Abstract
Postpartum depression (PPD) is a potentially debilitating disorder that develops in a significant percentage of women during the first year after giving birth. Women afflicted with PPD experience long-term consequences, including sadness, guilt, and despair. Offspring may be affected as well. Several investigators have tested psychosocial risk factors for the development of PPD; however, substantial amounts of variance in PPD have gone unexplained with regression on psychosocial variables alone. Likewise, interventions for PPD that have focused on psychosocial risk factors alone have been largely unsuccessful. The unexplained variance and disappointing treatment success could well be due to investigators’ failure to address relevant biological changes occurring during the postpartum period. Two biological systems that are affected significantly and remain in flux during the postpartum period are the innate immune system and the hypothalamic-pituitary-adrenal (HPA) axis. Bidirectional interactions between these two systems are well established, and it is generally acknowledged that dysfunction in either system can lead to depression in nonpregnant, nonpostpartum populations. To date, little research has pursued the contribution of these interacting systems to the development of PPD. The purpose of this paper is to review the psychoneuroimmunology of PPD. The central hypothesis presented is that dysregulation in either system individually or in their bidirectional interaction is associated with the development of PPD.
Introduction
Postpartum depression (PPD) is a debilitating disorder that develops in new mothers during the first year after giving birth. Long-term sequelae, including maternal sadness, guilt, and despair, may occur, as may consequences for offspring.1 Despite much research, the mechanisms underlying PPD remain elusive. Psychosocial variables are insufficient as the sole explanatory variables, and interventions focused on altering psychosocial risk factors alone have yielded disappointing results.2 Biological mechanisms have also been explored, but research on the role of gonadal hormone fluxes, thyroid dysregulation, and abnormal neurotransmitter levels likewise have been inconclusive.3,4
The purpose of this paper is to review the psychoneuroimmunology of PPD, wherein the contributions of two other, interconnected biological systems, both significantly altered during pregnancy and childbirth, are reviewed in regard to the development of PPD. Specifically, we review how pregnancy-related changes in the innate immune system and the hypothalamic-pituitary-adrenal (HPA) axis may contribute to the development of PPD if they do not return to normal function after delivery. This perspective is an extrapolation of data indicating that overstimulation of the innate immune system and HPA axis dysregulation are both associated with depression in adults who are not in the postpartum state.
First, a brief overview of the epidemiological, clinical, and physiological characteristics of PPD is presented. The next two sections review data in support of innate immune system dysfunction and HPA axis dysregulation as possible etiological factors in the development of PPD. The paper concludes with recommendations for future research to clarify these associations. Our goal throughout is to highlight the significance of these two systems in postpartum women in light of their known relationships with depression, in order to encourage future research to improve the health of postpartum women.
Overview of Postpartum Depression
The birth of a child is usually a time of great joy. For some women, however, the stressors of the postpartum period combine to reduce the ability of a new mother to meet her role demands. Of particular concern is the development of PPD, which occurs in 15%–20% of women during the first year after giving birth.5–7
PPD is a moderate to severe mood disorder comparable to a major depressive episode in the DSM-IV-TR. PPD carries significant and dangerous implications for a new mother's health and the health of her infant.1,8,9 It may interfere with maternal role development and mother-infant bonding and may cause physical risk to mother and child alike. The effects on children may include behavioral, developmental, and cognitive delay and may last years beyond infancy.10–13
The development of PPD is often insidious, with symptoms including sadness, an inability to take pleasure in most activities, anxiety, irritability, fatigue, poor sleep, and recurrent thoughts of suicide or death, beginning as early as 2 weeks to as late as several months after delivery.7,14 PPD is diagnosed by careful history and physical examination followed by application of one of many screening tools; women who score at high risk may receive a follow-up interview to confirm the diagnosis.1 Major psychosocial risk factors for PPD include prenatal depression, previous history of depression, low self-esteem, inadequate social support, child care stress, a fussy infant, and life stressors.15 Financial hardship more than triples the risk of PPD, partially explaining the higher prevalence of PPD in black and Hispanic mothers.16
Physiological risk factors for PPD have been proposed as well, including hypothyroidism and acute change in reproductive hormone levels.17–19 Although depressed mood is common in those suffering a thyroid abnormality, most women with PPD have thyroid hormone levels in the normal range. Similarly, although acute changes in estrogen levels may precipitate PPD, this appears to affect only a subpopulation of women with a history of depression.20
A possible biological mechanism that has received little attention to date is the bidirectional innate immune system-HPA axis association. This is surprising, given the profound changes that occur within these systems during pregnancy and delivery and their associations with depression in other populations.
