Abstract
Background
Migraine is a highly disabling condition for the individual and also has wide‐reaching implications for society, healthcare services, and the economy. Sumatriptan is an abortive medication for migraine attacks, belonging to the triptan family. It is available for administration by four different routes: oral, subcutaneous, intranasal, and rectal.
Objectives
To summarise evidence from four Cochrane intervention reviews on the efficacy and tolerability of sumatriptan in the treatment of acute migraine attacks in adults by four routes of administration (oral, subcutaneous, intranasal, and rectal) compared with both placebo and active comparators.
Methods
The included reviews were written by the authors of this overview; no additional searching was carried out. All included reviews were conducted according to a standard protocol and reported a standard set of outcomes. From each individual review we extracted results for pain relief at different levels, and adverse events. No additional statistical comparison was undertaken as part of the overview. We focused on the most important findings for doses and routes licensed in North America or Europe (oral 25 mg, 50 mg, 100 mg; subcutaneous 4 mg, 6 mg; intranasal 5 mg, 10 mg, 20 mg; rectal 25 mg).
Main results
Included reviews provided data for 18 different dose and route of administration combinations in 52,236 participants. Data for the primary outcomes sought were generally well reported, and involved adequate numbers of participants to give confidence in the results, except for the rectal route of administration, where numbers were low.
Subcutaneous administration was the most effective, with pain reduced from moderate or severe to none by two hours in almost 6 in 10 people (59%) taking 6 mg sumatriptan, compared with approximately 1 in 7 (15%) taking placebo; the number needed to treat (NNT) was 2.3 (95% confidence interval 2.1 to 2.4) with 2522 participants in the analysis. The most commonly used doses of oral, rectal, and intranasal sumatriptan also provided clinically useful pain relief, with the oral 50 mg dose providing complete relief of pain in almost 3 in 10 people (28%) compared with about 1 in 10 (11%) after placebo (NNT 6.1 (5.5 to 6.9) in 6447 participants). Subcutaneous administration provided more rapid pain relief than the other routes. Taking medication early, when pain was mild, was more effective than waiting until the pain was moderate or severe.
The most effective dose of sumatriptan for each route of administration for the outcome of headache relief (pain reduced from moderate or severe to none or mild) at two hours was oral 100 mg (NNT 3.5 (3.2 to 3.7) in 7811 participants), subcutaneous 6 mg (NNT 2.1 (2.0 to 2.2) in 2738 participants), intranasal 20 mg (NNT 3.5 (3.1 to 4.1) in 2020 participants), and rectal 25 mg (NNT 2.4 (1.9 to 3.4) in 240 participants).
Adverse events were generally of mild or moderate severity, of short duration, and more common with subcutaneously administered sumatriptan and higher doses of oral and intranasal sumatriptan than with other dose and route combinations.
Authors' conclusions
Sumatriptan is an effective abortive treatment for acute migraine attacks, but is associated with increased adverse events relative to placebo. The route of administration influences efficacy, particularly within the first hour after administration. Subcutaneous sumatriptan shows the greatest efficacy in terms of pain relief, but at the expense of relatively high levels of adverse events, and with a high financial cost compared with other routes. Information about the relative efficacy of the different routes of administration for different outcomes should help to inform decisions about the suitability of sumatriptan as a migraine treatment, as well as about the most appropriate way to administer the treatment for individual patients.
Plain language summary
Sumatriptan (all routes of administration) for acute migraine attacks in adults
Migraine is a complex condition with a wide variety of symptoms. For many people, the main feature is a painful, and often disabling, headache. Other symptoms include disturbed vision; sensitivity to light, sound, and smells; feeling sick; and vomiting. Migraine affects about 1 person in 8, mainly women, and mainly in the age range of 30 to 50 years.
Sumatriptan is one of the triptan family of drugs used to treat migraine attacks. It can be given by four different routes: by mouth (oral), by injection under the skin (subcutaneous), by nasal spray (intranasal), and by suppositories (rectal). Separate Cochrane reviews for each of these routes provided information on how well sumatriptan worked at reducing headache pain in over 50,000 people with migraine. For oral, subcutaneous, and intranasal sumatriptan there was a large amount of information from good quality trials, but there was relatively little information about rectal administration.
This overview found that a single dose administered via any of these routes was effective in relieving migraine headache pain.
The subcutaneous route provided the best pain relief, with pain reduced from moderate or severe to none by two hours in almost 6 in 10 people (59%) taking the 6 mg dose, compared with approximately 1 in 7 (15%) taking placebo. The most commonly used doses of oral, rectal, and intranasal sumatriptan also provided useful pain relief. The oral 50 mg dose (the least effective of the commonly used dose and route combinations) provided complete relief of pain in almost 3 in 10 people (28%) compared with about 1 in 10 (11%) after placebo. Subcutaneous sumatriptan was also the fastest acting, providing more people with pain relief within one hour of treatment than any other route of administration.
Adverse events, which were mostly of mild or moderate severity and of short duration, were more common with subcutaneously administered sumatriptan and higher doses of oral and intranasal sumatriptan than with other dose and route combinations.
Background
Description of the condition
Migraine is a common, disabling headache disorder, ranked seventh highest among specific causes of disability globally (Steiner 2013), and with considerable social and economic impact (Hazard 2009). Recent reviews found a one‐year prevalence of 15% globally (Vos 2012) and for adults in European countries (Stovner 2010), 13% for all ages in the USA (Victor 2010), 21% in Russia (Ayzenberg 2012) and 9% for adults in China (Yu 2012). Migraine is more prevalent in women than in men (by a factor of two to three), and in the age range 30 to 50 years.
The International Headache Society (IHS) classifies two major subtypes (IHS 2013). Migraine without aura is the most common subtype. It is characterised by attacks lasting 4 to 72 hours that are typically of moderate to severe pain intensity, unilateral, pulsating, aggravated by normal physical activity, and associated with nausea and/or photophobia and phonophobia. Migraine with aura is characterised by reversible focal neurological symptoms that develop over a period of at least five minutes and last for less than 60 minutes, followed by headache with the features of migraine without aura. In some cases the headache may lack migrainous features or be absent altogether (IHS 2013).
A large prevalence study in the USA found that over half of migraineurs had severe impairment or required bed rest during attacks. Despite this high level of disability and a strong desire for successful treatment, only a proportion of migraine sufferers sought professional advice for the treatment of attacks. The majority were not taking any preventive medication, although one‐third met guideline criteria for offering or considering it. Nearly all (98%) migraineurs used acute treatments for attacks, with 49% using over‐the‐counter (OTC) medication only, 20% using prescription medication, and 29% using both. OTC medication included aspirin, other non‐steroidal anti‐inflammatory drugs (NSAIDs), paracetamol (acetaminophen) and paracetamol with caffeine (Bigal 2008; Diamond 2007; Lipton 2007). Similar findings have been reported from other large studies in France and Germany (Lucas 2006; Radtke 2009).
The significant impact of migraine with regard to pain, functional health, and well‐being is well documented (Buse 2011; Leonardi 2005); it is ranked in the top 10 disorders for global years lived with disability (Vos 2012). A cross‐sectional survey of eight European Union (EU) countries (representing 55% of the adult population) has estimated an annual direct and indirect cost of migraine per person of EUR 1222, and a total annual cost for the EU of EUR 111 billion for adults aged 18 to 65 years (Linde 2012). Costs vary between countries, probably due to differences in available therapies and the way they are delivered, and structural differences in healthcare systems (Bloudek 2012). In the USA, the average annual direct cost per person has been estimated at USD 1757 for episodic migraine and USD 7750 for chronic migraine (Munakata 2009). Whatever the exact direct and indirect costs are for each country, it is clear that migraine presents a significant economic burden. Successful treatment of acute migraine attacks not only benefits patients by reducing their disability and improving health‐related quality of life, but also has the potential to reduce the need for healthcare resources and increase economic productivity.
Description of the interventions
The symptomatic treatment of migraine advanced significantly with the development of the triptan class of drugs, of which sumatriptan was the first, in 1991. It is available as a standard oral tablet, nasal spray, subcutaneous injection, and rectal suppository. Different formulations may offer benefits to individuals in terms of speed of onset of relief or adverse events, and non‐oral formulations may be particularly useful for those who experience severe nausea or vomiting with their attacks. Each route of administration has been evaluated in a separate Cochrane intervention review, and this overview summarises evidence from those reviews.
Sumatriptan is available only by prescription in most countries, but in the UK packs of 2 x 50 mg oral tablets are available OTC as Imigran Recovery for individuals with previously diagnosed migraine. Other countries in which sumatriptan is available OTC include Germany and Sweden. Generic (non‐proprietary) formulations are available for the standard tablets and subcutaneous injections in many countries. In primary care in the UK in 2012 there were over 1,150,000 prescriptions for sumatriptan, of which 64% and 23% were for generic 50 mg and 100 mg oral formulations (PCA 2013).
In order to establish whether sumatriptan is effective in reducing pain at specified doses in acute migraine attacks, it is necessary to study its effects in circumstances that permit detection of pain relief. Such studies are carried out in individuals with established pain of moderate to severe intensity, using single doses of the interventions. Participants who experience an inadequate response with either placebo or active treatment are permitted to use rescue medication, and the intervention is considered to have failed in those individuals. In clinical practice, however, individuals would not normally wait until pain is of at least moderate severity, and may take a second dose of medication if the first dose does not provide adequate relief. Once analgesic efficacy is confirmed in studies using single doses in established pain, further studies may investigate different treatment strategies and patient preferences. These are likely to include treating the migraine attack early while pain is mild, and using a low dose initially, with a second dose if response is inadequate.
How the intervention might work
Sumatriptan is a 5‐HT1 agonist, selectively targeting the 5‐HT (serotonin) 1B and 1D receptors. It has three putative mechanisms of therapeutic action (Ferrari 2002; Goadsby 2007).
Vasoconstriction of dilated meningeal blood vessels.
Inhibition of the release of vasoactive neuropeptides from perivascular trigeminal sensory neurons.
Reduction of pain signal transmission in the trigeminal dorsal horn.
Sumatriptan is used for acute treatment, having no efficacy in preventing future attacks.
Why it is important to do this overview
Sumatriptan was the first marketed triptan and is by far the most used triptan worldwide. Since it came off patent, generic formulations have greatly increased its availability, and sumatriptan has become the standard against which new acute migraine treatments are compared. An earlier Cochrane review of oral sumatriptan for acute migraine headaches searched for studies to the end of 2001 (McCrory 2003). Many more studies have been published since that time, and updates were needed to include these new studies and consider the other routes of administration. Owing to the very large amount of information now available, particularly for the oral formulation, we carried out separate reviews for each route of administration (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d).
This overview summarises the main findings from those four reviews so that readers can understand the benefits and harms of sumatriptan, by all routes of administration, without necessarily having to read all four individual reviews. The method of presenting results (in tabular format) is intended to facilitate informal comparison across the various routes of administration, but we did not conduct any formal meta‐analyses of these (indirect) comparisons.
Objectives
The objective of this overview was to summarise evidence from four Cochrane intervention reviews on the efficacy and tolerability of sumatriptan in the treatment of acute migraine attacks in adults by four routes of administration (oral, subcutaneous, intranasal, rectal) compared with both placebo and with active comparators. We limited this overview to doses of sumatriptan licensed in North America or Europe.
Methods
Criteria for considering reviews for inclusion
We included four Cochrane intervention reviews of sumatriptan administered by oral, intranasal, subcutaneous, and rectal routes (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d). Reviews were required to address doses of sumatriptan licensed in North America or Europe. These were:
oral sumatriptan 25 mg, 50 mg, 100 mg;
subcutaneous sumatriptan 4 mg, 6 mg;
intranasal sumatriptan 5 mg, 10 mg, 20 mg;
rectal sumatriptan 25 mg.
A fuller listing of results from any dose of sumatriptan for which there was evidence of efficacy or harm, and by any route of administration, can be found in the individual reviews.
Search methods for identification of reviews
Included reviews were known to the authors and published in The Cochrane Library; there was no additional searching.
Data collection and analysis
We used a tabular format to summarise results for a number of IHS‐preferred outcomes (IHS 2000; both efficacy and harm) for sumatriptan administered by different routes, at different doses, and at different levels of baseline pain. These outcomes include:
-
pain‐free outcomes: participants could have either moderate or severe, or mild pain when medication was taken, reduced to no pain at the time of assessment
pain‐free at two hours, without the use of rescue medication;
pain‐free at one hour, without the use of rescue medication;
sustained pain‐free during the 24 hours postdose (pain‐free within two hours, with no use of rescue medication or recurrence of moderate to severe pain within 24 hours).
-
headache relief outcomes: participants had moderate or severe pain when medication was taken, reduced to mild or no pain at the time of assessment
headache relief at two hours, without the use of rescue medication;
headache relief at one hour, without the use of rescue medication;
sustained headache relief during the 24 hours postdose (headache relief at two hours, sustained for 24 hours, with no use of rescue medication or a second dose of study medication).
any adverse event within 24 hours of dosing.
Summaries of other outcomes can be found in Appendix 1. These include:
use of rescue medication;
relief of headache‐associated symptoms at two hours: nausea, photophobia, and phonophobia;
relief of functional disability at two hours: partial relief, complete relief.
As in the individual reviews, we report results for each outcome in three ways in the summary tables under Effects of interventions.
First, together with the number of studies and participants, we give the actual number of participants with the outcome, and the total treated, as well as the percentage of participants achieving the outcome. This is done for the active drug and for the comparator (placebo, or a different active drug)
Second, we present the relative risk (RR) together with a 95% confidence interval (CI). Where the CI does not include 1, the result is taken to be statistically significant. If the RR is less than 1, then the rate of events is lower with the active drug than with placebo; if the RR is greater than 1, then the rate of events is higher with the active drug than with placebo
Third, where the RR is statistically significant, we report the number needed to treat to benefit (NNT), number needed to treat to harm or cause one adverse event (NNH), or number needed to treat to prevent one adverse event (NNTp; Cook 1995)
Comparisons involving fewer than 200 participants or fewer than two studies are highly susceptible to random effects of chance, and were not included in the individual reviews (Moore 1998).
Selection of reviews
Included reviews were carried out by the same authors and covered the four routes of administration available for sumatriptan (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d).
Data extraction and management
One review author collated results from the four reviews, and another checked them.
Assessment of methodological quality of included reviews
Quality of included reviews
All included reviews were carried out according to a standard protocol which satisfied the criteria specified in the 'assessment of multiple systematic reviews' (AMSTAR) measurement tool (Shea 2007) for rigorous methodological quality.
