Abstract
Background and Purpose: The inadequacy of white-light cystoscopy to detect flat bladder tumors is well recognized. Great interest exists in developing other imaging technologies to augment or supplant conventional cystoscopy. Fibered confocal microscopy offers the promise of providing in vivo histopathologic information to help distinguish malignant from benign bladder lesions. We report the initial use of this technology to visualize tumors in the human bladder.
Materials and Methods: We performed ex vivo fibered confocal imaging of fresh radical cystectomy specimens using the Mauna Kea Technologies Cellvizio® system. The findings were compared with results from standard histopathology.
Results: The bladders of four patients were imaged using the fibered confocal microscope. Normal and neoplastic urothelium manifested differences in cellular and vascular density.
Conclusion: This study demonstrates the feasibility of using fibered confocal microscopy to detect histologic differences between normal and neoplastic urothelium, and establishes a foundation for the use of fiber-based confocal microscopy in clinical studies.
Introduction
Diagnostic cystoscopy is an indispensable part of the urologist's armamentarium to evaluate the lower urinary tract. Nevertheless, conventional white-light cystoscopy has numerous well-recognized shortcomings, including operator variability, uncertainty when differentiating inflammatory from malignant lesions, and difficulty identifying flat lesions, such as carcinoma in situ (CIS). Indeed, conventional cystoscopy may fail to visualize up to one third of tumors.1
For pathologic confirmation, suspected lesions must be biopsied with a delay of several days to obtain the result. To sample the remainder of the bladder, random biopsies may be performed, but this remains controversial in part because of the relatively low yield.2–5 Furthermore, biopsy entails an associated risk of bleeding and perforation, and provides a relatively small sample size.
Strong interest exists in the development of imaging technologies to augment conventional white-light cystoscopy. Examples include fluorescence cystoscopy with hexyl aminolevulinate (HAL), optical coherence tomography (OCT), and autofluorescent flexible cystoscopy. Multiple studies have shown that HAL fluorescence cystoscopy enables the visualization of more tumors in the bladder urothelium than can be seen by white-light cystoscopy alone.6,7 Preliminary clinical studies of OCT demonstrate that noninvasive “optical sectioning” of bladder tumors may provide cancer staging information.8–10 An initial report of autofluo-rescent cystoscopy indicates that this technique can help distinguish CIS from normal urothelium without the need for an intravesical fluorescent agent.11
None of these evolving technologies, however, provides sufficient resolution to differentiate cellular details with molecular contrast. The ability to obtain noninvasive, real-time, in vivo urinary tract histopathologic information during cystoscopy would prove useful for early diagnosis, surveillance, and image-guided biopsy.
Technology
First developed in 1957, confocal microscopy is a powerful imaging tool that provides high resolution, dynamic, subsurface imaging of biological systems.12–14 In contrast to conventional fluorescence microscopy, confocal microscopy images are not significantly contaminated by light scattered from other focal planes, thereby resulting in the ability to optically section tissues, improved localization of signals, and enhanced contrast.
In conventional confocal microscopy, a low-powered laser is focused onto a single point of the specimen, and the microscope then refocuses the emitted light from the specimen. Any out-of-focus light is removed from the image by passing through a pinhole, so only a thin optical section of the specimen is formed.15 The illumination and detection systems are in the same focal plane and are termed confocal.16
Detection of only the light within the focal plane greatly improves image quality and allows for visualization of signals originating from greater tissue depths. Given the large size of conventional confocal microscopes, this approach has been largely restricted to research, rather than clinical, applications.
Recently, new instrument designs and advances in instrument miniaturization17,18 have made possible the development of flexible, fiberoptic confocal microscopes that can be passed through the working channel of standard endoscopes. This enables in vivo microscopy and is referred to as “confocal endomicroscopy” or “fibered confocal microscopy.”