Innate Immune System
Overview
The innate immune response is stimulated by infection, injury, malignancy, autoimmune disease, and stress. It is rapid in onset and serves to acutely limit tissue damage and the spread of infection. It is orchestrated by the synthesis and release of proinflammatory and anti-inflammatory cytokines. Proinflammatory cytokines, including interleukin-1-beta (IL-1β), IL-2, IL-6, tumor necrosis factor-alpha (TNF-α), interferon-alpha (IFN-α), and interferon-gamma (IFN-γ), are proteins released from activated white blood cells in response to the conditions noted.21 Release of proinflammatory cytokines initiates a systemic inflammatory response characterized by fever, increased sleep, reduction in activity, increased fatigue, decreased food intake, decreased exploration, diminished sexual activity, and, in humans, depressed mood.22
Because of the potential for unabated inflammation to cause undesirable side effects in the host, the proinflammatory response normally is kept in check through the reciprocal production of anti-inflammatory cytokines, including IL-4 and IL-10, which inhibit the production of proinflammatory cytokines.23,24 Occasionally, the balance between proinflammatory and anti-inflammatory cytokines shifts, impacting an individual's mental health.
Inflammation and depression
Research suggests that prolonged or excessive activation of the proinflammatory immune response may be a mechanism for depression.25 Administration of even nanomolar concentrations of proinflammatory cytokines to patients with cancer, hepatitis C, or other diseases induces symptoms of depression.26,27 In individuals already suffering from depression, proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, are elevated.28,29
HPA Axis
The HPA axis has far-reaching effects on immunity, metabolism, and reproduction. These are accomplished through frequent pulsatile secretion of cortisol, accompanied by large surges of the same hormone in response to changes or threats in the environment. The secretion of cortisol is the final product in a secretory pathway initiated by the release of corticotrophin-releasing hormone (CRH) from the hypothalamus. CRH stimulates the secretion of adrenocorticotrophic hormone (ACTH) from the anterior pituitary, which serves as the stimulus for adrenal cortisol secretion. HPA axis function is predominantly regulated by a negative feedback loop. Control of HPA axis activity is essential in protecting the organism from the catabolic, lipogenic, antireproductive, and immunosuppressive effects of prolonged exposure to glucocorticoids.
When exposed to changes in the environment perceived to be threatening or those which are physically stressful, the HPA axis secretes a larger amount of cortisol than the frequent pulses that comprise the basal circadian rhythm.30 The stress response produces an increase in alertness and provides additional energy through gluconeogenesis, accompanied by suppression of nearly all components of the immune response, including all the key steps of proinflammatory cytokine production and secretion.31–33 The system usually returns to baseline within a few hours after stress exposure, but if chronic exposure continues, the system can become dysregulated, manifest by various patterns of hyperactivity or hypoactivity.34
HPA axis dysfunction and depression
Chrousos and Gold35 originally hypothesized that dysregulation in HPA axis function is best characterized by a U-shaped curve. With HPA axis function on either end of the curve, depression, dysphoria, suicidal ideation, and all the neurovegetative symptoms of depression are possible.31,36 Clinically, hyperactivation of the stress response is associated with melancholic depression, anorexia nervosa, attachment disorder of infancy, and childhood maltreatment.35,36 Hypoactivation of the HPA, in turn, has been linked to seasonal and atypical depression, rheumatoid arthritis, fibromyalgia, and chronic fatigue syndrome.31 Chrousos32 included in this group depression that develops during the postpartum period, as described later.
