Abstract
The pathogenesis of rhegmatogenous retinal detachment depends on three factors, namely, retinal rupture, vitreous liquefaction and traction causing the retina to separate from the pigment epithelium, among which retinal rupture is the most important. Retinopathy is caused by a gap between the neurosensory retina and the retinal pigment epithelium, which severely damages the visual function of the patient. Therefore, early clinical discovery, prevention and selection of an appropriate treatment are important. This article reviews progress in the treatment of retinal detachment.
Keywords: rhegmatogenous retinal detachment, treatment progress, sclera external-route surgery, retinal laser photocoagulation, pars plana vitrectomy
INTRODUCTION
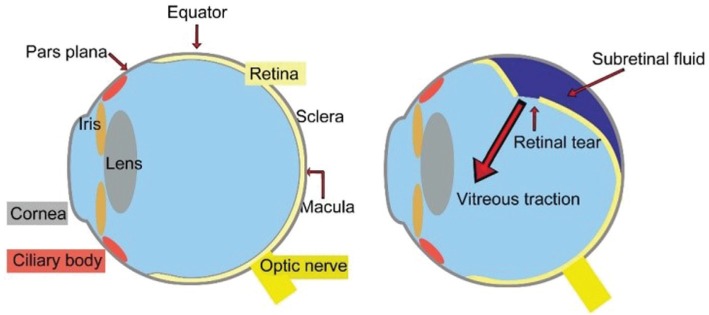
Rhegmatogenous retinal detachment (RRD) is the most common type of retinal detachment, with an incidence of 1 in 10 000 persons per year. Blindness in the affected eye may result via different mechanisms without proper and timely treatment. Various surgery options are available for RRD, but individualized surgery options may be the key to cure this disease. RRD is characterized by the formation of a retinal hole and is a pathological process in which the retina and vitreous degenerate and interact with each other. RRD is diagnosed when the retinal neurosensory layer (RNL) is isolated from retinal pigment epithelium (RPE) and retinopathy caused by ocular lesions or systemic diseases is excluded[1]. The anatomical changes of RRD are shown in Figures 1, 2 and 3. After retinal detachment occurs, nutrition to the photoreceptor cells is impaired. Once the posterior pole is involved, the photoreceptor cells of the retina will undergo apoptosis and degeneration; the pigment cells and fibroblasts will then abnormally and ectopically proliferate, thereby resulting in irreversible damage to visual function. If not treated quickly, the rate of blindness is nearly 100%.
Figure 1. The anatomical changes of rhegmatogenous retinal detachment.
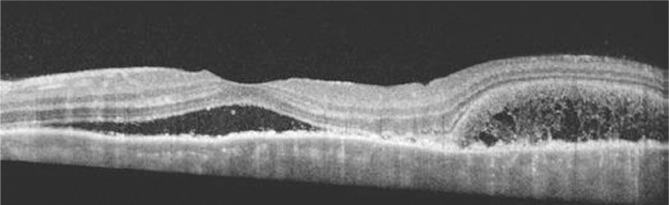
Figure 2. SD-OCT of the left eye demonstrates extensive serous retinal detachments involving the macular.
The image is derived from The Retinal Atlas[2].
Figure 3. Ultrasound image showing retinal detachment (arrowhead) and choroidal thickening (arrow).
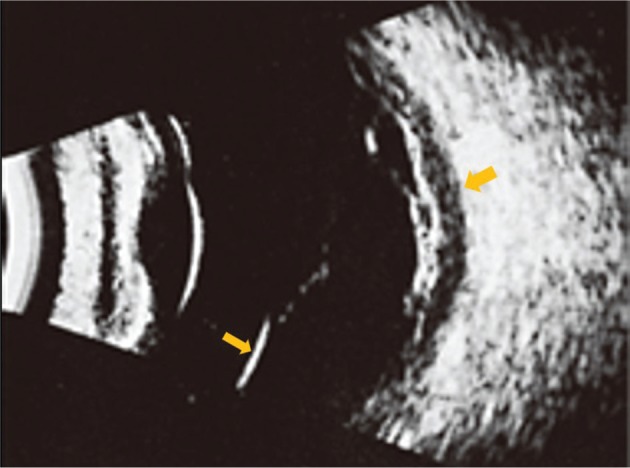
The image is derived from The Retinal Atlas[2].
Significant studies undertaken to understand the risk factors of RRD provide the basis for prevention and treatment. Since Gonin proposed that retinal holes are the cause of retinal detachment in1929, the identification and closing of all of these holes have been the basic principles of treatment. Decades of clinical practice have proven this theory to be correct[3], but according to the different positions and conditions of these holes, corresponding treatment options are available. Treatment involves one of two changes in retinal reposition: a type of evolution from whole retinal detachment surgery to confinement to the retina during surgery or a type of evolution from the eye to a change in intraocular surgery. Since the beginning of the 21st century, the key to the treatment of RRD has been to identify and solve the cause of primary retinal detachment; retinal detachment will repeat if the causes are left untreated. This approach is not associated with operation extended to the whole area or outside of the area and is unrelated to areas outside the eye or intraocular surgery. At the same time, retinal detachment treatment has been carried out under the following three assumptions: 1) one operation should be performed to achieve retinal reduction; 2) surgery should not cause complications that threaten later vision regaining; 3) surgery should be more cost-effective and local anesthesia are preferred.
This article reviews progress and indications in different surgeries for the treatment of RRD. The advantages and disadvantages of the surgeries are discussed, which may help ophthalmologists make treatment decisions.
Scleral Buckling
Scleral buckling (SB) surgery is considered the best surgical treatment for RRD. It has been reported that the postoperative anatomical reduction rate from SB is as high as 97.3%[4]. There are various advantages of SB surgery, such as ease of operation, reliable localization, low infection rate, and enhanced control of condensation time and condensation intensity[5]. At present, the most commonly used external scleral surgery involves a scleral ring, scleral pad and minimal external pressure. The diagnosis of retinal detachment and subsequent intervention through the application of circumferential bands and buckles and minimally invasive techniques can improve postoperative morbidity and the effectiveness of visual rehabilitation (Figure 4). The results of this conventional treatment of retinal detachment justify its application in treating the disease[6]. However, this treatment is particularly suitable for peripheral RRD and not for posterior polar retinal detachment.
