Abstract
Resistance to conventional lines of therapy develops in approximately 20% of all patients with lymphoma. These patients have a dismal prognosis, with an expected median survival of 6.3 months. In recent years, T-cell immunotherapy has demonstrated a remarkable capacity to induce complete and durable clinical responses in patients with chemotherapy-refractory lymphoma. A major contributor to the success of immunotherapy has been the advent of genetic engineering technologies that introduce a chimeric antigen receptor (CAR) into T cells to focus their killing activity on tumor cells. The adoptive transfer of autologous CAR T-cell products specific for the pan–B-cell antigen CD19 have now received approval from the US Food and Drug Administration (FDA) for the treatment of relapsed or chemotherapy-resistant B-cell non-Hodgkin lymphoma. This review is designed to showcase the clinical efficacy and unique toxicities of individually developed CAR T-cell products for the treatment of lymphomas and their evolution from the laboratory bench to commercialization.
Keywords: CAR T cells, CD19, CD20, lymphoma
Introduction
Lymphoma is the most common hematologic malignancy and is responsible for 3.5% of all deaths from cancer in the United States. According to the cell of origin, lymphomas can be broadly classified as B-cell, T-cell, or natural killer/T-cell lymphomas. B-cell lymphomas are the most common type, making up more than 70% of the approximately 80,000 newly diagnosed cases of lymphoma each year in the United States.1 The B-cell type can be further stratified into Hodgkin lymphoma (HL; ~10% of all cases) and non-Hodgkin lymphoma (NHL; ~90% of all cases), both of which comprise many subtypes. NHL subtypes can be grouped into indolent forms, such as follicular lymphoma (FL), and aggressive forms, such as diffuse large B-cell lymphoma (DLBCL).
Standard therapies for lymphoma include combination immunotherapy/chemotherapy, radiation therapy, and hematopoietic stem cell transplant (HSCT). Overall, resistance to conventional lines of therapy will develop in approximately 20% of all patients with lymphoma.2–6 The prognosis in this setting remains grim, especially for patients with DLBCL—the most common aggressive subtype—in which the overall survival is 6.3 months from the last treatment failure.7 Thus, novel therapies that can improve the outcomes for patients with relapsed or treatment-refractory lymphoma are clearly needed.
It has long been postulated that the curative graft-versus-tumor effect mediated by T cells following allogeneic HSCT can be replicated without HSCT by the adoptive transfer of T cells that are specific for tumor-expressed proteins. In early proof-of principle studies, infusions of T cells targeting Epstein-Barr virus (EBV) proteins through their native receptors eliminated chemotherapy-refractory EBV-driven lymphomas.8 However, most cancers do not express immunogenic viral proteins that can be easily targeted with T cells. As a result, many centers experimented with redirecting T cells to tumor targets by genetically engineering them to express a chimeric antigen receptor (CAR).9,10 A CAR is a molecule that consists of 2 critical components: (1) a single-chain variable fragment (scFv) derived from an immunoglobulin that has affinity to a cell surface tumor target antigen, and (2) an intracellular signaling moiety. These components are connected to each other by linker and transmembrane domains. The genetic sequence for this molecule is loaded into viral or nonviral vectors, which are then used to transduce T cells, enabling them to target tumors.11
The full implications of this technology have only recently been realized, with the striking efficacy of CD19-specific CAR T cells directed against treatment-resistant B-cell malignancies demonstrated in early-phase clinical trials. Because of these results, 2 products based on this technology have recently been licensed by the Food and Drug Administration (FDA) as standard-of-care therapies. Clinical trials using CD19 CAR T cells first reported unprecedented efficacy in patients with B-cell acute lymphoblastic leukemia (ALL), a highly aggressive B-cell malignancy. B-cell lymphomas were a natural extension for the application of CD19 CAR T-cell therapy because most B-cell NHLs also express CD19. The overall clinical efficacy of CD19 CAR T cells in patients with lymphoma appears to be less striking than in those with ALL; for example, cumulative 6-month complete response (CR) rates are 24% to 54% in B-cell lymphoma, compared with a 70% molecular CR rate in patients with ALL in reported clinical trials.12–15 The reasons for these differences are not immediately clear, although ongoing correlative studies may be able to provide some answers. Nonetheless, many patients with lymphoma for whom standard-of-care approaches were exhausted have exhibited dramatic responses.
Although it is tempting to combine efficacy and toxicity data from distinct CD19 CAR trials in lymphoma, this is likely unwise because a wide range of variables may affect the activity of CAR T-cell products, even when they target the same antigen. These variables include the following: (1) the vectors used for transduction (retroviral vs lentiviral vs nonviral); (2) the costimulatory domains included in the CAR (CD28 vs 4–1BB); and (3) the manufacturing processes (eg, cytokines, ratios of T-cell subsets). Indeed, distinct CAR T-cell products specific for the same cancer antigen have been developed independently. The purpose of this review is to outline the evolution of clinical trials of CAR T cells in patients with NHLs and to highlight unique aspects of using CAR T cells to treat these patients.
Early-Phase Trials of CD19 CAR T Cells for Lymphoma
CD19 CAR T Cells
Table 1 details the outcomes of patients with lymphoma in selected US early-phase clinical trials of the adoptive transfer of CAR-modified T cells. Although CAR T cells had shown excellent preclinical antitumor potential for a couple of decades, it was not until 2006 that their use as a therapy for patients with cancer was first reported.16 Disappointingly, no clinical responses were seen in this pilot clinical trial, and the T cells did not persist for more than a week after infusion despite systemic interleukin 2 (IL-2) infusion support. In lymphoma, similar findings of only modest efficacy were subsequently reported in phase 1 clinical trials of adoptively transferred CD20 or CD19 CAR-redirected T cells for patients with NHL and B-cell ALL,17,18 respectively, and it was clear that poor in vivo T-cell persistence was a contributor to these disappointing results. As a result, a second generation of CARs was developed, which in addition to providing T-cell activation mediated by CD3z (or “signal 1” of the 3 necessary for full activation of T cells) provided costimulation through extra domains derived from a molecule such as CD28 or 4–1BB (“signal 2”). The inclusion of costimulatory domains within the transgene construct did significantly improve the persistence of T cells from less than 2 weeks to more than 6 weeks; the best evidence for this improvement was demonstrated in a clinical trial that directly compared the persistence of first- and second-generation CD19 CAR T cells administered concomitantly to patients with B-cell malignancies.19 Thus, second-generation CAR T cells are now the basis of the majority of clinical trials in patients with lymphomas.
