Abstract
The nature of warfare in the 21st century has led to a significant increase in primary blast or over-pressurization injuries to the whole body and head, which manifest as a complex of neuro-somatic damage, including traumatic brain injury (TBI). Identifying relevant pathogenic pathways in reproducible experimental models of primary blast wave exposure is therefore vital to the development of biomarkers for diagnostics of blast brain injury. Comparative analysis of mechanisms and putative biomarkers of blast brain injury is complicated by a deficiency of experimental studies. In this article, we present an overview of current TBI biomarkers, as well as outline experimental strategies to investigate molecular signatures of blast neurotrauma and to develop a pathway network map for novel biomarker discovery. These biomarkers will be effective for triaging and managing both combat and civilian causalities.
Key words: biomarkers, blast, brain injury, molecular pathways, traumatic brain injury
Introduction
An understanding of the biological mechanisms, and particularly the biomarkers of blast-induced neurotrauma, remain elusive. Symptoms of blast brain injury often do not manifest themselves until sometime after the injury has occurred (Cernak et al., 1999a; Yilmaz and Pekdemir, 2007). Mild or moderate brain injuries in particular often go undiagnosed and untreated because emergency medical attention is directed toward more visible injuries such as penetrating flesh wounds (Belanger et al., 2005; Lew, 2005). However, even mild and moderate brain injuries can produce significant deficits, and when repeated can lead to sustained neuro-somatic damage and neurodegeneration (Lew, 2005; Lew et al., 2005).
Over the last several decades, a number of experimental animal models have been implemented to study the mechanisms of blast wave impact and include rodents and larger animals such as sheep (Savic et al., 1991; Stuhmiller et al., 1996). Shock tubes have been used as the fundamental research tool for the past several decades. However, because of the rather generic nature of the blast generators used in the different studies, the data on injury mechanisms, including brain damage, have been difficult to analyze and compare (Jaffin et al., 1987; Elsayed 1997; Guy et al., 1998). Thus, there is still a lack of relevant reproducible models within the blast injury framework, including the development of generators that precisely control parameters of the blast wave, similarly to those present in classical mechanical controlled cortical impact (CCI) models. This makes it difficult to investigate the blast injury mechanisms and to develop relevant biomarkers for diagnostics and mitigation. However, preliminary analysis of the data on blast brain injury mechanisms suggests that it can be compared to the CCI injury model and potential biomarkers suggested by the latter can be examined.
The goals of this review are to examine potential biochemical/pathophysiological pathways and possible biomarkers of blast brain injury.
Review of Existing Biomarker Candidates
According to a generally accepted definition, a biomarker has the characteristic that it can be objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses for therapeutic intervention (Biomarkers Definitions Working Group, 2001). In contrast, a “surrogate biomarker” or “surrogate endpoint” is expected to predict clinical effects based on therapeutic, pathophysiologic, or other scientific correlations. Despite the apparent consensus on definition, controversy still exists about how to clearly delineate these two groups that have been associated with different experimental approaches that employ biomarkers for discovery and validation (Lescuyer et al., 2007). Ideally, brain injury biomarkers should be biological substrates unique to the brain and should provide information on injury mechanisms, a criterion used to distinguish biochemical markers from surrogate markers of injury, since surrogate markers usually do not provide information on injury mechanisms. Although there are currently no biomarkers with proven clinical utility for the diagnosis of brain injury, whether caused by blast, mechanical trauma, stroke, or other acute brain injuries, research has uncovered several valuable candidates that have shown preclinical potential. The ones currently generating the most interest include S-100β, neuron specific enolase (NSE), glial fibrillary acid protein (GFAP), and myelin basic protein (MBP). Although these proteins are still being assessed, they appear to lack either the necessary sensitivity or brain specificity (except perhaps GFAP) to be used effectively alone (Bazarian et al., 2006; Piazza et al., 2007). However, the combination of these markers can effectively detect TBI and provide outcome predictions (Berger et al., 2005; Berger et al., 2007). Below is a brief analysis of these generally accepted putative biomarkers of TBI.
S-100β protein
S-100β is among the most well studied proteins for TBI and is considered a promising, non-proprietary brain injury biomarker. S-100β, a small dimeric calcium binding protein, is most abundant in glial cells of the central nervous system (astrocytes) and peripheral nervous system (Schwann cells). A weakness of this marker is that it is not exclusive to the brain; S-100β has been found to be expressed in melanocytes, adipocytes, and chondrocytes (Michetti and Gazzolo, 2002).
A number of studies have demonstrated S-100β's relationship to injury magnitude and outcome in TBI (Pelinka et al., 2004; Berger et al., 2005; Berger et al., 2007; Korfias et al., 2007). This suggests that S-100β may be useful in assessing the efficacy of treatment after severe TBI (Korfias et al., 2007). Other studies, however, reported a poor value of S100β as a predictor of outcome after brain injury, particularly mild and pediatric TBI (Bazarian et al., 2006; Kleindienst and Bullock, 2006; Piazza et al., 2007). Also, several studies raised concern whether the serum level of S100β can be considered a true biochemical marker of brain damage given the definition of “biomarker” discussed above (see Kleindienst et al., 2007 for review). For example, in several studies a poor correlation was found between serum and brain S100β values, suggesting that the serum levels may depend primarily on the integrity of the blood–brain barrier and do not reflect the S100β levels in the brain (Kleindienst and Bullock, 2006; Piazza et al., 2007). Despite apparent controversy, S-100β still has potential as a brain injury biomarker, and its preclinical and clinical utility should be further explored, especially after blast wave exposures.
Neuron specific enolase
NSE initially held promise as a brain injury biomarker, as it was originally believed to be strictly neuronal. The protein is located in the neuronal cytoplasm and is involved in regulating intracellular chloride levels (Vinores et al., 1984). However, follow-up research found that NSE is also present in red blood cells and platelets, decreasing its diagnostic utility as a marker due to possible cross-contamination that could occur in blood samples (Johnsson et al., 2000). In clinical studies, serum NSE levels have been frequently studied alongside S-100β. The sensitivity and specificity of serum NSE after pediatric TBI as determined by ROC curves was found to be 71% and 64%, respectively (Berger et al., 2005). After multiple trauma, elevated NSE levels have been observed, but systemic NSE increased by similar degrees with and without TBI, limiting its ability to discriminate brain injury magnitude (Pelinka et al., 2005). Additionally, NSE has a long half-life, more than 20 h in serum, and this may account for its limitation for use as a TBI biomarker (Ingebrigtsen and Romner, 2003). Reports on correlations of serum NSE levels alone with clinical and neurological measures of brain injury magnitude and overall outcome have been controversial (Berger et al., 2005; Berger et al., 2007; Ingebrigtsen and Romner, 2003; Pelinka et al., 2005), although assay of serum NSE together with S100β have been valuable in predicting TBI outcome (Berger et al., 2006; Berger et al., 2007).