Pilot clinical studies in gastrointestinal endoscopy19–29 and bronchoscopy30 have demonstrated excellent histologic resolution, producing images that resemble standard analyses via histopathology. In addition, a single in vivo study of fibered confocal microscopy in rat bladders has been reported.31
Contrast in confocal microscopy is generated through the use of fluorescent dyes and markers. In clinical studies, this has largely been provided by the dyes fluorescein19,32,33 and indocyanin green.34
To our knowledge, application of in vivo confocal microscopy in the human urinary tract has not been previously demonstrated. We hypothesized that fibered confocal microscopy would be an adjunct to conventional white-light cystoscopy, providing images of normal and pathologic urothelium with cellular resolution that would improve the accuracy of cystoscopy.
We report a study of fresh cystectomy specimens with biopsy proven bladder cancer using fibered confocal microscopy. The primary objectives of this study were to prove the feasibility of the technology and to document the presence of any morphologic differences between normal and cancerous bladder mucosa using a generalized stain and a confocal microscope.
Materials and Methods
The research protocol was approved by the Stanford University Medical Center and Veterans Affairs Palo Alto Health Care System Institutional Review Board. Informed consent was obtained from patients with biopsy proven bladder cancer who were deemed suitable candidates for radical cystectomy. Immediately after cystectomy, the bladder specimens were brought to our laboratory for evaluation with the Cellvizio® fibered confocal microscope (Mauna Kea Technologies, Paris, France). To obtain optical contrast, 0.05% fluorescein sodium (Sigma-Aldrich, St. Louis, MO) was applied topically to the bladder mucosa, either by directly spraying it over the regions of interest after opening the bladder or by instilling 200-300 mL using a Foley catheter into the intact bladder. After instillation, fluorescein was left in contact with the tissue for 5 minutes, followed by rinsing or irrigation with phosphate buffered saline.
The Cellvizio fibered confocal microscope is composed of a 2.6-mm diameter Confocal MiniprobeTM attached to the Laser Scanning Unit (Fig. 1). Images were acquired at a rate of 12 frames per second through direct contact of the urothelium with the probe tip (Fig. 2A). The probes produce images 60 μm in depth, with a lateral resolution down to 1 μm, and a field of view of 240 μm in diameter. Both normal and abnormal appearing areas of the bladder mucosa were imaged.