Table 1.
Single-Center Early-Phase Clinical Trials of CAR T Cells for Lymphomas
| Study | Target | Type (n) | Construct | Vector | CTX | Total CTX Dose, mg/m2 | ORR/CR Rate, % | CRS/Neuro Grade ≥3, % | Biomarkers |
|---|---|---|---|---|---|---|---|---|---|
| Till,17 2008 | CD20 | FL (6) MCL (1) |
CD3z | Plasmid | None | None | 14/0 | 0/0 |
|
| Jensen,18 2010 | CD20 CD19 |
FL (1) DLBCL (1) |
CD3z | Plasmid | Flu | 125 | 0/0 | 0/0 |
|
| Kochender- fer,21 2010 | CD19 | FL (1) | CD3.28z | γRv | Flu/Cy | 125/120 (mg/kg) | 100/0 | NR/0 |
|
| Savoldo,19 2011 | CD19 | DLBCL (4) FL (1) CNS-L (1) |
CD3z (1st) + CD3.28z (2nd) | γRv | None | None | 0/0 | 0/0 |
|
| Till,47 2012 | CD20 | MCL (4) FL (1) |
CD3.4–1BBz (3rd) | Plasmid | Cy | 1000 | 0/0 | 33/0 |
|
| Kochender- fer,22 2012 | CD19 | FL (4) MZL (1) CLL (4) |
CD3.28z | γRv | Flu/Cy | 125/120 (mg/kg) | 87/12 | 75/37 |
|
| Kochender- fer,23 2015 | CD19 | DLBCL (9) CLL (4) FL (1) SMZL (1) |
CD3.28z | γRv | Flu/Cy | 125/60–120 (mg/kg) | 89/56 | 84/40 |
|
| Turtle,25 2016 | CD19 | MCL (4) FL (6) DLBCL (22) |
CD3.4–1BBz | Lenti-v | Cy Cy/E Flu/Cy |
4000 4000/200 125/60 (mg/kg) |
72/50 | 12.5/28 |
|
| Ramos,45 2016 | K | DLBCL (4) LPL (2) CLL (2) MCL (1) | CD3.28z | γRv | Cy in selected cases | 1000 | 33/22 | None/ none |
|
| Kochender- fer,24 2017 | CD19 | DLBCL (19) FL (2) MCL (1) |
CD3.28z | γRv | Flu/Cy | 125/1500 | 73/55 | 62/55 |
|
| Ramos,46 2017 | CD30 | ALCL (2) HL (7) | CD3. CD28z | γRv | None | None | 33/22 | None/none |
|
| Schuster,15 2017 | CD19 | DLBCL (14) FL (14) |
CD3.4–1BBz | Lenti-v | Benda Cy/Flu Cy Cy/E Others |
180 800/80 1800 800/200 |
67/50 | 18/11 |
|
ALCL, anaplastic large cell lymphoma; AUC, area under the curve; Benda, bendamustine; CAR, chimeric antigen receptor; CLL, chronic lymphocytic leukemia; CNS-L, primary CNS lymphoma; CR, complete remission; CRS, cytokine release syndrome; CTX, lymphocyte-depleting chemotherapy; Cy, cyclophosphamide; Cy/E, cyclophosphamide/etoposide; d, day(s); DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; Flu, fludarabine; gen, generation; h, hour(s); HL, Hodgkin lymphoma; IFN-γ, interferon γ; IL, interleukin; κ, kappa; Lenti-v, lentivirus; LPL, lymphoplasmacytic lymphoma; Max, maximum; MCL, mantle cell lymphoma; mo, month(s); MZL, marginal zone lymphoma; Neuro, neurotoxicity; NR, not reported; ORR, overall response rate; γRv, gamma retrovirus; pts, patients; SMZL, splenic marginal zone lymphoma; wk, week(s); y, year(s).
Efficacy of Second-Generation CD19 CAR T Cells for Lymphomas
The best clinical responses, however, as well as the highest rates of T-cell expansion and persistence, were reported in clinical trials in which patients received some form of lymphocyte-depleting (“lymphodepleting”) chemo-therapy immediately before the infusion of CAR T cells. This concept of preconditioning is borrowed from allogeneic HSCT, in which the chemotherapy is designed to achieve 2 broad purposes beyond its cytotoxic effect: (1) limit rejection of the infused product and (2) create “space” to allow infused T cells to expand preferentially in an environment rich in homeostatic T-cell cytokines (providing “signal 3”).20
Kochenderfer and colleagues from the National Cancer Institute (NCI) delivered one of the earliest examples of the importance of providing lymphodepleting chemo-therapy in a report of a single patient with chemotherapy-refractory FL. This patient had a partial response after infusion of a second-generation CD28-containing CD19 CAR T-cell product.21 Although the patient received high-dose lymphodepletion in the form of cyclophosphamide (120 mg/kg) and fludarabine (125 mg/m2) over 5 days, the response could not be attributed to chemo-therapy alone because CAR T cells could be detected in the peripheral blood for more than 6 months that correlated with normal B-cell aplasia, indicating biological activity of the infused T cells. In addition to this patient, the clinical trial went on to enroll another 8 subjects with indolent B-cell neoplasms (3 with FL, 4 with chronic lymphocytic leukemia [CLL], and 1 with marginal zone lymphoma [MZL]), all of whom received 0.3 to 3.3 × 107 CAR-positive (CAR+) T cells per kilogram after being treated with similar conditioning therapy.22 Most patients (6 of 9) had a partial response, and 1 patient entered a long-term (>15 months) CR after treatment; however, all patients experienced significant toxicities.
The NCI has now completed 2 larger dose-finding clinical trials of the same CAR T-cell product in patients with aggressive lymphomas in which a modified lymphocyte-depleting regimen was used. In both trials, more than 50% of the treated patients—most of whom had chemo-therapy-refractory DLBCL—entered a CR, in parallel with prolonged persistence of the CAR T cells in vivo (>6 months).23,24 The investigators also determined that a T-cell dose of 2 × 106/kg after lymphodepletion with low doses of cyclophosphamide (total dose <1.5 g/m2) and fludarabine (total dose <75 mg/m2) was sufficient to facilitate a high peak expansion level and long-term persistence of the infused T cells with the most acceptable toxicity profile.