Glial fibrillary acidic protein
Glial fibrillary acidic protein (GFAP), a filament protein found in the astroglial cytoskeleton, is not found outside the CNS (Galea et al., 1995). It has only recently gained attention as a TBI biomarker, when it was shown to be as good as if not more promising than S-100β. In a study of 92 severe TBI patients 1 S-100β were shown to be equally good predictors of mortality, with sensitivity between 70% and 84% depending on the time they were measured after injury (Pelinka et al., 2004; Nylen et al., 2006). In addition to discriminating survival after TBI, GFAP, but not S-100β, could predictably discriminate between severe disability and vegetative state versus good and moderate outcomes as evaluated by the Glasgow Outcome Scale (GOS). Another study of 114 severe TBI patients confirmed its ability to predict mortality (Pelinka et al., 2004), and it was found to further discriminate outcome categories of the GOS. This study also indicated that GFAP had the ability to discriminate injury severity based on the Marshall scale classification of computed tomography (CT) scans. It could discriminate between patients that had intracranial pressure (ICP) greater or less than 25 mm Hg, patients that had cerebral perfusion pressure greater or less than 60 mm Hg, and patients with mean arterial pressure greater or less than 60 mm Hg. In a third study of 85 patients with severe TBI, levels of GFAP, NSE, and S-100β taken on hospital admission correlated with outcome measured by GOS at 6 months. Biomarker levels also correlated moderately with other indices of injury magnitude and were better correlated with Glasgow Coma Scale scores than was CT scan analysis. GFAP shows good diagnostic potential to predict outcome after injury, and may also be valuable for diagnosing injury magnitude, although it has not been studied in mild and moderate injury. Since it appears to be specific for brain tissue and is not affected by hemorrhagic shock or extracranial trauma, it may be a better diagnostic tool than S-100β.
Myelin basic protein
Myelin basic protein (MBP), an abundant protein in white matter, has been examined as a marker of axonal damage in several insults germane to neurointensive care. In TBI, CSF and serum levels of MBP have demonstrated excellent specificity, but limited sensitivity (Berger et al., 2005). It was recently shown that serum MBP, as well as NSE and S100β, concentrations obtained at the time of TBI may be useful in predicting outcome after pediatric TBI (Berger et al., 2006; Berger et al., 2007).
More recently a number of new candidate biomarkers have been discovered. The emerging data suggest TAU (c-tau) proteins (Zemlan et al., 2002; Gabbita et al., 2005), αII-spectrin protein breakdown products (SBDPs) (Pike et al., 1998a; Pike et al., 1998b; Pike et al., 2001; Pineda et al., 2004; Haskins et al., 2005; Wang et al., 2005; Kobeissy et al., 2006; Ottens et al., 2006; Pineda et al., 2007), and other proteins have strong potential. Currently, Banyan Biomarkers, Inc. is performing ELISA validation on some of these markers, and clinical validation of the biomarkers with human serum samples is in progress.
Microtubule associated protein-tau (c-Tau)
The same enzymes that cleave the cytoskeletal protein αII-spectrin cleave the microtubule associated protein-tau, releasing breakdown products (BDPs) with molecular weights that profile cleavage activity as being induced by the pro-necrotic calpains or the pro-apoptotic caspases. Tau cleavage by calpains include a BDP at 17 kDa that has been noted in cells following β-amyloid peptide treatment (Park and Ferreira, 2005; Park et al., 2007). Caspases have been shown to produce a tau breakdown product (TBDP) around 45 kDa (Chung et al., 2001). TBDPs were found to be present after TBI in rats (Zemlan et al., 2002; Gabbita et al., 2005). In a study whose primary objective was to determine the relationship between serum S-100β and c-tau levels and long-term outcome after mild TBI, S-100β and c-tau levels in serum taken 6 h after injury for 35 mild TBI subjects were compared to 3-month post-TBI Rivermead Post Concussion Questionnaire (RPCQ) scores and post-concussive syndrome. The results showed a weak linear correlation between marker levels and RPCQ scores for both markers, and that there was no statistically significant correlation between marker levels and 3-month post-concussive syndrome. Thus, serum S-100β and c-tau levels are apparently poor predictors of 3-month outcome after mild TBI (Bazarian et al., 2006). Further studies are required to ascertain whether the MAP-tau protein is a valid biomarker for brain trauma.
αll-Spectrin breakdown products
αII-spectrin is a major structural component of the neuron axonal cytoskeleton and a major proteolytic substrate for cysteine proteases (calpain and caspase-3) involved in oncotic (necrotic) and apoptotic cell death, respectively (Wang et al., 1998). Considerable evidence has been accumulated by our laboratory that αII-spectrin possesses signature cleavage products with molecular weights of 150 (SBDP150) and 145 kDa (SBDP145) due to cleavage by calpain, and a transient fragment of 150 kDa (SBDP150i) and a major cleavage product of 120 kDa (SBDP120) by caspase-3 (Nath et al., 1998; Pike et al., 1998a; Pike et al., 1998b). Since calpains and caspases become hyperactivated after traumatic and ischemic brain injury, we have proposed that SBDPs would be useful biomarkers of brain injury. Indeed, we and others found that these signature cleavage products are apparent in brain tissue and CSF after brain injury in rats (Pike et al., 1998a; Beer et al., 2000; Pike et al., 2001), brain ischemia (Zhang et al., 2002), and in human CSF following TBI (Pineda et al., 2004; Ringger et al., 2004). While MBP and c-Tau are relatively specific to the brain, the cytoskeleton αII-spectrin and its cleavage products SBDP150, 145, and 120 are not. Thus, plasma/serum levels of αII-spectrin SBDPs reflect the magnitude of multi-organ damage (including brain) rather than TBI per se.
NMDA-R fragments
Potential biomarkers for brain injury may include the fragmented form of the glutamate-N-methyl-D-aspartate (NMDA) receptor (NR2A/2B subtype). Dambinova and colleagues investigated the diagnostic accuracy of serum auto-antibodies to NR2A/2B, a subtype of NMDA receptor, in assessing transient ischemic attack (TIA) and ischemic stroke (IS), and its ability to distinguish cerebral ischemia from intracerebral hemorrhage (ICH). They showed that patients with TIA (n = 56) and acute IS (n = 31) had significantly higher NR2A/2B autoantibody concentrations than controls. In addition, levels of NR2A/2B autoantibodies within 72 h in the IS group differed significantly from those in ICH patients (p < 0.001). This was confirmed by magnetic resonance imaging and CT (Dambinova et al., 2003). It was concluded that NR2A/2B autoantibodies are independent and sensitive serologic markers capable of detecting TIA with a high post-test probability, and this correlates well with neurologic observation and neuroimaging, thereby ruling out ICH. Also, this biomarker may help assess the risk of TIA in routine general practice, and may potentially be useful in assisting diagnosis of acute IS in the emergency setting.
