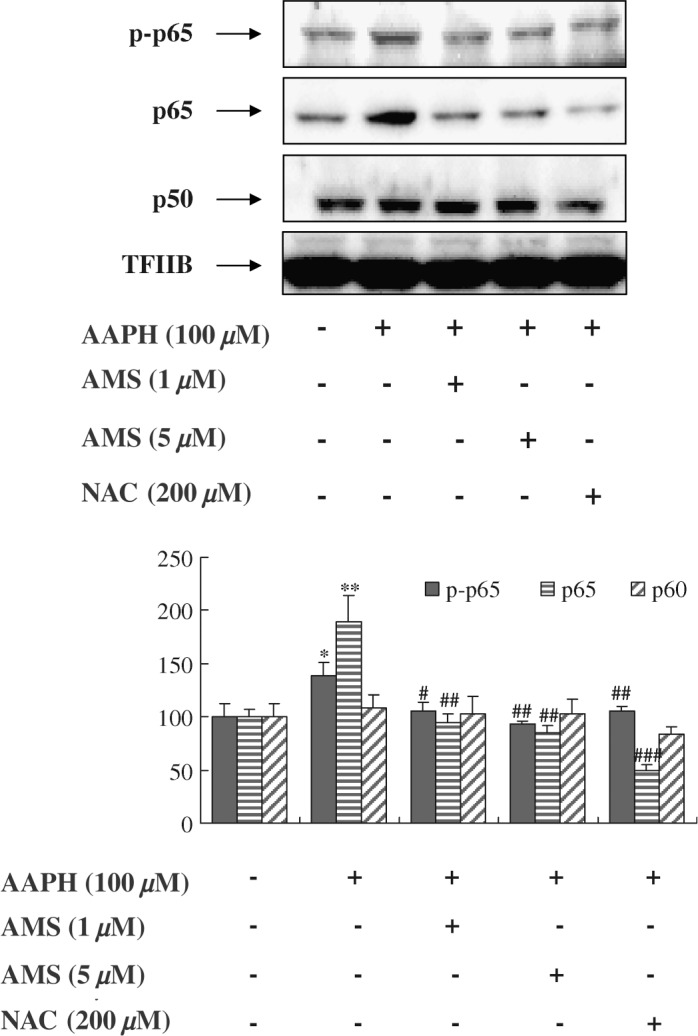
Effect of AMS on NF-κB activation in HEK293T cells. HEK293T cells were grown to 80% confluence in 100-mm-diameter dishes in Dulbecco's Modified Eagle's Medium and then stimulated with AAPH with or without AMS (1 μM or 5 μM) or N-acetylcysteine (NAC) (200 μM) for 6 hours. (Top panel) Western blot was performed to detect p-p65, p65, and p50 protein levels in nuclei (30 μg of protein) from HEK293T cells. Levels were normalized to transcription factor ΠB (TFΠB). One representative blot of each protein is shown from three experiments that yielded similar results. (Bottom panel) Values are the relative optical intensity of each band normalized as a percentage of the untreated control. Results of one-factor ANOVA followed by Fisher's protected LSD post hoc test were used: *P < .05, **P < .01 versus untreated control; #P < .05, ##P < .01, ###P < .001 versus AAPH-treated cells, respectively.
Inhibitory effects of AMS on lipid peroxidation induced by X-rays
To follow up on our in vitro observations, we tested the effect of AMS on the X-ray-induced oxidative stress in an animal model using C57/BL6 mice. One of the hallmarks of X-ray-induced oxidative stress is the formation of oxidized macromolecules, including lipid peroxidation.30 We therefore used lipid peroxidation as a marker of radiation-related oxidative damage. Measurement of MDA and HAE has been used as an indicator of lipid peroxidation. To elucidate the inhibitory effects of AMS on MDA/HAE formation induced by X-rays, MDA/HAE was measured with an assay kit on kidney homogenates. When mice were exposed to X-rays, the level of lipid peroxidation was increased to 1.48-fold (P < .001) of the levels in control (non–X-ray-exposed) mice. Supplementing with AMS resulted in significant inhibition of the levels of lipid peroxidation induced by X-rays (Fig. 4).
FIG. 4.