According to these criteria, each review should:
provide an a priori design;
carry out duplicate study selection and data extraction;
carry out a comprehensive literature search;
include published and unpublished studies irrespective of language of publication;
provide a list of studies (included and excluded);
assess and document the scientific quality of the included studies;
use the scientific quality of the included studies appropriately in formulating conclusions;
use appropriate methods to combine the findings of studies; and
state conflicts of interests.
Quality of evidence in included reviews
We assessed the strength of evidence for different outcomes according to the methodological quality of the primary studies as reported in the individual reviews, the total number of participants contributing data, and whether it was sensitive to potential publication bias.
Individual reviews included only randomised, double blind, placebo or active‐controlled trials, with a minimum of 10 participants in each treatment arm. The majority of included studies did not adequately report the methods used to generate the random sequence or to maintain allocation concealment and blinding; this may reflect the age of the studies and reporting deficiencies rather than methodological inadequacy. A minority of studies were judged at high risk of bias because they enrolled fewer than 50 participants per treatment arm (Nuesch 2010).
Individual reviews assessed the potential sensitivity to publication bias of the primary outcomes of pain‐free and headache relief at two hours by examining the number of participants in trials with zero effect (RR 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008). Reviews specified a clinically useful level as an NNT ≤ 8 for pain‐free at two hours, and an NNT ≤ 6 for headache relief at two hours, and judged outcomes to be potentially susceptible to publication bias if fewer than 400 additional participants were required to increase the NNT beyond this clinically useful level. We used this method because statistical tests for presence of publication bias have been shown to be unhelpful (Thornton 2000).
Data synthesis
There was no pooling of data beyond what was reported in the individual reviews. Specifically, we did not conduct any formal statistical analysis of data from indirect comparisons of one route of administration versus another.
Results
We included four Cochrane intervention reviews providing data on 18 different dose and route of administration combinations for sumatriptan administered orally, subcutaneously, intranasally, or rectally, in the treatment of acute migraine attacks in adults (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d). All of the reviews used the same methodological approach and assessed the same efficacy and safety outcomes. The total number of participants included in the 111 individual studies in the four reviews was 52,236.
| Route of administration | Studies | Participants |
| Oral | 61 | 37,250 |
| Subcutaneous | 35 | 9,365 |
| Intranasal | 12 | 4,755 |
| Rectal | 3 | 866 |
This overview summarises the most important findings from the four individual reviews for formulations and doses of sumatriptan licensed in North America or Europe (oral 25 mg, 50 mg, 100 mg; subcutaneous 4 mg, 6 mg; intranasal 5 mg, 10 mg, 20 mg; rectal 25 mg).
Description of included reviews
Included reviews each had the same structure and organisation. They used identical methods that were based on criteria established by extensive analysis and validation, using individual patient data.
All the reviews used the same criteria for inclusion; typically, these were:
adult participants with a history of migraine;
single dose administration of sumatriptan, active comparator, or placebo (with additional analgesia available, typically after one to two hours);
randomised, double blind studies;
pain assessed by using standard pain intensity and pain relief scales.
All the reviews used the same process to identify and select studies for inclusion; these were:
searching electronic searches (including CENTRAL, MEDLINE, EMBASE, and manufacturers' databases);
no language restriction on included reports;
assessment of study quality according to established criteria, with minimum criteria for inclusion (randomised, double blind, 10 or more participants per treatment group).
Methodological quality of included reviews
All four reviews satisfied the criteria specified in the AMSTAR measurement tool (Shea 2007) for rigorous methodological quality. Notably, each review used appropriate methods to combine findings of studies, and importantly provided analyses according to drug dose. The scientific quality of the included studies was used appropriately in formulating conclusions, because only studies with minimal risk of bias were included. A particular issue was the number of participants contributing data to some analyses, but conclusions were not drawn from inadequate data sets, based on previously established criteria (Moore 1998).
Effect of interventions
A common set of outcomes has arisen in randomised controlled trials (RCTs) of migraine medication, based around the features migraineurs want from their migraine treatment (Lipton 1999). The four reviews included in this overview addressed this set of outcomes for different routes of administration and doses separately.
In this overview, we focused on outcomes relating to pain relief (pain‐free and headache relief at various time points) and adverse events. All of the studies providing data for the included reviews measured pain intensity on a 4‐point scale, typically 0 = none, 1 = mild, 2 = moderate, and severe = 3, or equivalent terms. The vast majority of data were for comparisons of sumatriptan with placebo; where available we have commented on comparisons with other active treatments.
For each outcome we have reported dose and route of administration combinations for which sufficient data were available to carry out pooled analysis. We have presented the results in the form of a summary of results table to facilitate informal comparison across the various routes of administration and doses, and highlight those for which the results were considered to be robust (not susceptible to publication bias) and clinically relevant (NNT below a defined threshold).
All extracted information for all outcomes is provided in appendices in the individual reviews (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d).
Sumatriptan versus placebo
Pain‐free at two hours
Pooled analyses were performed on 10 dose, route of administration, and baseline pain intensity combinations for which sufficient data were available (Summary of results A). Eight treatments were administered to participants with moderate or severe baseline pain, while two (oral 50 mg and 100 mg) were specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results A: Pain‐free at two hours in placebo‐controlled studiesa | ||||||||||
| Route of administration | Dose (mg) | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | Susceptibility to publication biasb | |||
| Studies | Participants | Active | Placebo | Active | Placebo | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 25 | 3 | 1108 | 201/809 | 26/299 | 25 | 9 | 2.7 (1.8 to 4.0) | 6.2 (4.9 to 8.5) | 322 |
| Oral | 50 | 13 | 6447 | 1080/3922 | 282/2525 | 28 | 11 | 2.7 (2.4 to 3.1) | 6.1 (5.5 to 6.9) | 2008 |
| Oral | 100 | 16 | 6571 | 1291/4017 | 272/2554 | 32 | 11 | 3.2 (2.8 to 3.6) | 4.7 (4.3 to 5.1) | 4614 |
| Subcutaneous | 4 | 2 | 664 | 201/411 | 23/253 | 49 | 9 | 4.8 (3.2 to 7.2) | 2.5 (2.2 to 3.0) | 1461 |
| Subcutaneous | 6 | 13 | 2522 | 799/1351 | 174/1171 | 59 | 15 | 3.9 (3.3 to 4.5) | 2.3 (2.1 to 2.4) | 6250 |
| Intranasal | 10 | 5 | 1115 | 157/655 | 47/460 | 24 | 10 | 2.5 (1.8 to 3.4) | 7.3 (5.5 to 11) | 107 |
| Intranasal | 20 | 6 | 1379 | 283/891 | 52/488 | 32 | 11 | 3.1 (2.4 to 4.1) | 4.7 (4.0 to 5.9) | 968 |
| Rectal | 25 | 2 | 240 | 60/146 | 16/94 | 41 | 17 | 2.4 (1.5 to 3.9) | 4.2 (2.9 to 7.7) | 217 |
| In participants with mild baseline pain | ||||||||||
| Oral | 50 | 7 | 1514 | 357/783 | 168/731 | 46 | 23 | 2.0 (1.7 to 2.4) | 4.4 (3.7 to 5.6) | 1239 |
| Oral | 100 | 5 | 1240 | 358/618 | 151/622 | 58 | 24 | 2.4 (2.1 to 2.8) | 3.0 (2.6 to 3.5) | 2067 |
|
aResults shown in bold font are those considered to be the most robust and clinically relevant (see text for explanation) bNumber of participants in studies with no effect needed to change NNT to >8 | ||||||||||
Figure 1 shows the calculated NNTs for pain‐free at two hours for the five most widely used dose and route of administration combinations (oral 50 mg, oral 100 mg, subcutaneous 6 mg, intranasal 20 mg, rectal 25 mg) in patients with moderate or severe baseline pain.
1.
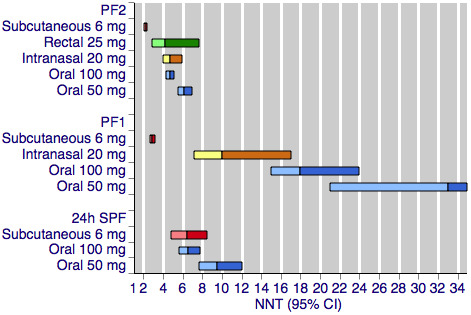
Sumatriptan versus placebo. Calculated NNTs for a pain‐free response after a specified time, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
PF2: pain‐free at two hours; PF1: pain‐free at one hour; 24h SPF: 24‐hour sustained pain‐free.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.
All dose and route combinations provided superior levels of pain relief to placebo. For moderate or severe baseline pain, calculated NNTs for this outcome ranged from 6 to 7 with 10 mg intranasal sumatriptan and 25 mg and 50 mg oral sumatriptan, through 4 to 5 for higher doses of intranasal, oral, and rectal treatments, and 2.3 for high‐dose subcutaneous treatment. The proportion of participants pain‐free two hours after treatment ranged from approximately 25% with low doses of oral and intranasal treatments to 59% with the higher dose of the subcutaneous treatment. The proportion of placebo‐treated participants pain‐free at two hours ranged from 9% to 17%. In general, higher doses of sumatriptan resulted in lower (better) NNTs, but in many cases the differences between NNTs were not statistically significant (overlapping confidence intervals), suggesting a relatively minor dose response relationship. The original reviews showed that the oral 100 mg dose was superior to the 50 mg dose (P = 0.0001), and that the 20 mg intranasal dose was superior to the 10 mg dose (P = 0.015).
The two doses of oral sumatriptan administered to participants with mild baseline pain showed significantly improved efficacy compared with the same doses administered to participants with moderate or severe pain (P = 0.014 for the 50 mg dose, and P < 0.00006 for the 100 mg dose) in indirect comparisons. The calculated NNTs for the 50 mg and 100 mg oral doses were 4.4 and 3.0 after treatment of mild pain, compared with 6.1 and 4.7 after treatment of moderate or severe pain. As with the participants treating moderate or severe headache, the 100 mg dose gave a significantly lower (better) NNT (P = 0.002) than the 50 mg dose. Response with placebo (24%) was greater with mild, compared with moderate or severe, baseline pain.
In the review of subcutaneous sumatriptan (Derry 2012b), three of the included studies provided data on the efficacy of a second dose of sumatriptan in case of an inadequate response to the initial dose. Participants with insufficient pain relief after one hour were offered a second dose of subcutaneous sumatriptan 6 mg and the pain‐free response at two hours recorded. There was no significant difference between the number of participants pain‐free at two hours with this alternative dosing strategy and with the standard single dose strategy.
Pain‐free at one hour
Pooled analyses were performed on seven dose, route of administration, and baseline pain intensity combinations for which sufficient data were available (Summary of results B). Five treatments were administered to participants with moderate or severe baseline pain, while two (oral 50 mg and 100 mg) were specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results B: Pain‐free at one hour in placebo‐controlled studies | |||||||||
| Route of administration | Dose (mg) | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 50 | 5 | 1735 | 45/902 | 16/833 | 5 | 2 | 2.6 (1.5 to 4.7) | 33 (21 to 73) |
| Oral | 100 | 6 | 3176 | 158/2216 | 15/960 | 7 | 2 | 4.0 (2.3 to 6.8) | 18 (15 to 24) |
| Subcutaneous | 4 | 2 | 664 | 134/411 | 16/253 | 33 | 6 | 4.7 (2.8 to 7.7) | 3.8 (3.2 to 4.8) |
| Subcutaneous | 6 | 16 | 3592 | 905/2198 | 99/1394 | 41 | 7 | 5.6 (4.6 to 6.8) | 2.9 (2.7 to 3.2) |
| Intranasal | 20 | 2 | 499 | 39/320 | 4/179 | 12 | 2 | 6.2 (2.2 to 18) | 10 (7.1 to 17) |
| In participants with mild baseline pain | |||||||||
| Oral | 50 | 5 | 1246 | 161/624 | 87/622 | 26 | 14 | 1.9 (1.5 to 2.4) | 8.5 (6.2 to 13) |
| Oral | 100 | 5 | 1240 | 189/618 | 87/622 | 31 | 14 | 2.2 (1.8 to 2.8) | 6.0 (4.7 to 8.3) |
Figure 1 shows the calculated NNTs for pain‐free at one hour for four of the five most widely used dose and route of administration combinations in patients with moderate or severe baseline pain (no information was available for rectal 25 mg for this outcome).
All dose and route combinations provided statistically superior levels of pain relief to placebo. When baseline pain was moderate or severe, subcutaneous sumatriptan (4 mg or 6 mg) was far superior to oral or intranasal formulations, with NNTs for this outcome of 3 to 4, compared to 10 to 33. The proportion of participants pain‐free at one hour ranged from 5% to 12% for all oral and intranasal doses, compared to 33% to 41% for the subcutaneous doses. Placebo response rates varied from 2% to 7%. Higher doses of oral sumatriptan resulted in lower (better) NNTs, but the differences between NNTs were not statistically significant (overlapping confidence intervals). The subcutaneous review (Derry 2012b) showed that the subcutaneous 6 mg dose was superior to the 4 mg dose (P = 0.011).
Oral sumatriptan administered to participants with mild baseline pain showed significantly improved efficacy compared with the same doses administered to participants with moderate or severe pain in indirect comparisons, with NNTs of 8.5 and 6.0 compared with 33 and 18 for the 50 mg and 100 mg doses (P = 0.014 for 50 mg, and P < 0.00006 for 100 mg). Response with both sumatriptan and placebo was greater with mild baseline pain than with moderate or severe baseline pain.
Sustained pain‐free during the 24 hours postdose
The 24‐hour sustained pain‐free outcome requires participants to be pain‐free at two hours, with no use of rescue medication and no recurrence of pain within 24 hours.
Pooled analyses were performed on five dose, route of administration, and baseline pain intensity combinations for which sufficient data were available (Summary of results C). Three treatments were administered to participants with moderate or severe baseline pain, while two (oral 50 mg and 100 mg) were specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results C: Sustained pain‐free during the 24 hours postdose in placebo‐controlled studies | |||||||||
| Route of administration | Dose (mg) | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 50 | 4 | 2526 | 226/1309 | 82/1217 | 17 | 7 | 2.6 (2.1 to 3.4) | 9.5 (7.7 to 12) |
| Oral | 100 | 6 | 2891 | 374/1590 | 106/1301 | 24 | 8 | 2.8 (2.3 to 3.4) | 6.5 (5.6 to 7.8) |
| Subcutaneous | 6 | 5 | 1336 | 222/713 | 91/623 | 31 | 15 | 2.2 (1.8 to 2.8) | 6.1 (4.8 to 8.2) |
| In participants with mild baseline pain | |||||||||
| Oral | 50 | 4 | 866 | 124/436 | 44/430 | 28 | 10 | 2.8 (2.1 to 3.9) | 5.5 (4.3 to 7.6) |
| Oral | 100 | 3 | 771 | 127/389 | 39/382 | 33 | 10 | 3.2 (2.3 to 4.5) | 4.5 (3.6 to 5.9) |
Figure 1 shows the calculated NNTs for sustained pain‐free during the 24 hours postdose for three of the five most widely used dose and route combinations in patients with moderate or severe baseline pain (no information was available for the intranasal 20 mg or rectal 25 mg formulations for this outcome).