Neuro-inflammatory cytokine markers
The role of inflammation in various CNS injuries has been shown in numerous studies (see Lucas et al., 2006 for review). Hayakata and associates investigated early changes in the concentrations of CSF S-100β and various cytokines after TBI, and evaluated the relations of both to ICP and prognosis. CSF concentrations of S-100β and CSF and serum concentrations of five cytokines (IL-1β, TNF-α, IL-6, IL-8, and IL-10) were measured and compared. The CSF concentrations of S-100β and the cytokines peaked within 24 h after severe TBI, and decreased gradually thereafter. The authors concluded that CSF S-100β and IL-1β may be useful as outcome predictors in cases of severe TBI (Hayakata et al., 2004). In a subsequent study, they compared the CSF and serum levels of pro- and anti-inflammatory cytokines in 35 patients with severe TBI with and without additional injury. CSF and serum concentrations of two pro-inflammatory mediators (IL-1β and TNF-α), and three anti-inflammatory mediators (IL-1 receptor antagonist [IL-1ra], soluble TNF receptor-I [sTNFr-I], and IL-10) were measured and compared at 6 h after injury. CSF concentrations of pro-inflammatory mediators were much higher than the corresponding serum concentrations in both patient groups. In contrast, serum concentrations of anti-inflammatory mediators were much higher than the paired CSF concentrations in patients with additional injury, but serum concentrations were lower than or equal to the corresponding CSF concentrations in patients without additional injury. CSF concentrations of IL-1β, IL-1ra, sTNFr-I, and IL-10 were significantly higher in patients with high ICP than in patients with low ICP, and were also significantly higher in patients with an unfavorable outcome than in patients with a favorable outcome. These studies suggest that increased serum concentrations of anti-inflammatory mediators after severe TBI were mainly due to the additional extracranial injury/polytrauma. In contrast, anti-inflammatory mediators in CSF may be useful indicators of the severity of brain damage in terms of ICP, as well as overall prognosis of patients with severe TBI (Shiozaki et al., 2005).
General Approaches for Studies of Specific Mechanisms of Blast-Related Injuries
In the blast injury model, experimental animals are exposed to blasts that result from the detonation of known quantities of explosives at varying distances from the source, at different body orientations towards the blast wave, and in open or confined fields (Elsayed, 1997; Axelsson et al., 2000; Saljo et al., 2000). Also, a number of investigations have employed compressed air-driven shock tubes and nitrogen-driven blast wave generators to expose various animals to blast injury (e.g., rats, mice, and rabbits) to analyze the mechanisms of injury (Jaffin et al., 1987).
The majority of the blast injury–related work has been done using animals that have been exposed to total body blasts (Cernak et al., 1991a; Cernak et al., 1991b; Cernak et al., 1995; Stuhmiller et al., 1996; Elsayed, 1997; Elsayed et al., 1997; Gorbunov et al., 1997; Januszkiewicz et al., 1997; Mayorga, 1997; Cernak et al., 1999c; Cernak et al., 2001a; Elsayed and Gorbunov, 2003; Chavko et al., 2006; Elsayed and Gorbunov, 2007). Several groups reported that animals exhibited lung and abdominal injuries when they were exposed to a directed shock tube–generated blast. Guy and co-workers showed that rats exposed to moderate thoracic blast exhibited increased apnea, bradycardia, and hypotension before they returned to pre-blast values. No significant cardiovascular or respiratory changes were seen in animals subjected to abdominal blast (Guy et al., 1998). The most noticeable damage after blast exposure was found in the inner ear, lungs, and other gas- or fluid-containing interfaces such as the gastrointestinal tract (Cernak et al., 1991a; Cernak et al., 1991b; Elsayed, 1997; Elsayed et al., 1997; Januszkiewicz et al., 1997; Chavko et al., 2006; Elsayed and Gorbunov, 2007).
Several reports suggest that free radical–mediated injury in lung and blood of rats, rabbits, and sheep occurs in response to the blast (Elsayed et al., 1996, 1997). The biochemical changes included an increase in lipid peroxidation and hemoglobin associated with decreased Pao2, and antioxidant depletion and impairment of calcium transport across the cell membrane. These changes were found to correlate well with blast peak overpressure (Elsayed et al., 1996).
Hemoglobin and especially either of its oxidation products (metHb and oxoferrylHb) or its degradation products (heme and free iron) promote injury due to free radical–mediated consequences (Elsayed et al., 1996; Gorbunov et al., 1997). This may explain the continued pathological response after the blast wave exposure has ceased. Nitric oxide (NO) released from endothelial cells can form ligands that complex the active iron on the hemoglobin molecules rendering it unreactive (i.e., NO may function as an antioxidant by quenching the hemoglobin-mediated free-radical reactions) (Gorbunov et al., 1996). On the other hand, following blast exposure, endogenous NO was observed to increase in some rat tissues including lung and brain tissue.
Identification of brain-specific blast injury mechanisms and potential biomarkers
Several decades of medical literature reports provide a number of cases in which brain injuries are likely to have resulted from primary blast forces (Cramer et al., 1949; Murthy et al., 1979). Reported neuropathological changes have included small hemorrhages within the white matter, chromatolytic changes in the neurons (due to degeneration of Nissl bodies, an indication of neuronal damage), diffuse brain injury, and subdural hemorrhage. It is still controversial whether primary blast forces directly damage the brain, and if they do, what the mechanisms are that mediate the injury (Taber et al., 2006).
Shear and stress waves from the primary blast could potentially cause TBI directly (e.g., concussion, hemorrhage, edema, and diffuse axonal injury). On the other hand, the primary blast can also cause formation of gas emboli, leading to infarction (Guy et al., 2000a; Guy et al., 2000b).
The most common types of TBI are diffuse axonal injury, contusion, and subdural hemorrhage (Vander Vorst et al., 2007). Diffuse axonal injuries are very common following closed head injuries. They result when shearing, stretching, and/or angular forces pull on axons and small vessels. Impaired axonal transport leads to focal axonal swelling and after several hours may result in axonal disconnection (Hurley et al., 2004). The most common locations are the corticomedullary (gray matter-white matter) junction (particularly in the frontal and temporal areas), the internal capsule, the deep gray matter, the upper brainstem, and the corpus callosum. However, it has been shown that severely injured axons do not necessarily swell. The presence of focal axonal swellings, the most commonly used neuropathological marker for TBI, may therefore seriously underestimate the magnitude of injury present. It has also been noted that the insensitivity of presently used neuropathological markers to the unmyelinated fine-caliber axons that make up 30% of the corpus callosum may also contribute to inaccurate injury evaluation. After rats had been exposed to a total body blast, a phosphorylated epitope of the heavy subunit of the neurofilament proteins (p-NFH) normally restricted to axons, accumulated in the neuronal perikarya in layers II–IV of the temporal cortex, and in the piriform cortices, the dentate gyrus, and the CA1 region of the hippocampus. At the same time, the p-NFH immunoreactivity disappeared from the axons and dendrites of cerebral cortex neurons. These findings suggested a dephosphorylation of NFHs in axons and dendrites, and an accumulation of p-NFHs in the perikarya due to disturbed axonal transport (Saljo et al., 2000). The authors noted that these changes are likely the result of disturbed anterograde axonal transport.