Innate System and HPA Axis Function
There is a well-documented bidirectional association between the innate immune response and the HPA axis.33,37 IL-1, IL-2, IL-6, and TNF-α stimulate cortisol secretion, directly by acting on the cells of the adrenal cortex and indirectly via stimulation of CRH from the hypothalamus and ACTH from the anterior pituitary.38 IL-2 is a more potent known stimulator of ACTH release than CRH on a molar-to-molar basis. Chronically elevated proinflammatory cytokines appear to induce a decrease in central nervous system (CNS) glucocorticoid receptor function, decreasing the sensitivity of hypothalamic CRH-secreting cells to rising cortisol and blunting the normal negative feedback response of the hypothalamus to cortisol.21,39
HPA activation, in turn, has profound inhibitory effects on the immune/inflammatory response, acting to block virtually each step of the proinflammatory process.31 The glucocorticoids, of which cortisol is the primary type in humans, inhibit the production of proinflammatory cytokines at the level of the DNA by blocking the genes responsible for their production and by inducing the production of NFkB, a protein that binds to and neutralizes important cytokine transcription factors. Cytokines known to be downregulated by cortisol and other glucocorticoids include IL-1, IL-2, TNF-α, and IFN-γ. Moreover, glucocorticoids favor the production of the anti-inflammatory cytokines,40 especially at low concentrations of glucocorticoids.
Innate Immune System, HPA Axis Function, and PPD
The implication that dysregulation in the immune system or the HPA axis or both may contribute to the development of PPD is conceptually based on the psychoneuroimmunology model originally suggested by Chrousos and Gold as early as 199235 and expanded upon more recently.31,32,41 The impact of the innate immune response and HPA axis function on postpartum emotional regulation is depicted as occurring both independently and via their bidirectional relationships. In Figure 1, normal immune and HPA axis responses are proposed, which individually and together contribute to postpartum emotional regulation. In Figure 2, dysfunction in either system alone or in their bidirectional interactions is shown to destabilize emotional regulation and contribute to PPD.
FIG. 1.
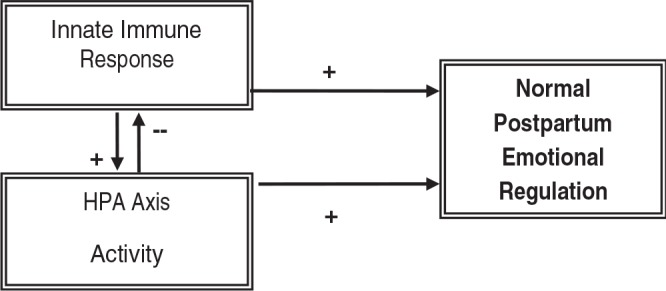
Conceptual framework depicting normal relationship between the inflammatory response and the HPA axis.
FIG. 2.
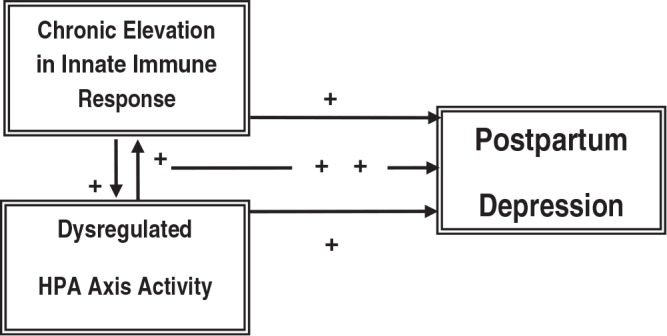
Conceptual framework for the proposed study, depicting dysregulated relationships among the HPA axis, the inflammatory response, and PPD.