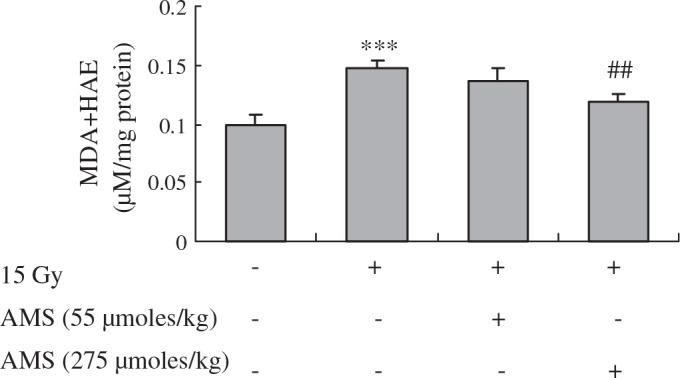
Influence of AMS on lipid peroxidation in X-ray-exposed mouse kidney. MDA+HAE assays were performed as described in Materials and Methods. Data are shown for kidneys of mice 6 hours after 15 Gy irradiation. Data are mean ± SE values representing triplicate assays of five mice from each group (n = 5). Results of one-factor ANOVA followed by Fisher's protected LSD post hoc test were used: ***P < .001 versus nonexposed mouse kidney; ##P < .01 versus X-ray-exposed mouse kidney, respectively.
Inhibitory effects of AMS on X-ray-induced phosphorylation of MAPKs
X-ray-induced oxidative stress has been implicated in phosphorylation of MAPKs.31–33 We determined the effect of AMS on X-ray-induced phosphorylation of the JNK, ERK1/2, and p38 proteins of the MAPK family in mouse kidney using western blotting. Western blot results indicated that exposure of mice to X-rays enhanced the phosphorylation of JNK protein compared with non–X-ray-exposed mouse kidney (Fig. 5). However, supplementing with AMS (55 or 275 μmol/kg) inhibited X-ray-induced phosphorylation of JNK in the mouse kidney compared with non–AMS-supplied but X-ray-exposed mice. The inhibitory effect of AMS at the dose of 275 μmol/kg on X-ray-induced phosphorylation of JNK was significantly greater than the dose of 55 μmol/kg AMS. Also, supplementing with AMS inhibited X-ray-induced phosphorylation of ERK1/2 and p38 in mouse kidney.
FIG. 5.
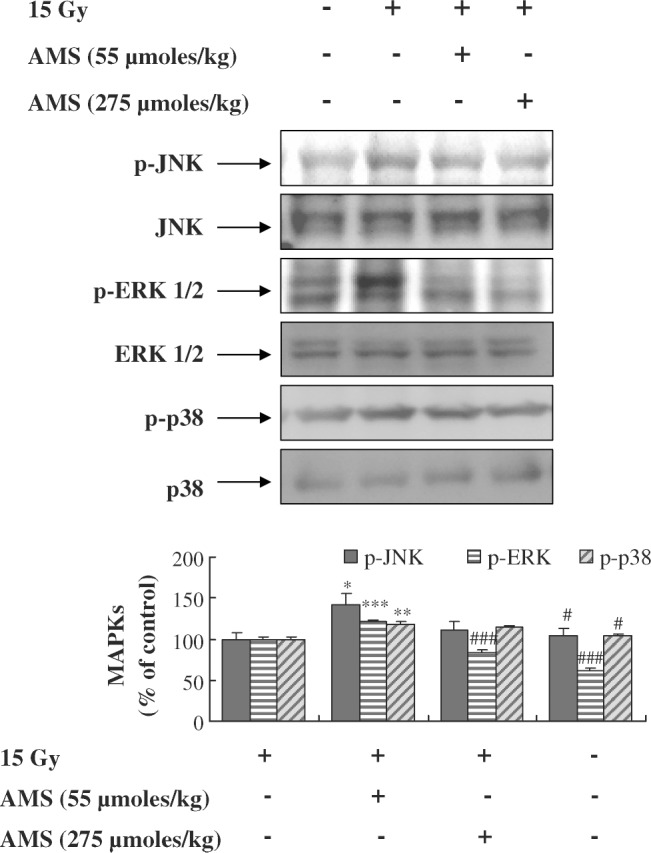
Effect of AMS on X-ray-exposed phosphorylation of MAPKs in mouse kidney. Mice were treated as described in Materials and Methods. Kidney homogenates were prepared to determine the phosphorylated levels of JNK, ERK, and p38 using western blot. A representative blot is shown from three independent experiments with identical observations (n = 5), and equivalent protein loading was confirmed by probing stripped blots for total JNK, EKR1/2, and p38. Blot density was detected with FluorChem and standardized to a percentage of the untreated group. Results of one-factor ANOVA followed by Fisher's protected LSD post hoc test were used: *P < .05, **P < .01, ***P < .001 versus nonexposed mouse kidney; #P < .05, ###P < .001 versus X-ray-exposed mouse kidney, respectively.