Cernak and co-workers studied the effect on the brain of exposure to primary blast (delivered by a shock tube) and noted that it was sufficient to cause a moderate level of lung injury in rats (Cernak et al., 1999b; Cernak et al., 2001a, 2001b). In some cases, the effect of exposing the whole body was compared to exposing only the thoracic region (with the head protected) (Cernak et al., 2001b). Both types of blast exposure resulted in ultrastructural evidence of neuronal injury (expanded perineuronal spaces, cytoplasmic vacuoles, myelin deformation, and axoplasmic shrinkage) in the areas examined (hippocampus and brainstem reticular formation). The authors noted that the pattern of neuronal abnormalities is similar to those seen in diffuse axonal injury. Biochemical changes indicative of oxidative stress were also present. The degree of neuronal damage correlated with impaired performance on an active avoidance task (Cernak et al., 1999b; Cernak et al., 2001a, 2001b).
In a recent controversial paper, Kato and co-workers exposed rats to a direct single shock-wave shot (produced by a silver azide explosion) after craniotomy. This high-overpressure (>10 MPa) shock-wave exposure resulted in hemorrhage and a significant increase in TUNEL-positive neurons exhibiting chromatin condensation, nuclear segmentation, and apoptotic bodies. The maximum increase was seen at 24 h after the shockwave application. Low-overpressure (1 MPa) shock-wave exposure resulted in spindle-shaped changes in neurons and elongation of nuclei without marked neuronal injury. The administration of the caspase-3 antagonist Z-VAD-FMK significantly reduced the number of TUNEL-positive cells observed 24 h after high-overpressure shock-wave exposure. High-overpressure shock-wave exposure results in brain injury, including neuronal apoptosis mediated by a caspase-dependent pathway. The authors speculated that the threshold for shock-wave–induced brain injury is under 1 MPa, a level that is lower than the threshold for other organs including lungs (Kato et al., 2007). However, the model of blast injury employed in this study is very similar to a controlled cortical impact (CCI) model, which our group has been using for a number of years in rat TBI studies (Pineda et al., 2004; Wang et al., 2005; Ottens et al., 2006). Specifically, Kato and colleagues imposed the pressure of blast wave locally on dura mater after craniotomy instead of using mechanical piston impact at various depths as is typical in our studies. It is not clear from the study whether the magnitude and duration of overpressure blast was measured at the surface of the dura mater. The morphological pattern of brain tissue injury in that model closely resembled the damage observed after CCI (Kato et al., 2007).
In a number of studies using a CCI model of TBI in rats, we have convincingly demonstrated the important role for caspase-3-mediated apoptotic pathways in neuronal injury, as well as the calpain-mediated oncosis/necrosis mechanisms (Pineda et al., 2004; Wang et al., 2005; Ottens et al., 2006). Several pathways and target proteins have been identified as potential biomarkers of TBI (Kobeissy et al., 2006). Moreover, a systems biology approach was implemented to construct a network map of proteins and pathways involved in the pathophysiology of TBI (Kobeissy et al., 2006). Whether these pathways are equally critical after blast-wave exposure of total body and/or direct head targeting without imposing mechanical/traumatic forces remains to be elucidated.
Importantly, the rats exposed to blast injury exhibited significant deficits in performance of an active avoidance task that persisted up to 5 days post-injury. Electron microscopy findings in several brain structures showed swellings of neurons, glial reaction, myelin debris, and increased pinocytotic activity on the fifth day following trauma. In blast-injured rats, there was also a significant elevation in total nitrite/nitrate levels 3 and 24 h following injury, which were comparable with the changes in the expression of inducible nitric oxide synthase mRNA. The results indicate that blast injury–induced neurotrauma is able to cause cognitive deficits (Cernak et al., 2001a). In a later study, performance tests of coordination, balance, and strength were significantly impaired by exposure to a more intense explosion. An increase in the number of degenerating neurons was seen in cerebral cortex following these more intense explosions (Moochhala et al., 2004). An important finding was that administration of aminoguanidine, an antagonist of inducible NO synthase, before or after blast exposure ameliorated the blast-evoked behavioral changes (Moochhala et al., 2004).
On the other hand, as indicated above, NO may function as an antioxidant by quenching the hemoglobin-mediated free-radical reactions (Gorbunov et al., 1996). Thus the roles of NO and NO synthase, and particularly iNOS, in blast brain injury remains unclear. Moreover, recent studies using spin trap techniques and nitrosylation markers in iNOS knockout mice indicated that NO may play a dual role after TBI, that is both injurious and protective, depending on the time after traumatic impact (Bayir et al., 2005; Foley et al., 2008).
For rats subjected to an explosive-generated blast, Kaur and co-workers showed generalized activation of microglia and astrocytes 1 and 7 days after the blast, particularly in the superficial layers of the cerebral and cerebellar cortices, while the oligodendrocyte profile remained intact (Kaur et al., 1995, 1996, 1997a, 1997b, 1999). It was concluded from this study that the blast may have disrupted the integrity of the blood–brain barrier, resulting in possible abnormal entry of serum-derived substances, thereby leading to microglia/astrocytic activation.
Approaches for the development of specific biomarkers of blast-induced brain injury
Integrating the existing data obtained from different models of blast brain injury, as well as from classical CCI-like models, we propose that exposures to blast waves cause brain injury with distinct biochemical pathophysiological signatures, which when defined and integrated with systems biology tools, can be exploited to discover diagnostic biomarkers and blast brain injury mitigation strategies. For example, we recently employed Pathway Studio software for creation of a functional interaction map of proteins affected during controlled cortical impact TBI (Kobeissy et al., 2006). We propose the term BLAST-OMICS for the similar functional interaction map of proteins impacted by blast-wave exposure, which will be created during current and future studies.
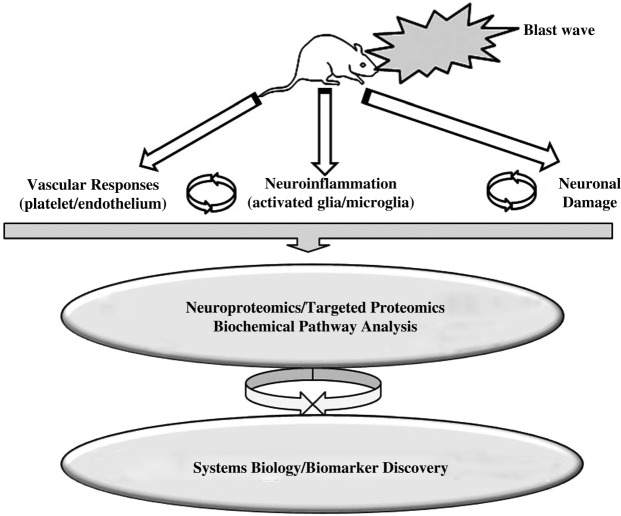
We postulate that the following molecular pathways and/or targets are identifiable upon blast wave exposures of experimental animals (Fig. 1).