These findings are in close agreement with the reported clinical efficacy of a CD19 CAR T-cell product developed and clinically tested at the Fred Hutchinson Cancer Research Center (FHCRC).25 Although the manufacturing platform (enforcing a CD4-to-CD8 ratio of 1:1 in infused products) and the CD19 transgene construct (containing a 4–1BB costimulatory domain and a CD28 transmembrane domain) used at this center differed from those at the NCI, the response rate seen in a cohort of 32 patients with B-cell lymphomas and a similarly poor prognosis was comparable (CR rate of 50% when cytarabine/fludarabine was used for lymphodepletion along with cell doses ranging from 2 × 106/kg to 2 × 107/kg). Importantly, they identified a ceiling dose level (2 × 107/kg) at which the toxicity and death rate (>20%) from their product was unacceptably high.
More recently, the University of Pennsylvania (UPenn), which had already demonstrated the remarkable efficacy of CD19 CAR T cells in a series of landmark reports of 3 patients (1 with relapsed ALL and the other 2 with chemotherapy-resistant CLL),26,27 extended its experience with this therapy to 28 patients with relapsed and/or chemorefractory DLBCL (n=14) or FL (n=14).15,28 Impressively, the overall response rate was 67% at 6 months after infusion (71% in patients with FL and 43% in those with DLBCL) when they were treated with a single dose across a range of 3.08 × 106 to 8.87 × 106 CD19 CAR+ cells per kilogram after various lymphodepleting chemotherapy regimens. The most common of these regimens (n=8) consisted of 2 doses of bendamustine at 90 mg/m2 (other regimens used are listed in Table 1). There was a trend toward a higher level of expression of immune checkpoint markers (programmed death ligand 1 [PD-L1], TIM3, LAG3, and programmed death 1 [PD-1]) in 5 patients with DLBCL who did not respond compared with 5 who entered a CR after infusion. Of 16 patients who entered a CR, 14 had detectable circulating CD19 CAR T cells more than 6 months after infusion that lasted up to 24 months. Importantly, relapse had developed in none of the patients in CR by the time of publication, which was up to 3 years after treatment.
An emerging feature of CD19 CAR T-cell therapy is that those patients with any type of B-cell malignancy who enter a CR are likely to have long-term remissions. With these impressive clinical responses, selected CD19 CAR T-cell products that were originally developed at individual centers have now entered larger, multicenter, efficacy-focused clinical trials, which are discussed below (see section “Late-Phase Multicenter Trials”).
Toxicity of CD19 CAR T Cells
Cytokine release syndrome.
Notably, reports of striking anticancer efficacy came with cautionary tales of significant toxicity syndromes—some unexpected—associated with this form of immunotherapy. For example, all 9 patients in the initial report from the NCI experienced serious toxicities (grade ≥3), which we now know comprise a constellation of findings that has come to be known as cytokine release syndrome (CRS).22 This syndrome was also seen in concurrent clinical trials of patients with B-cell ALL, some of whom received other types of CD19 CAR T cells.12 The severity of CRS correlated with the peak expansion level of the infused CAR T cells.29 Correlative analysis of data from patients with CRS at the NCI and other centers demonstrated that the development of CRS was directly related to a rapid rise in the frequency of CAR T cells, large pretreatment tumor burden, high peak CAR T-cell expansion levels, and the upregulation of several inflammatory cytokines after infusion. These cytokines include interferon γ (IFN-γ), tumor necrosis factor α (TNF-α), IL-6, IL-15, IL-10, monocyte chemoattractant protein 1 (MCP-1), and macrophage inflammatory protein 1β (MIP-1β); of these, IL-6 has consistently been associated with CRS across multiple trials of CD19 CAR T cells.23–26
Our current understanding of CRS is that (much as in macrophage activation syndrome or hemophagocytic lymphohistiocytosis) massive, supraphysiologic T-cell expansion in response to antigenic stimulation causes macrophages and other immune cells to degranulate rapidly and secrete cytokines that result in high fever, hypotension, renal failure, and coagulopathy. To mitigate CRS, most centers have now modified the dose of T cells administered to patients, in some cases reducing the dose by 1 log from their maximum tested dose levels.25 Despite these modifications, 20% to 50% of patients treated still experience a severe form of CRS. A grading scale has been established to document the severity of symptoms and direct immunosuppressive therapy for CRS accordingly.30
In most patients, peak CRS toxicities develop by day 7 following infusion. Most cases are transient and resolve completely by 4 weeks after infusion with supportive therapy and a possible brief stay in the intensive care unit. Higher grades of CRS (grade ≥3) should trigger treatment with immunosuppressive therapy. To this end, IL-6 has emerged not only as a major biomarker of toxicity but also as a major target for the management of severe CRS. Although corticosteroids are effective in mitigating CRS, they can affect T-cell expansion and persistence. As a result, direct targeting of the IL-6 axis—which likely spares expanding T cells—has become the management of choice.
Tocilizumab (Actemra, Genentech), an IL-6 receptor antagonist, has been used successfully in clinical trials of CAR T cells to treat CRS. In most cases, a single dose is sufficient to induce a rapid (within 4 hours) decrease of symptoms. For instance, all 3 patients in whom severe CRS developed in one of the NCI reports were effectively managed with tocilizumab.24 As well as at the NCI, tocilizumab was successfully used in the single-center studies at both FHCRC and UPenn for rapid control of the symptoms of severe CRS.25,31 Tocilizumab is now FDA-approved for the treatment of severe CRS in patients with B-cell ALL receiving CD19-directed CAR T cells because of its demonstrated efficacy in controlling CRS in pivotal registration clinical trials. Therefore, the FDA has mandated that referral centers offering FDA-approved CD19 CAR T cells have at least 2 doses of tocilizumab available before any patients are infused with these products.32
In preparation for CAR T-cell infusions becoming routine practice, levels of traditional inflammatory markers (eg, C-reactive protein and ferritin, which are known to increase in CRS) and coagulation panels could be substituted for cytokine levels at centers where these measurements are not available in a timely fashion.