Innate Immunity, HPA Axis Function, and Pregnancy
Pregnancy presents a unique immunological challenge to the body. To carry a pregnancy to term, a woman must not reject her developing fetus despite its antigenic incompatibility. At the same time, she must maintain her own immunocompetence. Studies suggest that maternal anti-inflammatory cytokines favorable for immunosuppression and pregnancy maintenance are elevated during pregnancy42,43 and proinflammatory cytokines are downregulated.42–44
With the cessation of pregnancy and delivery of the infant and placenta, the anti-inflammatory mileu abruptly, that is, within hours, shifts to a proinflammatory state.42 This occurs for several reasons: (1) a woman who has given birth often has experienced significant perineal tissue injury,45 (2) uterine involution occurs in all postpartum women and is characterized by ischemia, autolysis, and phagocytosis, processes that in animal and human studies are shown to involve significant inflammation and cytokine participation,46 and (3) pain, physical exertion, and emotional stress—all hallmarks of childbirth—stimulate the proinflammatory response.21
Several studies have described an increase in proinflammatory cytokines in healthy women with the onset of labor and lasting at least for the first 72 hours after delivery.47,48 There are, however, only a few reports, often conflicting, that follow cytokine changes beyond the first few days after parturition. In our laboratory, we found that IL-1β levels are elevated within 24 hours after giving birth and remain significantly elevated throughout the first month postpartum compared with levels measured in control, nonpregnant, nonpostpartum women.49 Other authors variously report a decrease in anti-inflammatory cytokines in the first several weeks postpartum,40 an increase in proinflammatory cytokines from 2 to 11 months postpartum with an overlapping increase in IL-4 after 6 months,50 and an increase in both anti-inflammatory and proinflammatory cytokines at 3 months postpartum compared with levels immediately after infant delivery.51 The conflicting nature of these results may be at least partially explained by the fact that two of the studies fail to exclude or identify women with such pregnancy-associated complications as hemorrhage, surgery, and infection.42,50 Moreover, none collected data on immunizations or antibiotic use, both of which affect cytokines.
Significant changes in HPA axis function also occur during pregnancy and delivery. Maternal levels of CRH, ACTH, and cortisol increase dramatically during parturition, attaining peak levels in the third trimester.37 After delivery, HPA axis hormones drop within the first 3 days, with central axis suppression similar to that occurring after abrupt discontinuation of exogenous steroids.37
The fetal adrenal gland is critical in understanding HPA axis physiology during pregnancy and the perinatal period. It secretes significant amounts of cortisol, which under the influence of progesterone, stimulates cells of the trophoblast and placenta to increase (not decrease) their production of CRH.52 Placental CRH is required for successful implantation and maintenance of early pregnancy and may play a role in initiating parturition.53 Production of CRH by the placenta leads to a self-propagating cycle wherein elevated CRH stimulates further fetal cortisol production, causing continued CRH secretion. Importantly, although fetal cortisol stimulates placental CRH secretion, it suppresses maternal CRH secretion. Maternal CRH suppression continues after delivery of the infant.52 Maternal HPA axis suppression may last for several weeks in healthy postpartum women.
In synthesizing these two lines of research, we propose that for some women, PPD might be a psychoneuroimmunological disorder. In healthy postpartum women, the innate immune response is stimulated by labor and delivery and causes an increase in the production of proinflammatory cytokines. Over the next weeks to months, a woman recovers from childbirth, and inflammation regresses. HPA axis function, although elevated early on, is suppressed, and the cytokines do not stimulate the secretion of HPA axis hormones. Focusing on the HPA axis, following delivery of an infant and the placenta, a woman's levels of CRH, ACTH, and cortisol drop from pregnancy levels, and the axis becomes hyporesponsive, normalizing by about 12 weeks.52 With recovery, the HPA axis hormones return and assist in limiting inflammation. Together, these steps assure normal postpartum emotional regulation.