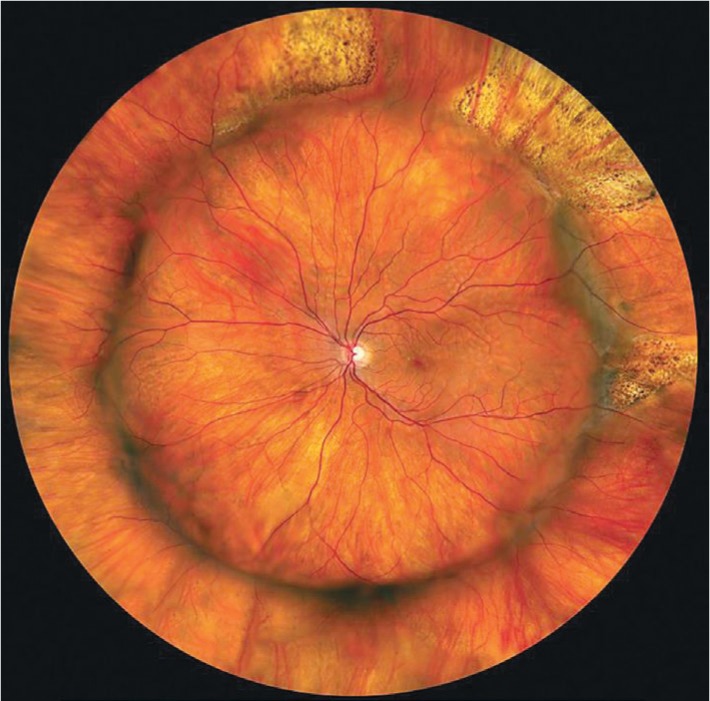
Figure 4. A panoramic image of the fundus following reattachment surgery with an encircling band.
Chorioretinal degenerative changes, likely a result of cryotherapy for retinal breaks, can be observed superiorly, superotemporally, and temporally. The image is derived from The Retinal Atlas[2].
Scleral ligation
Scleral ligation is an important method for the treatment of RRD. A ligation band is fixed under the four rectus muscles such that the retinal hiatus is located just in front of or on the scleral ridge. The band can be combined with local pressurization, drainage, and vitreous condensation, with or without injection of fluid to seal the retinal tear (Figure 5). If the location of the tear is appropriate, scleral ligation can have certain preventive effects. Scleral ring ligation is often used to ensure the successful reduction of retinal detachment involving multiple holes or a large area of detachment with no obvious holes. However, this procedure requires a 360° cut along the bulbar conjunctiva at the edge of the sclera, which can affect the microenvironment of the ocular surface, cause damage to the goblet cells and tear ducts, and reduce the production of mucin and basic tear secretion[7]–[8]. The patient may then have an acidic burning sensation, swelling, discomfort, dry eye, or eye-winking sensation after the operation. Wong et al[9] performed scleral ring ligation on 30 patients with RRD and concluded that the treatment can indeed affect the ocular surface microenvironment, thereby resulting in decreased tear secretion, shortened tear break-up time (BUT), and increased positive rates of corneal fluorescein (FL) staining, all of which take time from which to recover. Therefore, the scope of surgery and the degree of injury should be minimized during operation to avoid unnecessary complications.
Figure 5. Another panoramic image of RRD.
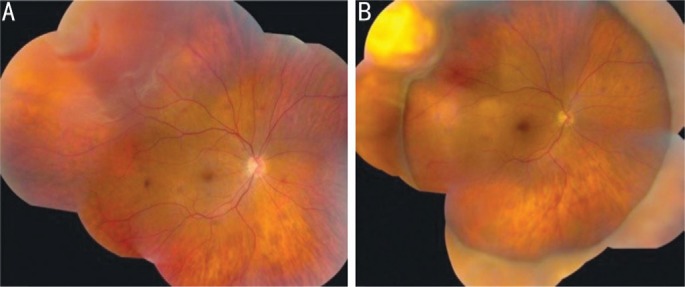
A: Preoperative image; B: The retinal tear site was treated superotemporally with cryotherapy, and an encircling band was placed. The image shows complete retinal reattachment with cryotherapy-induced atrophy at the site of the retinal tear, which is positioned on the crest of the encircling buckle. The image is derived from The Retinal Atlas[2].
Episcleral compression
In episcleral compression, the general scleral location of a hole is marked, and then the sclera is pressed towards the center of the eye with a pressure device to determine the exact location of the hole on the scleral surface. The ridge caused by the pressure is positioned at the posterior margin of the hiatus, and a mattress suture is used to fix the ridge in place, which effectively seals the hole. The purpose of developing external surgery for RRD is to seal the hiatus with minimal surgical invasion, to improve repositioning rates and to reduce surgical complications[3]. This simple external compression surgery was pioneered by Lincoff and Kreissig[10] and yielded good clinical results for the treatment of RRD using segmented scleral compression without drainage; the treatment reduced complications such as refractive changes, anterior ischemia, suprachoroidal hemorrhage, and visual field reduction[6],[11]–[13]. Clinical practice has shown that minimally invasive retinal surgery, including vitreous condensation, which does not impact discharge, optic circulation, scleral injection, or scleral extravasation, has the merits of accurate positioning, reliability in minimal surgical trauma and avoidance of certain complications from traditional scleral surgery while maintaining a high success rate. Therefore, the approach is a good alternative surgical method for the treatment of RRD[14]–[15].
Scleral Condensation
Scleral condensation, which has the advantages of simplicity, minimal scleral damage, external compression and a definite curative effect, is the most commonly used external operation for sealing the retinal hiatus in retinal detachment. Condensation is a safe and effective sealing method. However, scleral condensation can lead to cell death and aseptic necrosis of tissues, which can cause local inflammatory reactions in the choroid and retina, ultimately resulting in the adhesion of the RNL to the RPE and the formation of scar tissue that seals the retinal hiatus. Many complications, such as iatrogenic retinal tear, choroidal ischemia, proliferative vitreous retinopathy (PVR), and macular edema, are related to overly extensive and excessive condensation. The key is to master the proper amount of condensation needed, which can be achieved by direct operation.