Neurotoxicity.
A type of neurologic toxicity, called CAR T-cell–related encephalopathy syndrome, tends to develop in patients with severe forms of CRS.33 This syndrome manifests as a spectrum of encephalopathy, aphasia, hemiparesis, seizures, and—in rare cases—fatal cerebral edema and central nervous system (CNS) hemorrhage. In most cases, symptoms develop within the first 2 weeks after T-cell infusion, usually in the background of ongoing CRS and thrombocytopenia. Most cases resolve spontaneously within 7 to 14 days after onset. Treatment with tocilizumab may be beneficial in reducing the severity of the toxicity, although this has not been seen in all settings. Correlates of neurotoxicity in initial reports mirror those of CRS, such as high peak T-cell expansion levels and a large pretreatment tumor burden. However, the key mechanisms that underlie the development of this syndrome remain to be determined.
Although many centers have demonstrated the trafficking of CD19 CAR T cells to the cerebrospinal fluid in patients with neurotoxicity, CD19 expression is consistently negative in CNS tissues.29,31 Therefore, the leading hypothesis is that a cohort of vasoactive cytokines disrupts the blood-brain barrier by promoting endothelial cell activation and microthrombus formation, causing the leakage of T cells and perhaps other toxins into the CNS. In support of this theory, Gust and colleagues demonstrated that severe CNS toxicity (grade ≥3), which occurred in more than 20% of 123 patients treated with CD19 CAR T cells at their center, correlated with the absence of high-molecular-weight von Willebrand factor multimers and low levels of platelets and ADAMSTS13 activity, indicating their consumption in the formation of microthrombi.34 These observations are supported by the results of experiments with an in vitro endothelial system exposed to several cytokines that are upregulated in patients with CRS, and by the finding of widespread microthrombi and disrupted CNS endothelium at the autopsy of a patient who died of severe neurotoxicity. Other centers using other CD19 CAR T cells have reported similar rates of CNS toxicity after CAR T-cell infusion, highlighting the need to identify strategies to prevent and effectively treat these complications.
Preventing CRS/neurotoxicity.
Because of the correlation between the dose of infused T cells and the incidence of severe toxicity syndromes, reducing the infused dose of T cells, in particular in patients who have a larger disease burden, has been proposed.35 Furthermore, in the dose-finding clinical trial performed at FHCRC, the highest dose level was considered unsafe (64% rate of neurotoxicity) when both fludarabine and cyclophosphamide were used for lymphodepletion. By contrast, no neurotoxicity was seen when only cyclophosphamide was used for lymphodepletion, albeit at the cost of lower response rates (8% CR compared with 50% when fludarabine was added), leading to the notion that the therapeutic window for the combination of lymphodepletion and T-cell doses may be narrow.25 Thus, doses of each lymphodepletion agent have now been optimized for each CAR product in the ongoing registration clinical trials. Administering tocilizumab prophylactically,36 incorporating suicide systems into the T cells,37 and achieving transient CAR expression by T cells by using mRNA transfection38 are all being tested in ongoing clinical trials as additional strategies to limit overall toxicities.
B-cell aplasia.
B-cell aplasia is one of the expected toxicities of CD19 CAR T cells because CD19 is a pan–B-cell marker. Most patients with lymphomas who have been heavily pretreated with chemoimmunotherapy already have low levels of circulating normal B cells and consequent hypogammaglobulinemia. CD19 CAR T cells, however, which persist long term, can induce and maintain severe B-cell aplasia. Residual B-cell or immunoglobulin levels can serve as surrogate markers for the persistence of biologically active CD19 CAR T cells because polyclonal B-cell recovery occurs only when CD19 CAR T cells are no longer detected in the peripheral blood. It is plausible that the hypogammaglobulinemia induced by CD19 CAR T cells is partly responsible for the high rate of infectious complications noted after CD19 CAR T-cell therapy (>30% of patients experiencing bacterial, viral, or fungal infections, according to one group),39 although many of these patients will have pre-existing hypogammaglobulinemia owing to previous therapies. The administration of intravenous immunoglobulin to those in whom severe hypogammaglobulinemia develops might prevent these infectious complications.
Biomarkers of the Efficacy of CD19 CAR T Cells
As expected, most centers have confirmed the notion that a higher T-cell expansion level and longer persistence are related to each other and to the development of durable responses. For specific CD19 CAR T-cell products, the peak CAR T-cell expansion level (98 CAR+ cells per microliter of peripheral blood for NCI, >10 CAR copies per microgram of DNA for FHCRC, peak of >11% CD3+ CD19 CAR+ cells in peripheral blood mononuclear cells for UPenn) and the pretreatment levels of cytokines such as IL-15 and IL-6 have been independently identified as correlated with a clinical response. Furthermore, specific characteristics of infused products have been identified that predict better outcomes, such as the presence of a subset of central memory cells in the infused product, which consistently induces CRs in CLL patients treated with the CAR developed at UPenn and improves in vivo persistence in NHL patients treated with a different CAR at Baylor College of Medicine.19 Similar subsets have been identified in other products (Table 1). Therefore, strategies to improve the quality of infused products are being attempted, including, for example, generating products from patients who are currently receiving ibrutinib (Imbruvica, Pharmacyclics),40 which has been shown to increase the frequencies of certain memory subsets in CD19 CAR T cells.
Improving the Efficacy of CD19 CAR T Cells for Lymphomas
Despite treatment with similar CD19 CAR products, there remain differences in responses across CD19-expressing tumor types. Some of these may be the consequence of the immune-inhibitory microenvironment present in most B-cell lymphomas, which recruits immune-suppressive cells and secretes immune-inhibitory cytokines, allowing tumor inhibition of CAR T-cell activity. For example, in one report published in abstract form, more than 33% of all patients with DLBCL who failed to respond to or had a relapse after an infusion of CD19 CAR T cells had high levels of PD-L1 expression on tumor cells.41 Moreover, 25% of the remaining nonresponders had tumors in which the expression of CD19 was downregulated. In one case report, the repeated administration of PD-1 inhibitor antibody therapy resulted in a CR after treatment with CD19 CAR T cells, when previously each treatment alone had failed to induce a response.42 Therefore, the combination of CD19 CAR T cells with immune checkpoint inhibitors is likely to enhance their efficacy and is currently being tested in at least 2 ongoing clinical trials (NCT02650999, NCT03208556).