For women who develop PPD, however, we suggest that the inflammatory response after labor and delivery is exaggerated, the HPA axis function is not adequately suppressed, or both conditions occur. With an exaggerated proinflammatory response, the systemic inflammatory response syndrome would occur, characterized by fatigue, poor sleep, poor appetite, and depressed mood. HPA axis hyperactivity would be associated with agitation, dysphoria, insomnia, and anorexia. One would expect an exaggerated inflammatory or stress response after prolonged or difficult labor, excessive blood loss, perineal damage, clinical or subclinical infection, surgery, or a negative emotional delivery.
There are some data to support this hypothesis. Increased IL-6 levels have been reported in women with PPD early in the perinatal period and in women with past histories of depression.54–56 In addition, one study has examined both cytokines and cortisol, although the authors did not address this link.57 This study was cross-sectional, involving women visited in their homes once between postpartum weeks 4 and 6. Serum IFN-γ, IL-10, and cortisol were measured and evaluated in light of perceived stress and dysphoric mood. Findings suggested that depressed mothers had significantly lower salivary but not serum cortisol concentrations and a lower serum ratio of IFN-γ/IL-10. IL-6 levels, in contrast, were nonsignificantly higher by 3-fold in depressed women. This study suggests that hypoactivation rather than hyperactivation of the HPA axis might contribute to PPD and supports a mixed role for the proinflammatory immune response. It should be noted, however, that depression was determined using a nonspecific measure (the Profile of Mood States) and was not validated clinically. Also, women who delivered vaginally or surgically were included, perhaps impacting cytokine and stress hormone levels, and the women scoring as depressed were significantly more likely to smoke, again potentially impacting cytokine levels58 and postpartum depression.59
Hyperactivity of the HPA axis has been reported in women with PPD by some researchers,17,60 although others have failed to replicate these findings.18,61 Still others have found a correlation between low cortisol levels and PPD.57,62
It is important to note that few of the studies of either system controlled for demographic factors, breastfeeding status, hormonal contraception, resumption of menses, vaginal vs. surgical delivery, or season, all of which will affect cytokine levels and HPA axis function. Very few collected data on past psychiatric history, which may be an indicator of chronic dysregulation of the immune system, the HPA axis, or both.
Future Studies
Additional research controlling for a number of external variables needs to be performed to clarify the psychoneuroimmunology of PPD. These studies should involve, at first, only women delivering vaginally, without complications, and without antibiotic or antidepressant therapy. Circadian function will need to be evaluated, as it may be that very subtle derangements in cytokines or HPA axis hormones are at play. Both proinflammatory and antiinflammatory cytokines will need to be measured, as the balance between these is the variable of most significance. Likewise, measuring cortisol alone tells less about the HPA axis feedback than including CRH and ACTH in analysis, as well as diurnal variation. And finally, women screened as depressed must be clinically diagnosed as well.
If dysregulation of the bidirectional interactions between the innate immune system and the HPA axis does play a role in the development of PPD, we would expect that studies such as those outlined here would demonstrate that women clinically diagnosed with PPD have a higher ratio of proinflammatory cytokines/anti-inflammatory cytokines prior to and during their episodes of depression compared with women who do not develop PPD. We would also expect that women diagnosed with PPD have higher levels of CRH, ACTH, and cortisol prior to and during their episodes of depression compared with women who do not develop PPD. Finally, we would expect that women diagnosed with PPD will demonstrate a cytokine-driven hyperactivity of the HPA axis compared with women who do not develop PPD, as indicated by significant differences in the slopes of the relationships between cytokines and HPA axis hormones in nondepressed women compared with women diagnosed with PPD.
Summary
PPD is a significant problem for a substantial number of women and families. By investigating reciprocal changes in cytokine and HPA axis functioning over time in nondepressed and depressed postpartum women, researchers will have the opportunity to address an etiology for PPD not previously studied. Insights gained from such research will significantly influence the prevention, diagnosis, and treatment of depression afflicting women during this vulnerable and important time of their lives.