FIG. 1.
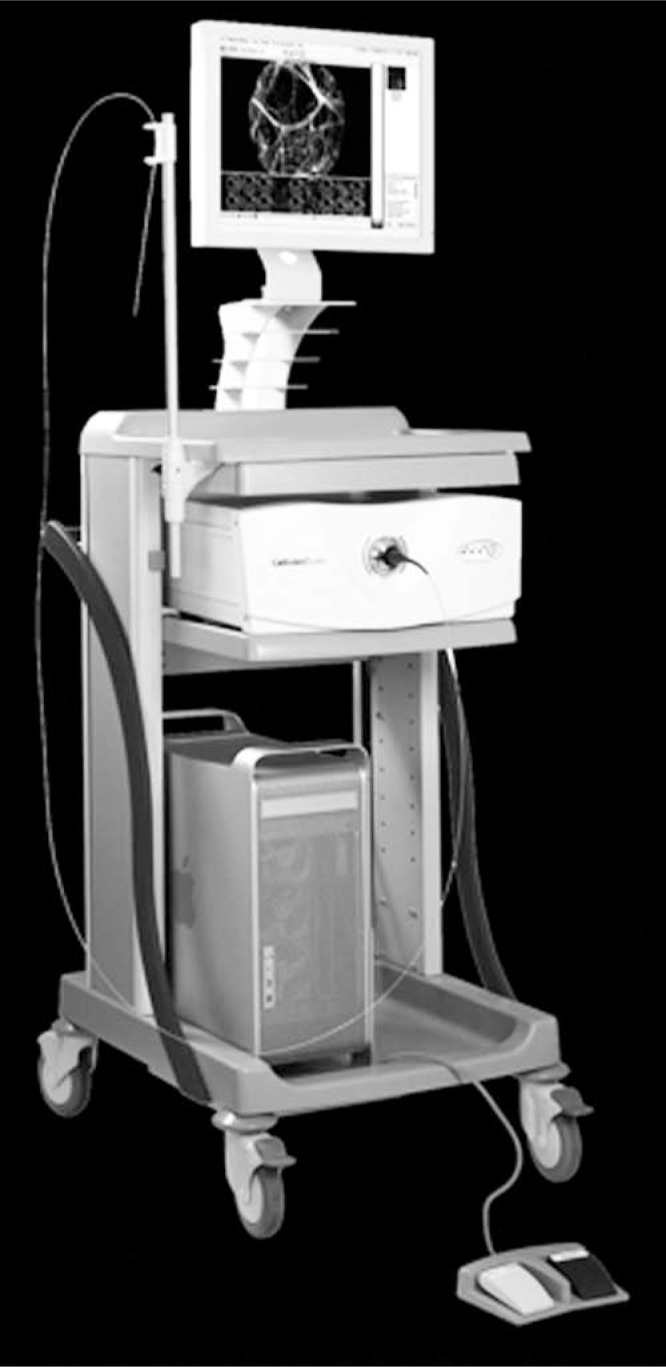
Fibered confocal microscope, composed of a Confocal Miniprobe™ attached to the Laser Scanning Unit. (Image courtesy of Mauna Kea Technologies.)
FIG. 2.
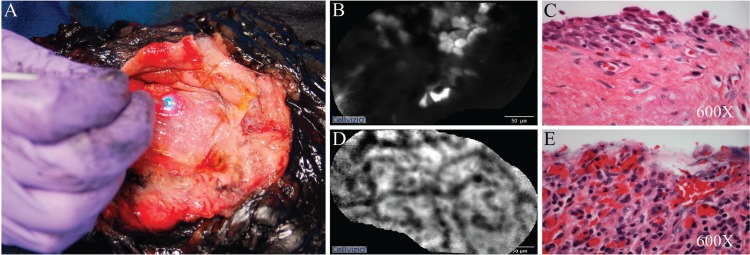
Representative images from a patient with multifocal carcinoma in situ (CIS). (A) Images being acquired through direct contact of the Confocal MiniprobeTM tip with the urothelium of the inked cystectomy specimen. (B) Cellular appearance of a normal area of bladder urothelium near the dome. (C) Corresponding normal bladder urothelium near the dome as seen on hematoxylin and eosin (H&E) stain. (D) Dense, arborized urothelial architecture suggestive of hemorrhagic small vessels later found by standard histology to represent CIS. (E) Corresponding appearance of the area seen on H&E stain showing denuded mucosa commonly seen with CIS. Scale bar represents 50 μm in (B) and (D).
The specimens were then sent for standard pathologic assessment with hematoxylin and eosin (H&E) staining. Image processing was performed using the proprietary software from Mauna Kea Technologies. Confocal images of normal and abnormal areas of bladder mucosa were compared with the H&E sections of the same tissues.
Results
Four patients were recruited for the study. On final pathologic evaluation, two specimens had high-grade invasive transitional cell carcinoma (TCC), one had high-grade invasive primary bladder adenocarcinoma, and one had multifocal CIS. Instillation of fluorescein through the Foley catheter yielded images of better quality than topically applied fluorescein alone. We noted microarchitectural differences between normal-appearing and tumor-bearing areas with respect to cellular density and vascular density.
Figures 2B–E show representative images from the patient with multifocal CIS, comparing a grossly normal area at the dome with a suspicious appearing erythematous patch near the trigone. In areas that appeared grossly normal, sheets of uniform cellular structures with uneven uptake of fluorescein were observed (Fig. 2B). These likely correspond to the intermediate cells seen in the H&E staining (Fig. 2C). In contrast, a dense arborized network in a background of irregular cellular structures was seen at the region of the erythematous patch (Fig. 2D). This region was confirmed to be CIS, characterized by denuded mucosa, local hemorrhage, and lymphocytic infiltrate (Fig. 2E). These arborized structures, which we speculate represent increased vascular density, were seen consistently in the suspicious regions of the bladders with TCC.