Scleral Electrocoagulation
Scleral electrocoagulation involves the application of high electric heat, or diathermy, to critical areas of the sclera, thereby resulting in an inflammatory reaction, scar adhesion and closure of the retinal hiatus. The adhesion is relatively firm and has a highly reliable, curative effect. However, if this method is performed in an area that cannot be directly seen by the surgeon, it may result in damage to the sclera and choroid vitreous body. Excessive electrocoagulation is more likely to produce extensive scleral necrosis, scleral expansion and staphyloma, which increases the difficulty of reoperation. Therefore, at present, scleral condensation has displaced electrocoagulation and has been widely used[16].
Retinal Laser Photocoagulation
The use of lasers is an important method for treating and preventing retinal detachment, mainly for treating peripheral retinal degeneration and closed retinal hiatuses, preventing retinal detachment in denatured areas and promoting retinal restoration. The light energy of a laser is absorbed by the hemoglobin or uveal pigment in the retina and vascular tissues and transformed into heat energy, resulting in tissue degeneration and coagulation. At the same time, the RPE in the area of photocoagulation is temporarily damaged, and the passive motion of the subretinal fluids is accelerated, causing a decrease in uveal effusion and increased retinal adhesion, which finally close the hiatus. Research has demonstrated that laser photocoagulation has the same anatomical reduction rate as does condensation, but the extent of retinal damage is lower and complications are fewer[17]–[18]. Intraoperative denaturing of certain areas in the retina and the protruding edges of the hiatus can be accomplished by a laser, unlike the extensive ligation and condensation procedures previously used to prevent detachment in other sites. The lasers used are mainly the 532 semiconductor fundus laser (BVI, French), the argon laser (ZEISS, Germany) and the multiwavelength krypton laser (Qioptiq, England). Li[19] studied 89 patients with retinal holes treated by surrounding the holes with spots of photocoagulation using a 532 nm frequency-doubled laser. The results of the study showed that after 6-30mo, 97 of the treated eyes (99%) were sealed, and the subretinal fluid was absorbed completely after laser photocoagulation. This finding demonstrates that photocoagulation with a 532 nm frequency-doubled laser is a convenient method for treating retinal holes with less tissue damage and great therapeutic effect. Li[20] also researched seventy-two cases (78 eyes) of retinal holes treated with a 532 nm frequency-doubled laser to show that photocoagulation with this laser is effective and safe. Gao et al[21] retrospectively analyzed the data of 210 retinal detachment patients (224 eyes) with retinal holes who underwent argon laser therapy and compared the results with the data of 173 retinal detachment patients (198 eyes) without retinal holes who underwent the same therapy; the authors concluded that the effect of prophylactic argon laser therapy in retinal detachment patients with retinal holes, but not retinal detachment, is satisfactory. Compared with vitreoretinal surgery and conventional SB surgery, retinal laser photocoagulation quickly seals retinal holes after diagnosis with RRD (Figure 6). The requirements of the therapeutic environment and equipment for retinal laser photocoagulation are less restrictive, as it is a relatively simple technique and inexpensive treatment. However, treatmmust be followed up immediately[22].
Figure 6. A horseshoe retinal tear surrounded by a triple-row laser photocoagulation.
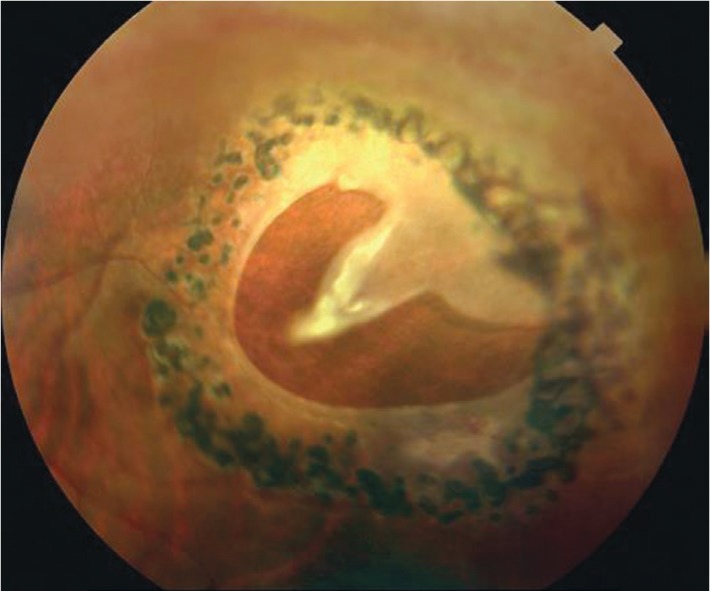
Laser retinopexy decreases the risk of retinal detachment to less than 5%. The image is derived from The Retinal Atlas[2].
Pneumatic Retinopexy
In 1986, Hilton and Grizzard[23] first introduced pneumatic retinopexy to treat RRD. The operation procedure begins with cryotherapy to freeze and seal the area around the retinal tear. Then, a gas bubble is injected through the sclera into the vitreous cavity to press against the retinal tear for reattachment[23]–[24]. This approach treats noncomplex RRD, which is retinal detachment caused by a small retinal hiatus at the top and back of the retina. Pneumatic retinopexy (PR) is the least invasive form of RRD surgery and can reduce recurrence rates and shorten recovery time[24]. However, postoperatively, patients must remain in certain positions to ensure retinal fixation, attend follow-up visits soon after surgery and avoid immediate air travel[25]. PR does not relieve vitreous traction on the retina, thereby increasing the risk that the sealed hiatus may be reopened and a new hiatus could form, which might result in retinal detachment. PR is not appropriate for inferior RRD. Nevertheless, the increased use of pneumatic retinopexy would achieve significant cost savings while maintaining outcomes[26]. Additionally, in the case of recurrence after PR, SB or vitrectomy can be successfully performed.
Subretinal Drainage
The drainage of subretinal fluid brings the RNL close to the RPE, which facilitates rapid restoration of the retina and shortens the course of the disease. Subretinal drainage can locate the retinal hiatus, thereby providing space for a large scleral ridge and preventing high intraocular pressure. Risks during the operation include low intraocular pressure, explosive suprachoroidal hemorrhage, accidental scleral perforation, massive choroid vascular hemorrhage, iatrogenic rupture, and retinal incarceration. However, case studies performed by Kang et al[27] suggest that subretinal aspiration and injection device provides an efficient and safe means of gaining access to the subretinal space.