References
- 1. Cooper PJ, Murray L. Postnatal depression. BMJ 1998;316: 1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boath E, Bradley E, Henshaw C. The prevention of postnatal depression: A narrative systematic review. J Psychosom Obstet Gynaecol 2005;26:185. [DOI] [PubMed] [Google Scholar]
- 3. McCoy SJ, Beal JM, Watson GH. Endocrine factors and postpartum depression. A selected review. J Reprod Med 2003;48:402. [PubMed] [Google Scholar]
- 4. Wisner KL, Stowe ZN. Psychobiology of postpartum mood disorders. Semin Reprod Endocrinol 1997;15:77. [DOI] [PubMed] [Google Scholar]
- 5. Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression A systematic review of prevalence and incidence. Obstet Gynecol 2005;106:1071. [DOI] [PubMed] [Google Scholar]
- 6. Wisner KL, Chambers C, Sit DK. Postpartum depression: A major public health problem. JAMA 2006;296:2616. [DOI] [PubMed] [Google Scholar]
- 7. Munk-Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders: A population-based register study. JAMA 2006;296:2582. [DOI] [PubMed] [Google Scholar]
- 8. Martins C, Gaffan EA. Effects of early maternal depression on patterns of infant-mother attachment: A meta-analytic investigation. J Child Psychol Psychiatry 2000;41:737. [PubMed] [Google Scholar]
- 9. Beck CT. The effects of postpartum depression on maternal-infant interaction: A meta-analysis. Nurs Res 1995;44:298. [PubMed] [Google Scholar]
- 10. Beck CT. The effects of postpartum depression on child development: A meta-analysis. Arch Psychiatr Nurs 1998;12:12. [DOI] [PubMed] [Google Scholar]
- 11. Field T. Infants of depressed mothers. Infant Behav Dev 1995;18:1. [DOI] [PubMed] [Google Scholar]
- 12. Hay DF, Pawlby S, Angold A, Harold GT, Sharp D. Pathways to violence in the children of mothers who were depressed postpartum. Dev Psychol 2003;39:1083. [DOI] [PubMed] [Google Scholar]
- 13. Logsdon MC, Wisner KL, Pinto-Foltz MD. The impact of postpartum depression on mothering. J Obstet Gynecol Neonatal Nurs 2006;35:652. [DOI] [PubMed] [Google Scholar]
- 14. Wisner KL, Perel JM, Peindl KS, Hanusa BH, Piontek CM, Findling RL. Prevention of postpartum depression: A pilot randomized clinical trial. Am J Psychiatry 2004;161:1290. [DOI] [PubMed] [Google Scholar]
- 15. Bloch M, Rotenberg N, Koren D, Klein E. Risk factors for early postpartum depressive symptoms. Gen Hosp Psychiatry 2006;28:3. [DOI] [PubMed] [Google Scholar]
- 16. Rich-Edwards JW, Kleinman K, Abrams A, et al. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J Epidemiol Community Health 2006;60:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pedersen CA, Stern RA, Pate J, Senger MA, Bowes WA, Mason GA. Thyroid and adrenal measures during later pregnancy and the puerperium in women who have been depressed or who become dysphoric postpartum. J Affect Disord 1993;29:201. [DOI] [PubMed] [Google Scholar]
- 18. Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry 2003;44:234. [DOI] [PubMed] [Google Scholar]
- 19. Lucas A, Pizarro E, Granada ML, Salinas I, Fox M, Sanmarti A. Postpartum thyroiditis: Epidemiology and clinical evolution in a non-selected population. Thyroid 2000;10:71. [DOI] [PubMed] [Google Scholar]
- 20. Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000;157:924. [DOI] [PubMed] [Google Scholar]
- 21. Watkins LR, Nguyen KT, Lee JE, Maier SF. Dynamic regulation of proinflammatory cytokines. Adv Exp Med Biol 1999;461:153. [DOI] [PubMed] [Google Scholar]
- 22. Anisman H, Merali Z. Cytokines, stress and depressive illness: Brain-immune interactions. Ann Med 2003;35:2. [DOI] [PubMed] [Google Scholar]