Suppressive effects of AMS on NF-κB activation and the degradation of IκBα induced by X-rays in mouse kidney
NF-κB activation is mediated by MAPK signaling pathways.13 Our western blot analysis indicated that exposure of mice to X-rays resulted in markedly greater activation of p50 and p65 translocation to the nucleus than non–X-ray-exposed control mice. However, supplementing mice with AMS (55 μmol/kg and 275 μmol/kg) resulted in inhibition of translocation of p65 and activation of p50 to the nucleus compared with non–AMS-supplied but X-ray-exposed mice (Fig. 6A). This finding was consistent with our data that oxidative stress and phosphorylation of MAPKs are higher in X-ray-irradiated mice.
FIG. 6.
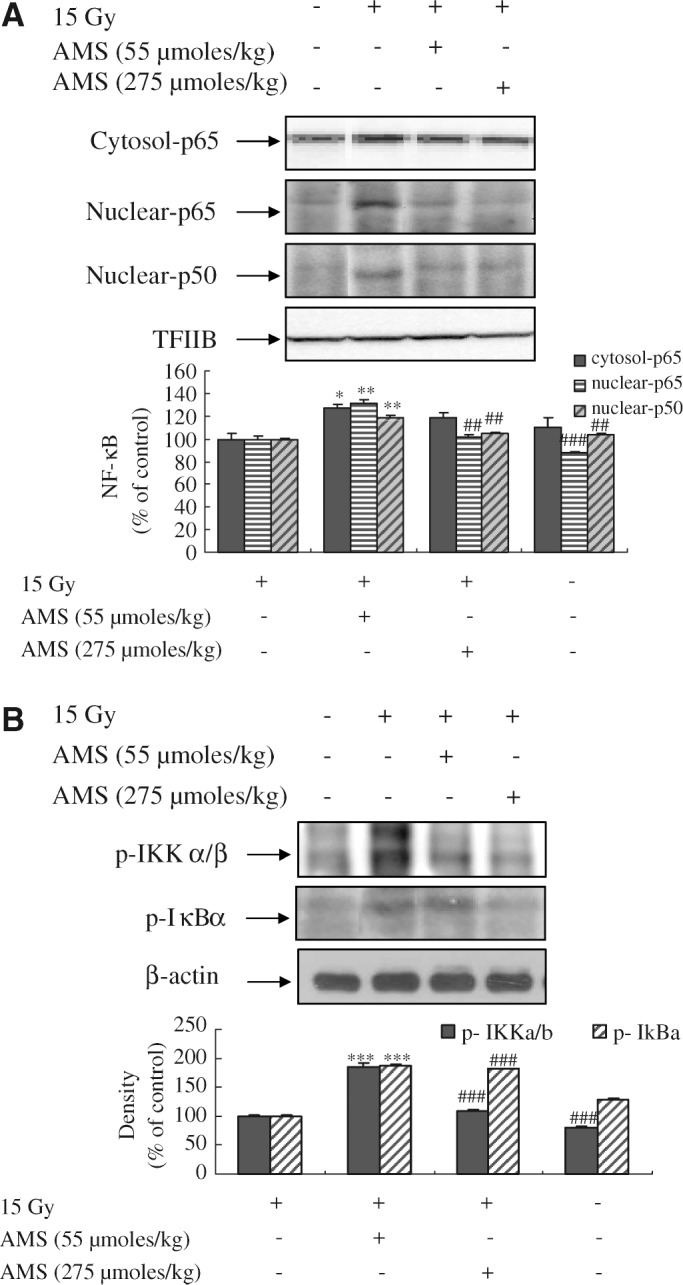
Effect of AMS on X-ray-induced activation of NF-κB, phospho-IκBα, and phospho-IKKα/β in mouse kidney. Mice were treated as described in Materials and Methods. The activations of (A) NF-κB and (B) p-IκBα and p-IKKα/β were determined using western blot. One representative blot of each protein is shown from three experiments that yielded similar results (n = 5). Equivalent protein loading was confirmed by probing stripped blots for transcription factor ΠB (TFΠB) and β-actin. Blot density was detected with FluorChem and standardized as a percentage of the untreated group. Results of one-factor ANOVA followed by Fisher's protected LSD post hoc test were used: *P < .05, **P < .01, ***P < .001 versus nonexposed mouse kidney; ##P < .01, ###P < .001 versus X-ray-exposed mouse kidney, respectively.
X-ray irradiation also resulted in activation of IKKα/β in the kidney compared with non–X-ray-exposed control mice. However, supplementing with AMS inhibited the activation levels of IKKα/β in cytosol compared with non–AMS-supplied but X-ray-exposed mice (Fig. 6B). The induction of IKKα/β has been shown to be essential for X-ray-induced phosphorylation and degradation of IκBα. Phosphorylation of IκBα was higher in X-ray-exposed mice compared with non–X-ray-exposed mice, whereas supplementing with AMS at doses of 55 μmol/kg and 275 μmol/kg inhibited X-ray-induced phosphorylation of IκBα. These data indicate that AMS modulated X-ray-exposed IKKα/β activity.