Joint Vitreous Retinal Surgery
In the 1970s, Machemer pioneered closed vitrectomy. Pars plana vitrectomy (PPV) is a preferred surgical procedure for complex retinal detachment, such as a posterior pole retinal hiatus, vitreous hemorrhage, and PVR[28]. Not only does this procedure remove the vitreous traction on the retina, but it also removes any opaque mesenchyme, thereby optimizing conditions for examination and treatment. In addition, as with PR, SB, and laser prophylaxis, PPV is cost-effective[29]. After vitreous excision, a substitute substance is injected to restore the retina. Clinical vitreous substitutes include certain liquids and gases. Silicone oil, perfluorocarbon solution, and other liquids have been used as substitutes[30]. The condition in which silicone oil is filled in the vitreous is shown in Figure 7. The gas substitutes include air and perfluorocarbon gas, as shown in Figure 8. A liquid substitute restores the content of the eye, separates any membranes adhering to the retina, and flattens the detached retina[31]. Although air can successfully fill the vitreous cavity and contribute to volume reduction[31], it is readily absorbed after a few days, thereby affecting its long-term ability to hold the retina in place. A procedure was developed in 1980 in which perfluorinated carbon is injected into the vitreous cavity and then expanded; due to the long compression time of perfluorinated carbon[32], a high intraocular pressure is achieved, and when the patient maintains a certain position, the pressure on the retina can be maximized. The application of perfluorocarbon liquids has been well established in vitreoretinal surgery. The unique physical properties of these liquids make them an ideal intraoperative tool for improving the efficiency and safety of surgical procedures in complicated cases[33]–[34]. Scheer et al[35] used a heavy silicone oil substitute in 66 vitrectomies in patients with retinal detachment to reduce the rate of retinal reposition and incidence of complications; the authors found that many silica gels were well tolerated and did not appear to promote inflammation. The reset rate of silicon oil is 30%-75%[36], and the reset rate of heavy silicon oil is 45.8%-92.3%[37]–[39]. Gartry et al[40] reported that RRD without proliferative retinopathy was successfully treated with a single surgery in 74% of complex cases, and 92% were successful after further treatment. Additionally, a stain can be used to visualize the subretinal fluid and help reveal hidden retinal tears. The key features to the success of vitreous retinal surgery for RRD are the complete release of vitreous traction around the anterior retinal membrane and the retinal hole, the complete sealing of all retinal tears and the effective filling of the vitreous cavity. However, retinal leakage after PPV surgery is still common[40], thus highlighting the importance of preoperative fundus examination. With the development of science and technology, new machines, such as VISALIS 500 (ZEISS, Germany), Constellation (Alcon, America), and MidLabs (AI-TEST, China), are achieving higher cutting speeds and have more in-built facilities compared to previously developed machines.
Figure 7. This patient underwent vitreoretinal surgery with the use of silicone oil as a long-term tamponade.
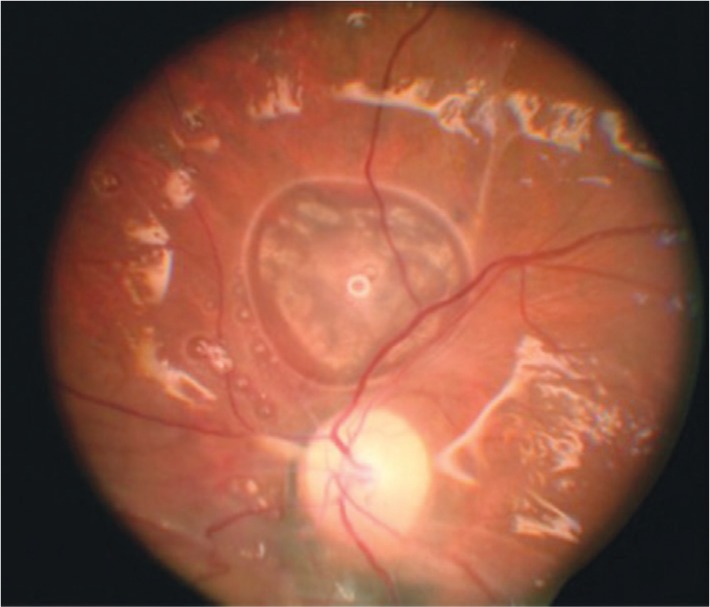
The glistening reflectance off the retinal surface is characteristic of vitrectomized eyes filled with silicone oil. In the center of the photograph, just superior to the optic nerve, note the elevated area where silicone oil has migrated into the subretinal space. The image is derived from The Retinal Atlas[2].
Figure 8. This patient underwent a pars plana vitrectomy with a long-acting gas tamponade to repair a retinal detachment.
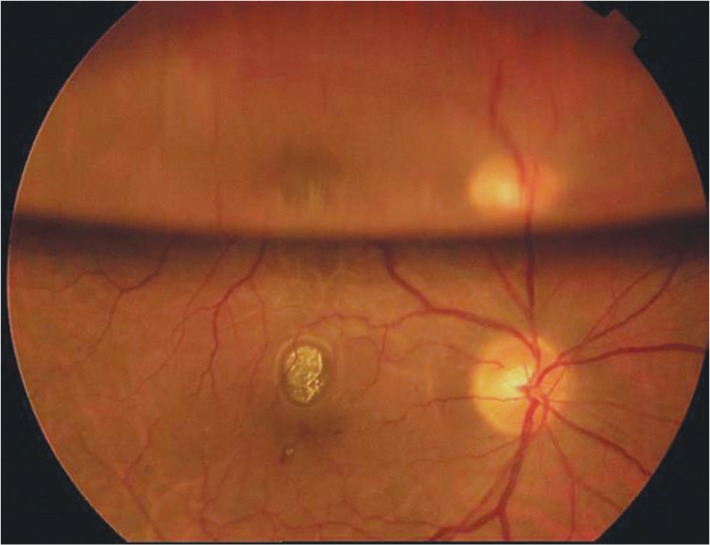
Note the reflection created by the gas-fluid interface superiorly. This reflection off the surface of the gas bubble creates a split image, giving the false appearance of a double optic nerve. The image is derived from The Retinal Atlas[2].