- 23. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001;19:683. [DOI] [PubMed] [Google Scholar]
- 24. Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest 2000;117:1162–1172 [DOI] [PubMed] [Google Scholar]
- 25. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol 2006;27:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alpha therapy in patients with chronic hepatitis C: Evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry 2005;66:1050. [DOI] [PubMed] [Google Scholar]
- 27. Wichers MC, Kenis G, Koek GH, Robaeys G, Nicolson NA, Maes M. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. J Psychosom Res 2007;62:207. [DOI] [PubMed] [Google Scholar]
- 28. Dentino AN, Pieper CF, Rao MK, et al. Association of interleukin-6 and other biologic variables with depression in older people living in the community. J Am Geriatr Soc 1999;47:6. [DOI] [PubMed] [Google Scholar]
- 29. Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry 2005;29:201. [DOI] [PubMed] [Google Scholar]
- 30. Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res 2002;53:865. [DOI] [PubMed] [Google Scholar]
- 31. Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol 2005;67:259. [DOI] [PubMed] [Google Scholar]
- 32. Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 1995;332:1351. [DOI] [PubMed] [Google Scholar]
- 33. Kiecolt-Glaser JK, Glaser R. Depression and immune function: Central pathways to morbidity and mortality. J Psychosomat Res 2002;53:873. [DOI] [PubMed] [Google Scholar]
- 34. Nemeroff CB. The neurobiology of depression. Sci Am 1998;278:42. [DOI] [PubMed] [Google Scholar]
- 35. Chrousos GP, Gold PW. The concepts of stress and stress system disorders. JAMA 1992;267:1244. [PubMed] [Google Scholar]
- 36. Shea A, Walsh C, Macmillan H, Steiner M. Child maltreatment and HPA axis dysregulation: Relationship to major depressive disorder and posttraumatic stress disorder in females. Biol Psychiatry 2005;30:162. [DOI] [PubMed] [Google Scholar]
- 37. Chrousos GP, Kino T. Interactive functional specificity of the stress and immune responses: The ying, the yang, and the defense against 2 major classes of bacteria. J Infect Dis 2005;192:551. [DOI] [PubMed] [Google Scholar]
- 38. McCann SM, Kimura M, Karanth S, Yu WH, Mastronardi CA, Rettori V. The mechanism of action of cytokines to control the release of hypothalamic and pituitary hormones in infection. Ann NY Acad Sci 2000;917:4. [DOI] [PubMed] [Google Scholar]
- 39. Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun 2007;21:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and anti-inflammatory cytokines, and autoimmunity. Ann NY Acad Sci 2002;966:290. [DOI] [PubMed] [Google Scholar]
- 41. Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation 2005;12:255. [DOI] [PubMed] [Google Scholar]
- 42. Østensen M, Förger F, Nelson JL, Schuhmacher A, Hebisch G, Villiger PM. Pregnancy in patients with rheumatic disease: Anti-inflammatory cytokines increase in pregnancy and decrease postpartum. Ann Rhuem Dis 2005;64:839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saito S, Sakai M, Sasaki Y, Tanebe K, Tsuda H, Michimata T. Interleukin-12 secretion by peripheral blood mononuclear cells is decreased in normal pregnant subjects and increased in preeclamptic patients. Clin Exp Immunol 1999;117:550. [DOI] [PubMed] [Google Scholar]
- 44. Veenstra van Nieuwenhoven AL, Bouman A, Moes H, et al. Endotoxin-induced cytokine production of monocytes of third-trimester pregnant women compared with women in the follicular phase of the menstrual cycle. Obstet Gynecol 2003;188:1073. [DOI] [PubMed] [Google Scholar]