Suppressive effects of AMS on expression of NF-κB responsive proteins in X-ray-irradiated mouse kidney
We next examined whether AMS has the ability to inhibit the induction of NF-κB-responsive proteins, such as VCAM-1, inducible nitric oxide synthase (iNOS), and COX-2, in X-ray-exposed mouse kidney. As shown in Figure 7, exposure of mice to X-rays markedly enhanced the expression of pro-inflammatory VCAM-1, iNOS, and COX-2 proteins compared with control non–X-ray-exposed mice. The supplementing AMS at doses of 55 μmol/kg and 275 μmol/kg resulted in marked inhibition of X-ray-induced expression of VCAM-1, iNOS, and COX-2 proteins. These data suggest that AMS suppressed pro-inflammatory gene expression induced by X-rays.
FIG. 7.
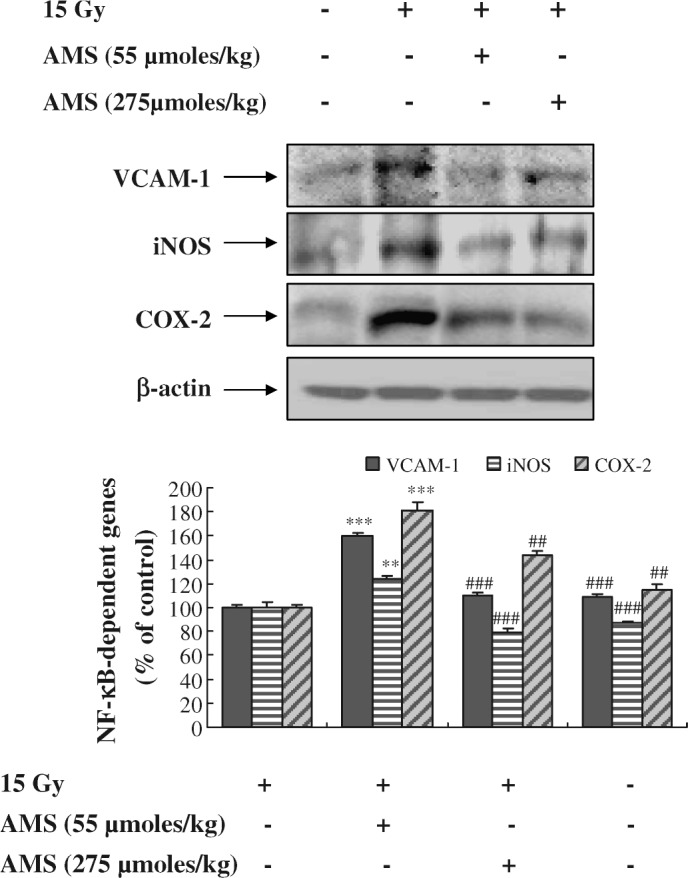
Effect of AMS on X-ray-induced activation of NF-κB-dependent genes in mouse kidney. Mice were treated as described in Materials and Methods. Kidney homogenates were prepared to determine the levels of VCAM-1, iNOS, and COX-2 using western blot. A representative blot is shown from three independent experiments with identical observations (n = 5), and equivalent protein loading was confirmed by probing for β-actin. Blot density was detected with FluorChem and standardized as a percentage of the untreated group. Results of one-factor ANOVA followed by Fisher's protected LSD post hoc test were used: **P < 0.01, ***P < .001 versus nonexposed mouse kidney; ##P < .01, ###P < .001 versus X-ray-exposed mouse kidney, respectively.
Discussion
Ionizing radiation induces inflammation in most tissues, and NF-κB activation is one of the important inflammatory mediators in radiation-exposed tissues.7,15 Over the past few decades, a number of active compounds with potential radioprotective effects have been studied at the cellular level and in animal models.34–36 In particular, sulfur compounds have been tested in animal models for their possible protective effects against inflammation induced by radiation.37,38 In the present study, we used AMS, an organic sulfur compound from garlic, that is known to act as an antitumor agent by inhibiting cytochrome P450 2E1 protein as well as an anti-inflammatory agent by suppressing nitric oxide production in LPS-stimulated macrophages.39,40 However, the radioprotective effects of AMS on X-ray-induced NF-κB activation in mouse kidney have not been thoroughly examined.