The feasibility and effectiveness of simultaneously targeting multiple antigens to mitigate relapses in patients with CD19-negative tumors, which is now an accepted mechanism of immune escape, is also currently being tested in the clinic as a means to improve outcomes. At least for ALL, the use of CD22 CAR T cells in one clinical trial43 and of CD123 CAR T cells in a preclinical report44 was effective in eliminating CD19-negative ALL relapses after CD19 CAR T-cell therapy. In lymphomas as well, CAR T cells specific for targets other than CD19, such as kappa (κ),45 CD30,46 and CD20,47 have demonstrated safety and, in some cases, anti-lymphoma efficacy (Table 1). Thus, a CAR T-cell product specific for multiple antigens is an attractive advance for this therapy and is currently in preclinical development.
Late-Phase Multicenter Trials of CAR T Cells for Lymphomas
Axicabtagene Ciloleucel (KTE-C19)
Axicabtagene ciloleucel (Yescarta, Kite Pharma) is a centrally manufactured second-generation CD19-directed CAR T-cell product that has a CD28 costimulatory domain. The parent construct was developed and first tested in at least 3 phase 1/2 investigator-initiated trials at the NCI. With each iteration, several modifications were made to the clinical trial design, dosages, type of lymphomas targeted, and type of lymphodepleting chemotherapy administered before a pivotal clinical trial (ZUMA-1, A Phase 1–2 Multi-Center Study Evaluating Axicabtagene Ciloleucel in Subjects With Refractory Aggressive Non-Hodgkin Lymphoma) was begun.33 So far, in the latest results reported from this trial,41 108 patients with relapsed/refractory (R/R) NHL (95% with DLBCL) had received lymphodepleting chemotherapy (total fludarabine dose of 90 mg/m2 and total cyclophosphamide dose of 1500 mg/m2), followed by an infusion of up to 2 × 106 CAR T cells per kilogram. With impressive efficacy, this product met its prespecified endpoint at 3 months of an objective response rate of 82%, compared with a historical rate of 20%. More than half of the treated patients had a CR at the time of the report, and the expected rates of CRS (93% any grade, 13% grade ≥3) and neurotoxicity (64% any grade, 28% grade ≥3) identified in earlier-phase clinical trials were replicated. Of the enrolled patients, 43% required tocilizumab and 27% received glucocorticoids to manage the toxicities. Again, as predicted from early-phase studies, higher peak CAR expansion levels and persistence were associated with both response and the development of toxicities (area under the curve for responders 5.4 times that for nonresponders). This product is now licensed by the FDA for the treatment of “relapsed or refractory large B-cell lymphoma after 2 or more lines of systemic therapy, including DLBCL not otherwise specified, primary mediastinal large B-cell lymphoma, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma” and is thus commercially available to patients as standard of care.
Tisagenlecleucel (CTL019)
Tisagenlecleucel (Kymriah, Novartis) was the first gene-modified T-cell immunotherapy to receive FDA approval, although only for the treatment of B-cell ALL. This product is currently being tested in a registration trial (JULIET, Study of Efficacy and Safety of CTL019 in Adult DLBCL Patients) for the treatment of B-cell NHL.28 It was developed at UPenn and first clinically tested in patients with CLL. In the ongoing pivotal trial, 147 patients with chemotherapy-refractory DLBCL have been enrolled, and 99 of them have been treated with variable lymphodepletion regimens (commonly cyclophosphamide at 0.75 g/m2 and fludarabine at 75 mg/m2 in 3 divided doses, or bendamustine at 90 mg/m2 for 2 doses) plus an infusion of CD19 CAR T cells.15 The reported durable CR rate at 6 months is approximately 30% in all those patients who received an infusion, matching closely the outcomes of both axicabtagene ciloleucel and lisocabtagene maraleucel. The rates of neurotoxicity (12%) are lower than those in early-phase trials, possibly because of optimization of the doses of T cells and lymphodepletion agents used. With these findings, tisagenlecleucel is anticipated to meet its primary endpoint and to be licensed by the FDA in 2018.
Lisocabtagene Maraleucel (JCAR017)
Lisocabtagene maraleucel is the commercial CD19 CAR T cell originally developed at FHCRC. The biggest difference in the manufacturing process compared with the manufacture of the other commercial products is that the CD4 and CD8 subsets are transduced independently to express a CD19 CAR with a 4–1BB costimulatory domain; they are then normalized to a 1:1 ratio before administration to the patient. The pivotal study was preceded by a multicenter dose-finding feasibility study (TRANSCEND-NHL-001, Study Evaluating the Safety and Pharmacokinetics of JCAR017 in B-cell Non-Hodgkin Lymphoma) in which 74 patients (69 with DLBCL) who had had a relapse after a median of 3 lines of prior therapy were enrolled and treated with either of 2 flat dose levels of CAR+ cells: 0.5 × 108 or 1 × 108 cells.14 Impressively, the toxicity rates were lower than expected, with grade 3 or higher CRS limited to 1% of the patients and severe neurotoxicity occurring in 15% of the treated patients. At 6 months after infusion, 37% remained in CR, including 1 patient with CNS lymphoma. With precise dosing ensured, the investigators believe that a fixed ratio of CD4+ and CD8+ CAR T cells limits variability in the in vivo biological effects of the CAR T cells and the associated toxicities. The lower rates of toxicity reported in this trial could be related to the more gradual expansion of T cells, with peak expansion occurring at approximately 15 days vs 5 to 7 days in earlier-phase studies of the same product. The pivotal registration phase of this trial has now accrued 67 patients, and the responses mirror those of the feasibility phase (Table 2).
Table 2.
Multicenter Clinical Trials of CD19-CAR T Cells for Lymphomas
| Study | Type | N | Construct | Vector | CTX | Total Dose, mg/m2 | Max CellDose | ORR/CRb, % | DOR, mo | CRS/Neuro Grade ≥3, % | Correlative Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Neeiapua,132017 | DLBCL (96) TFL (5) |
101 | CD3.28z | γRv | Flu/Cy | 90/1500 | 2 × 106/kg | 82/40 | 8.2 | 12/31 |
|
| Schustera,15 2017 | DLBCL (99) | 99 | CD3.4–1BBz | Lenti-v | Various | Variable | 0.6–6 × 108 | 53/30 | NR | 23/12 |
|
| Abramsona,14 2017 | DLBCL (67) | 67 | CD3.4–1BBz | Lenti-v | Flu/Cy | 90/900 | 0.5–1 × 108 | 80/42 | NR | 1/15 |
|
Published, or data presented in abstract form.