FIG. 1.
Schematic presentation of a proposed research algorithm to elucidate molecular signatures and pathophysiological pathways of blast brain injury to develop a network map for blast brain injury.
-
1.
Vascular responses and dysregulation of cell adhesion molecules as bridges connecting vascular-endothelialneural tissue disturbances, including but not limited to nitric oxide metabolism assessed in brain tissues, CSF, and blood by Western blot, sandwich ELISA, and immunohistochemical means.
-
2.
Neuroinflammation as a further development of impaired vascular reaction in the brain, resulting in enhancement of endothelial permeability/leakage, infiltration of macrophages, and activation of microglia that will involve cytokines and markers of activated microglia.
-
3.
Neuron-glia interactions and cell death pathways, including apoptosis, oncosis/necrosis, and autophagy.
-
4.
Additional pathways and biomarkers, such as dendrite/axonal disturbances including axonal transport dysfunction and damage to microtubules and neurofilaments.
The successful development of true biochemical markers of blast brain injury should help to distinguish between minor brain damage, particularly mild TBI, and post-traumatic stress disorder (Kennedy et al., 2007; King, 2008). The differentiation of PTSD and mild TBI is especially important for soldiers returning from Iraq, as mild TBI (e.g., concussion) that occurs among these soldiers has a strong association with PTSD and physical health problems 3 to 4 months after the soldiers return home (Hoge et al., 2008).
Perspectives
Despite a long history, critical need, and a vast array of recent data, the mechanisms of primary blast neurotrauma, particularly brain injury and potential biomarkers, remain elusive. A lack of unified models with defined blast-wave signatures has contributed significantly to existing controversies. Given data from both our laboratory and others, it has become increasingly clear that brain pathology, the underlying mechanisms and potential biomarkers associated with primary blast exposures, may be different from those imposed by focal mechanical head trauma (Bhattacharjee, 2008). This exciting new area of research clearly requires more adequate models and in-depth studies of biochemical/pathophysiological signatures of blast neurotrauma. Thus the design and implementation of a relevant experimental framework is of particular importance for elucidation of the mechanisms of injury, the identification of biomarkers, and eventually the development of strategies for mitigating blast-induced brain injury.
Acknowledgments
The authors wish to thank Ms. Qiushi Tang for her excellent technical assistance. This work was supported by grants N14-06-1-1029, W81XWH-8-1-0376 and X81XWH-07-01-0701 from Department of Defense.
Author Disclosure Statement
Dr. Svetlov, Dr. Larner, Dr. Hayes, and Dr. Wang have financial interest in Banyan Biomarkers Inc. Dr. Kirk and Dr. Atkinson have no conflict of interests.
References
- Axelsson H., Hjelmqvist H., Medin A., Persson J.K., and Suneson A. (2000). Physiological changes in pigs exposed to a blast wave from a detonating high-explosive charge. Mil. Med. 165, 119–126 [PubMed] [Google Scholar]
- Bayir H., Kagan V.E., Borisenko G.G., Tyurina Y.Y., Janesko K.L., Vagni V.A., Billiar T.R., Williams D.L., and Kochanek P.M. (2005). Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. J. Cereb. Blood Flow Metab. 25, 673–684 [DOI] [PubMed] [Google Scholar]
- Bazarian J.J., Zemlan F.P., Mookerjee S., and Stigbrand T. (2006). Serum S-100B and cleaved-tau are poor predictors of long-term outcome after mild traumatic brain injury. Brain Inj. 20, 759–765 [DOI] [PubMed] [Google Scholar]
- Beer R., Franz G., Srinivasan A., Hayes R.L., Pike B.R., Newcomb J.K., Zhao X., Schmutzhard E., Poewe W., and Kampfl A. (2000). Temporal profile and cell subtype distribution of activated caspase-3 following experimental traumatic brain injury. J. Neurochem. 75, 1264–1273 [DOI] [PubMed] [Google Scholar]
- Belanger H.G., Scott S.G., Scholten J., Curtiss G., and Vanderploeg R.D. (2005). Utility of mechanism-of-injury-based assessment and treatment: Blast Injury Program case illustration. J. Rehabil. Res. Dev. 42, 403–412 [DOI] [PubMed] [Google Scholar]
- Berger R.P., Adelson P.D., Pierce M.C., Dulani T., Cassidy L.D., and Kochanek P.M. (2005). Serum neuron-specific enolase, S100B, and myelin basic protein concentrations after inflicted and noninflicted traumatic brain injury in children. J. Neurosurg. 103(1 Suppl), 61–68 [DOI] [PubMed] [Google Scholar]
- Berger R.P., Beers S.R., Richichi R., Wiesman D., and Adelson P.D. (2007). Serum biomarker concentrations and outcome after pediatric traumatic brain injury. J. Neurotrauma. 24, 1793–1801 [DOI] [PubMed] [Google Scholar]
- Berger R.P., Dulani T., Adelson P.D., Leventhal J.M., Richichi R., and Kochanek P.M. (2006). Identification of inflicted traumatic brain injury in well-appearing infants using serum and cerebrospinal markers: a possible screening tool. Pediatrics 117, 325–332 [DOI] [PubMed] [Google Scholar]
- Bhattacharjee Y. (2008). Neuroscience. Shell shock revisited: solving the puzzle of blast trauma. Science 319, 406–408 [DOI] [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group. (2001). Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95 [DOI] [PubMed] [Google Scholar]
- Cernak I., Ignjatovic D., Andelic G., and Savic J. (1991a). [Metabolic changes as part of the general response of the body to the effect of blast waves]. Vojnosanit. Pregl. 48, 515–522 [PubMed] [Google Scholar]
- Cernak I., Radosevic P., Malicevic Z., and Savic J. (1995). Experimental magnesium depletion in adult rabbits caused by blast overpressure. Magnes. Res. 8, 249–259 [PubMed] [Google Scholar]