The present study was designed to define the radioprotective mechanism of AMS against X-ray irradiation. For this purpose, we used the HEK293T cell line and C57/BL6 mouse models. We found that AMS has powerful antioxidant effects that can modulate the effects of AAPH-induced redox imbalance in a cell culture system and also suppress activation of NF-κB, a key inflammatory transcription factor. Ionizing radiation interacting with water in cells results in the production of ROS, such as hydroxyl radicals, hydrogen radicals, and hydrogen peroxide, and thus can induce cell damage and, potentially, cell dysfunction and death.41 Therefore, screening of radioprotective agents should include the examination of their ability to ameliorate free radical production and related inflammation. Moreover, we speculated that AMS would exert protective effects against X-ray-exposed kidney inflammation. Based on the results from the cell culture system, we expected that AMS supplementation may protect against X-ray-irradiation-induced kidney damage.
X-ray-induced oxidation of lipids has been used as a marker of oxidative stress.29 In the present study, we confirmed that lipid peroxidation was increased in the X-ray-exposed kidney and also showed that formation of MDA, a lipid peroxidation product, was significantly inhibited by AMS supplementation in X-ray-exposed kidney. The formation and accumulation of lipid peroxidation products have been reported to result in tissue damage, inflammation, and malignancies.42 Thus, inhibition of X-ray-induced lipid peroxidation by AMS in mouse kidney would likely reduce risk factors associated with oxidative stress-mediated pro-inflammatory effects of X-ray irradiation.
Several studies have recently reported that low-dose ionizing radiation from 0.02 to 0.05 Gy causes phenomenon such as activity of cell metabolism or proliferation of normal human diploid cells through the MAPK signaling pathways, which suggests that the activation of the MAPK pathways has cytoprotective effects.43,44 In contrast, high-dose ionizing radiation from 2 to 50 Gy is well established to exert pro-inflammatory effects.45 In addition, the MAPK signaling pathway is known to become activated upon high-dose ionizing radiation in many cells, including macrophages.10 MAPK signaling cascades have been identified to be ERK activated by tumor promoters and agonists for tyrosine kinase-encoded receptors,46 JNK and p38 MAPK, in response to pro-inflammatory cytokines and other environmental stresses.11,12 Our data show that X-ray-induced phosphorylations of ERK, JNK, and p38 in mouse kidney were prevented by AMS supplementation. It is well known that X-rays induce oxidative stress production in target cells, which in turn initiates phosphorylation of MAPKs, activation of downstream signals, and expression of genes involved in the inflammatory response.47 Therefore, we confirmed that AMS supplementation prevents phosphorylation of MAPKs by inhibiting X-ray-induced oxidative stress.
Inhibition of NF-κB activation appears to be a promising target for reducing radiation-induced inflammation.9 One of the most effective protectors developed was WR-2721 or amifostine, which is a synthetic thiol compound.37,38 Amifostin has been used in clinical trials, and it is approved by the Food and Drug Administration as a radioprotector. Although amifostine or WR-2721 showed good radioprotection effects, its toxicity at optimum protective doses has motivated the search for natural compounds that have low toxicity and can be administrated easily.37
The activation of NF-κB by extracellular stimuli depends on the phosphorylation and subsequent degradation of IκBα, through IKKα/β, serine/threonine kinases.14 Based on our western blot results, we observed that AMS inhibits phosphorylation of IκBα, activation of IKKα/β, and translocation into the nucleus of p65 in X-ray-exposed mice kidney. Therefore, our findings involving X-ray-irradiated kidney inflammation suggest AMS is a potential radioprotector. Activation of NF-κB is important for the initiation of inflammation, which up-regulates the expression of pro-inflammatory cytokines and inflammatory genes. Consequently, ionizing radiation-induced expression of various inflammatory genes has been proposed to contribute to radiation injury in normal tissues.48,49 Moreover, other radioprotectors, such as polyphenols and green tea, have shown almost similar mechanisms of action against ionizing radiation-induced biological effects.50,51 Therefore, our findings regarding the effects of AMS on this pathway suggest that it represents a key molecular target of AMS.
NF-κB regulates the transcription of pro-inflammatory molecules, including COX-2, iNOS, and VCAM-1.52 Most of these genes have been shown in radiation-induced inflammation, and expression of these genes was suppressed through NF-κB inhibition.13 In contrast, inhibition of COX-2 stimulates apoptosis in cancer models. However, our models focused on inflammation induction by radiation, and COX-2 has been shown to be up-regulated in cells exposed to radiation.
The present results showed that enhanced levels of NF-κB-responsive proteins in X-ray-exposed mouse kidney were suppressed by AMS supplement. Based on our results, we suggest that the NF-κB pathway in X-ray-exposed kidney represents a key molecular target of AMS, a natural compound.