Discussion
We report the first application of fibered confocal microscopy in the human urinary tract using a Confocal Miniprobe from Mauna Kea Technologies. Using fresh surgical specimens from patients with bladder cancer and topical fluorescein as contrast, we observed morphologic differences between normal bladder mucosa and cancerous areas that were confirmed using standard H&E sectioning. The data were obtained rapidly from relatively intact tissues, offering the prospect of using this microscope in the clinic.
The possibility of acquiring real-time histopathologic information of urinary tract pathology (ie, “optical biopsy”), if realized, would represent an important advance in endourology. While bladder biopsy only obtains a static, histologic snapshot of small pieces of tissue, fibered confocal microscopy offers a dynamic survey of selected areas of the urothelium.
While promising, our pilot ex vivo study has several limitations, particularly the small sample size and the nonquantitative, descriptive nature of our image interpretation. Our preliminary experience with fibered confocal microscopy precludes us from making conclusive statements regarding the observed confocal images beyond empiric pattern recognition.
These observations argue that contrast agents with molecular specificity would be detectable using this microscope, and such agents may have a significant impact on detection and diagnosis. Such studies have been reported using peptide probes in the colon.35 In addition, the recently devascularized bladder specimens, resulting in varying degrees of cell death, may explain the inconsistent staining of fluorescein.
Comparison with standard histopathologic analysis is limited because the fibered confocal microscope produces images of horizontal planes, parallel to the surface of the bladder, while vertical sections are displayed in routine histopathologic analysis.
Based on our ex vivo experience, we are initiating an in vivo study to assess bladder tumors using fibered confocal microscopy with fluorescein as a contrast agent. By imaging selected normal or abnormal-appearing areas of the bladder before surgical biopsy, this technology may improve the rate of positive findings in “random” bladder biopsy and reduce unnecessary biopsies.
At this time, the Cellvizio probe fits within the working channel of a standard resectoscope. Further advances in miniaturization, however, may enable future compatibility with flexible cystoscopes for office use.
Conclusion
While the depth of penetration of fibered confocal microscopy will likely remain insufficient to provide information about muscle invasiveness, and the requirement for direct tissue contact with the probe makes imaging of the entire urothelium impractical, we anticipate that confocal endomicroscopy may complement other novel imaging modalities to improve conventional white-light cystoscopy, and that additional advances in the technology will increase its utility.
Abbreviations Used
- CIS
carcinoma in situ
- HAL
hexyl aminolevulinate
- H&E
hematoxylin and eosin
- OCT
optical coherence tomography
- TCC
transitional-cell carcinoma
Disclosure Statement
Funding support has been provided by the Stanford Cancer Center Developmental Cancer Research Award. Technical support has been provided by Mauna Kea Technologies.