- Cernak I., Savic J., Ignjatovic D., and Jevtic M. (1999a). Blast injury from explosive munitions. J. Trauma 47, 96–103; discussion 103–104. [DOI] [PubMed] [Google Scholar]
- Cernak I., Savic J., Mrsulja B., and Duricic B. (1991b). [Pathogenesis of pulmonary edema caused by blast waves]. Vojnosanit. Pregl. 48, 507–514 [PubMed] [Google Scholar]
- Cernak I., Savic J., Zunic G., Pejnovic N., Jovanikic O., and Stepic V. (1999b). Recognizing, scoring, and predicting blast injuries. World J. Surg. 23, 44–53 [DOI] [PubMed] [Google Scholar]
- Cernak I., Savic V.J., Lazarov A., Joksimovic M., and Markovic S. (1999c). Neuroendocrine responses following graded traumatic brain injury in male adults. Brain Inj. 13, 1005–1015 [DOI] [PubMed] [Google Scholar]
- Cernak I., Wang Z., Jiang J., Bian X., and Savic J. (2001a). Cognitive deficits following blast injury-induced neurotrauma: possible involvement of nitric oxide. Brain Inj. 15, 593–612 [DOI] [PubMed] [Google Scholar]
- Cernak I., Wang Z., Jiang J., Bian X., and Savic J. (2001b). Ultrastructural and functional characteristics of blast injury-induced neurotrauma. J. Trauma 50, 695–706 [DOI] [PubMed] [Google Scholar]
- Chavko M., Prusaczyk W.K., and McCarron R.M. (2006). Lung injury and recovery after exposure to blast overpressure. J. Trauma 61, 933–942 [DOI] [PubMed] [Google Scholar]
- Chung C.W., Song Y.H., Kim I.K., Yoon W.J., Ryu B.R., Jo D.G., Woo H.N., Kwon Y.K., Kim H.H., Gwag B.J., Mook-Jung I.H., and Jung Y.K. (2001). Proapoptotic effects of tau cleavage product generated by caspase-3. Neurobiol. Dis. 8, 162–172 [DOI] [PubMed] [Google Scholar]
- Cramer F., Paster S., and Stephenson C. (1949). Cerebral injuries due to explosion waves, cerebral blast concussion; a pathologic, clinical and electroencephalographic study. Arch. Neurol. Psychiatry 61, 1–20 [DOI] [PubMed] [Google Scholar]
- Dambinova S.A., Khounteev G.A., Izykenova G.A., Zavolokov I.G., Ilyukhina A.Y., and Skoromets A.A. (2003). Blood test detecting autoantibodies to N-methyl-D-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin. Chem. 49, 1752–1762 [DOI] [PubMed] [Google Scholar]
- Elsayed N.M. (1997). Toxicology of blast overpressure. Toxicology 121, 1–15 [DOI] [PubMed] [Google Scholar]
- Elsayed N.M., and Gorbunov N.V. (2003). Interplay between high energy impulse noise (blast) and antioxidants in the lung. Toxicology 189, 63–74 [DOI] [PubMed] [Google Scholar]
- Elsayed N.M., and Gorbunov N.V. (2007). Pulmonary biochemical and histological alterations after repeated low-level blast overpressure exposures. Toxicol. Sci. 95, 289–296 [DOI] [PubMed] [Google Scholar]
- Elsayed N.M., Gorbunov N.V., and Kagan V.E. (1997). A proposed biochemical mechanism involving hemoglobin for blast overpressure-induced injury. Toxicology 121, 81–90 [DOI] [PubMed] [Google Scholar]
- Elsayed N.M., Tyurina Y.Y., Tyurin V.A., Menshikova E.V., Kisin E.R., Kagan V.E. (1996). Antioxidant depletion, lipid peroxidation, and impairment of calcium transport induced by air-blast overpressure in rat lungs. Exp. Lung Res. 22, 179–200 [DOI] [PubMed] [Google Scholar]
- Foley L.M., Hitchens T.K., Melick J.A., Bayir H., Ho C., and Kochanek P.M. (2008). Effect of inducible nitric oxide synthase on cerebral blood flow after experimental traumatic brain injury in mice. J. Neurotrauma 25, 299–310 [DOI] [PubMed] [Google Scholar]
- Gabbita S.P., Scheff S.W., Menard R.M., Roberts K., Fugaccia I., and Zemlan F.P. (2005). Cleaved-tau: a biomarker of neuronal damage after traumatic brain injury. J. Neurotrauma 22, 83–94 [DOI] [PubMed] [Google Scholar]
- Galea E., Dupouey P., and Feinstein D.L. (1995). Glial fibrillary acidic protein mRNA isotypes: expression in vitro and in vivo. J. Neurosci. Res. 41, 452–461 [DOI] [PubMed] [Google Scholar]
- Gorbunov N.V., Elsayed N.M., Kisin E.R., Kozlov A.V., and Kagan V.E. (1997). Air blast-induced pulmonary oxidative stress: interplay among hemoglobin, antioxidants, and lipid peroxidation. Am. J. Physiol. 272, L320–L334 [DOI] [PubMed] [Google Scholar]
- Gorbunov N.V., Osipov A.N., Sweetland M.A., Day B.W., Elsayed N.M., Kagan V.E. (1996). NO-redux paradox: direct oxidation of alpha-tocopheral and alpha-tocopherol mediated oxidation of ascorbate. Biochem. Biophys. Res. Commun. 219, 835–841 [DOI] [PubMed] [Google Scholar]
- Guy R.J., Glover M.A., and Cripps N.P. (2000a). Primary blast injury: pathophysiology and implications for treatment. Part III: Injury to the central nervous system and the limbs. J. R. Nav. Med. Serv. 86, 27–31 [PubMed] [Google Scholar]
- Guy R.J., Kirkman E., Watkins P.E., and Cooper G.J. (1998). Physiologic responses to primary blast. J. Trauma 45, 983–987 [DOI] [PubMed] [Google Scholar]
- Guy R.J., Watkins P.E., and Edmondstone W.M. (2000b). Electrocardiographic changes following primary blast injury to the thorax. J. R. Nav. Med. Serv. 86, 125–133 [PubMed] [Google Scholar]
- Haskins W.E., Kobeissy F.H., Wolper R.A., Ottens A.K., Kitlen J.W., McClung S.H., O'Steen B.E., Chow M.M., Pineda J.A., Denslow N.D., Hayes R.L., and Wang K.K. (2005). Rapid discovery of putative protein biomarkers of traumatic brain injury by SDS-PAGE-capillary liquid chromatographytandem mass spectrometry. J. Neurotrauma 22, 629–644 [DOI] [PubMed] [Google Scholar]
- Hayakata T., Shiozaki T., Tasaki O., Ikegawa H., Inoue Y., Toshiyuki F., Hosotubo H., Kieko F., Yamashita T., Tanaka H., Shimazu T., and Sugimoto H. (2004). Changes in CSF S100B and cytokine concentrations in early-phase severe traumatic brain injury. Shock 22, 102–107 [DOI] [PubMed] [Google Scholar]
- Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 [DOI] [PubMed] [Google Scholar]
- Hurley R.A., McGowan J.C., Arfanakis K., and Taber K.H. (2004). Traumatic axonal injury: novel insights into evolution and identification. J. Neuropsychiatry Clin. Neurosci. 16, 1–7 [DOI] [PubMed] [Google Scholar]