In conclusion, the present study indicates that AMS has the potential to attenuate the activation of the NF-κB pathway through MAPKs and IKKα/β in X-ray-irradiation-induced kidney inflammation. Based on the current findings, we propose that AMS mediates radioprotective effects through (a) prevention of oxidative damage of lipids and (b) suppression of activation of MAPKs, IKKα/β, and NF-κB signaling pathways in response to X-ray-irradiation-induced inflammation in kidney. Moreover, our findings suggest that AMS might be used to improve deleterious conditions related to X-ray-exposed kidney and other diseases.
Acknowledgments
This work was supported by Korea Science and Engineering Foundation grant 2007-00376 funded by the Korean Government. We also thank the Aging Tissue Bank for the supply of the X-ray-exposed kidney tissue.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Kim GJ, Fiskum GM, Morgan WF: A role for mitochondrial dysfunction in perpetuating radiation-induced genomic instability. Cancer Res 2006;66:10377–10383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider LA, Dissemond J, Brenneisen P, Hainzl A, Briviba K, Wlaschek M, Scharffetter-Kochanek K: Adaptive cellular protection against UVA-1-induced lipid peroxidation in human dermal fibroblasts shows donor-to-donor variability and is glutathione dependent. Arch Dermatol Res 2006;297:324–328 [DOI] [PubMed] [Google Scholar]
- 3. Punnonen K, Puntala A, Jansén CT, Ahotupa M: UVB irradiation induces lipid peroxidation and reduces antioxidant enzyme activities in human keratinocytes in vitro. Acta Derm Venereol 1991;71:239–242 [PubMed] [Google Scholar]
- 4. Kharbanda S, Yuan ZM, Rubin E, Weichselbaum R, Kufe D: Activation of Src-like p56/p53lyn tyrosine kinase by ionizing radiation. J Biol Chem 1994;269:20739–20743 [PubMed] [Google Scholar]
- 5. Mikkelsen RB, Wardman P: Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene 2003;22:5734–5754 [DOI] [PubMed] [Google Scholar]
- 6. Sutherland BM, Bennett PV, Sutherland JC, Laval J: Clustered DNA damages induced by X-rays in human cells. Radiat Res 2002;157:611–616 [DOI] [PubMed] [Google Scholar]
- 7. Hallahan DE, Virudachalam S, Kuchibhotla J: Nuclear factor kappaB dominant negative genetic constructs inhibit X-rays induction of cell adhesion molecules in the vascular endothelium. Cancer Res 1998;58:5484–5488 [PubMed] [Google Scholar]
- 8. Quarmby S, Kumar P, Kumar S: Radiation-induced normal tissue injury: role of adhesion molecules in leukocyte-endothelial cell interactions. Int J Cancer 1999;30;82:385–395 [DOI] [PubMed] [Google Scholar]
- 9. Sharma SD, Meeran SM, Katiyar SK: Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-kappaB signaling in in vivo SKH-1 hairless mice. Mol Cancer Ther 2007;6:995–1005 [DOI] [PubMed] [Google Scholar]
- 10. Dent P, Yacoub A, Fisher PB, Hagan MP, Grant S: MAPK in radiation responses. Oncogene 2003;22:1–12 [DOI] [PubMed] [Google Scholar]
- 11. Tomita M, Suzuki N, Matsumoto Y, Enomoto A, Yin HL, Hosoi Y, Hirano K, Sakai K: Wortmannin-enhanced X-ray-induced apoptosis of human T-cell leukemia MOLT-4 cells possibly through the JNK/SAPK pathway. Radiat Res 2003;160:467–477 [DOI] [PubMed] [Google Scholar]
- 12. Lee MR, Dominguez C: MAP kinase p38 inhibitors: clinical results and an intimate look at their interactions with p38alpha protein. Curr Med Chem 2005;12:2979–2994 [DOI] [PubMed] [Google Scholar]
- 13. Muselet-Charlier C, Roque T, Boncoeur E, Chadelat K, Clement A, Jacquot J, Tabary O: Enhanced IL-1beta-induced IL-8 production in cystic fibrosis lung epithelial cells is dependent of both mitogen-activated protein kinases and NF-kappaB signaling. Biochem Biophys Res Commun 2007;357:402–407 [DOI] [PubMed] [Google Scholar]
- 14. Chung HY, Sung B, Jung KJ, Zou Y, Yu BP: The molecular inflammatory process in aging. Antioxid Redox Signal 2006;8: 572–581 [DOI] [PubMed] [Google Scholar]