References
- 1. Zaak D, Kriegmair M, Stepp H, et al. Endoscopic detection of transitional cell carcinoma with 5-aminolevulinic acid: Results of 1012 fluorescence endoscopies. Urology 2001;57:690–694 [DOI] [PubMed] [Google Scholar]
- 2. Swinn MJ, Walker MM, Harbin LJ, Adshead JM, Witherow RO, Vale JA, Patel A. Biopsy of the red patch at cystoscopy: Is it worthwhile? Eur Urol 2004;45:471–474 [DOI] [PubMed] [Google Scholar]
- 3. van der Meijden A, Oosterlinck W, Brausi M, Kurth KH, Sylvester R, de Balincourt C. Significance of bladder biopsies in Ta,T1 bladder tumors: A report from the EORTC Genito-Urinary Tract Cancer Cooperative Group. EORTC-GU Group Superficial Bladder Committee. Eur Urol 1999;35:267–271 [DOI] [PubMed] [Google Scholar]
- 4. May F, Treiber U, Hartung R, Schwaibold H. Significance of random bladder biopsies in superficial bladder cancer. Eur Urol 2003;44:47–50 [DOI] [PubMed] [Google Scholar]
- 5. Fujimoto N, Harada S, Terado M, Sato H, Matsumoto T. Multiple biopsies of normal-looking urothelium in patients with superficial bladder cancer: Are they necessary? Int J Urol 2003;10:631–635 [DOI] [PubMed] [Google Scholar]
- 6. Grossman HB, Gomella L, Fradet Y, Morales A, Presti J, Ritenour C, Nseyo U, Droller MJ. A phase III, multicenter comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of superficial papillary lesions in patients with bladder cancer. J Urol 2007; 178:62–67 [DOI] [PubMed] [Google Scholar]
- 7. Schmidbauer J, Witjes F, Schmeller N,Donat R, Susani M, Marberger M. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol 2004;171:135–138 [DOI] [PubMed] [Google Scholar]
- 8. Manyak MJ, Gladkova ND, Makari JH, et al. Evaluation of superficial bladder transitional-cell carcinoma by optical coherence tomography. J Endourol 2005;19:570-574 [DOI] [PubMed] [Google Scholar]
- 9. Lerner SP, Goh AC, Tresser NJ, Shen SS. Optical coherence tomography as an adjunct to white light cystoscopy for intravesical real-time imaging and staging of bladder cancer. Urology 2008;72:133–137 [DOI] [PubMed] [Google Scholar]
- 10. Hermes B, Spöler F, Naami A, Bornemann J, Först M, Grosse J, Jakse G, Knüchel R. Visualization of the basement membrane zone of the bladder by optical coherence tomography: Feasibility of noninvasive evaluation of tumor invasion. Urology 2008;72:677–681 [DOI] [PubMed] [Google Scholar]
- 11. Golijanin DJ, Singer EA, Messing EM. Management of carcinoma in situ of the bladder. AUA News. 2008; pp 11
- 12. Minsky M. Microscopy apparatus. US. Patent 3013467. United States. 1961
- 13. Minsky M. Memoir on inventing the confocal scanning microscope. Scanning 1988;10:128–138 [Google Scholar]
- 14. Pawley JB. Handbook of Biological Confocal Microscopy. New York: Springer; 2006
- 15. Robinson JP. Principles of confocal microscopy. Methods Cell Biol 2001;63:89–106 [DOI] [PubMed] [Google Scholar]
- 16. Kiesslich R, Goetz M, Neurath MF. Confocal laser endomicroscopy for gastrointestinal diseases. Gastrointest Endosc Clin N Am 2008;18:451–466 [DOI] [PubMed] [Google Scholar]
- 17. Helmchen F. Miniaturization of fluorescence microscopes using fibre optics. Exp Physiol 2002;87:737–745 [DOI] [PubMed] [Google Scholar]
- 18. Yelin D, Rizvi I, White WM, Motz JT, Hasan T, Couma BE, Tearney GJ. Three-dimensional miniature endoscopy. Nature 2006;443:765. [DOI] [PubMed] [Google Scholar]