All dose and route combinations provided superior levels of pain relief to placebo. When baseline pain was moderate or severe, calculated NNTs for this outcome ranged from 9.5 with 50 mg oral sumatriptan through to 6.5 and 6.1 with the higher oral dose and subcutaneous treatment, respectively. The proportion of participants with a 24‐hour sustained pain‐free response ranged from approximately 17% with the lower dose oral treatment to 31% with subcutaneous treatment. The proportion of placebo‐treated participants with a 24‐hour sustained pain‐free response ranged from 7% to 15%. Data on more than one dose of sumatriptan were available only for the oral route of administration, for which the 100 mg dose was shown to result in a lower (better) NNT than the 50 mg dose (P = 0.008).
Oral sumatriptan administered to participants with mild baseline pain showed significantly improved efficacy compared with the same doses administered to participants with moderate or severe pain in indirect comparisons, with NNTs of 5.5 and 4.5 compared with 9.5 and 6.5 for the 50 mg and 100 mg doses (P = 0.008 for 50 mg, and P = 0.024 for 100 mg). Response with placebo was similar to that with initially moderate or severe pain.
Headache relief at two hours
Pooled analyses were performed on nine dose and route of administration combinations for which sufficient data were available (Summary of results D).
| Summary of results D: Headache relief at two hours in placebo‐controlled studiesa | ||||||||||
| Route of administration | Dose (mg) | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | Susceptibility to publication biasb | |||
| Studies | Participants | Active | Placebo | Active | Placebo | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 25 | 5 | 1580 | 638/1143 | 140/437 | 56 | 32 | 1.7 (1.4 to 1.9) | 4.2 (3.5 to 5.4) | 677 |
| Oral | 50 | 19 | 8102 | 2822/4955 | 1007/3147 | 57 | 32 | 1.8 (1.7 to 1.9) | 4.0 (3.7 to 4.4) | 4051 |
| Oral | 100 | 21 | 7811 | 2877/4751 | 967/3060 | 61 | 32 | 1.9 (1.8 to 2.0) | 3.5 (3.2 to 3.7) | 5579 |
| Subcutaneous | 4 | 2 | 664 | 286/411 | 56/253 | 70 | 22 | 3.1 (2.4 to 4.0) | 2.1 (1.8 to 2.5) | 1233 |
| Subcutaneous | 6 | 14 | 2738 | 1152/1459 | 395/1279 | 79 | 31 | 2.5 (2.3 to 2.7) | 2.1 (2.0 to 2.2) | 5085 |
| Intranasal | 5 | 4 | 830 | 236/496 | 114/334 | 48 | 34 | 1.4 (1.2 to 1.7) | 7.4 (5.0 to 15) | Not calculated (NNT > 6) |
| Intranasal | 10 | 8 | 1755 | 510/1025 | 230/730 | 50 | 32 | 1.6 (1.4 to 1.8) | 5.5 (4.4 to 7.3) | 160 |
| Intranasal | 20 | 9 | 2020 | 767/1262 | 245/758 | 61 | 32 | 1.9 (1.7 to 2.2) | 3.5 (3.1 to 4.1) | 1443 |
| Rectal | 25 | 2 | 240 | 104/146 | 28/94 | 71 | 30 | 2.3 (1.7 to 3.2) | 2.4 (1.9 to 3.4) | 360 |
|
aResults shown in bold font are those considered to be the most robust and clinically relevant (see text for explanation) bNumber of participants in studies with no effect needed to change NNT to >6 | ||||||||||
Figure 2 shows the calculated NNTs for headache relief at two hours with the five most widely used dose and route of administration combinations.
2.
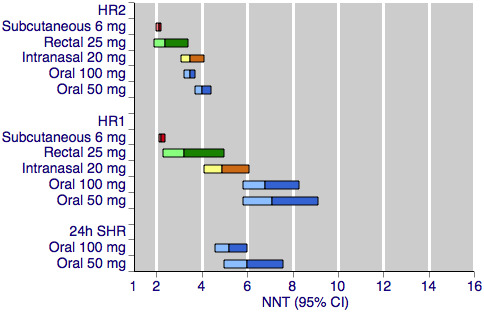
Sumatriptan versus placebo. Calculated NNTs for headache relief after a specified time, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
HR2 (headache relief at two hours); HR1 (headache relief at one hour); 24h SHR (24‐hour sustained headache relief).
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.
All dose and route combinations provided statistically superior levels of headache relief to placebo, with all but one also resulting in clinically useful NNTs for this outcome. The 5 mg intranasal dose gave an NNT of 7.4, which lies outside of the range we consider to be clinically useful for this outcome. Other NNTs ranged from 4.2 with 25 mg oral sumatriptan, through 3.5 to 2.1 for the higher doses of oral, intranasal, rectal, and subcutaneous sumatriptan. The proportion of participants with headache relief at two hours ranged from 48% to 56% with low doses of intranasal and oral treatments, to 79% with the higher dose of the subcutaneous treatment. The proportion of placebo‐treated participants with headache relief after two hours was fairly constant across the doses and routes of administration, ranging from 22% to 34%. In general, higher doses of sumatriptan resulted in lower (better) NNTs, but in many cases the differences between doses were not statistically significant (overlapping confidence intervals), suggesting a relatively minor dose response relationship. The included reviews further showed that the oral 100 mg dose was superior to the 50 mg dose (P = 0.010; Derry 2012a), and that the 20 mg intranasal dose was superior to the 10 mg dose (P = 0.002; Derry 2012c).
In the review of subcutaneous sumatriptan (Derry 2012b), six of the included studies provided data on the efficacy of a second dose of sumatriptan in case of an inadequate response to the initial dose. Participants with insufficient pain relief after one hour were offered a second dose of subcutaneous sumatriptan 6 mg and the number of participants with headache relief at two hours recorded. There was no significant difference between the NNTs for headache relief at two hours with this alternative dosing strategy and with the standard single dose strategy.
Headache relief at one hour
Pooled analyses were performed on nine dose and route of administration combinations for which sufficient data were available (Summary of results E).
| Summary of results E: Headache relief at one hour in placebo‐controlled studies | |||||||||
| Route of administration | Dose (mg) | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 25 | 2 | 472 | 95/334 | 16/138 | 28 | 12 | 2.3 (1.4 to 3.7) | 5.9 (4.2 to 10) |
| Oral | 50 | 8 | 2492 | 406/1474 | 137/1018 | 28 | 13 | 1.9 (1.6 to 2.3) | 7.1 (5.8 to 9.1) |
| Oral | 100 | 10 | 3983 | 795/2709 | 187/1274 | 29 | 15 | 1.9 (1.6 to 2.2) | 6.8 (5.8 to 8.3) |
| Subcutaneous | 4 | 2 | 664 | 271/411 | 64/253 | 66 | 25 | 2.6 (2.0 to 3.2) | 2.5 (2.1 to 3.0) |
| Subcutaneous | 6 | 24 | 5177 | 2229/3139 | 532/2038 | 71 | 26 | 2.7 (2.5 to 2.9) | 2.2 (2.1 to 2.4) |
| Intranasal | 5 | 4 | 830 | 193/496 | 95/334 | 39 | 28 | 1.4 (1.1 to 1.7) | 9.6 (5.9 to 25) |
| Intranasal | 10 | 8 | 1755 | 392/1025 | 180/730 | 38 | 25 | 1.6 (1.4 to 1.9) | 7.4 (5.6 to 11) |
| Intranasal | 20 | 9 | 2020 | 579/1262 | 192/758 | 46 | 25 | 1.9 (1.6 to 2.2) | 4.9 (4.1 to 6.1) |
| Rectal | 25 | 2 | 240 | 74/146 | 18/94 | 51 | 19 | 2.7 (1.7 to 4.2) | 3.2 (2.3 to 5.0) |
Figure 2 shows the calculated NNTs for headache relief at one hour for the five most widely used dose and route of administration combinations.
All dose and route combinations provided superior levels of headache relief to placebo. Calculated NNTs for this outcome were highly dependent on the route of administration, ranging from 7.1 to 5.9 for oral administration; 9.6 to 4.9 for intranasal administration; 2.5 to 2.2 for subcutaneous administration,and 3.2 for rectal administration. Similarly, the proportion of participants with headache relief at one hour varied substantially between the different routes of administration. Between 28% and 29% of oral sumatriptan‐treated participants had headache relief by one hour, compared with between 38% and 51% of intranasal or rectal sumatriptan‐treated participants, and 66% to 71% of subcutaneous sumatriptan‐treated participants. The proportion of placebo‐treated participants with headache relief after one hour varied slightly according to the route of administration, at 12% to 15% with oral treatments, 19% with rectal treatment, and 25% to 28% with subcutaneous and intranasal treatments. The general trend for higher doses of sumatriptan giving lower (better) NNTs remains apparent, although again in many cases the differences between NNTs were not statistically significant (overlapping confidence intervals), suggesting a relatively minor dose response relationship.
Sustained headache relief during the 24 hours postdose
The 24‐hour sustained headache relief outcome requires participants to have headache relief at two hours, and then to sustain this relief for a further 22 hours without the use of rescue medication.
Pooled analyses were performed on two dose and route of administration combinations for which sufficient data were available (Summary of results F). There were no data provided for any other combinations for this outcome.
| Summary of results F: Sustained headache relief during the 24 hours postdose in placebo‐controlled studies | |||||||||
| Route of administration | Dose (mg) | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95%CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 50 | 4 | 2526 | 454/1309 | 220/1217 | 35 | 18 | 1.9 (1.7 to 2.2) | 6.0 (5.0 to 7.6) |
| Oral | 100 | 6 | 4116 | 922/2538 | 270/1578 | 36 | 17 | 2.1 (1.9 to 2.4) | 5.2 (4.6 to 6.0) |
Figure 2 shows the calculated NNTs for 24‐hour sustained headache relief for two of the five most widely used dose and route of administration combinations (no information was available for subcutaneous 6 mg, intranasal 20 mg, or rectal 25 mg for this outcome).
Both doses of oral sumatriptan provided superior levels of sustained headache relief to placebo. Calculated NNTs were 6 with the lower dose and 5 with the higher, with 35% and 36% of participants respectively achieving the outcome with sumatriptan, and 18% and 17% with placebo. There was no significant difference between the NNTs for each of the two doses.
Any adverse event within 24 hours
This outcome captures the number of participants experiencing at least one adverse event during the 24 hours following administration of study medication; it does not attempt to take into consideration the relative severity of different adverse events, the number of individual events experienced, or any relationship between the study medication and the event as judged by the original study investigators.
All four reviews found adverse event reporting in the included studies to be highly variable and often of poor quality. Inconsistencies were found in the duration over which adverse event data were collected, assignment of a causal relationship to the study medication, and the continued collection of adverse data after a second dose of study medication or alternative rescue medication had been administered. Despite these inconsistencies, we included as much data as possible in the analyses in order to be more inclusive and conservative, but analyses of pooled data on adverse events should be interpreted cautiously.
Treatments were generally described as well tolerated, with most adverse events being of mild or moderate severity and self‐limiting.
Pooled analyses were performed on eight dose, route of administration, and baseline pain severity combinations for which sufficient data were available (Summary of results G). Six treatments were administered to participants with moderate or severe baseline pain, while two (oral 50 mg and 100 mg) were specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results G: Any adverse event within 24 hours in placebo‐controlled studies | |||||||||
| Route of administration | Dose (mg) | Number of | Number with outcome/total | Percent with outcome | Relative harm (95% CI) | NNH (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 25 | 4 | 1550 | 371/956 | 220/594 | 39 | 37 | 1.1 (1.0 to 1.3) | Not calculated |
| Oral | 50 | 10 | 3728 | 667/2114 | 389/1614 | 32 | 24 | 1.3 (1.2 to 1.4) | 13 (9.7 to 22) |
| Oral | 100 | 12 | 3257 | 931/2171 | 255/1086 | 43 | 23 | 1.7 (1.5 to 1.9) | 5.2 (4.4 to 6.2) |
| Subcutaneous | 4 | 3 | 720 | 313/442 | 113/278 | 71 | 41 | 1.8 (1.6 to 2.2) | 3.3 (2.7 to 4.4) |
| Subcutaneous | 6 | 9 | 1342 | 341/767 | 137/575 | 44 | 24 | 2.1 (1.8 to 2.5) | 4.9 (3.9 to 6.4) |
| Intranasal | 20 | 2 | 516 | 125/331 | 27/185 | 38 | 15 | 2.9 (2.0 to 4.2) | 4.3 (3.3 to 6.3) |
| In participants with mild baseline pain | |||||||||
| Oral | 50 | 6 | 1242 | 104/642 | 43/600 | 16 | 7 | 2.3 (1.6 to 3.2) | 11 (8.0 to 18) |
| Oral | 100 | 4 | 941 | 89/471 | 32/470 | 19 | 7 | 2.7 (1.9 to 4.0) | 8.3 (6.1 to 13) |
Figure 3 shows the calculated NNHs for any adverse event within 24 hours of dosing for four of the five most widely used dose and route of administration combinations (no information was available for rectal 25 mg for this outcome).
3.
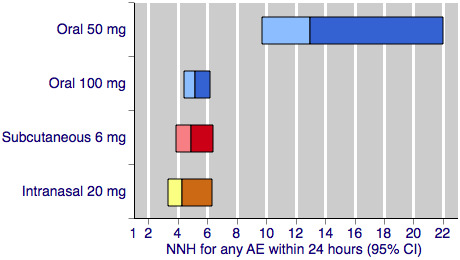
Sumatriptan versus placebo. Calculated NNHs for any adverse event within 24 hours of dosing, in participants treating moderate or severe migraine pain. Results for four of the five most commonly used dose and route of administration combinations (adverse event information for rectal sumatriptan not available), listed in rank order.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, and intranasal doses are shown with yellow bars.
With the exception of the 25 mg oral dose, all of the dose and route combinations resulted in significantly more harm with sumatriptan than placebo. For participants with moderate or severe baseline pain, calculated NNHs for this outcome ranged from 13 with oral 50 mg, to 3.3 with subcutaneous 4 mg. For oral administration, higher doses of sumatriptan resulted in lower (worse) NNHs, with 100 mg significantly worse than 50 mg (P < 0.00006). There was no apparent dose response relationship for the two doses of subcutaneous sumatriptan.