Vitreoretinal Surgery Combined with Scleral Ring Ligation
A scleral ring can relieve the traction of the vitreous on the retina and close the retinal hiatus. Therefore, use of a scleral ring was once considered a necessary surgical method. However, it is still unknown whether a scleral ring is necessary for RRD patients undergoing vitrectomy. It was reported that both the SB and PPV surgical techniques yielded high success rates and low complication rates in the treatment of primary RRD, effectively demonstrating the effect of scleral ring ligation in vitrectomy[41]. Macula-off status was associated with a lower success rate in the SB group, although lesion location and lens status had no significant effect on success rates in either group[42]. A study by Heimann et al[43] showed that compared with PPV, SB surgery was more beneficial for the improvement of BCVA in the eye with the lens. Some studies indicate that vitrectomy combined with an encircling scleral buckle in patients with RRD can improve patient comfort and grant a more stable refractive status after surgery[41]. However, studies have demonstrated no significant differences in the surgical success rates in the treatment of RRD between PPV, SB and the combined procedure of vitrectomy and buckling[44]–[45]. More information on comparative studies of SB and PPV is presented in Table 1. Table 1 shows that vitrectomy and SB surgery are the most commonly used surgeries. Each procedure has its own advantages and disadvantages. On the one hand, SB surgery in subretinal drainage may cause intraocular pressure fluctuations, and cingulate may cause retinal ischemia. On the other hand, injection of gas or silicone oil during vitrectomy can have certain effects on intraocular pressure fluctuations and the visual field in PPV. Both methods produce phototoxicity to the retina: SB surgery requires microscopic or external laser damage, while vitrectomy requires intraocular laser irradiation and intraocular lighting.
Table 1. More information on comparative studies of SB and PPV.
| First author (year) | Design | Main observational index | SB (n) | PPV (n) | Effects |
| Oshima (2000)[46] | Retrospective, 2 centers | BCVA | 55 | 47 | P<0.05 |
| Miki (2001)[47] | Retrospective, single center | Success rate, incidence of postoperative | 138 | 87 | PPV merit to SB |
| Schmidt (2003)[48] | Retrospective, single center | Reattachment rate, complications | 60 | 40 | Different |
| Le Rouic (2002)[49] | Retrospective, single center | BCVA, success rate | 40 | 32 | Similar |
| Afrashi (2004)[50] | Retrospective, single center | Success rate, complications | 30 | 22 | Different |
| Sharma (2005)[51] | Prospective, randomized, single center | BCVA, reattachment rate | 25 | 25 | PPV merit to SB |
| Ahmadieh (2005)[52] | Prospective, randomized, multicenter | BCVA, reattachment rate | 125 | 125 | Different |
| Brazitikos (2005)[53] | Prospective, single center | Proliferative vitreoretinopathy | 75 | 75 | Similar |
| Kobashi (2014)[42] | Prospective, randomized, multicenter | BCVA, reattachment rate | 133 | 132 | Different |
BCVA: Best corrected visual acuity; SB: Scleral buckling; PPV: Pars plana vitrectomy.
Drug Therapy
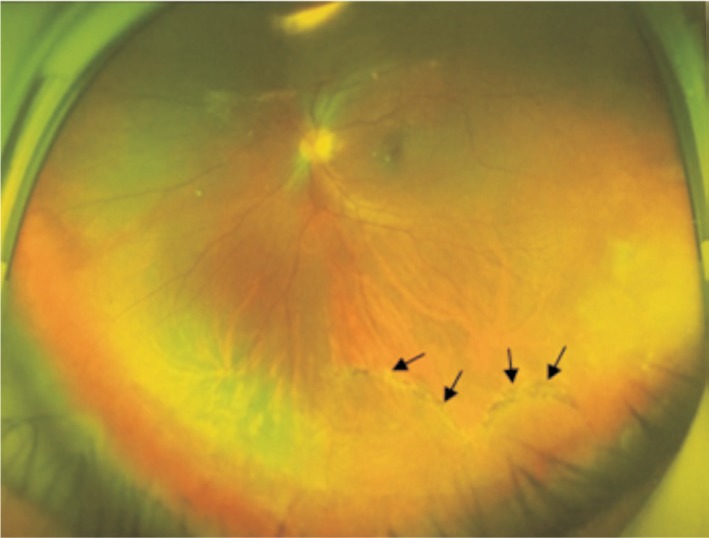
Currently, drugs are mainly used as an auxiliary therapy for retinal detachment. Western medicines used in treatment include tenecteplase, acetazolamide, and triamcinolone acetonide. Traditional Chinese medicines include decoctions of Kangwangling, Lishuifang, Wangfutang, and Fumingpian. It was found that an intracavitary, intravitreal injection of tenecteplase could induce vitreous posterior detachment and reduce the occurrence of PVR and the recurrence of retinal detachment[54]. Triamcinolone can be used to help locate hidden retinal hiatuses, thus improving the detection of retinal hiatuses and reducing the recurrence of retinal detachment[55]–[56]. Tuuminen et al[57]–[59] conducted a retrospective observational study of 14 patients operated on for RRD while on statins compared to patients without statin medication (n=82). The findings of the study suggested that administration of statins may be effective in preventing inflammation-related PVR formation. In some cases, RRD tends to be self-limiting and self-healing. Currently, more observations are needed. The fundus of a patient after self-healing is shown in Figure 9.
Figure 9. A male patient seeking laser surgery found a detached retina in the fundus of his eye.
To summarize, at present, RRD is still one of the most common and complex diseases in the clinic. Although anatomical reduction is obtained after treatment, good postoperative visual acuity cannot always be guaranteed. Therefore, the choice of a suitable surgical method is particularly important. Each method has advantages and disadvantages, and each case has its corresponding indications, requiring that the appropriate surgical methods be selected accordingly[60]. For complex retinal detachment, there are no reliable data to prove whether SB surgery or vitrectomy is more advantageous, and a standard treatment is still lacking, generally leaving surgical treatment options to be selected according to the experience of the attending doctors[61]–[62]. Therefore, a simple, adaptable, safe and effective method is still needed, and we should choose the most suitable treatment according to the conditions faced.