- 19. Wang TD, Friedland S, Sahbaie P, Soetikno R, Hsiung PL, Liu JT, Crawford JM, Contag CH. Functional imaging of colonic mucosa with a fibered confocal microscope for real-time in vivo pathology. Clin Gastroenterol Hepatol 2007; 5:1300–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Becker V, von Delius S, Bajbouj M, Karagianni A, Schmid RM, Meining A. Intravenous application of fluorescein for confocal laser scanning microscopy: Evaluation of contrast dynamics and image quality with increasing injection-to-imaging time. Gastrointest Endosc 2008;68:319–323 [DOI] [PubMed] [Google Scholar]
- 21. Meining A, Wallace MB. Endoscopic imaging of angiogenesis in vivo. Gastroenterology 2008;134:915–918 [DOI] [PubMed] [Google Scholar]
- 22. Meining A, Frimberger E, Becker V, Von Delius S, Von Weyhern CH, Schmid RM, Prinz C. Detection of cholangio-carcinoma in vivo using miniprobe-based confocal fluorescence microscopy. Clin Gastroenterol Hepatol 2008;6:1057–1060 [DOI] [PubMed] [Google Scholar]
- 23. Becker V, Vercauteren T, von Weyhern CH, Prinz C, Schmid RM, Meining A. High-resolution miniprobe-based confocal microscopy in combination with video mosaicing (with video). Gastrointest Endosc 2007;66:1001–1007 [DOI] [PubMed] [Google Scholar]
- 24. Meining A, Bajbouj M, Schmid RM. Confocal fluorescence microscopy for detection of gastric angiodysplasia. Endoscopy 2007;39(suppl 1):E145. [DOI] [PubMed] [Google Scholar]
- 25. Meining A, Saur D, Bajbouj M, et al. In vivo histopathology for detection of gastrointestinal neoplasia with a portable, confocal miniprobe: An examiner blinded analysis. Clin Gastroenterol Hepatol 2007;5:1261–1267 [DOI] [PubMed] [Google Scholar]
- 26. Meining A, Schwendy S, Becker V, Schmid RM, Prinz C. In vivo histopathology of lymphocytic colitis. Gastrointest Endosc 2007;66:398–400 [DOI] [PubMed] [Google Scholar]
- 27. Miehlke S, Morgner A, Aust D, Madisch A, Vieth M, Baretton G. Combined use of narrow-band imaging magnification endoscopy and miniprobe confocal laser microscopy in neoplastic Barrett's esophagus. Endoscopy 2007;39(suppl 1):E316. [DOI] [PubMed] [Google Scholar]
- 28. Morgner A, Stolte M, Miehlke S. Visualization of lymphoepithelial lesions in gastric mucosa-associated lymphoid tissue-type lymphoma by miniprobe confocal laser microscopy. Clin Gastroenterol Hepatol 2007;5:e37. [DOI] [PubMed] [Google Scholar]
- 29. von Delius S, Feussner H, Wilhelm D, Karagianni A, Henke J, Schmid RM, Meining A. Transgastric in vivo histology in the peritoneal cavity using miniprobe-based confocal fluorescence microscopy in an acute porcine model. Endoscopy 2007;39:407–411 [DOI] [PubMed] [Google Scholar]
- 30. Thiberville L, Moreno-Swirc S, Vercauteren T, Peltier E, Cavé C, Bourg Heckly G. In vivo imaging of the bronchial wall microstructure using fibered confocal fluorescence microscopy. Am J Respir Crit Care Med 2007;175:22–31 [DOI] [PubMed] [Google Scholar]
- 31. D'Hallewin MA, El Khatib S, Leroux A, Bezdetnaya L, Guillemin F. Endoscopic confocal fluorescence microscopy of normal and tumor bearing rat bladder. J Urol 2005;174: 736–740 [DOI] [PubMed] [Google Scholar]
- 32. Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc 2005;62:686–695 [DOI] [PubMed] [Google Scholar]
- 33. Kiesslich R, Gossner L, Goetz M, et al. In vivo histology of Barrett's esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol 2006;4: 979–987 [DOI] [PubMed] [Google Scholar]
- 34. Mueller AJ, Bartsch DU, Folberg R, et al. Imaging the microvasculature of choroidal melanomas with confocal indocyanine green scanning laser ophthalmoscopy. Arch Ophthalmol 1998;116:31–39 [DOI] [PubMed] [Google Scholar]
- 35. Hsiung PL, Hardy J, Friedland S, et al. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med 2008;14:454–458 [DOI] [PMC free article] [PubMed] [Google Scholar]