- Ingebrigtsen T., and Romner B. (2003). Biochemical serum markers for brain damage: a short review with emphasis on clinical utility in mild head injury. Restor. Neurol. Neurosci. 21, 171–176 [PubMed] [Google Scholar]
- Jaffin J.H., McKinney L., Kinney R.C., Cunningham J.A., Moritz D.M., Kraimer J.M., Graeber G.M., Moe J.B., Salander J.M., and Harmon J.W. (1987). A laboratory model for studying blast overpressure injury. J. Trauma 27, 349–356 [DOI] [PubMed] [Google Scholar]
- Januszkiewicz A.J., Mundie T.G., and Dodd K.T. (1997). Maximal exercise performance-impairing effects of simulated blast overpressure in sheep. Toxicology 121, 51–63 [DOI] [PubMed] [Google Scholar]
- Johnsson P., Blomquist S., Luhrs C., Malmkvist G., Alling C., Solem J.O., and Stahl E. (2000). Neuron-specific enolase increases in plasma during and immediately after extracorporeal circulation. Ann. Thorac. Surg. 69, 750–754 [DOI] [PubMed] [Google Scholar]
- Kato K., Fujimura M., Nakagawa A., Saito A., Ohki T., Takayama K., and Tominaga T. (2007). Pressure-dependent effect of shock waves on rat brain: induction of neuronal apoptosis mediated by a caspase-dependent pathway. J. Neurosurg. 106, 667–676 [DOI] [PubMed] [Google Scholar]
- Kaur C., Singh J., Lim M.K., Ng B.L., and Ling E.A. (1997a). Macrophages/microglia as ‘sensors’ of injury in the pineal gland of rats following a non-penetrative blast. Neurosci. Res. 27, 317–322 [DOI] [PubMed] [Google Scholar]
- Kaur C., Singh J., Lim M.K., Ng B.L., Yap E.P., and Ling E.A. (1995). The response of neurons and microglia to blast injury in the rat brain. Neuropathol. Appl. Neurobiol. 21, 369–377 [DOI] [PubMed] [Google Scholar]
- Kaur C., Singh J., Lim M.K., Ng B.L., Yap E.P., and Ling E.A. (1996). Studies of the choroid plexus and its associated epiplexus cells in the lateral ventricles of rats following an exposure to a single non-penetrative blast. Arch. Histol. Cytol. 59, 239–248 [DOI] [PubMed] [Google Scholar]
- Kaur C., Singh J., Lim M.K., Ng B.L., Yap E.P., and Ling E.A. (1997b). Ultrastructural changes of macroglial cells in the rat brain following an exposure to a non-penetrative blast. Ann. Acad. Med. Singapore 26, 27–29 [PubMed] [Google Scholar]
- Kaur C., Singh J., Moochhala S., Lim M.K., Lu J., and Ling E.A. (1999). Induction of NADPH diaphorase/nitric oxide synthase in the spinal cord motor neurons of rats following a single and multiple non-penetrative blasts. Histol. Histopathol. 14, 417–425 [DOI] [PubMed] [Google Scholar]
- Kennedy J.E., Jaffee M.S., Leskin G.A., Stokes J.M., Leal F.O., and Fitzpatrick P.J. (2007). Posttraumatic stress disorder and posttraumatic stress disorder-like symptoms and mild traumatic brain injury. J. Rehabil. Res. Dev. 44, 895–920 [DOI] [PubMed] [Google Scholar]
- King N.S. (2008). PTSD and traumatic brain injury: folklore and fact? Brain Inj. 22, 1–5 [DOI] [PubMed] [Google Scholar]
- Kleindienst A., Hesse F., Bullock M.R., and Buchfelder M. (2007). The neurotrophic protein S100B: value as a marker of brain damage and possible therapeutic implications. Prog. Brain Res. 161, 317–325 [DOI] [PubMed] [Google Scholar]
- Kleindienst A., and Ross Bullock M. (2006). A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J. Neurotrauma 23, 1185–1200 [DOI] [PubMed] [Google Scholar]
- Kobeissy F.H., Ottens A.K., Zhang Z., Liu M.C., Denslow N.D., Dave J.R., Tortella F.C., Hayes R.L., and Wang K.K. (2006). Novel differential neuroproteomics analysis of traumatic brain injury in rats. Mol. Cell Proteomics 5, 1887–1898 [DOI] [PubMed] [Google Scholar]
- Korfias S., Stranjalis G., Boviatsis E., Psachoulia C., Jullien G., Gregson B., Mendelow A.D., and Sakas D.E. (2007). Serum S-100B protein monitoring in patients with severe traumatic brain injury. Intensive Care Med. 33, 255–260 [DOI] [PubMed] [Google Scholar]
- Lescuyer P., Hochstrasser D., and Rabilloud T. (2007). How shall we use the proteomics toolbox for biomarker discovery? J. Proteome Res. 6, 3371–3376 [DOI] [PubMed] [Google Scholar]
- Lew H.L. (2005). Rehabilitation needs of an increasing population of patients: Traumatic brain injury, polytrauma, and blast-related injuries. J. Rehabil. Res. Dev. 42, xiii–xvi [PubMed] [Google Scholar]
- Lew H.L., Poole J.H., Alvarez S., and Moore W. (2005). Soldiers with occult traumatic brain injury. Am. J. Phys. Med. Rehabil. 84, 393–398 [DOI] [PubMed] [Google Scholar]
- Lucas S.M., Rothwell N.J., and Gibson R.M. (2006). The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 147(Suppl 1), S232–S240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga M.A. (1997). The pathology of primary blast overpressure injury. Toxicology 121, 17–28 [DOI] [PubMed] [Google Scholar]
- Michetti F., and Gazzolo D. (2002). S100B protein in biological fluids: a tool for perinatal medicine. Clin. Chem. 48, 2097–2104 [PubMed] [Google Scholar]
- Moochhala S.M., Md S., Lu J., Teng C.H., and Greengrass C. (2004). Neuroprotective role of aminoguanidine in behavioral changes after blast injury. J. Trauma 56, 393–403 [DOI] [PubMed] [Google Scholar]
- Murthy J.M., Chopra J.S., and Gulati D.R. (1979). Subdural hematoma in an adult following a blast injury. Case report. J. Neurosurg. 50, 260–261 [DOI] [PubMed] [Google Scholar]
- Nath R., Probert A., Jr., McGinnis K.M., and Wang K.K. (1998). Evidence for activation of caspase-3-like protease in excitotoxin-and hypoxia/hypoglycemia-injured neurons. J. Neurochem. 71, 186–195 [DOI] [PubMed] [Google Scholar]
- Nylen K., Ost M., Csajbok L.Z., Nilsson I., Blennow K., Nellgard B., and Rosengren L. (2006). Increased serum-GFAP in patients with severe traumatic brain injury is related to outcome. J. Neurol. Sci. 240, 85–91 [DOI] [PubMed] [Google Scholar]
- Ottens A.K., Kobeissy F.H., Golden E.C., Zhang Z., Haskins W.E., Chen S.S., Hayes R.L., Wang K.K., and Denslow N.D. (2006). Neuroproteomics in neurotrauma. Mass Spectrom. Rev. 25, 380–408 [DOI] [PubMed] [Google Scholar]