CR rate at 6 months after infusion.
AE, adverse event; CAR, chimeric antigen receptor; CR, complete response; day(s); CRS, cytokine release syndrome; CTX, lymphocyte-depleting chemotherapy; Cy, cyclophosphamide; d, day(s); DLBCL, diffuse large B-cell lymphoma; DOR, duration of response; Flu, fludarabine; Lenti-v, lentivirus; mo, month(s); Neuro, neurotoxicity; NR, not reached at last follow-up; ORR, overall response rate; PFS, progression-free survival; pts, patients; PR: partial remission, γRv, gamma retrovirus; TFL, transformed follicular lymphoma; y, year(s).
Chimeric Antigen Receptors for T-Cell Lymphomas
With the emerging success of anti-CD19 CAR T cells in treating B-cell malignancies, there is now an intense interest in expanding this therapeutic modality to the treatment of T-cell malignancies, such as peripheral T-cell lymphomas (PTCLs). The PTCLs are a heterogeneous group of lymphoid malignancies that make up 10% to 15% of NHLs and tend to behave aggressively.48–50 The prognosis for patients with T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) or a PTCL remains poor compared with that of patients with similarly aggressive B-cell malignancies.50 Unfortunately, the development of novel targeted agents (eg, monoclonal antibodies, bispecific T-cell engagers, CAR T-cell therapy) for the treatment of T-cell malignancies has lagged behind that of the agents developed for B-cell malignancies. Despite the success seen with CD19 CAR T cells for B-cell malignancies, targeting T-cell malignancies with CAR T cells has proved more challenging owing to shared antigen expression between normal and malignant T cells. Although CD19 is a pan–B-cell marker, the resultant depletion of normal B cells as a consequence of CD19 CAR T-cell therapy is considered an acceptable and treatable side effect.23 However, the major concern with the loss of normal T cells is the subsequent profound immunodeficiency due to T-cell aplasia, which is not easily corrected.
Several commonly recognized leukocyte differentiation markers have been identified on T lymphocytes, including CD2, CD3, CD4, CD5, CD7, CD8, and the T-cell receptor.48,51 The currently available data on the use of CAR T cells in T-cell malignancies come from preclinical studies targeting various several of the previously noted antigens. CD5 is a surface marker expressed by approximately 80% of T-cell ALLs and PTCLs, in addition to normal T lymphocytes and a small subpopulation of normal B lymphocytes.52 Mamonkin and colleagues created a second-generation CD5 CAR T-cell construct that led to transient in vitro fratricide, which was likely limited by downregulation of the CD5 protein from the cell surface of CD5 CAR T cells, followed by normal expansion.52 The CAR T cells had significant antitumor activity against CD5+ T-cell lines in vitro. The CD5-specific CAR T cell was also able to recognize and eliminate malignant T cells in vivo in 2 different murine models of T-cell ALL, with minimal reduction in the number of normal human T cells. A phase 1 trial of autologous T cells expressing a second-generation CAR for the treatment of CD5+ T-cell malignancies was recently registered (NCT03081910) and opened for enrollment at the end of 2017.
CD7 is a transmembrane protein on T cells and natural killer cells that is expressed in most T-cell leukemias/lymphomas and a subset of PTCLs.53 The same authors were able to produce a CD7-knockout CD7 CAR T cell by using clustered, regularly interspaced, short palindromic repeats (CRISPR)/Cas9 genome editing to prevent CAR T-cell fratricide.54 The CD7 knockdown did not appear to affect the function of the CD7 CAR T cells significantly; they were able to expand normally, exhibited cytotoxicity against malignant T-cell lines, and were able to control the progression of T-ALL in a xenograft mouse model. These data demonstrate that CD7 might reasonably be a targetable antigen in T-cell malignancies.
Other potential targets that have been identified in preclinical models include C-C chemokine receptor 4 (CCR4) and the T-cell receptor beta chain. CCR4 is a transmembrane cell surface receptor molecule that is expressed on multiple T-cell subsets, including T-regulatory cells, T-helper 2 cells, T-helper 17 cells, natural killer cells, and platelets.55–57 It is also highly expressed on the surface of several T-cell lymphomas.58,59 Mogamulizumab, a humanized monoclonal antibody against CCR4, has been approved in Japan for the treatment of R/R adult T-ALL/lymphoma.60 On the basis of the promising results seen with this agent,61,62 a group from the Lymphoid Malignancies Branch of the NCI Center for Cancer Research developed an autologous lentivirus-based CAR T cell that targets CCR4.59 The product exhibited ex vivo cytotoxicity against multiple patient-derived cell lines representing adult T-cell leukemia, cutaneous T-cell lymphoma, anaplastic large cell lymphoma, and a subset of HL with T-cell markers. To assess in vivo efficacy, the CCR4 CAR T cells were introduced into a murine xenograft model of adult T-cell leukemia, where they eradicated the tumor cells. Given the potential hematologic toxicities, specifically thrombocytopenia, the authors plan to undertake a nonhuman primate study to better evaluate the potential toxicities and efficacy of CCR4 CAR T cells before initiating clinical trials in humans.
More recently, Maciocia and colleagues showed that specifically targeting the T-cell receptor beta-chain constant domain (TRBC), type 1 or 2, could lead to the killing of T-cell malignancies exclusively expressing TRBC1 or TRBC2 while sparing an adequate number of normal T cells to maintain cellular immunity.63 In a series of experiments, they demonstrated that the normal T-cell population contains a mixture of TRBC1+ and TRBC2+ cells, whereas the populations in T-cell malignancies are restricted to one. With the use of anti-TRBC1+ CAR T cells, both in vitro and in a mouse model of T-cell leukemia, TRBC1+ normal and malignant T cells were eradicated, whereas TRBC2+ normal T cells were spared. The results of this study are encouraging, as they may provide a targeted immunotherapy for T-cell malignancies without the resultant T-cell aplasia and subsequent severe, profound immunosuppression.