The two doses of oral sumatriptan administered to participants with mild baseline pain also resulted in significantly more participants with at least one adverse event than placebo. Calculated NNHs were 11 and 8.3 for the 50 and 100 mg doses respectively, with about 15% to 20% of participants experiencing an adverse event after sumatriptan, compared with 7% after placebo. Statistical comparison between the treatment effects in mild and moderate or severe baseline pain were not performed for incidence of adverse events due to the inconsistencies described previously in the contributing data.
Other outcomes
The individual reviews also provided information on use of rescue medication, relief of headache‐associated symptoms (nausea, photophobia, and phonophobia), and relief of functional disability in placebo‐controlled studies. Summaries of these outcomes are available in Appendix 1.
Sumatriptan versus active comparators
Only the oral route of administration provided sufficient data to allow pooled analysis of any dose of sumatriptan versus another active migraine treatment. Several individual studies comparing sumatriptan, delivered by other routes, with active treatments were included in the relevant reviews, but the amount of data was insufficient to allow pooled analysis (fewer than two studies or 200 participants, or both, contributing data). Detailed descriptions of all head‐to‐head comparisons can be found in the individual intervention reviews (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d). Using the available information, it is not possible to compare the relative efficacies of the different routes of administration of sumatriptan versus other active treatments, so we have simply summarised the findings from the review of oral sumatriptan. Full summary tables for each individual outcome are provided in Appendix 2.
Of the active comparators tested against oral sumatriptan, only rizatriptan 5 mg and 10 mg, effervescent acetylsalicylic acid 1000 mg, zolmitriptan 2.5 mg and 5 mg, eletriptan 40 mg and 80 mg, almotriptan 12.5 mg, paracetamol 1000 mg plus metoclopramide 10 mg, and acetylsalicylic acid 900 mg plus metoclopramide 10 mg provided sufficient data for analysis of any particular outcome. Results, in brief, were as follows.
Rizatriptan 5 mg was superior to sumatriptan 25 mg for pain‐free at two hours and headache relief at two hours, but there was no significant difference between the treatments for headache relief at one hour, and there was no difference between rizatriptan 5 mg and sumatriptan 50 mg for headache relief at two hours.
Rizatriptan 10 mg was superior to sumatriptan 25 mg, 50 mg, and 100 mg for all reported outcomes, including pain‐free at two hours and headache relief at one and two hours.
Zolmitriptan 2.5 mg and 5 mg showed no significant difference to sumatriptan 50 mg for headache relief at one or two hours.
Almotriptan 12.5 mg showed no significant difference to sumatriptan 100 mg for either pain‐free at two hours or 24‐hour sustained pain‐free.
Eletriptan in both doses (40 mg and 80 mg) was superior to sumatriptan in both doses (50 mg and 100 mg) for most reported primary outcomes, including pain‐free and headache relief at two hours. However, there was no significant difference between sumatriptan 50 mg and eletriptan 40 mg for headache relief at one hour, or between sumatriptan 100 mg and eletriptan 40 mg for pain‐free at one hour. Eletriptan was also generally superior in terms of the relief of headache‐associated symptoms and need for rescue medication.
Effervescent acetylsalicylic acid 1000 mg was more effective than sumatriptan 50 mg for headache relief at one hour, but there was no difference between the treatments for pain‐free at one or two hours, and sumatriptan 50 mg was significantly superior for headache relief at two hours.
There was no significant difference between sumatriptan 100 mg and either paracetamol 1000 mg plus metoclopramide 10 mg or acetylsalicylic acid 900 mg plus metoclopramide 10 mg for headache relief at two hours. Sumatriptan 100 mg was, however, significantly superior to acetylsalicylic acid plus metoclopramide for pain‐free at two hours.
There was no significant difference in the incidence of adverse events between any of the analysed doses of sumatriptan and their active comparators, with the exception of acetylsalicylic acid 900 mg plus metoclopramide 10 mg, which caused significantly fewer adverse events than sumatriptan 100 mg.
Discussion
A number of features of anti‐migraine treatments come together to determine overall performance and success. Individual patients will prioritise some of these features over others, depending on what aspect of their headache affects them most. The four reviews included in this overview particularly addressed the extent, speed of onset, and maintenance of pain relief, and the incidence of adverse events after treatment with sumatriptan administered via four alternative routes. Other aspects like relief of phonophobia, photophobia, or other symptoms were also reported, and are covered in Appendix 1. In this overview we bring together the information for different doses of sumatriptan and different route of administration to allow indirect comparison. In the context of treating individual patients this information may then be used to inform decisions about the use of sumatriptan to treat acute migraine.
Summary of main results
Extent of pain relief—results two hours after dosing
For the IHS preferred outcome of pain‐free at two hours, the 4 mg and 6 mg doses of subcutaneous sumatriptan, given when pain was moderate or severe, showed the greatest efficacy with 50% to 60% of participants achieving the response, compared with about 13% with placebo. NNTs were 2.5 and 2.3 for the 4 mg and 6 mg doses, respectively. All other routes of administration, at all analysed doses, resulted in reduced efficacy compared with the two subcutaneous doses. NNTs ranged from 4.2 to 7.3, and with overlapping confidence intervals, there was little difference between the these three routes of administration. Efficacy was significantly improved if treatment was taken early, while pain was still mild.
For headache relief at two hours, the two subcutaneous doses analysed (4 mg and 6 mg) again showed the greatest efficacy, with 70% to 80% of participants achieving the response, compared with about 30% of placebo‐treated participants, giving an NNT of 2.1 for both doses. All other routes of administration resulted in reduced efficacy at all analysed doses, with NNTs ranging from 2.4 to 7.4. For the most commonly used doses (oral 50 mg and 100 mg, intranasal 20 mg, and rectal 25 mg), there was very little difference between oral, rectal, and intranasal sumatriptan.
Speed of onset of pain relief—results one hour after dosing
For some migraineurs, rapid pain relief is a priority, and some studies have assessed pain relief outcomes at the earlier time of one hour after administration. There were limited data for the outcome of pain‐free at one hour, and only the subcutaneous route provided clinically useful levels of efficacy. The calculated NNTs for pain‐free at one hour with subcutaneous sumatriptan were 3.8 and 2.9 for 4 mg and 6 mg, respectively, with 33% and 41% of participants achieving the response after sumatriptan compared with 6% and 7% after placebo.
More participants achieved the less stringent outcome of headache relief at one hour, and again the subcutaneous route showed the greatest efficacy. NNTs were 2.5 and 2.2 for subcutaneous sumatriptan 4 mg and 6 mg, respectively, with 66% and 71% of participants achieving the response with sumatriptan compared with 25% and 26% after placebo. Rectal (25 mg) and intranasal (20 mg) treatment had NNTs of 3.2 and 4.9, respectively. A lower intranasal dose, and all analysed doses of orally administered sumatriptan, showed limited efficacy, with NNTs of 5.9 to 9.6, with only about 30% to 40% of participants achieving the response.
Sustained pain relief during the 24 hours postdose
Recurrence of headache following an initial response has been reported as a problem with sumatriptan. Two of the specified outcomes addressed the efficacy of sumatriptan for sustaining initial pain relief (at two hours) over the following 22 hour period, without the use of additional medication. Many studies did not report data for the 24‐hour sustained efficacy measures, so only limited comparison between routes of administration was possible.
For sustained pain‐free response during the 24 hours following the dose of sumatriptan, there was little difference between the subcutaneous dose analysed (6 mg) and the higher of the two oral doses (100 mg). About 25% to 30% of sumatriptan‐treated participants were pain‐free at two hours and sustained this level of pain relief up to 24 hours after administration (compared with about 10% of placebo‐treated participants), giving NNTs of 6.1 and 6.5 for the subcutaneous and oral treatments, respectively. The lower dose of oral sumatriptan (50 mg) showed reduced sustained efficacy, with only 17% of participants achieving this response, and an NNT of 9.5.
Information on sustained headache relief during the 24 hours following the dose of sumatriptan was available only for orally administered sumatriptan, which gave NNTs of about 5 to 6 for the 50 mg and 100 mg doses, respectively (about 35% of participants achieving response after sumatriptan, compared with 17% after placebo).
Safety and tolerability
There was a considerable degree of inconsistency in the reporting of adverse events in all four reviews, and while attempts were made to analyse the available data, results from pooled analyses must be interpreted with caution. A further consideration is that these data are largely obtained from single dose studies and may not represent clinical practice, where single doses may be taken repeatedly at differing time intervals, sometimes over many years.
There was little difference in the number of participants experiencing at least one adverse event within 24 hours of treatment between the subcutaneous dose of 6 mg, the intranasal dose (20 mg), and the highest oral dose analysed (100 mg). Approximately 40% of sumatriptan‐treated participants experienced at least one adverse event within 24 hours, compared with only 15% to 20% of placebo‐treated participants. For the 4 mg subcutaneous dose, there was a high response rate in both the active and placebo treatment arms (71% and 41%, respectively), but the relative benefit was similar. The calculated NNHs for these treatments ranged from 3.3 to 5.2. Fewer participants experienced adverse events with 50 mg (NNH 13) and 25 mg (not significantly different from placebo) oral sumatriptan.
Adverse events were generally described as of mild or moderate severity and self‐limiting. Few events were considered serious, and there was no strong evidence for cardiovascular problems with sumatriptan.
Treating early, when pain is mild
Results discussed up to this point were obtained by treating participants with a single dose of sumatriptan when pain intensity was moderate or severe. Studies have been done in this way, primarily for regulatory approval, to determine whether the drug has efficacy in this condition; the presence of at least moderate pain gives sensitivity to detect a change with treatment. In clinical practice many people are able to recognise the onset of a migraine attack and treat their headache during the initial phase, when pain is usually mild. There is some evidence suggesting that treating attacks in the early stages in this way may be beneficial (Gendolla 2008; Pascual 2002), and recently a number of studies have been carried out to investigate treatment of mild baseline pain.
Data for participants treating mild baseline pain were available only for the oral route of administration. The two doses analysed (50 mg and 100 mg) in these participants provided superior pain relief compared to placebo at all three time points investigated. NNTs for a pain‐free response at one and two hours and for a 24‐hour sustained pain‐free response in comparisons with placebo were all found to be significantly lower (better) in participants who treated attacks early, while pain was still mild, compared with waiting until pain was at least moderate (indirect comparisons). Both doses resulted in similar (or possibly slightly reduced in the case of the 100 mg dose) numbers of participants experiencing at least one adverse event after treatment (NNH values of 11 and 8.3 for the 50 mg and 100 mg doses, respectively). No statistical comparisons were performed due to inconsistencies and uncertainty in the data contributing to pooled analyses of adverse events.
Repeat dosing for inadequate response
Some individuals experience an inadequate response to a single dose of sumatriptan. In clinical practice, it is not uncommon to take a second dose in these circumstances, and a few studies have investigated this as a treatment strategy. Information on the efficacy of repeat dosing strategies was limited to the subcutaneous route of administration. Giving of a second dose of 6 mg subcutaneous sumatriptan if the participant had insufficient relief at one hour did not have a significant effect; the number of participants with either headache relief or a pain‐free response at two hours after two doses was not significantly different to that found after the single 6 mg subcutaneous dose.
Other outcomes
Additional information (provided in Appendix 1) shows that sumatriptan reduces the need for additional medication, relieves headache‐associated symptoms (nausea, photophobia, and phonophobia), and relieves functional disability. Generally, the subcutaneous and intranasal routes of administration, and the 100 mg oral dose gave better results, with clinically useful (< 6) NNTs.
Comparison with other active migraine treatments
Sufficient data were available to perform pooled analyses directly comparing sumatriptan with other active treatments only for the oral route of administration. Oral sumatriptan was compared with rizatriptan, effervescent acetylsalicylic acid, eletriptan, almotriptan, acetylsalicylic acid + metoclopramide, zolmitriptan, and paracetamol + metoclopramide. In general there was little difference between sumatriptan and these active comparators at the doses tested. Only eletriptan (particularly the 80 mg dose) provided consistently superior pain relief and relief of headache‐associated symptoms. Rizatriptan provided better relief when compared with low dose sumatriptan (25 mg), but not when compared with higher doses (50 mg or 100 mg) where the difference was far less substantial and often not significant. More participants experienced the outcome of pain‐free at two hours, but not headache relief at two hours, with sumatriptan 100 mg than with acetylsalicylic acid 900 mg plus metoclopramide 10 mg. There was no difference between sumatriptan 50 mg and acetylsalicylic acid 1000 mg.
Data for other outcomes, such as relief of headache‐associated symptoms, were mostly limited to comparisons with eletriptan. Generally eletriptan 80 mg gave better results than oral sumatriptan 100 mg, but NNTs were of borderline clinical utility (≥ 6). Oral sumatriptan 100 mg gave equivalent relief of nausea to oral acetylsalicylic acid 900 mg plus metoclopramide 10 mg.
Variation in the placebo response
It is possible that route of administration could influence the response to placebo and affect comparisons between different routes of administration of the same drug. It is commonly stated that intravenous or subcutaneous drug administration elicits a greater placebo response than oral administration in the treatment of migraine; however, the evidence is limited and only a few studies have rigorously addressed this question (Bendtsen 2003; de Craen 2000; Diener 2008; Macedo 2006).
This large and clinically homogeneous data set permits investigation; Figure 4 shows pooled placebo response rates, with 95% confidence intervals, for the four routes of administration and each of the primary efficacy outcomes. Visual comparison between the oral and subcutaneous treatments shows that the placebo response after subcutaneous treatment is, in nearly all cases, higher than after oral treatment. The response after intranasal treatment is slightly more variable, but tends to fall between oral and subcutaneous responses, while the confidence intervals (due to insufficient data) are too large to draw meaningful conclusions about rectal treatment. The only outcome that does not show any variability in placebo response is headache relief at two hours, for which all four routes of administration show a consistent response of around 30%. This outcome, of course, has the largest amount of data for placebo, and it is not unlikely that the similarity in placebo response rates across routes of administration is a refection of limiting random play of chance (Moore 1998).
4.
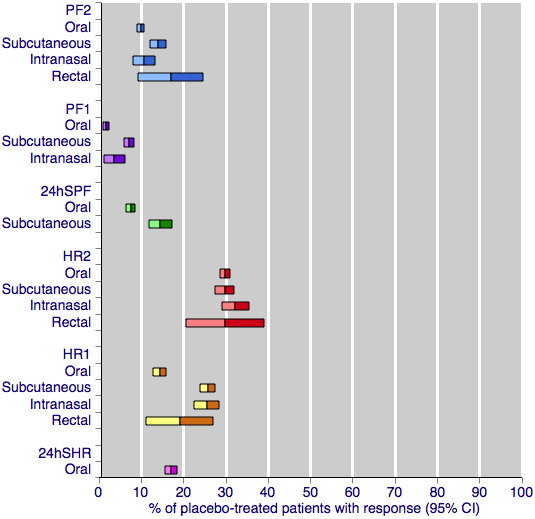
Placebo response rates for the primary efficacy outcomes, by route of administration. Response rates of each outcome are grouped by colour to facilitate comparison between different routes of administration. Proportion of placebo‐treated participants pain‐free at two hours are shown with blue bars, pain‐free at one hour with purple bars, 24‐h sustained pain‐free with green bars, headache relief at two hours with red bars, headache relief at one hour with yellow bars, and 24‐h sustained headache relief with a pink bar.