- 15. Berking C: Photocarcinogenesis. Molecular mechanisms and preventive strategies. Hautarzt 2007;58:398–405 [DOI] [PubMed] [Google Scholar]
- 16. Borek C: Garlic reduces dementia and heart-disease risk. J Nutr 2006;136:810S–812S [DOI] [PubMed] [Google Scholar]
- 17. McMahon FG, Vargas R: Can garlic lower blood pressure? A pilot study. Pharmacotherapy 1993;13:406–407 [PubMed] [Google Scholar]
- 18. Eilat S, Oestraicher Y, Rabinkov A, Ohad D, Mirelman D, Battler A, Eldar M, Vered Z: Alteration of lipid profile in hyperlipidemic rabbits by allicin, an active constituent of garlic. Coron Artery Dis 1995;6:985–990 [PubMed] [Google Scholar]
- 19. Gonen A, Harats D, Rabinkov A, Miron T, Mirelman D, Wilchek M, Weiner L, Ulman E, Levkovitz H, Ben-Shushan D, Shaish A: The antiatherogenic effect of allicin: possible mode of action. Pathobiology 2005;72:325–334 [DOI] [PubMed] [Google Scholar]
- 20. Ishikawa H, Saeki T, Otani T, Suzuki T, Shimozuma K, Nishino H, Fukuda S, Morimoto K: Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J Nutr 2006;136:816S–820S [DOI] [PubMed] [Google Scholar]
- 21. Chang HP, Chen YH: Differential effects of organosulfur compounds from garlic oil on nitric oxide and prostaglandin E2 in stimulated macrophages. Nutrition 2005;21:530–536 [DOI] [PubMed] [Google Scholar]
- 22. Son EW, Mo SJ, Rhee DK, Pyo S: Inhibition of ICAM-1 expression by garlic component, allicin, in gamma-irradiated human vascular endothelial cells via downregulation of the JNK signaling pathway. Int Immunopharmacol 2006;6:1788–1795 [DOI] [PubMed] [Google Scholar]
- 23. Kunkler PB, Farr RF, Luxton RW: The limit of renal tolerance to X-rays. Br J Radiol 1952;25:190–201 [DOI] [PubMed] [Google Scholar]
- 24. Go EK, Jung KJ, Kim JM, Lim H, Lim HK, Yu BP, Chung HY: Betaine modulates age-related NF-kappaB by thiol-enhancing action. Biol Pharm Bull 2007;30:2244–2249 [DOI] [PubMed] [Google Scholar]
- 25. Hissin PJ, Hilif R: A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem 1976;74:214–226 [DOI] [PubMed] [Google Scholar]
- 26. Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685 [DOI] [PubMed] [Google Scholar]
- 27. Loertzer H, Bauer S, Mörke W, Fornara P, Brömme HJ: Formation of ascorbate radicals as a measure of oxidative stress: an in vitro electron spin resonance-study using 2,2-azobis (2-amidinopropane) dihydrochloride as a radical generator. Transplant Proc 2006;38:674–678 [DOI] [PubMed] [Google Scholar]
- 28. Viatour P, Merville MP, Bours V, Chariot A: Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci 2005;30:43–52 [DOI] [PubMed] [Google Scholar]
- 29. Park LJ, Ju SM, Song HY, Lee JA, Yang MY, Kang YH, Kwon HJ, Kim TY, Choi SY, Park J: The enhanced monocyte adhesiveness after UVB exposure requires ROS and NF-kappaB signaling in human keratinocyte. J Biochem Mol Biol 2006; 30;39:618–625 [DOI] [PubMed] [Google Scholar]
- 30. Enginar H, Cemek M, Karaca T, Unak P: Effect of grape seed extract on lipid peroxidation, antioxidant activity and peripheral blood lymphocytes in rats exposed to X-radiation. Phytother Res 2007;21:1029–1035 [DOI] [PubMed] [Google Scholar]
- 31. Silasi G, Diaz-Heijtz R, Besplug J, Rodriguez-Juarez R, Titov V, Kolb B, Kovalchuk O: Selective brain responses to acute and chronic low-dose X-ray irradiation in males and females. Biochem Biophys Res Commun 2004;325:1223–1235 [DOI] [PubMed] [Google Scholar]
- 32. Choi CH, Xu H, Bark H, Lee TB, Yun J, Kang SI, Oh YK: Balance of NF-kappaB and p38 MAPK is a determinant of radiosensitivity of the AML-2 and its doxorubicin-resistant cell lines. Leuk Res 2007;31:1267–1276 [DOI] [PubMed] [Google Scholar]
- 33. Hamasu T, Inanami O, Tsujitani M, Yokoyama K, Takahashi E, Kashiwakura I, Kuwabara M: Post-irradiation hypoxic incubation of X-irradiated MOLT-4 cells reduces apoptotic cell death by changing the intracellular redox state and modulating SAPK/JNK pathways. Apoptosis 2005;10:557–567 [DOI] [PubMed] [Google Scholar]