Exploiting CAR T-cell technology to develop targeted therapies for T-cell malignancies would satisfy an unmet need in the treatment of these disorders, for which currently no curative options exist other than HSCT in patients who can achieve an adequate remission. The studies illustrate the potential utility of this modality in the treatment of a wide spectrum of T-cell malignancies, evidenced by the activity in various cell lines representing the many subtypes of T-cell malignancies. Patients with B-cell malignancies have greatly benefitted from immunotherapies, and if the right target is identified, similar results could be achieved in treating T-cell malignancies.
How Accessible Is CAR T-Cell Therapy to Patients With Lymphoma?
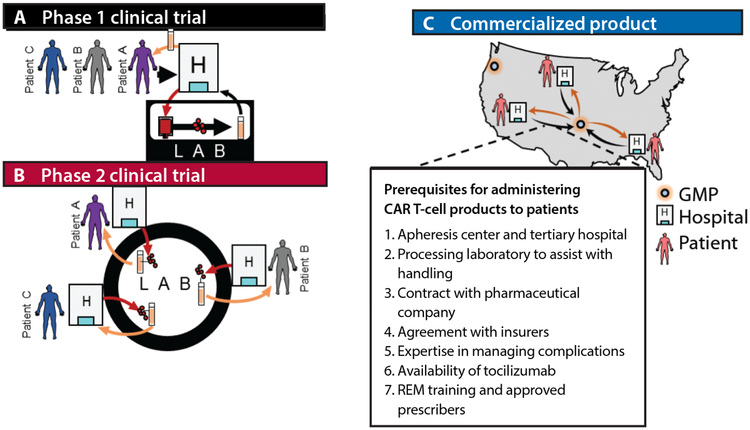
The conventional management of lymphomas uses off-the-shelf medications that can be made available rapidly at most centers with a chemotherapy pharmacy. Although at least 3 CD19 CAR T-cell products are positioned to become standard lines of therapy for B-cell lymphomas, accessibility remains a challenge. In addition to a chemotherapy pharmacy, centers must be equipped with an apheresis center and tertiary hospital facilities, and preferably have access to a cell-processing facility. Initial clinical translation of CAR T-cell products has occurred at academic centers with established HSCT clinical programs that meet these requirements (Figure, A), although centralized good manufacturing practice (GMP) facilities outside academia were necessary for the simultaneous handling of enrolled patients at multiple sites in pivotal multicenter trials (Figure, B). Yet, to date only approximately 300 patients with lymphomas have been enrolled and treated in registration clinical trials, a number dwarfed by the estimated 10,000 patients in the United States with R/R B-cell lymphomas who could benefit from treatment with CD19 CAR T cells. To meet this demand safely, commercial companies with FDA-licensed products are currently contracting for product administration sites that have established HSCT programs, with the initial “rollout,” as directed by the FDA, limited to a finite number of sites that have experience with T-cell therapies. It is evident from toxicity profiles that patient selection in the early adoption of standard-of-care CAR T-cell therapies will be critical in limiting treatment-related complications. Each “approved” site will undergo training in a risk evaluation and mitigation strategy (REM) mandated by the FDA, will have in place a contracting process with an apheresis center and a local cell-processing facility, and will implement an established ordering process limited to selected prescribers in each institution. As with HSCT, contracts with health insurance companies based on unique coding for T-cell therapies may also have to be established before the product can be ordered (preauthorization). Thus, initially, access to standard-of-care CAR T-cell therapies will be limited to referral centers that are contracted with the manufacturing pharmaceutical companies (Figure, C). Nonetheless, the eventual goal in the coming years is to expand access to a large number of sites across the country.
Figure.
Evolution of patient access to CAR T cells.
CAR, chimeric antigen receptor; FDA, US Food and Drug Administration; REM, risk evaluation and mitigation strategy.
A, Early-phase, investigator-initiated trials of CAR T cells at academic centers. B, Multicenter pivotal-phase trials with a centralized manufacturing laboratory. C, Prerequisites for administering FDA-approved CAR T-cell products to patients.
Footnotes
Disclosures
Drs Lulla and Hill have no relevant disclosures. Dr Ramos has served as an advisory board member for Novartis and Celgene, and Dr Heslop has served as an advisory board member for Novartis and received research support from Cell Medica.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. (eds). SEER cancer statistics review, 1975–2014. National Cancer Institute; https://seer.cancer.gov/csr/1975_2014/. Posted April 2017. Accessed March 14, 2018. [Google Scholar]
- 2.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuruvilla J The role of autologous and allogeneic stem cell transplantation in the management of indolent B-cell lymphoma. Blood. 2016;127(17):2093–2100. [DOI] [PubMed] [Google Scholar]
- 4.Arcaini L, Rossi D, Paulli M. Splenic marginal zone lymphoma: from genetics to management. Blood. 2016;127(17):2072–2081. [DOI] [PubMed] [Google Scholar]
- 5.Thieblemont C, Molina T, Davi F. Optimizing therapy for nodal marginal zone lymphoma. Blood. 2016;127(17):2064–2071. [DOI] [PubMed] [Google Scholar]
- 6.Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127(17):2055–2063. [DOI] [PubMed] [Google Scholar]
- 7.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bollard CM, Gottschalk S, Torrano V, et al. Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol. 2014;32(8):798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell. 2017;168(4):724–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadelain M, Rivière I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545(7655):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3(4):388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abramson JS, Palomba ML, Gordon LI, et al. High durable CR rates in relapsed/refractory (R/R) aggressive B-NHL treated with the CD19-directed CAR T cell product JCAR017 (TRANSCEND NHL 001): defined composition allows for dose-finding and definition of pivotal cohort [ASH abstract 581]. Blood. 2017;130(suppl 1). [Google Scholar]
- 15.Schuster SJ, Bishop MR, Tam CS, et al. Primary analysis of Juliet: a global, pivotal, phase 2 trial of CTL019 in adult patients with relapsed or refractory diffuse large B-cell lymphoma [ASH abstract 577]. Blood. 2017;130(suppl 1). [Google Scholar]