While Figure 4 shows a small degree of variation between the placebo responses with different routes of treatment for most outcomes, a much stronger determinant appears to be the outcome measured. There is a substantial difference between different levels of pain relief at the same time point, for example pain‐free and headache relief at two hours, and even greater differences between different levels of pain relief at different time points, for example pain‐free at one hour and headache relief at two hours. These data show clearly that the more stringent or exacting outcomes (those that are harder to achieve) result in lower placebo response rates. This is consistent with previous studies, which have shown the placebo response for pain‐free outcomes to be much lower than for headache relief outcomes Diener 2008; Macedo 2006; Oldman 2002), and parallels what is seen with active treatment. For any given route and outcome, the placebo response rates in this overview of sumatriptan alone are remarkably similar to those in Macedo 2006, which reviewed all acute migraine treatments, and Oldman 2002 which provided an overview across many treatments.
It may be that only the more exacting outcomes (which fewer patients will achieve) can provide the necessary sensitivity to expose small differences in placebo response rates between different routes of administration. This idea that more exacting outcomes provide a greater degree of discrimination and expose relatively small differences between pooled results has been described before (Moore 2011), in the context of active treatments in acute pain trials. Here we show that the same idea can be applied to identifying small differences between placebo responses.
Investigation and discussion of placebo is of academic importance. It could be argued that patients want complete pain relief (Lipton 1999), and that placebo is important relating to complete pain relief. There is a wealth of evidence suggesting that patients generally consider 'no worse than mild pain' (in the present context, the result of achieving 'headache relief' (pain reduced from moderate or severe to none or mild)) a useful outcome (Moore 2013). At two hours after dosing, there is little meaningful difference between placebo response rates using either pain‐free or headache relief (Figure 4).
Overall completeness and applicability of evidence
The four individual reviews involved 52,236 participants, and all used the same methodological approach and assessed the same efficacy and safety outcomes. The outcomes were chosen because they are of known importance to patients who suffer acute migraine attacks (Lipton 1999). Not all of the studies reported results for all of these outcomes, particularly that of sustained pain relief and incidence of adverse events. This inconsistency of reporting limited analysis of these outcomes for some dose and route of administration combinations. For example, only the oral 50 mg and 100 mg doses, and the subcutaneous 6 mg dose provided sufficient data to carry out any analysis of sustained pain relief during the 24 hours postdose.
The vast majority of studies included in each of the four reviews specifically treated participants with moderate or severe baseline pain intensity, and only a small number of studies included in the review of oral sumatriptan provided any efficacy data for sumatriptan in participants with mild baseline pain intensity, which may more closely reflect what happens in clinical practice. Although more participants experienced a pain‐free outcome when treating mild pain, more studies reporting consistently on early treatment and different dosing strategies are needed to inform the best clinical use of sumatriptan.
Several new routes of sumatriptan administration are currently being considered, including needle‐free injection systems, buccal patches, and transdermal patches. Investigations into the possible clinical utility of these new routes are still largely preliminary in nature, and no Phase III RCTs making use of them were found for inclusion in individual reviews. Recently a needle‐free delivery system for subcutaneous sumatriptan has been approved for use. Sumavel DosePro uses compressed gas to create a stream of medication that passes through the skin into the subcutaneous tissue. Bioequivalence with traditional injected subcutaneous sumatriptan has been demonstrated for this novel method of administration (Brandes 2009), but no studies were found specifically addressing its efficacy, safety and tolerability. Similarly, a novel iontophoretic transdermal patch, known as Zelrix, has recently reached Phase III clinical trials, after a phase I study demonstrated good tolerability and equivalent plasma levels to the oral tablet, subcutaneous, and intranasal routes of administration (Pierce 2009). More clinical trial data are required to make adequate assessments of the relative merits of these novel routes for sumatriptan administration.
Quality of the evidence
The quality of the evidence was largely excellent. Each of the included reviews met all of the AMSTAR criteria, including the use of wide searching strategies and no language limitation, and incorporated only studies that were both randomised and double‐blind, and had a low risk of bias from any major source. Where identified, potential sources of bias in the included studies have been discussed in depth in each of the individual reviews, but in each case removal of these data was found to have no significant effect on the calculated results. Perhaps the most important source of potential bias is that of publication bias, where there is a risk that unpublished data not included in the review may be sufficient to overturn any positive effect identified in the review. This is particularly relevant to those doses and routes of administration for which less evidence was available. Susceptibility to publication bias has been assessed for the two most widely reported IHS efficacy outcomes (pain‐free and headache relief at two hours), and taken into consideration when commenting on the robustness of the evidence for a particular dose and route of administration combination. For each route of administration, publication bias was considered unlikely to affect the result for licensed doses, with the exception of 25 mg oral, 10 mg intranasal, and 25 mg rectal for pain‐free at two hours, and of 5 mg intranasal, 10 mg intranasal, and 25 mg rectal for headache relief at two hours.
Potential biases in the overview process
The purpose of this overview was simply to bring together the evidence reported in four separate reviews on the use of sumatriptan to treat acute migraine. Each review used the same methodology to address the same set of outcomes for each of the different routes of administration of sumatriptan currently available. No statistical analyses were performed within this overview, and only informal comparisons were made between the various routes of administration and doses. There was therefore no opportunity to introduce bias through the methods used in the overview process.
Agreements and disagreements with other studies or reviews
The results for each route of administration were found to be consistent with previous studies and reviews using the same route of delivery. The specific agreements and disagreements are described in the appropriate individual reviews.
No previous systematic reviews encompassing or summarising all four routes of administration of sumatriptan could be found to compare with this overview. Several studies (for example, Bigal 2003; Johnston 2010) providing a narrative evaluation of the different routes have reported findings consistent with those reported here. That is, that subcutaneous sumatriptan provides the highest clinical efficacy and the fastest onset of effect, but is associated with a large number of adverse events; and that the oral and intranasal routes provide a similar level of efficacy, albeit with slower onset and for significantly fewer participants than the subcutaneous route. These narrative reports do not provide a systematic assessment with pooled analyses of all the available data, and therefore detailed comparison with this overview is not possible. One review (Oldman 2002) did provide quantitative measures of efficacy for three of the four routes of administration of sumatriptan: subcutaneous, intranasal and oral. Interestingly, despite the fact that the calculations in our up‐to‐date reviews were based on data from many more participants (at least half as many again, and for some dose, route, and outcome combinations, up to six times as many participants), there are no major differences between the NNTs calculated. The table below summarises, for the outcomes that are directly comparable, the NNTs calculated for the four dose and route of administration combinations originally analysed in Oldman 2002.
| Comparison of the calculated NNTs for four different route of administration/dose combinations of sumatriptan in two different reviewsa | |||
| Route of administration/dose combination | Headache relief at 2 hours | Headache relief at 1 hour | Pain‐free at 2 hours |
| Subcutaneous 6 mg | 2.0 (1.8 to 2.2) | 2.1 (1.9 to 2.2) | 2.1 (1.9 to 2.4 |
| 2.1 (2.0 to 2.2) | 2.2 (2.1 to 2.4) | 2.3 (2.1 to 2.4) | |
| Intranasal 20 mg | 3.4 (2.9 to 4.1) | 5.6 (4.3 to 8.0) | 4.6 (3.6 to 6.1) |
| 3.5 (3.1 to 4.1) | 4.9 (4.1 to 6.1) | 4.7 (4.0 to 5.9) | |
| Oral 50 mg | 4.1 (3.4 to 5.2) | 11 (7.1 to 22) | 7.8 (6.1 to 11) |
| 4.0 (3.7 to 4.3) | 7.1 (5.8 to 9.1) | 6.1 (5.5 to 6.9) | |
| Oral 100 mg | 3.3 (3.0 to 3.7) | 7.6 (5.9 to 10) | 4.7 (4.1 to 5.5) |
| 3.5 (3.2 to 3.7) | 6.8 (5.8 to 8.3) | 4.7 (4.3 to 5.1) | |
| aResults in bold are the most up‐to‐date from the four reviews included in this overview. Non‐bold results are from the Oldman 2002 review. | |||
Authors' conclusions
Implications for practice.
Sumatriptan is an effective treatment for acute migraine in adults. Subcutaneous administration provides clinically useful outcomes for more individuals, and more rapidly, than other routes, but with increased adverse events. Other routes can provide largely the same outcomes for a smaller number of individuals, and with a slower onset of action. In practice, choosing the most appropriate route of administration of sumatriptan for the treatment of acute migraine involves balancing the strengths and weaknesses of each of the treatments discussed here, along with other practical considerations outside the scope of this review. These include questions of availability, patient preference, convenience of use, and cost. In the UK, a single dose of subcutaneous sumatriptan (6 mg) costs three to four times that of a single dose of oral (50 mg or 100 mg, branded) or intranasal formulations (20 mg), and over 60 times that of a single oral dose of a generic equivalent (data from BNF 2013). In the absence of other deciding factors, it seems likely that oral sumatriptan 50 mg will remain a starting point for triptan therapy, although it is imperative to recognise that for a substantial number of patients different doses, drugs, or routes of administration will be needed to ensure satisfactory results.
Generally, for the oral route of administration, commonly used doses of sumatriptan and other active comparators have equivalent efficacy for most outcomes, with higher doses being marginally better than lower doses. Eletriptan was the only comparator that consistently outperformed sumatriptan. It is likely that for oral administration, sumatriptan will remain a first‐line triptan therapy, with alternatives tried in the event of intolerance or inadequate response. Patients should be encouraged to treat their migraine attacks earlier, rather than wait until pain has become more severe, in order to have the best chance of successful treatment.
Implications for research.
The quantity of information available on sumatriptan is good, with the exception of rectal treatment and long‐term (24 hour) outcomes for all routes of administration. No given dose and route of administration provides adequate results in all individuals. Future trials should investigate whether, in the case of treatment failure, increasing the dose, or switching formulation or drug can increase the proportion who benefit, and benefit consistently.
Another useful line of research would be to investigate whether sumatriptan is a useful second‐line treatment for individuals who fail to get an adequate response with analgesics such as aspirin, ibuprofen, or paracetamol. Fixed‐dose combinations of sumatriptan and analgesics might also be more extensively explored, as combinations of drugs with different modes of action provide better pain relief in acute pain and migraine (Law 2013; Moore 2012).
Oral sumatriptan has become a standard against which a large number of newer migraine treatments are now tested, and hence a pool of information involving commonly used doses of oral sumatriptan is now available. However, there are still very few data comparing sumatriptan delivered via alternative routes with other active migraine treatments. It may be possible to derive some of the information needed to compare different treatments and routes of administration from network meta‐analysis of the data in these four reviews, which would reduce the need for new trials.
More complete and more consistent reporting of adverse events is necessary to properly assess their impact and make comparisons between different treatments.
What's new
| Date | Event | Description |
|---|---|---|
| 28 May 2019 | Amended | Contact details updated. |
| 16 December 2016 | Review declared as stable | See Published notes. |
Notes
This overview is part of a series on sumatriptan for acute migraine attacks in adults (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d) which replaces an earlier Cochrane review of oral sumatriptan (McCrory 2003).
At December 2016, this overview has been stabilised following discussion with the authors and editors. If appropriate, we will update the overview if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We would like to acknowledge the helpful comments and editorial expertise of Timothy Steiner, Douglas McCory, and Rebecca Gray.
Lifting The Burden: the Global Campaign against Headache and the International Headache Society provided financial support for the editorial process (see Sources of support).
Appendices
Appendix 1. Additional data from placebo‐controlled studies
Use of rescue, or additional, medication
Rescue medication (usually a different analgesic, or in some studies a second dose of test medication) was available to participants whose symptoms were not adequately controlled in the vast majority of studies included in the four reviews. Participants were asked to wait, usually for two hours, before taking rescue medication in order to give the test medication enough time to have an effect. Ideally, the number of participants requiring rescue medication because of failure of the initial dose of test medication should be recorded soon after the first primary efficacy time point (two hours) (Tfelt‐Hansen 2012). Delay beyond six hours in recording this outcome risks conflating the use of rescue medication and treatment of recurrence of the headache. In practice, most of the studies recorded it at 24 hours, without always clearly differentiating between primary failure of the test medication and recurrence following initial response. Despite this shortcoming, we felt that use of additional medication within 24 hours remained a useful measure of treatment failure if one considers treatment success to be adequate pain relief that is sustained for 24 hours. Use of rescue medication at or after a defined time point is, therefore, a useful measure of treatment failure (lack of efficacy).
Pooled analyses were performed on five doses, route of administration, and baseline pain severity combinations for which sufficient data were available (Summary of results H). Four treatments were administered to participants with moderate or severe baseline pain, while one (oral 50 mg) was specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results H: Use of additional medication within 24 hours of dosing in placebo‐controlled studies | |||||||||
| Route of administration | Dose (mg) | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNTp (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 50 | 4 | 2079 | 266/1339 | 309/740 | 20 | 42 | 0.77 (0.68 to 0.87) | 4.6 (3.8 to 5.6) |
| Oral | 100 | 6 | 2810 | 621/1877 | 543/933 | 33 | 58 | 0.57 (0.52 to 0.62) | 4.0 (3.5 to 4.7) |
| Subcutaneous | 6 | 5 | 987 | 168/621 | 176/366 | 27 | 48 | 0.52 (0.45 to 0.60) | 4.8 (3.7 to 6.7) |
| Intranasal | 20 | 2 | 642 | 136/422 | 108/220 | 32 | 49 | 0.66 (0.55 to 0.79) | 5.9 (4.0 to 11) |
| In participants with mild baseline pain | |||||||||
| Oral | 50 | 2 | 384 | 66/221 | 94/163 | 30 | 58 | 0.54 (0.43 to 0.69) | 3.6 (2.7 to 5.5) |
All dose and route combinations of sumatriptan resulted in significantly fewer participants needing additional medication than after placebo. When baseline pain was moderate or severe, calculated NNTps ranged from 12 with the lowest dose of intranasal sumatriptan, to 4 with the highest dose of oral sumatriptan. The proportion of participants requiring additional medication ranged from 20% to 33% with sumatriptan, compared with 42% to 58% with placebo. For both the oral and intranasal routes of administration, where more than one dose was analysed, the higher dose appeared to produce a lower (better) NNTp. The significant overlap between the 95% confidence intervals does not suggest any clinically important dose response relationship.