- 45. Salamonsen LA. Tissue injury and repair in the female human reproductive tract. Reproduction 2003;125:301. [DOI] [PubMed] [Google Scholar]
- 46. Kayisli UA, Mahutte NG, Arici A. Uterine chemokines in reproductive physiology and pathology. Am J Reprod Immunol 2002;47:213. [DOI] [PubMed] [Google Scholar]
- 47. Hebisch G, Neumaier-Wagner PM, Huch R. Maternal serum interleukin-1b, -6 and -8 levels and potential determinants in pregnancy and peripartum. J Perinat Med 2004;32:475. [DOI] [PubMed] [Google Scholar]
- 48. Vassiliadis S, Ranella A, Papadimitriou L, Makrygiannakis A, Athanassakis I. Serum levels of pro- and anti-inflammatory cytokines in non-pregnant women, during pregnancy, labour and abortion. Mediators Inflamm 1998;7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Corwin EJ, Bozoky I, Pugh LC, Johnston N. Interleukin-1beta elevation during the postpartum period. Ann Behav Med 2003;25:41. [DOI] [PubMed] [Google Scholar]
- 50. Shimaoka Y, Hidaka Y, Tada H, et al. Changes in cytokine production during and after normal pregnancy. Am J Reprod Immunol 2000;44:143. [DOI] [PubMed] [Google Scholar]
- 51. Keski-Nisula L, Hirvonen MR, Roponen M, Heinonen S, Pekkanen J. Maternal and neonatal IL-4 and IFN-gamma production at delivery and 3 months after birth. J Reprod Immunol 2003;60:25. [DOI] [PubMed] [Google Scholar]
- 52. Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann NY Acad Sci 2003;997:136. [DOI] [PubMed] [Google Scholar]
- 53. Makrigiannakis A, Zoumakis E, Kalantaridou S, Chrousos G. Endometrial and placental CRH as regulators of human embryo implantation. J Reprod Immunol 2004;62:53. [DOI] [PubMed] [Google Scholar]
- 54. Maes M, Lin AH, Ombelet W, et al. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology 2000;25:121. [DOI] [PubMed] [Google Scholar]
- 55. Maes M, Ombelet W, De Jongh R, Kenis G, Bosmans E. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J Affect Disord 2001;63:85. [DOI] [PubMed] [Google Scholar]
- 56. Schmeelk KH, Granger DA, Susman EJ, Chrousos GP. Maternal depression and risk for postpartum complications: Role of prenatal corticotropin-releasing hormone and interleukin-1 receptor antagonist. Behav Med 1999;25:88. [DOI] [PubMed] [Google Scholar]
- 57. Groer MW, Morgan K. Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology 2007;32:133. [DOI] [PubMed] [Google Scholar]
- 58. Gennaro S, Dunphy P, Dowd M, Fehder W, Douglas SD. Postpartum smoking behaviors and immune response in mothers of term and preterm infants. Res Nurs Health 2001;24:9. [DOI] [PubMed] [Google Scholar]
- 59. McCoy SJ, Beal JM, Shipman SB, Payton ME, Watson GH. Risk factors for postpartum depression: A retrospective investigation at 4 weeks postnatal and a review of the literature. J Am Osteopath Assoc 2006;106:193. [PubMed] [Google Scholar]
- 60. Harris B, Lovett L, Smith J, Read G, Walker R, Newcombe R. Cardiff puerperal mood and hormone study. III. Postnatal depression at 5 to 6 weeks postpartum, and its hormonal correlates across the peripartum period. Br J Psychiatry 1996;168:739. [DOI] [PubMed] [Google Scholar]
- 61. Magiakou MA, Mastorakos G, Rabin D, Dubbert B, Gold PW, Chrousos GP. Hypothalamic corticotropin-releasing hormone suppression during the postpartum period: Implications for the increase in psychiatric manifestations at this time. J Clin Endocrinol Metab 1996;81:1912. [DOI] [PubMed] [Google Scholar]
- 62. Parry BL, Sorenson DL, Meliska CJ, et al. Hormonal basis of mood and postpartum disorders. Curr Womens Health Rep 2003;3:230. [PubMed] [Google Scholar]