- 16.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 pt 1):6106–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112(6):2261–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen MC, Popplewell L, Cooper LJ, et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol Blood Marrow Transplant. 2010;16(9):1245–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochenderfer JN, Wilson WH, Janik JE, et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116(20):4099–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kochenderfer JN, Somerville RPT, Lu T, et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol. 2017;35(16):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor–modified T cells. Sci Transl Med. 2016;8(355):355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teachey DT, Lacey SF, Shaw PA, et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016;6(6):664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turtle CJ, Hay KA, Hanafi L-A, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35(26):3010–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bach PB, Giralt SA, Saltz LB. FDA approval of tisagenlecleucel: promise and complexities of a $475 000 cancer drug. JAMA. 2017;318(19):1861–1862. [DOI] [PubMed] [Google Scholar]
- 33.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gust J, Hay KA, Hanafi L-A, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics. 2016;3(suppl):16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Locke FL, Neelapu SS, Bartlett NL, et al. Preliminary results of prophylactic tocilizumab after axicabtagene ciloleucel (axi-cel; KTE-C19) treatment for patients with refractory, aggressive non-Hodgkin lymphoma (NHL) [ASH abstract 1547]. Blood. 2017;130(suppl 1). [Google Scholar]
- 37.Bayle JH, Duong MT, Lu A, et al. Dual-switch CAR-T cells: orthogonal molecular switches to control activation and elimination of CAR-T cells to target CD123+ cancer [ASH abstract 3184]. Blood. 2017;130(suppl 1). [Google Scholar]
- 38.Svoboda J, Rheingold SR, Gill SI, et al. Pilot study of non-viral, RNA-redirected autologous anti-CD19 chimeric antigen receptor modified T-cells in patients with refractory/relapsed Hodgkin lymphoma (HL) [ASH abstract 653]. Blood. 2017;130(suppl 1). [Google Scholar]
- 39.Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T cell immunotherapy. Blood. 2018;131(1): 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest. 2017;127(8):3052–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neelapu SS, Locke FL, Bartlett NL, et al. Long-term follow-up ZUMA-1: a pivotal trial of axicabtagene ciloleucel (axi-cel; KTE-C19) in patients with refractory aggressive non-Hodgkin lymphoma (NHL) [ASH abstract 578]. Blood. 2017;130(suppl 1). [Google Scholar]
- 42.Chong EA, Melenhorst JJ, Svoboda J, et al. Phase I/II study of pembrolizumab for progressive diffuse large B cell lymphoma after anti-CD19 directed chimeric antigen receptor modified T cell therapy [ASH abstract 4121]. Blood. 2017;130(suppl 1). [Google Scholar]
- 43.Fry TJ, Shah NN, Orentas RJ, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruella M, Barrett DM, Kenderian SS, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest. 2016;126(10):3814–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos CA, Savoldo B, Torrano V, et al. Clinical responses with T lymphocytes targeting malignancy-associated k light chains. J Clin Invest. 2016;126(7):2588–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramos CA, Ballard B, Zhang H, et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J Clin Invest. 2017;127(9):3462–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Till BG, Jensen MC, Wang J, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4–1BB domains: pilot clinical trial results. Blood. 2012;119(17):3940–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foss F Evolving therapy of peripheral T-cell lymphoma: 2010 and beyond. Ther Adv Hematol. 2011;2(3):161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El-Mallawany NK, Frazer JK, Van Vlierberghe P, et al. Pediatric T- and NK-cell lymphomas: new biologic insights and treatment strategies. Blood Cancer J. 2012;2(4):e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pui CH, Behm FG, Crist WM. Clinical and biologic relevance of immunologic marker studies in childhood acute lymphoblastic leukemia. Blood. 1993;82(2):343–362. [PubMed] [Google Scholar]
- 52.Mamonkin M, Rouce RH, Tashiro H, Brenner MK. A T-cell-directed chimeric antigen receptor for the selective treatment of T-cell malignancies. Blood. 2015;126(8):983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campana D, van Dongen JJ, Mehta A, et al. Stages of T-cell receptor protein expression in T-cell acute lymphoblastic leukemia. Blood. 1991;77(7):1546–1554. [PubMed] [Google Scholar]
- 54.Gomes-Silva D, Srinivasan M, Sharma S, et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. 2017;130(3): 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Solari R, Pease JE. Targeting chemokine receptors in disease – a case study of CCR4. Eur J Pharmacol. 2015;763(pt B):169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishida T, Inagaki H, Utsunomiya A, et al. CXC chemokine receptor 3 and CC chemokine receptor 4 expression in T-cell and NK-cell lymphomas with special reference to clinicopathological significance for peripheral T-cell lymphoma, unspecified. Clin Cancer Res. 2004;10(16):5494–5500. [DOI] [PubMed] [Google Scholar]
- 57.Ishida T, Utsunomiya A, Iida S, et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res. 2003;9(10 Pt 1):3625–3634. [PubMed] [Google Scholar]
- 58.Ishii T, Ishida T, Utsunomiya A, et al. Defucosylated humanized anti-CCR4 monoclonal antibody KW-0761 as a novel immunotherapeutic agent for adult T-cell leukemia/lymphoma. Clin Cancer Res. 2010;16(5):1520–1531. [DOI] [PubMed] [Google Scholar]
- 59.Perera LP, Zhang M, Nakagawa M, et al. Chimeric antigen receptor modified T cells that target chemokine receptor CCR4 as a therapeutic modality for T-cell malignancies. Am J Hematol. 2017;92(9):892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subramaniam JM, Whiteside G, McKeage K, Croxtall JC. Mogamulizumab: first global approval. Drugs. 2012;72(9):1293–1298. [DOI] [PubMed] [Google Scholar]
- 61.Ogura M, Ishida T, Hatake K, et al. Multicenter phase II study of mogamulizumab (KW-0761), a defucosylated anti-cc chemokine receptor 4 antibody, in patients with relapsed peripheral T-cell lymphoma and cutaneous T-cell lymphoma. J Clin Oncol. 2014;32(11):1157–1163. [DOI] [PubMed] [Google Scholar]
- 62.Duvic M, Pinter-Brown LC, Foss FM, et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood. 2015;125(12):1883–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maciocia PM, Wawrzyniecka PA, Philip B, et al. Targeting the T cell receptor β-chain constant region for immunotherapy of T cell malignancies. Nat Med. 2017;23(12):1416–1423. [DOI] [PubMed] [Google Scholar]