The 50 mg dose of oral sumatriptan administered to participants with mild baseline pain did not result in a significantly different NNTp when compared with the same dose administered to participants with moderate or severe pain. The calculated NNTp was 3.6 after treatment of mild pain, compared with 4.6 after treatment of moderate or severe pain.
Relief of headache‐associated symptoms
In addition to relief of headache pain, relief of headache‐associated symptoms is an important part of any anti‐migraine treatment. The majority of studies do not comment on the severity of associated symptoms, and relief is therefore defined as the complete resolution of any symptom present at baseline by a defined time after administration. Since it is common for individual migraine sufferers to regularly experience the same associated symptom(s), while others do not, we have chosen to express the proportion of participants experiencing relief as a fraction of participants with the symptom at baseline rather than as a fraction of the total treated population. This increases the relevance of the information to those patients who regularly suffer from associated symptoms.
Nausea
Pooled analyses were performed on eight dose, route of administration, and baseline pain severity combinations for which sufficient data were available (Summary of results I). Six treatments were administered to participants with moderate or severe baseline pain, while two (oral 50 mg and 100 mg) were specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results I: Relief of nausea within two hours in placebo‐controlled studies | |||||||||
| Route of administration | Dose (mg) | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 25 | 4 | 550 | 172/357 | 66/193 | 48 | 34 | 1.5 (1.2 to 1.9) | 7.2 (4.5 to 18) |
| Oral | 50 | 7 | 973 | 268/596 | 123/377 | 45 | 33 | 1.4 (1.2 to 1.7) | 8.1 (5.4 to 16) |
| Oral | 100 | 14 | 2996 | 880/1955 | 317/1041 | 45 | 30 | 1.5 (1.4 to 1.7) | 6.9 (5.5 to 9.1) |
| Subcutaneous | 6 | 5 | 667 | 276/364 | 103/303 | 76 | 34 | 2.2 (1.9 to 2.6) | 2.4 (2.1 to 2.9) |
| Intranasal | 5 | 2 | 476 | 140/294 | 58/182 | 48 | 32 | 1.5 (1.2 to 1.9) | 6.4 (4.1 to 15) |
| Intranasal | 20 | 5 | 1272 | 484/825 | 153/447 | 59 | 34 | 1.7 (1.5 to 2.0) | 4.1 (3.3 to 5.3) |
| In participants with mild baseline pain | |||||||||
| Oral | 50 | 3 | 280 | 78/145 | 10/135 | 54 | 7 | 6.9 (3.8 to 13) | 2.2 (1.8 to 2.7) |
| Oral | 100 | 3 | 265 | 58/130 | 10/135 | 45 | 7 | 5.9 (3.2 to 11) | 2.7 (2.1 to 3.6) |
Figure 5 shows the calculated NNTs for relief of nausea at two hours for four of the five most widely used dose and route of administration combinations in patients with moderate or severe baseline pain (no information was available for rectal 25 mg for this outcome).
5.
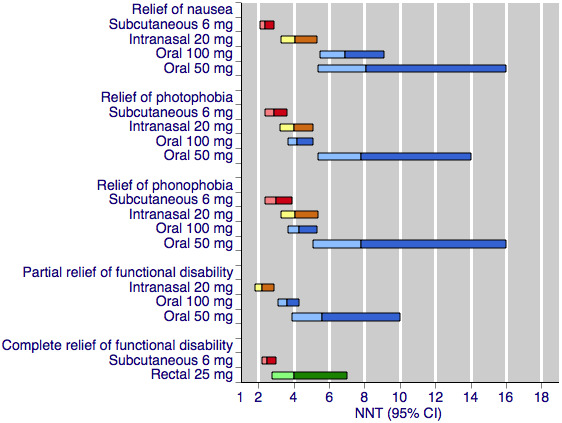
Sumatriptan versus placebo. Calculated NNTs for relief of migraine‐associated symptoms and functional disability after two hours, in participants treating moderate or severe migraine pain. Results for the five most commonly used dose and route of administration combinations, listed in rank order.
Oral doses are shown with blue bars, subcutaneous doses are shown with red bars, intranasal doses are shown with yellow bars, and rectal doses are shown with green bars.
All dose and route combinations provided superior relief of nausea compared with placebo. For participants with moderate or severe baseline pain, calculated NNTs were about 7 to 8 for the oral doses, 4 to 6 for the intranasal doses, and 2.4 for the subcutaneous dose. The proportion of participants with relief of nausea within two hours after oral sumatriptan was about 45% to 50%, about 50% to 60% after intranasal sumatriptan, and 76% after subcutaneous sumatriptan. Placebo response rates were consistently around 30% to 35% across all routes of administration.
The two doses of oral sumatriptan administered to participants with mild baseline pain also provided significant relief of nausea. Calculated NNTs were 2.2 and 2.7 for the 50 and 100 mg doses respectively. The proportion of participants with relief of nausea after treatment with sumatriptan was similar to that seen after sumatriptan treatment in participants with moderate or severe baseline pain; however, the proportion of placebo‐treated participants reporting relief of nausea was much lower amongst participants treating mild baseline pain. We did not perform statistical comparisons between the treatment effects in mild and moderate or severe baseline pain for relief of associated symptoms due to important differences between the two groups of participants. Participants treating mild baseline pain are less likely to have headache‐associated symptoms before treatment, and this significant difference in baseline incidence is likely to affect the relief obtained by these participants. In addition, any associated symptoms experienced by participants treating mild baseline pain are likely to be less severe than those experienced by participants treating moderate or severe attacks. Since we do not take into consideration the severity of symptoms when calculating relief, it is not meaningful to compare the relief in these two very different starting populations.
Photophobia
Pooled analyses were performed on seven dose, route of administration, and baseline pain intensity combinations for which sufficient data were available (Summary of results J). Five treatments were administered to participants with moderate or severe baseline pain, while two (oral 50 mg and 100 mg) were specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results J: Relief of photophobia within two hours in placebo‐controlled studies | |||||||||
| Route of administration | Dose (mg) | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 25 | 3 | 411 | 97/240 | 35/171 | 40 | 20 | 1.8 (1.3 to 2.5) | 5.0 (3.5 to 8.9) |
| Oral | 50 | 6 | 1144 | 284/638 | 160/506 | 45 | 32 | 1.4 (1.2 to 1.7) | 7.8 (5.4 to 14) |
| Oral | 100 | 9 | 2494 | 834/1703 | 201/791 | 49 | 25 | 1.9 (1.6 to 2.1) | 4.2 (3.7 to 5.1) |
| Subcutaneous | 6 | 3 | 631 | 245/343 | 105/288 | 71 | 36 | 1.9 (1.6 to 2.2) | 2.9 (2.4 to 3.6) |
| Intranasal | 20 | 3 | 1021 | 314/643 | 89/378 | 49 | 24 | 2.1 (1.7 to 2.5) | 4.0 (3.2 to 5.1) |
| In participants with mild baseline pain | |||||||||
| Oral | 50 | 3 | 483 | 125/237 | 44/246 | 53 | 18 | 3.0 (2.2 to 4.0) | 2.9 (2.3 to 3.7) |
| Oral | 100 | 3 | 475 | 131/229 | 44/246 | 57 | 18 | 3.2 (2.4 to 4.3) | 2.5 (2.1 to 3.2) |
Figure 5 shows the calculated NNTs for relief of photophobia at two hours for four of the five most widely used dose and route of administration combinations in patients with moderate or severe baseline pain (no information was available for rectal 25 mg for this outcome).
All dose and route combinations provided superior relief of photophobia compared with placebo. For participants with moderate or severe baseline pain, calculated NNTs were about 4 to 8 for the oral and intranasal doses, and 3 for the subcutaneous dose. The proportion of participants with relief of nausea within two hours after oral sumatriptan was about 40% to 50%, compared with about 35% to 50% after intranasal sumatriptan, and 71% after subcutaneous sumatriptan. Placebo response rates were around 20% to 35% across all routes of administration.
The two doses of oral sumatriptan administered to participants with mild baseline pain also provided significant relief of photophobia. Calculated NNTs were 2.9 and 2.5 for the 50 and 100 mg doses, respectively. About 50% to 60% of participants experienced relief of photophobia after treatment with sumatriptan compared with about 20% after treatment with placebo. As discussed previously, statistical comparisons between the treatment effects in mild and moderate or severe baseline pain were not performed for relief of headache‐associated symptoms.
Phonophobia
Pooled analyses were performed on six dose, route of administration, and baseline pain severity combinations for which sufficient data were available (Summary of results K). Four treatments were administered to participants with moderate or severe baseline pain, while two (oral 50 mg and 100 mg) were specifically administered to participants early in the migraine attack, while pain was still mild.
| Summary of results K: Relief of phonophobia within two hours in placebo‐controlled studies | |||||||||
| Route of administration | Dose (mg) | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 50 | 4 | 852 | 244/490 | 134/362 | 50 | 37 | 1.4 (1.2 to 1.6) | 7.8 (5.1 to 16) |
| Oral | 100 | 7 | 2118 | 736/1492 | 164/626 | 49 | 26 | 1.8 (1.6 to 2.1) | 4.3 (3.7 to 5.3) |
| Subcutaneous | 6 | 3 | 572 | 223/310 | 101/262 | 72 | 39 | 1.8 (1.5 to 2.2) | 3.0 (2.4 to 3.9) |
| Intranasal | 20 | 3 | 933 | 309/594 | 93/339 | 52 | 27 | 1.9 (1.6 to 2.3) | 4.1 (3.3 to 5.4) |
| In participants with mild baseline pain | |||||||||
| Oral | 50 | 3 | 413 | 105/202 | 37/211 | 52 | 18 | 3.0 (2.2 to 4.2) | 2.9 (2.3 to 3.9) |
| Oral | 100 | 3 | 400 | 120/189 | 37/211 | 63 | 18 | 3.7 (2.7 to 5.1) | 2.2 (1.8 to 2.7) |
Figure 5 shows the calculated NNTs for relief of phonophobia at two hours for four of the five most widely used dose and route of administration combinations in patients with moderate or severe baseline pain (no information was available for rectal 25 mg for this outcome).
All dose and route combinations provided superior relief of phonophobia compared with placebo. For participants with moderate or severe baseline pain, calculated NNTs were about 4 to 8 for the oral doses, 4 to 7 for the intranasal doses, and 3.0 for the subcutaneous dose. The proportion of participants with relief of nausea within two hours after oral sumatriptan was about 50%, about 40% to 50% after intranasal sumatriptan, and 72% after subcutaneous sumatriptan. Placebo response rates were around 25% to 40% across all routes of administration.
The two doses of oral sumatriptan administered to participants with mild baseline pain also provided significant relief of phonophobia. Calculated NNTs were 2.9 and 2.2 for the 50 and 100 mg doses respectively. About 50% to 60% of participants experienced relief of phonophobia after treatment with sumatriptan, compared with about 20% after treatment with placebo. As discussed previously, statistical comparisons between the treatment effects in mild and moderate or severe baseline pain were not performed for relief of headache‐associated symptoms.
Relief of functional disability
Functional disability provides a measure of the impact of a migraine on the capacity of the sufferer to work and carry out normal daily activities. It is typically assessed on a 4‐point scale, as follows: able to work and function normally (0 = none), working ability impaired to some degree (1 = mild), working ability severely impaired (2 = moderate), or bed rest required (4 = severe).
Relief of functional disability was defined in different ways by the studies included in each of the reviews. Some required complete relief of any functional disability (i.e. any disability at baseline reduced to none by two hours), while others required only partial relief (i.e. moderate or severe disability at baseline reduced to mild or none by two hours).
Partial relief of functional disability
Pooled analyses were performed on four dose and route of administration combinations for which sufficient data were available (Summary of results L). All of these treatments were administered to participants with moderate or severe baseline pain.
| Summary of results L: Partial relief of functional disability at two hours in placebo‐controlled studies | |||||||||
| Route of administration | Dose (mg) | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Oral | 25 | 3 | 381 | 107/220 | 51/161 | 49 | 32 | 1.4 (1.1 to 1.8) | 5.9 (3.7 to 14) |
| Oral | 50 | 4 | 607 | 186/378 | 72/229 | 49 | 31 | 1.5 (1.2 to 1.8) | 5.6 (3.9 to 10) |
| Oral | 100 | 6 | 1827 | 651/1113 | 220/714 | 58 | 31 | 1.9 (1.7 to 2.1) | 3.6 (3.1 to 4.3) |
| Intranasal | 20 | 2 | 225 | 89/144 | 13/81 | 62 | 16 | 3.8 (2.3 to 6.4) | 2.2 (1.8 to 2.9) |
Figure 5 shows the calculated NNTs for partial relief of functional disability at two hours for three of the five most widely used dose and route of administration combinations in patients with moderate or severe baseline pain (no information was available for subcutaneous 6 mg or rectal 25 mg for this outcome).
All dose and route combinations provided superior relief of functional disability compared with placebo. Calculated NNTs ranged from 5.9 to 3.6 with oral administration, to 2.2 with intranasal treatment. The proportion of sumatriptan‐treated participants with partial relief of functional disability at two hours ranged from about 50% to 60% with the low and high doses, respectively. The proportion of placebo‐treated participants with the same outcome was about 30% with the three doses of oral sumatriptan, and half that (16%) with intranasal sumatriptan. In general, higher doses of sumatriptan resulted in lower (better) NNTs, but the differences between NNTs were not statistically significant (overlapping confidence intervals), suggesting that any dose response relationship in not clinically significant.
Complete relief of functional disability
Pooled analyses were performed on two dose and route of administration combinations for which sufficient data were available (Summary of results M). Both these treatments were administered to participants with moderate or severe baseline pain.
| Summary of results M: Complete relief of functional disability at two hours in placebo‐controlled studies | |||||||||
| Route of administration | Dose (mg) | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Placebo | Active | Placebo | ||||
| In participants with moderate or severe baseline pain | |||||||||
| Subcutaneous | 6 | 3 | 750 | 213/377 | 62/373 | 56 | 17 | 3.4 (2.7 to 4.4) | 2.5 (2.2 to 3.0) |
| Rectal | 25 | 2 | 238 | 60/145 | 15/93 | 41 | 16 | 2.6 (1.6 to 4.3) | 4.0 (2.8 to 7.0) |
Figure 5 shows the calculated NNTs for complete relief of functional disability at two hours for these dose and route of administration combinations in patients with moderate or severe baseline pain (no information was available for oral 100 mg or 50 mg, or for intranasal 20 mg for this outcome).
Both dose and route combinations provided superior relief of functional disability compared with placebo. Calculated NNTs were 4.0 with the rectal administration, and 2.5 with the subcutaneous treatment, with 41% and 56% of participants respectively achieving this outcome with sumatriptan, and 16% and 17% with placebo.