Acknowledgments
Conflicts of Interest: Liao L, None; Zhu XH, None.
REFERENCES
- 1.Feltgen N, Walter P. Rhegmatogenous retinal detachment--an ophthalmologic emergency. Dtsch Arztebl Int. 2014;111(1-2):12–21. quiz 22. doi: 10.3238/arztebl.2014.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freund KB, Sarraf D, Mieler WF, Yannuzzi LA. The Retinal Atlas E-Book. 2nd ed. Philadelphia, PA: Elsevier; 2017. [Google Scholar]
- 3.Sodhi A, Leung LS, Do DV, Gower EW, Schein OD, Handa JT. Recent trends in the management of rhegmatogenous retinal detachment. Surv Ophthalmol. 2008;53(1):50–67. doi: 10.1016/j.survophthal.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhu L, Lin LI, Tian XY, Liu T, Chen M, Pei XU, Zou J, Xie AM. Correlative factors analysis of affecting anatomical reattachment and vision restoration after scleral buckling surgery. Recent Advances in Ophthalmology. 2017;37(2):167–171. [Google Scholar]
- 5.Zhong LX, Du Y, Liu W, Huang SY, Zhang SC. Using surgical microscope for sclera buckling and transscleral cryopexy: an alternative procedure of treatment for rhegmatogenous retinal detachment. Biomed Res Int. 2014;2014:364961. doi: 10.1155/2014/364961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H. Clinical experience of external-route retinal detachment surgery under a surgical microscope. Eye Sci. 2014;29(1):43–46. [PubMed] [Google Scholar]
- 7.Colligris B, Crooke A, Huete-Toral F, Pintor J. An update on dry eye disease molecular treatment: advances in drug pipelines. Expert Opinion on Pharmacotherapy. 2014;15(10):1371–1390. doi: 10.1517/14656566.2014.914492. [DOI] [PubMed] [Google Scholar]
- 8.Zhang XB, M VJ, Qu Y, He X, Ou SK, Bu JH, Jia CK, Wang JQ, Wu H, Liu ZG, Li W. Dry eye management: targeting the ocular surface microenvironment. Int J Mol Sci. 2017;18(7):pii: E1398. doi: 10.3390/ijms18071398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong CW, Ang M, Tsai A, Phua V, Lee SY. A prospective study of biometric stability after scleral buckling surgery. Am J Ophthalmol. 2016;165:47–53. doi: 10.1016/j.ajo.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Lincoff H, Kreissig I. Extraocular repeat surgery of retinal detachment. A minimal approach. Ophthalmology. 1996;103(10):1586–1592. doi: 10.1016/s0161-6420(96)30459-4. [DOI] [PubMed] [Google Scholar]
- 11.Wu TE, Rosenbaum AL, Demer JL. Severe strabismus after scleral buckling: multiple mechanisms revealed by high-resolution magnetic resonance imaging. Ophthalmology. 2005;112(2):327–336. doi: 10.1016/j.ophtha.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Kreissig I. Treatment of primary retinal detachment. Minimal extraocular or intraocular? Ophthalmologe. 2002;99(6):474–484. doi: 10.1007/s00347-001-0597-2. [DOI] [PubMed] [Google Scholar]
- 13.Kreissig I. Surgical techniques for repair of primary retinal detachment: Part II. Comparison of present techniques in relation to morbidity. Folia Med (Plovdiv) 2010;52(1):5–11. [PubMed] [Google Scholar]
- 14.Rumpf J. Jules Gonin. Inventor of the surgical treatment for retinal detachment. Surv Ophthalmol. 1976;21(3):276–284. doi: 10.1016/0039-6257(76)90125-9. [DOI] [PubMed] [Google Scholar]
- 15.Hadden B. Pioneering surgery for retinal detachment in Australasia: a review. Clin Exp Ophthalmol. 2016;44(7):618–623. doi: 10.1111/ceo.12776. [DOI] [PubMed] [Google Scholar]
- 16.Matthäus W, Dietze H, Huhle C, Pilz D. Comparative experimental studies on the degree of scleral shrinking in electrocoagulation and cryotherapy. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1969;178(2):147–151. doi: 10.1007/BF00414379. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Xia Y, Yang Z, Yang X, Wang X. The scleral buckling combined with argon laser photocoagulation for retinal detachment. Zhonghua Yan Ke Za Zhi. 2001;37(4):278–280. [PubMed] [Google Scholar]
- 18.Schwarze U, Laqua H. Retinoschisis with a giant central outer layer retinal tear and concomitant detachment. Klin Monbl Augenheilkd. 1990;197(1):50–52. doi: 10.1055/s-2008-1046243. [DOI] [PubMed] [Google Scholar]
- 19.Li H. Photocoagulation with 532nm fre-quency-doubled laser for 89 cases of retinal holes. Guoji Yanke Zazhi. 2005;5(2):360–361. [Google Scholar]
- 20.Li H. Clinical analysis of 532nm frequency-doubled laser in treating retinal holes of 78 eyes. Guoji Yanke Zazhi. 2013;13(9):1832–1834. [Google Scholar]
- 21.Gao XW, Guan HJ, Xu JY. Clinical characteristics of retinal degeneration with retinal holes and the therapeutic effect of argon laser therapy. Chin J Ocul Fundus Dis. 2006;22(1):39–41. [Google Scholar]
- 22.Velieva IA, Il'ina TS, Privivkova EA, Gurova IV, Gamidov AA. Efficiency of laser coagulation in the treatment of rhegmatogenous retinal detachment. Vestn Oftalmol. 2010;126(5):40–43. [PubMed] [Google Scholar]
- 23.Hilton GF, Grizzard WS. Pneumatic retinopexy. A two-step outpatient operation without conjunctival incision. Ophthalmology. 1986;93(5):626–641. doi: 10.1016/s0161-6420(86)33696-0. [DOI] [PubMed] [Google Scholar]
- 24.Hilton GF, Kelly NE, Salzano TC, Tornambe PE, Wells JW, Wendel RT. Pneumatic retinopexy. A collaborative report of the first 100 cases. Ophthalmology. 1987;94(4):307–314. [PubMed] [Google Scholar]