- 34. Brown DQ, Pittock JW 3rd, Rubinstein JS: Early results of the screening program for radioprotectors. Int J Radiat Oncol Biol Phys 1982;8:565–570 [DOI] [PubMed] [Google Scholar]
- 35. Landauer MR, Srinivasan V, Seed TM: Genistein treatment protects mice from ionizing radiation injury. J Appl Toxicol 2003;23:379–385 [DOI] [PubMed] [Google Scholar]
- 36. Kumar KS, Srinivasan V, Toles R, Jobe L, Seed TM: Nutritional approaches to radioprotection: vitamin E. Mil Med 2002;167:57–59 [PubMed] [Google Scholar]
- 37. Cassatt DR, Fazenbaker CA, Kifle G, Bachy CM: Preclinical studies on the radioprotective efficacy and pharmacokinetics of subcutaneously administered amifostine. Semin Oncol 2002;29:2–8 [DOI] [PubMed] [Google Scholar]
- 38. Cairnie AB: Adverse effects of radioprotector WR2721. Radiat Res 1983;94:221–226 [PubMed] [Google Scholar]
- 39. Davenport DM, Wargovich MJ: Modulation of cytochrome P450 enzymes by organosulfur compounds from garlic. Food Chem Toxicol 2005;43:1753–1762 [DOI] [PubMed] [Google Scholar]
- 40. Chang HP, Huang SY, Chen YH: Modulation of cytokine secretion by garlic oil derivatives is associated with suppressed nitric oxide production in stimulated macrophages. J Agric Food Chem 2005;53:2530–2534 [DOI] [PubMed] [Google Scholar]
- 41. Hosseinimehr SJ: Trends in the development of radioprotective agents. Drug Discov Today 2007;12:794–805 [DOI] [PubMed] [Google Scholar]
- 42. Bartsch H, Nair J: Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg 2006;391:499–510 [DOI] [PubMed] [Google Scholar]
- 43. Suzuki K, Kodama S, Watanabe M: Extremely low-dose ionizing radiation causes activation of mitogen activated protein kinase pathway and enhances proliferation of normal human diploid cells. Cancer Res 2001;61:5396–5401 [PubMed] [Google Scholar]
- 44. Kim CS, Kim JM, Nam SY, Yang KH, Jeong M, Kim HS, Lim YK, Kim CS, Jin YW, Kim J: Low-dose of ionizing radiation enhances cell proliferation via transient ERK1/2 and p38 activation in normal human lung fibroblasts. J Radiat Res (Tokyo) 2007;48:407–415 [DOI] [PubMed] [Google Scholar]
- 45. Brach MA, Hass ML, Sherman R, Gunji H, Weichselbaum RR, Kufe D: Ionising radiation induces expression and binding activity of the nuclear factor kappa B. Int J Radiat Biol 1991;88: 691–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schmitz KJ, Lang H, Wohlschlaeger J, Sotiropoulos GC, Reis H, Schmid KW, Baba HA: AKT and ERK1/2 signaling in intrahepatic cholangiocarcinoma. World J Gastroenterol 2007;13:6470–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Weichselbaum RR, Hallahan D, Fuks Z, Kufe D: Radiation induction of immediate early genes: effectors of the radiation-stress response. Int J Radiat Oncol Biol Phys 1994;30:229–234 [DOI] [PubMed] [Google Scholar]
- 48. Hallahan DE, Virudachalam S, Kuchibhotla J: Nuclear factor kappaB dominant negative genetic constructs inhibit X-ray induction of cell adhesion molecules in the vascular endothelium. Cancer Res 1998;58:5484–5488 [PubMed] [Google Scholar]
- 49. Linard C, Marquette C, Mathieu J, Pennequin A, Clarençon D, Mathé D: Acute induction of inflammatory cytokine expression after gamma-irradiation in the rat: effect of an NF-kappaB inhibitor. Int J Radiat Oncol Biol Phys 2004;58:427–434 [DOI] [PubMed] [Google Scholar]
- 50. Liao HF, Kuo CD, Yang YC, Lin CP, Tai HC, Chen YY, Chen YJ: Resveratrol enhances radiosensitivity of human non-small cell lung cancer NCI-H838 cells accompanied by inhibition of nuclear factor-kappa B activation. J Radiat Res (Tokyo) 2005;46:387–393 [DOI] [PubMed] [Google Scholar]
- 51. Afaq F, Ahmad N, Mukhtar H: Suppression of UVB-induced phosphorylation of mitogen-activated protein kinases and nuclear factor kappa B by green tea polyphenol in SKH-1 hairless mice. Oncogene 2003;22:9254–9264 [DOI] [PubMed] [Google Scholar]
- 52. Zou Y, Jung KJ, Kim JW, Yu BP, Chung HY: Alteration of soluble adhesion molecules during aging and their modulation by calorie restriction. FASEB J 2004;18:320–322 [DOI] [PubMed] [Google Scholar]