Appendix 2. Summary tables for sumatriptan versus active comparators
Pain‐free at two hours
Pooled analyses were performed on 12 dose and route of administration combinations for which sufficient data were available to evaluate the pain‐free response at two hours. All treatments were administered to participants with moderate or severe baseline pain.
| Pain‐free at two hours in active‐controlled studies | ||||||||||
| Route of administration | Dose (mg) | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 25 | Rizatriptan 5 mg | 2 | 2210 | 310/1117 | 363/1093 | 28 | 33 | 0.84 (0.74 to 0.95) | ‐18 (‐11 to ‐62) |
| Oral | 25 | Rizatriptan 10 mg | 2 | 2231 | 310/1117 | 440/1114 | 28 | 39 | 0.70 (0.62 to 0.79) | ‐8.5 (‐6.4 to ‐13) |
| Oral | 50 | Effervescent ASA 1000 mg | 2 | 726 | 116/359 | 97/367 | 32 | 26 | 1.2 (0.97 to 1.5) | Not calculated |
| Oral | 50 | Rizatriptan 5 mg | 2 | 2209 | 394/1116 | 363/1093 | 35 | 33 | 1.1 (0.94 1.2) | Not calculated |
| Oral | 50 | Rizatriptan 10 mg | 2 | 2230 | 394/1116 | 440/1114 | 35 | 39 | 0.89 (0.80 to 0.99) | ‐24 (‐12 to ‐560) |
| Oral | 50 | Eletriptan 40 mg | 2 | 721 | 64/362 | 86/359 | 18 | 24 | 0.74 (0.55 to 0.99) | ‐16 (‐8.2 to ‐270) |
| Oral | 50 | Eletriptan 80 mg | 2 | 706 | 64/362 | 104/344 | 18 | 30 | 0.58 (0.44 to 0.76) | ‐8.0 (‐5.3 to ‐ 16) |
| Oral | 100 | Almotriptan 12.5 mg | 2 | 754 | 129/387 | 102/367 | 33 | 28 | 1.2 (0.97 to 1.5) | Not calculated |
| Oral | 100 | Eletriptan 40 mg | 3 | 2263 | 271/1130 | 366/1133 | 24 | 32 | 0.74 (0.65 to 0.85) | ‐12 (‐8.3 to ‐22) |
| Oral | 100 | Eletriptan 80 mg | 2 | 604 | 55/299 | 103/305 | 18 | 34 | 0.54 (0.41 to 0.72) | ‐6.5 (‐4.5 to ‐12) |
| Oral | 100 | Rizatriptan 10 mg | 2 | 936 | 143/460 | 178/476 | 31 | 37 | 0.82 (0.69 to 0.98) | ‐16 (‐8.1 to ‐410) |
| Oral | 100 | ASA 900 mg + MCP 10 mg | 2 | 575 | 71/275 | 48/300 | 26 | 16 | 1.6 (1.2 to 2.3) | 10 (6.1 to 31) |
| Footnotes: ASA ‐ acetyl salicylic acid, aspirin; MCP ‐ metoclopramide | ||||||||||
Pain‐free at one hour
Pooled analyses were performed on three dose and route of administration combinations for which sufficient data were available to evaluate the pain‐free response at one hour. All treatments were administered to participants with moderate or severe baseline pain.
| Pain‐free at one hour in active‐controlled studies | ||||||||||
| Route of administration | Dose (mg) | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 50 | Effervescent ASA 1000 mg | 2 | 726 | 19/359 | 20/367 | 5 | 5 | 0.97 (0.53 to 1.8) | Not calculated |
| Oral | 100 | Eletriptan 40 mg | 3 | 2263 | 59/1130 | 75/1133 | 5 | 7 | 0.79 (0.57 to 1.1) | Not calculated |
| Oral | 100 | Eletriptan 80 mg | 2 | 604 | 19/299 | 40/305 | 6 | 13 | 0.48 (0.28 to 0.81) | ‐15 (‐8.7 to ‐48) |
| Footnotes: ASA ‐ acetyl salicylic acid, aspirin | ||||||||||
Sustained pain‐free during the 24 hours postdose
Pooled analyses were performed on one dose and route of administration combination for which sufficient data were available to evaluate the 24‐hour sustained pain‐free response. The treatments were administered to participants with moderate or severe baseline pain.
| Sustained pain‐free during the 24 hours postdose in active‐controlled studies | ||||||||||
| Route of administration | Dose (mg) | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 100 | Almotriptan 12.5 mg | 2 | 754 | 111/387 | 110/367 | 29 | 30 | 0.96 (0.77 to 1.2) | Not calculated |
Headache relief at two hours
Pooled analyses were performed on 13 dose and route of administration combinations for which sufficient data were available to evaluate the headache relief response at two hours. All treatments were administered to participants with moderate or severe baseline pain.
| Headache relief at two hours in active‐controlled studies | ||||||||||
| Route of administration | Dose (mg) | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 25 | Rizatriptan 5 mg | 2 | 2210 | 669/1117 | 731/1093 | 60 | 67 | 0.90 (0.84 to 0.96) | ‐14 (‐9.1 to ‐34) |
| Oral | 25 | Rizatriptan 10 mg | 2 | 2231 | 669/1117 | 780/1114 | 60 | 70 | 0.86 (0.81 to 0.91) | ‐9.9 (‐7.1 to ‐16) |
| Oral | 50 | Effervescent ASA 1000 mg | 2 | 726 | 191/359 | 153/367 | 53 | 42 | 1.3 (1.1 to 1.5) | 8.7 (5.3 to 23) |
| Oral | 50 | Zolmitriptan 2.5 mg | 2 | 1609 | 543/814 | 523/795 | 67 | 66 | 1.0 (0.94 to 1.1) | Not calculated |
| Oral | 50 | Zolmitriptan 5 mg | 2 | 1633 | 543/814 | 537/819 | 67 | 66 | 1.0 (0.95 to 1.1) | Not calculated |
| Oral | 50 | Rizatriptan 5 mg | 3 | 2911 | 949/1469 | 951/1442 | 65 | 66 | 0.98 (0.93 to 1.0) | Not calculated |
| Oral | 50 | Rizatriptan 10 mg | 2 | 2227 | 710/1113 | 780/1114 | 64 | 70 | 0.91 (0.86 to 0.96) | ‐16 (‐9.9 to ‐43) |
| Oral | 50 | Eletriptan 40 mg | 2 | 721 | 186/362 | 217/359 | 51 | 60 | 0.85 (0.75 to 0.97) | ‐11 (‐6.1 to ‐54) |
| Oral | 50 | Eletriptan 80 mg | 2 | 706 | 186/362 | 226/344 | 51 | 66 | 0.78 (0.69 to 0.88) | ‐7.0 (‐4.7 to ‐14) |
| Oral | 100 | Eletriptan 40 mg | 3 | 2263 | 622/1130 | 706/1133 | 55 | 62 | 0.88 (0.82 to 0.94) | ‐14 (‐8.8 to ‐31) |
| Oral | 100 | Eletriptan 80 mg | 2 | 604 | 151/299 | 198/305 | 51 | 65 | 0.78 (0.68 to 0.90) | ‐6.9 (‐4.5 to ‐15) |
| Oral | 100 | Paracetamol 1000 mg + MCP 10 mg | 2 | 1035 | 233/514 | 225/521 | 45 | 43 | 1.1 (0.92 to 1.2) | Not calculated |
| Oral | 100 | ASA 900 mg + MCP 10 mg | 2 | 575 | 137/275 | 138/300 | 50 | 46 | 1.1 (0.92 to 1.3) | Not calculated |
| Footnotes: ASA ‐ acetyl salicylic acid, aspirin; MCP ‐ metoclopramide | ||||||||||
Headache relief at one hour
Pooled analyses were performed on 12 dose and route of administration combinations for which sufficient data were available to evaluate the headache relief response at one hour. All treatments were administered to participants with moderate or severe baseline pain.
| Headache relief at one hour in active‐controlled studies | ||||||||||
| Route of administration | Dose (mg) | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 25 | Rizatriptan 5 mg | 2 | 2210 | 375/1117 | 404/1093 | 34 | 37 | 0.91 (0.81 to 1.0) | Not calculated |
| Oral | 25 | Rizatriptan 10 mg | 2 | 2231 | 375/1117 | 456/1114 | 34 | 41 | 0.82 (0.74 to 0.91) | ‐14 (‐8.8 to ‐30) |
| Oral | 50 | Effervescent ASA 1000 mg | 2 | 726 | 86/359 | 113/367 | 24 | 31 | 0.78 (0.61 to 0.99) | ‐15 (‐7.5 to ‐270) |
| Oral | 50 | Zolmitriptan 2.5 mg | 2 | 1609 | 330/814 | 318/795 | 41 | 40 | 1.0 (0.90 to 1.1) | Not calculated |
| Oral | 50 | Zolmitriptan 5 mg | 2 | 1633 | 330/814 | 320/819 | 41 | 39 | 1.0 (0.92 to 1.2) | Not calculated |
| Oral | 50 | Rizatriptan 5 mg | 2 | 2209 | 409/1116 | 404/1093 | 37 | 37 | 0.99 (0.89 to 1.1) | Not calculated |
| Oral | 50 | Rizatriptan 10 mg | 2 | 2230 | 409/1116 | 456/1114 | 37 | 41 | 0.90 (0.81 to 1.0) | Not calculated |
| Oral | 50 | Eletriptan 40 mg | 2 | 721 | 90/362 | 90/359 | 25 | 25 | 0.99 (0.77 to 1.3) | Not calculated |
| Oral | 50 | Eletriptan 80 mg | 2 | 706 | 90/362 | 119/344 | 25 | 35 | 0.72 (0.57 to 0.91) | ‐10 (‐6.1 to ‐33) |
| Oral | 100 | Eletriptan 40 mg | 3 | 2263 | 282/1130 | 368/1133 | 25 | 32 | 0.77 (0.68 to 0.88) | ‐13 (‐8.9 to ‐26) |
| Oral | 100 | Eletriptan 80 mg | 2 | 604 | 68/299 | 106/305 | 23 | 35 | 0.65 (0.50 to 0.84) | ‐8.3 (‐5.2 to ‐21) |
| Oral | 100 | Rizatriptan 10 mg | 2 | 936 | 120/460 | 163/476 | 26 | 34 | 0.76 (0.62 to 0.93) | ‐12 (‐7.1 to ‐43) |
| Footnotes: ASA ‐ acetyl salicylic acid, aspirin | ||||||||||
Sustained headache relief during the 24 hours postdose
Pooled analyses were performed on one dose and route of administration combination for which sufficient data were available to evaluate the 24‐hour sustained headache relief response. The treatments were administered to participants with moderate or severe baseline pain.
| Sustained headache relief during the 24 hours postdose in active‐controlled studies | ||||||||||
| Route of administration | Dose (mg) | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 100 | Eletriptan 40 mg | 2 | 1998 | 340/1001 | 430/997 | 34 | 43 | 0.79 (0.71 to 0.88) | ‐11 (‐7.5 to ‐20) |
Any adverse event during within 24 hours
Pooled analyses were performed on nine dose and route of administration combinations for which sufficient data were available to evaluate the incidence of adverse events within 24 hours of treatment. All treatments were administered to participants with moderate or severe baseline pain.
| Any adverse event within 24 hours in active‐controlled studies | ||||||||||
| Route of administration | Dose (mg) | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative harm (95% CI) | NNH (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 25 | Rizatriptan 5 mg | 2 | 1169 | 250/587 | 238/582 | 43 | 41 | 1.0 (0.91 to 1.2) | Not calculated |
| Oral | 25 | Rizatriptan 10 mg | 2 | 1186 | 250/587 | 276/599 | 43 | 46 | 0.92 (0.81 to 1.1) | Not calculated |
| Oral | 50 | Effervescent ASA 1000 mg | 2 | 730 | 64/361 | 55/369 | 18 | 15 | 1.2 (0.85 to 1.6) | Not calculated |
| Oral | 50 | Zolmitriptan 2.5 mg | 2 | 1771 | 290/893 | 283/878 | 32 | 32 | 1.0 (0.88 to 1.2) | Not calculated |
| Oral | 50 | Zolmitriptan 5 mg | 2 | 1790 | 290/893 | 322/897 | 32 | 36 | 0.91 (0.80 to 1.0) | Not calculated |
| Oral | 50 | Rizatriptan 5 mg | 2 | 1160 | 276/578 | 238/582 | 48 | 41 | 1.2 (1.0 to 1.3) | Not calculated |
| Oral | 50 | Rizatriptan 10 mg | 2 | 1177 | 276/578 | 276/599 | 48 | 46 | 1.0 (0.92 to 1.2) | Not calculated |
| Oral | 100 | Rizatriptan 10 mg | 2 | 856 | 217/421 | 203/435 | 52 | 47 | 1.1 (0.96 to 1.3) | Not calculated |
| Oral | 100 | ASA 900 mg + MCP 10 mg | 2 | 621 | 112/300 | 78/321 | 37 | 24 | 1.5 (1.2 to 2.0) | 7.7 (4.9 to 17 |
| Footnotes: ASA ‐ acetyl salicylic acid, aspirin; MCP ‐ metoclopramide | ||||||||||
Use of rescue medication
Pooled analyses were performed on two dose and route of administration combinations for which sufficient data were available to evaluate the use of rescue medication during the 24 hours postdose. All treatments were administered to participants with moderate or severe baseline pain.
| Use of rescue medication during the 24 hours postdose in active‐controlled studies | ||||||||||
| Route of administration | Dose (mg) | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNTp (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 100 | Eletriptan 40 mg | 2 | 1918 | 261/960 | 203/958 | 27 | 21 | 1.3 (1.1 to 1.5) | ‐17 (‐10 to ‐46) |
| Oral | 100 | Paracetamol 1000 mg + MCP 10 mg | 2 | 1243 | 198/606 | 245/637 | 33 | 38 | 0.86 (0.74 to 1.0) | Not calculated |
| Footnotes: MCP ‐ metoclopramide | ||||||||||
Relief of migraine‐associated symptoms
Nausea
Pooled analyses were performed on three dose and route of administration combinations for which sufficient data were available to evaluate the relief of nausea within two hours. All treatments were administered to participants with moderate or severe baseline pain.