- 25.Trillo M, Facino M, Terrile R, Corazza M, Mosci C, Baldi F, Trillo CA. Treatment of uncomplicated cases of rhegmatogenous retinal detachment with an expanding gas bubble. Ophthalmologica. 1993;207(3):140–143. doi: 10.1159/000310420. [DOI] [PubMed] [Google Scholar]
- 26.Goldman DR, Shah CP, Heier JS. Expanded criteria for pneumatic retinopexy and potential cost savings. Ophthalmology. 2014;121(1):318–326. doi: 10.1016/j.ophtha.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 27.Kang SW, Kwon HN, Lee HW, Ham DI, Ahn BH. A new instrument for drainage or injection of fluid within subretinal space. Retina. 2003;23(5):661–666. doi: 10.1097/00006982-200310000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Jackson TL, Johnston RL, Donachie PH, Williamson TH, Sparrow JM, Steel DH. The royal college of ophthalmologists' national ophthalmology database study of vitreoretinal surgery: report 6, diabetic vitrectomy. JAMA Ophthalmol. 2016;134(1):79–85. quiz 120. doi: 10.1001/jamaophthalmol.2015.4587. [DOI] [PubMed] [Google Scholar]
- 29.Chang JS, Smiddy WE. Cost-effectiveness of retinal detachment repair. Ophthalmology. 2014;121(4):946–951. doi: 10.1016/j.ophtha.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz SG, Flynn HW, Jr, Lee WH, Wang X. Tamponade in surgery for retinal detachment associated with proliferative vitreoretinopathy. Cochrane Database Syst Rev. 2014;(2):CD006126. doi: 10.1002/14651858.CD006126.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cekic O, Ohji M. Intraocular gas tamponades. Semin Ophthalmol. 2000;15(1):3–14. doi: 10.3109/08820530009037846. [DOI] [PubMed] [Google Scholar]
- 32.Wong D, Lois N. Perfluorocarbons and semifluorinated alkanes. Semin Ophthalmol. 2000;15(1):25–35. doi: 10.3109/08820530009037848. [DOI] [PubMed] [Google Scholar]
- 33.Yu Q, Liu K, Su L, Xia X, Xu X. Perfluorocarbon liquid: its application in vitreoretinal surgery and related ocular inflammation. Biomed Res Int. 2014;2014:250323. doi: 10.1155/2014/250323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theodoratou A, Jonas U, Loppinet B, Geue T, Stangenberg R, Keller R, Li D, Berger R, Vermant J, Vlassopoulos D. Semifluorinated alkanes at the air-water interface: tailoring structure and rheology at the molecular scale. Langmuir. 2016;32(13):3139–3151. doi: 10.1021/acs.langmuir.5b04744. [DOI] [PubMed] [Google Scholar]
- 35.Scheer S, Boni S, Barale PO, Bourhis A, Bonnel S, Tuil E, Girmens JF, Buil O, Baudouin C, Laroche L, Nordmann JP, Poisson F, Warnet JM, Sahel JA. Heavy silicone oil as internal tamponade for retinal detachment: efficacy and tolerance. J Fr Ophtalmol. 2006;29(2):129–135. doi: 10.1016/s0181-5512(06)73760-3. [DOI] [PubMed] [Google Scholar]
- 36.Jonas JB, Knorr HL, Rank RM, Budde WM. Retinal redetachment after removal of intraocular silicone oil tamponade. Br J Ophthalmol. 2001;85(10):1203–1207. doi: 10.1136/bjo.85.10.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizzo S, Genovesi-Ebert F, Belting C, Vento A, Cresti F. A pilot study on the use of silicone oil–RMN3 as heavier-than-water endotamponade agent. Graefe's Arch Clin Exp Ophthalmo. 2005;243(11):1153–1157. doi: 10.1007/s00417-005-0015-6. [DOI] [PubMed] [Google Scholar]
- 38.Sandner D, Engelmann K. First experiences with high-density silicone oil (Densiron) as an intraocular tamponade in complex retinal detachment. Graefe's Arch Clin Exp Ophthalmo. 2006;244(5):609–619. doi: 10.1007/s00417-005-0110-8. [DOI] [PubMed] [Google Scholar]
- 39.Prazeres J, Magalhães O, Jr, Lucatto LF, Navarro RM, Moraes NS, Farah ME, Maia A, Maia M. Heavy silicone oil as a long-term endotamponade agent for complicated retinal detachments. Biomed Res Int. 2014;2014:136031. doi: 10.1155/2014/136031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gartry DS, Chignell AH, Franks WA, Wong D. Pars plana vitrectomy for the treatment of rhegmatogenous retinal detachment uncomplicated by advanced proliferative vitreoretinopathy. Br J Ophthalmol. 1993;77(4):199–203. doi: 10.1136/bjo.77.4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Falkner-Radler CI, Graf A, Binder S. Vitrectomy combined with endolaser or an encircling scleral buckle in primary retinal detachment surgery: a pilot study. Acta Ophthalmol. 2015;93(5):464–469. doi: 10.1111/aos.12663. [DOI] [PubMed] [Google Scholar]
- 42.Kobashi H, Takano M, Yanagita T, Shiratani T, Wang GQ, Hoshi K, Shimizu K. Scleral buckling and pars plana vitrectomy for rhegmatogenous retinal detachment: an analysis of 542 eyes. Curr Eye Res. 2014;39(2):204–211. doi: 10.3109/02713683.2013.838270. [DOI] [PubMed] [Google Scholar]
- 43.Heimann H, Bartz-Schmidt KU, Bornfeld N, Weiss C, Hilgers RD, Foerster MH. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment. Ophthalmology. 2007;114(12):2142–2154.e4. doi: 10.1016/j.ophtha.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 44.Haugstad M, Moosmayer S, Bragadόttir R. Primary rhegmatogenous retinal detachment - surgical methods and anatomical outcome. Acta Ophthalmol. 2017;95(3):247–251. doi: 10.1111/aos.13295. [DOI] [PubMed] [Google Scholar]