- Park S.Y., and Ferreira A. (2005). The generation of a 17 kDa neurotoxic fragment: an alternative mechanism by which tau mediates beta-amyloid-induced neurodegeneration. J. Neurosci. 25, 5365–5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.Y., Tournell C., Sinjoanu R.C., and Ferreira A. (2007). Caspase-3- and calpain-mediated tau cleavage are differentially prevented by estrogen and testosterone in beta-amyloid-treated hippocampal neurons. Neuroscience 144, 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelinka L.E., Hertz H., Mauritz W., Harada N., Jafarmadar M., Albrecht M., Redl H., and Bahrami S. (2005). Nonspecific increase of systemic neuron-specific enolase after trauma: clinical and experimental findings. Shock 24, 119–123 [DOI] [PubMed] [Google Scholar]
- Pelinka L.E., Kroepfl A., Leixnering M., Buchinger W., Raabe A., and Redl H. (2004). GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J. Neurotrauma 21, 1553–1561 [DOI] [PubMed] [Google Scholar]
- Piazza O., Storti M.P., Cotena S., Stoppa F., Perrotta D., Esposito G., Pirozzi N., and Tufano R. (2007). S100B is not a reliable prognostic index in paediatric TBI. Pediatr. Neurosurg. 43, 258–264 [DOI] [PubMed] [Google Scholar]
- Pike B.R., Flint J., Dutta S., Johnson E., . Wang K.K., and Hayes R.L. (2001). Accumulation of non-erythroid alpha II-spectrin and calpain-cleaved alpha II-spectrin breakdown products in cerebrospinal fluid after traumatic brain injury in rats. J. Neurochem. 78, 1297–1306 [DOI] [PubMed] [Google Scholar]
- Pike B.R., Zhao X., Newcomb J.K., Posmantur R.M., Wang K.K., and Hayes R.L. (1998a). Regional calpain and caspase-3 proteolysis of alpha-spectrin after traumatic brain injury. Neuroreport 9, 2437–2442 [DOI] [PubMed] [Google Scholar]
- Pike B.R., Zhao X., Newcomb J.K., Wang K.K., Posmantur R.M., and Hayes R.L. (1998b). Temporal relationships between de novo protein synthesis, calpain and caspase 3-like protease activation, and DNA fragmentation during apoptosis in septo-hippocampal cultures. J. Neurosci. Res. 52, 505–520 [DOI] [PubMed] [Google Scholar]
- Pineda J.A., Lewis S.B., Valadka A.B., Papa L., Hannay H.J., Heaton S.C., Demery J.A., Liu M.C., Aikman J.M., Akle V., Brophy G.M., Tepas J.J., Wang K.K., Robertson C.S., and Hayes R.L. (2007). Clinical significance of alphaII-spectrin breakdown products in cerebrospinal fluid after severe traumatic brain injury. J. Neurotrauma 24, 354–366 [DOI] [PubMed] [Google Scholar]
- Pineda J.A., Wang K.K., and Hayes R.L. (2004). Biomarkers of proteolytic damage following traumatic brain injury. Brain Pathol. 14, 202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringger N.C., O'Steen B.E., Brabham J.G., Silver X., Pineda J., Wang K.K., Hayes R.L., and Papa L. (2004). A novel marker for traumatic brain injury: CSF alphaII-spectrin breakdown product levels. J. Neurotrauma 21, 1443–1456 [DOI] [PubMed] [Google Scholar]
- Saljo A., Bao F., Haglid K.G., and Hansson H.A. (2000). Blast exposure causes redistribution of phosphorylated neurofilament subunits in neurons of the adult rat brain. J. Neurotrauma 17, 719–726 [DOI] [PubMed] [Google Scholar]
- Savic J., Tatic V., Ignjatovic D., Mrda V., Erdeljan D., Cernak I., Vujnov S., Simovic M., Andelic G., and Duknic M. (1991). [Pathophysiologic reactions in sheep to blast waves from detonation of aerosol explosives]. Vojnosanit. Pregl. 48, 499–506 [PubMed] [Google Scholar]
- Shiozaki T., Hayakata T., Tasaki O., Hosotubo H., Fuijita K., Mouri T., Tajima G., Kajino K., Nakae H., Tanaka H., Shimazu T., and Sugimoto H. (2005). Cerebrospinal fluid concentrations of anti-inflammatory mediators in early-phase severe traumatic brain injury. Shock 23, 406–410 [DOI] [PubMed] [Google Scholar]
- Stuhmiller J.H., Ho K.H., Vander Vorst M.J., Dodd K.T., Fitzpatrick T., and Mayorga M. (1996). A model of blast overpressure injury to the lung. J. Biomech. 29, 227–234 [DOI] [PubMed] [Google Scholar]
- Taber K.H., Warden D.L., and Hurley R.A. (2006). Blast-related traumatic brain injury: what is known? J. Neuropsychiatry Clin. Neurosci. 18, 141–145 [DOI] [PubMed] [Google Scholar]
- Vander Vorst M., Ono K., Chan P., and Stuhmiller J. (2007). Correlates to traumatic brain injury in nonhuman primates. J. Trauma 62, 199–206 [DOI] [PubMed] [Google Scholar]
- Vinores S.A., Herman M.M., Rubinstein L.J., and Marangos P.J. (1984). Electron microscopic localization of neuron-specific enolase in rat and mouse brain. J. Histochem. Cytochem. 32, 1295–1302 [DOI] [PubMed] [Google Scholar]
- Wang K.K., Ottens A.K., Liu M.C., Lewis S.B., Meegan C., Oli M.W., Tortella F.C., and Hayes R.L. (2005). Proteomic identification of biomarkers of traumatic brain injury. Expert Rev. Proteomics 2, 603–614 [DOI] [PubMed] [Google Scholar]
- Wang K.K., Posmantur R., Nath R., McGinnis K., Whitton M., Talanian R.V., Glantz S.B., and Morrow J.S. (1998). Simultaneous degradation of alphaII- and betaII-spectrin by caspase 3 (CPP32) in apoptotic cells. J. Biol. Chem. 273, 22490–22497 [DOI] [PubMed] [Google Scholar]
- Yilmaz S., and Pekdemir M. (2007). An unusual primary blast injury: Traumatic brain injury due to primary blast injury. Am. J. Emerg. Med. 25, 97–98 [DOI] [PubMed] [Google Scholar]
- Zemlan F.P., Jauch E.C., Mulchahey J.J., Gabbita S.P., Rosenberg W.S., Speciale S.G., and Zuccarello M. (2002). C-tau biomarker of neuronal damage in severe brain injured patients: association with elevated intracranial pressure and clinical outcome. Brain Res. 947, 131–139 [DOI] [PubMed] [Google Scholar]
- Zhang C., Siman R., Xu Y.A., Mills A.M., Frederick J.R., and Neumar R.W. (2002). Comparison of calpain and caspase activities in the adult rat brain after transient forebrain ischemia. Neurobiol. Dis. 10, 289–205 [DOI] [PubMed] [Google Scholar]