| Relief of nausea within two hours in active‐controlled studies | ||||||||||
| Route of administration | Dose (mg) | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 100 | Eletriptan 40 mg | 3 | 1478 | 352/719 | 420/759 | 49 | 55 | 0.87 (0.79 to 0.96) | ‐16 (‐8.7 to ‐77) |
| Oral | 100 | Eletriptan 80 mg | 2 | 408 | 100/204 | 123/204 | 49 | 60 | 0.83 (0.69 to 0.99) | ‐8.9 (‐4.8 to ‐60) |
| Oral | 100 | ASA 900 mg + MCP 10 mg | 2 | 410 | 60/192 | 76/218 | 31 | 35 | 0.91 (0.69 to 1.2) | Not calculated |
| Footnotes: MCP ‐ metoclopramide | ||||||||||
Photophobia
Pooled analyses were performed on four dose and route of administration combinations for which sufficient data were available to evaluate the relief of photophobia within two hours. All treatments were administered to participants with moderate or severe baseline pain.
| Relief of photophobia within two hours in active‐controlled studies | ||||||||||
| Route of administration | Dose (mg) | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 50 | Eletriptan 40 mg | 2 | 528 | 107/261 | 132/267 | 41 | 49 | 0.83 (0.69 to 1.0) | Not calculated |
| Oral | 50 | Eletriptan 80 mg | 2 | 508 | 107/261 | 142/247 | 41 | 57 | 0.72 (0.60 to 0.86) | ‐6.1 (‐4.0 to ‐13) |
| Oral | 100 | Eletriptan 40 mg | 3 | 1692 | 438/855 | 500/837 | 51 | 60 | 0.85 (0.78 to 0.93) | ‐12 (‐7.6 to ‐26) |
| Oral | 100 | Eletriptan 80 mg | 2 | 457 | 110/232 | 142/225 | 47 | 63 | 0.76 (0.64 to 0.90) | ‐6.4 (‐4.1 to ‐15) |
Phonophobia
Pooled analyses were performed on three dose and route of administration combinations for which sufficient data were available to evaluate the relief of phonophobia within two hours. All treatments were administered to participants with moderate or severe baseline pain.
| Relief of phonophobia within two hours in active‐controlled studies | ||||||||||
| Route of administration | Dose (mg) | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 50 | Eletriptan 40 mg | 2 | 517 | 120/257 | 139/260 | 47 | 53 | 0.87 (0.73 to 1.0) | Not calculated |
| Oral | 50 | Eletriptan 80 mg | 2 | 508 | 120/257 | 145/251 | 47 | 58 | 0.81 (0.69 to 0.96) | ‐9.0 (‐5.1 to ‐41) |
| Oral | 100 | Eletriptan 40 mg | 2 | 1361 | 352/691 | 405/670 | 51 | 60 | 0.84 (0.76 to 0.92) | ‐11 (‐6.8 to ‐24) |
Relief of functional disability
Partial relief of functional disability
Pooled analyses were performed on four dose and route of administration combinations for which sufficient data were available to evaluate the partial relief of functional disability within two hours. All treatments were administered to participants with moderate or severe baseline pain.
| Partial relief of functional disability within two hours in active‐controlled studies | ||||||||||
| Route of administration | Dose (mg) | Comparator | Number of | Number with outcome/total | Percent with outcome | Relative benefit (95% CI) | NNT (95% CI) | |||
| Studies | Participants | Active | Comparator | Active | Comparator | |||||
| In participants with moderate or severe baseline pain | ||||||||||
| Oral | 50 | Eletriptan 40 mg | 2 | 590 | 153/298 | 180/292 | 51 | 62 | 0.83 (0.72 to 0.96) | ‐9.7 (‐5.5 to ‐43) |
| Oral | 50 | Eletriptan 80 mg | 2 | 570 | 153/298 | 168/272 | 51 | 62 | 0.84 (0.73 to 0.97) | ‐9.6 (‐5.4 to ‐43) |
| Oral | 100 | Eletriptan 40 mg | 3 | 1880 | 553/936 | 645/944 | 59 | 68 | 0.86 (0.80 to 0.92) | ‐11 (‐7.4 to ‐20) |
| Oral | 100 | Eletriptan 80 mg | 2 | 516 | 129/255 | 173/261 | 51 | 66 | 0.77 (0.66 to 0.89) | ‐6.4 (‐4.2 to ‐14) |
Complete relief of functional disability
There were insufficient data to perform any pooled analyses for the complete relief of functional disability.
Contributions of authors
All authors contributed to writing the protocol. CD collated data from the individual reviews and entered it into RevMan. SD checked the data. All authors were involved with writing the full review.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
Institutional support
External sources
-
Lifting The Burden: the Global Campaign against Headache, UK.
Funding for administrative costs associated with editorial review of the protocol
-
International Headache Society, UK.
Funding for administrative costs associated with editorial and peer review of the full review
Declarations of interest
The authors of this overview are also the authors of the four included individual reviews.
SD and RAM have received research support from charities, government, and industry sources at various times. RAM has consulted for various pharmaceutical companies and has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. CD has no interests to declare. Support for this review came from the Oxford Pain Relief Trust.
Stable (no update expected for reasons given in 'What's new')
References
References to included reviews
Derry 2012a
- Derry CJ, Derry S, Moore RA. Sumatriptan (oral route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008615] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012b
- Derry CJ, Derry S, Moore RA. Sumatriptan (subcutaneous route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012c
- Derry CJ, Derry S, Moore RA. Sumatriptan (intranasal route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009663] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012d
- Derry CJ, Derry S, Moore RA. Sumatriptan (rectal route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009664] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ayzenberg 2012
- Ayzenberg I, Katsarava Z, Sborowski A, Chernysh M, Osipova V, Tabeeva G, et al. The prevalence of primary headache disorders in Russia: a countrywide survey. Cephalalgia 2012;32(5):373‐81. [DOI: 10.1177/0333102412438977] [DOI] [PubMed] [Google Scholar]
Bendtsen 2003
- Bendtsen L, Mattsson P, Zwart JA, Lipton RB. Placebo response in clinical randomized trials of analgesics in migraine. Cephalalgia 2003;23(7):487‐90. [DOI] [PubMed] [Google Scholar]
Bigal 2003
- Bigal ME, Bordini CA, Antoniazzi AL, Speciali JG. The triptan formulations: a critical evaluation. Arquivos de Neuro‐Psiquiatria 2003;61(2A):313‐20. [DOI] [PubMed] [Google Scholar]
Bigal 2008
- Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology 2008;71(8):559‐66. [DOI: 10.1212/01.wn1.0000323925.29520.e7] [DOI] [PubMed] [Google Scholar]
Bloudek 2012
- Bloudek LM, Stokes M, Buse DC, Wilcox TK, Lipton RB, Goadsby PJ, et al. Cost of healthcare for patients with migraine in five European countries: results from the International Burden of Migraine Study (IBMS). Journal of Headache and Pain 2012;13(5):361‐78. [DOI: 10.1007/s10194-012-0460-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF 2013
- British National Formulary. http://www.medicinescomplete.com/mc/bnf/current/PHP2854‐sumatriptan.htm (Accessed 30 October 2013). London: BMJ Group and Pharmaceutical Press, 2013; Vol. October.
Brandes 2009
- Brandes JL, Cady RK, Freitag FG, Smith TR, Chandler P, Fox AW, et al. Needle‐free subcutaneous sumatriptan (Sumavel DosePro): bioequivalence and ease of use. Headache 2009;49(10):1435‐44. [DOI] [PubMed] [Google Scholar]
Buse 2011
- Buse D, Manack A, Serrano D, Reed M, Varon S, Turkel C, et al. Headache impact of chronic and episodic migraine: results from the American Migraine Prevalence and Prevention study. Headache 2012;52(1):3‐17. [DOI: 10.1111/j.1526-4610.2011.02046.x] [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Craen 2000
- Craen AJ, Tijssen JG, Gans J, Kleijnen J. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. Journal of Neurology 2000;247(3):183‐8. [DOI] [PubMed] [Google Scholar]
Diamond 2007
- Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47(3):355‐63. [DOI: 10.1111/j.1526-4610.2006.00631.x] [DOI] [PubMed] [Google Scholar]
Diener 2008
- Diener HC, Schorn CF, Bingel U, Dodick DW. The importance of placebo in headache research. Cephalalgia 2008;28(10):1003‐11. [DOI] [PubMed] [Google Scholar]
Ferrari 2002
- Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia 2002;22(8):633‐58. [DOI: 10.1046/j.1468-2982.2002.00404.x] [DOI] [PubMed] [Google Scholar]
Gendolla 2008
- Gendolla A. Early treatment in migraine: how strong is the current evidence?. Cephalalgia 2008;28(Suppl 2):28‐35. [DOI] [PubMed] [Google Scholar]
Goadsby 2007
- Goadsby PJ. Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends in Molecular Medicine 2007;13(1):39‐44. [DOI: 10.1016/j.molmed.2006.11.005] [DOI] [PubMed] [Google Scholar]
Hazard 2009
- Hazard E, Munakata J, Bigal ME, Rupnow MF, Lipton RB. The burden of migraine in the United States: current and emerging perspectives on disease management and economic analysis. Value in Health 2009;12(1):55‐64. [DOI: 10.1111/j.1524-4733.2008.00404.x] [DOI] [PubMed] [Google Scholar]
IHS 2000
- International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 2000;20(9):765‐86. [DOI] [PubMed] [Google Scholar]
IHS 2013
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33(9):629‐808. [DOI: 10.1177/0333102413485658] [DOI] [PubMed] [Google Scholar]
Johnston 2010
- Johnston MM, Rapoport AM. Triptans for the management of migraine. Drugs 2010;70(12):1505‐18. [DOI: 10.2165/11537990-000000000-00000] [DOI] [PubMed] [Google Scholar]
Law 2013
- Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008541.pub2] [DOI] [PubMed] [Google Scholar]
Leonardi 2005
- Leonardi M, Steiner TJ, Scher AT, Lipton RB. The global burden of migraine: measuring disability in headache disorders with WHO's Classification of Functioning, Disability and Health (ICF). Journal of Headache and Pain 2005;6(6):429‐40. [DOI: 10.1007/s10194-005-0252-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linde 2012
- Linde M, Gustavsson A, Stovner LJ, Steiner TJ, Barré J, Katsarava Z, et al. The cost of headache disorders in Europe: the Eurolight project. European Journal of Neurology 2012;19(5):703‐11. [DOI: 10.1111/j.1468-1331.2011.03612.x] [DOI] [PubMed] [Google Scholar]
Lipton 1999
- Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy?. Headache 1999;39(Suppl 2):S20‐S26. [Google Scholar]
Lipton 2007
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, AMPP Advisory Group, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68(5):343‐9. [DOI] [PubMed] [Google Scholar]
Lucas 2006
- Lucas C, Géraud G, Valade D, Chautard MH, Lantéri‐Minet M. Recognition and therapeutic management of migraine in 2004, in France: results of FRAMIG 3, a French nationwide population‐based survey. Headache 2006;46(5):715‐25. [DOI: 10.1111/j.1526-4610.2006.00430.x] [DOI] [PubMed] [Google Scholar]
Macedo 2006
- Macedo A, Farré M, Baños JE. A meta‐analysis of the placebo response in acute migraine and how this response may be influenced by some of the characteristics of clinical trials. European Journal of Clinical Pharmacology 2006;62(3):161‐72. [DOI] [PubMed] [Google Scholar]
McCrory 2003
- McCrory DC, Gray RN. Oral sumatriptan for acute migraine. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD002915] [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5] [Google Scholar]
Moore 2011
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: Examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI] [PubMed] [Google Scholar]
Moore 2012
- Moore RA, Derry CJ, Derry S, Straube S, McQuay HJ. A conservative method of testing whether combination analgesics produce additive or synergistic effects using evidence from acute pain and migraine. European Journal of Pain 2012;16(4):585‐91. [DOI: 10.1016/j.ejpain.2011.08.009] [DOI] [PubMed] [Google Scholar]
Moore 2013
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Munakata 2009
- Munakata J, Hazard E, Serrano D, Klingman D, Rupnow MF, Tierce J, et al. Economic burden of transformed migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2009;49(4):498‐508. [DOI: 10.1111/j.1526-4610.2009.01369.x] [DOI] [PubMed] [Google Scholar]
Nuesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI: 10.1136/bmj.c3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oldman 2002
- Oldman AD, Smith LA, McQuay HJ, Moore RA. Pharmacological treatments for acute migraine: quantitative systematic review. Pain 2002;97(3):247‐57. [DOI] [PubMed] [Google Scholar]
Pascual 2002
- Pascual J. Clinical benefits of early triptan therapy for migraine. Headache 2002;42 Suppl 1:10‐7. [DOI: 10.1046/j.1526-4610.2002.0420s1010.x] [DOI] [PubMed] [Google Scholar]
PCA 2013
- Prescribing and Primary Care team, Health and Social Care Information Centre. Prescription cost analysis, England 2012. http://data.gov.uk/dataset/prescription_cost_analysis_england [Accessed 17 Apr 2013]. Government Statistical Service: Health and Social Care Information Centre, 2013. [ISBN: 978‐1‐84‐636859‐2]
Pierce 2009
- Pierce M, Marbury T, O'Neill C, Siegel S, Du W, Sebree T. Zelrix: a novel transdermal formulation of sumatriptan. Headache 2009;49(6):817‐25. [DOI] [PubMed] [Google Scholar]
Radtke 2009
- Radtke A, Neuhauser H. Prevalence and burden of headache and migraine in Germany. Headache 2009;49(1):79‐89. [DOI: 10.1111/j.1526-4610.2008.01263.x] [DOI] [PubMed] [Google Scholar]
Shea 2007
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Medical Research Methodology 2007;7:10. [DOI: 10.1186/1471-2288-7-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steiner 2013
- Steiner TS, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. Journal of Headache and Pain 2013;14:1. [DOI: 10.1186/1129-2377-14-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stovner 2010
- Stovner LJ, Andree C. Prevalence of headache in Europe: a review for the Eurolight project. Journal of Headache and Pain 2010;11(4):289‐99. [DOI: 10.1007/s10194-010-0217-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tfelt‐Hansen 2012
- Tfelt‐Hansen P, Pascual J, Ramadan N, Dahlöf C, D'Amico D, Diener HC, et al. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia 2012;32(1):6‐38. [DOI: 10.1177/0333102411417901] [DOI] [PubMed] [Google Scholar]
Thornton 2000
- Thornton A, Lee P. Publication bias in meta‐analysis: its causes and consequences. Journal of Clinical Epidemiology 2000;53(2):207‐16. [DOI: 10.1016/S0895-4356(99)00161-4] [DOI] [PubMed] [Google Scholar]
Victor 2010
- Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life‐span study. Cephalalgia 2010;30(9):1065‐72. [DOI: 10.1177/0333102409355601] [DOI] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2012
- Yu S, Liu R, Zhao G, Yang X, Qiao X, Feng J, et al. The prevalence and burden of primary headaches in China: a population‐based door‐to‐door survey. Headache 2012;52(4):582‐91. [DOI: 10.1111/j.1526-4610.2011.02061.x] [DOI] [PubMed] [Google Scholar]