- 45.Ghoraba HH, Zaky AG, Ellakwa AF. Long-term follow-up of vitrectomy, with or without 360° encircling buckle, for rhegmatogenous retinal detachment due to inferior retinal breaks. Clin Ophthalmol. 2016;10:1145–1151. doi: 10.2147/OPTH.S102082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oshima Y, Yamanishi S, Sawa M, Motokura M, Harino S, Emi K. Two-year follow-up study comparing primary vitrectomy with scleral buckling for macula-off rhegmatogenous retinal detachment. Jpn J Ophthalmol. 2000;44(5):538–549. doi: 10.1016/s0021-5155(00)00205-7. [DOI] [PubMed] [Google Scholar]
- 47.Miki D, Hida T, Hotta K, Shinoda K, Hirakata A. Comparison of scleral buckling and vitrectomy for retinal detachment resulting from flap tears in superior quadrants. Jpn J Ophthalmol. 2001;45(2):187–191. doi: 10.1016/s0021-5155(00)00377-4. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt JC, Rodrigues EB, Hoerle S, Meyer CH, Kroll P. Primary vitrectomy in complicated rhegmatogenous retinal detachment: a survey of 205 eyes. Ophthalmologica. 2003;217(6):387–392. doi: 10.1159/000073067. [DOI] [PubMed] [Google Scholar]
- 49.Le Rouic JF, Behar-Cohen F, Azan F, Bertin S, Bettembourg O, Rumen F, Caudron C, Renard G, Chauvaud D. Vitrectomy without scleral buckle versus ab-externo approach for pseudophakic retinal detachment: comparative retrospective study. J Fr Ophtalmol. 2002;25(3):240–245. [PubMed] [Google Scholar]
- 50.Afrashi F, Erakgun T, Akkin C, Kaskaloglu M, Mentes J. Conventional buckling surgery or primary vitrectomy with silicone oil tamponade in rhegmatogenous retinal detachment with multiple breaks. Graefe's Arch Clin Exp Ophthalmol. 2004;242(4):295–300. doi: 10.1007/s00417-003-0842-2. [DOI] [PubMed] [Google Scholar]
- 51.Sharma YR, Karunanithi S, Azad RV, Vohra R, Pal N, Singh DV, Chandra P. Functional and anatomic outcome of scleral buckling versus primary vitrectomy in pseudophakic retinal detachment. Acta Ophthalmol Scand. 2005;83(3):293–297. doi: 10.1111/j.1600-0420.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- 52.Ahmadieh H, Moradian S, Faghihi H, Parvaresh MM, Ghanbari H, Mehryar M, Heidari E, Behboudi H, Banaee T, Golestan B, Pseudophakic and Aphakic Retinal Detachment (PARD) Study Group Anatomic and visual outcomes of scleral buckling versus primary vitrectomy in pseudophakic and aphakic retinal detachment: six-month follow-up results of a single operation: report no. 1. Ophthalmology. 2005;112(8):1421–1429. doi: 10.1016/j.ophtha.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 53.Brazitikos PD, Androudi S, Christen WG, Stangos NT. Primary pars plana vitrectomy versus scleral buckle surgery for the treatment of pseudophakic retinal detachment: a randomized clinical trial. Retina. 2005;25(8):957–964. doi: 10.1097/00006982-200512000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Tezel TH, del Priore LV, Kaplan HJ. Posterior vitreous detachment with dispase. Retina. 1998;18(1):7–15. doi: 10.1097/00006982-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Matsumoto H, Enaida H, Hisatomi T, Ueno A, Nakamura T, Yamanaka I, Sakamoto T, Ishibashi T. Retinal detachment in morning glory syndrome treated by triamcinolone acetonide-assisted pars plana vitrectomy. Retina. 2003;23(4):569–572. doi: 10.1097/00006982-200308000-00028. [DOI] [PubMed] [Google Scholar]
- 56.Mao JB, Wu SL, Chen YQ, Dong YG, Zheng B, Tao JW, Zhao SX, Fang D, Shen LJ. The efficiency of 23 G vitrectomy combined with preoperative subtenon injection of triamcinolone acetonide for treatment of retinal detachment associated with choroidal detachment. Zhonghua Yan Ke Za Zhi. 2018;54(4):252–257. doi: 10.3760/cma.j.issn.0412-4081.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 57.Tuuminen R, Haukka J, Loukovaara S. Statins in rhegmatogenous retinal detachment are associated with low intravitreal angiopoietin-2, VEGF and MMP-2 levels, and improved visual acuity gain in vitrectomized patients. Graefes Arch Clin Exp Ophthalmol. 2015;253(10):1685–1693. doi: 10.1007/s00417-014-2873-2. [DOI] [PubMed] [Google Scholar]
- 58.Tuuminen R, Loukovaara S. Statin medication in patients with epiretinal membrane is associated with low intravitreal EPO, TGF-beta-1, and VEGF levels. Clin Ophthalmol. 2016;10:921–928. doi: 10.2147/OPTH.S105686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tuuminen R, Sahanne S, Loukovaara S. Low intravitreal angiopoietin-2 and VEGF levels in vitrectomized diabetic patients with simvastatin treatment. Acta Ophthalmol. 2014;92(7):675–681. doi: 10.1111/aos.12363. [DOI] [PubMed] [Google Scholar]
- 60.García-Arumí J, Martínez-Castillo V, Boixadera A, Blasco H, Marticorena J, Zapata MÁ, Macià C, Badal J, Distéfano L, Rafart JM, Berrocal M, Zambrano A, Ruíz-Moreno JM, Figueroa MS. Rhegmatogenous retinal detachment treatment guidelines. Arch Soc Esp Oftalmol. 2013;88(1):11–35. doi: 10.1016/j.oftal.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 61.Loo A, Fitt AW, Ramchandani M, Kirkby GR. Pars plana vitrectomy with silicone oil in the management of combined rhegmatogenous retinal and choroidal detachment. Eye (Lond) 2001;15(Pt 5):612–615. doi: 10.1038/eye.2001.195. [DOI] [PubMed] [Google Scholar]
- 62.Furino C, Micelli Ferrari T, Boscia F, Cardascia N, Recchimurzo N, Sborgia C. Triamcinolone-assisted pars plana vitrectomy for proliferative vitreoretinopathy. Retina. 2003;23(6):771–776. doi: 10.1097/00006982-200312000-00004. [DOI] [PubMed] [Google Scholar]